Chapter 119 Chiari Malformations and Syringomyelia
History
John Cleland (1835–1925) was among the first to describe hindbrain herniation in a patient with myelodysplasia.1–3 In 1883, Cleland published “Contribution to the study of spina bifida, encephalocele and anencephalus,” which included an illustration and a description of patients with hindbrain herniation, in the Journal of Anatomy and Physiology. Julius Arnold (1835–1915) also discussed a patient with hindbrain herniation and myelodysplasia in 1894.1,4–7 In 1891 and 1896, Hans Chiari (1851–1916) provided descriptions of hindbrain herniation in postmortem specimens in which variations I through IV were described.5,8 Because Chiari performed the majority of the early work describing the condition, his name remains associated with the condition today.
The surgical treatment of Chiari malformations began with the technique described by Penfield and Coburn, who performed a posterior fossa decompression. In the case they described, the patient died, and subsequent early surgeries had a high rate of complications and death.9 Gardner et al.10–15 noted five deaths among 74 patients undergoing posterior fossa decompression. Williams16–18 reported 5 deaths in 41 cases and also described increased neurologic deficits, increased ventricular size, and arachnoiditis in his patients. Despite these inauspicious beginnings, modern surgical techniques make Chiari surgery much safer, with the chance of a serious, irreversible injury less than 2%.
Pathophysiology
The various types of Chiari malformations are difficult to explain with a unifying theory that accounts for all the cerebral and spinal anomalies and addresses the occurrence of hindbrain herniation. Instead, the various Chiari types likely result from different causative factors but share similar radiographic and symptomatic expression. It remains unclear whether defects are a result of embryonic missteps or of other pathologic processes. While clear associations can be made with Chiari II, myelomeningocele, and folate deficiency, few other clear associations can be garnered. Few studies have demonstrated a genetic predisposition to the condition.19
Although causation remains unclear, mechanistic theories abound to describe Chiari I and II malformations and the common finding of syringomyelia. Gardner11,12 posited the hydrodynamic theory, which attributes the development of hydromyelia to a “water hammer” effect caused by blockage of the foramen of Magendie whereby cerebrospinal fluid (CSF) transits the potential space of the central canal in the spinal cord, causing slow progressive dilation. Oldfield20,21 suggested that the downward pistoning of the cerebellar tonsils seen on cine-mode MRI (cine-MRI) forces CSF via perivascular and interstitial spaces into the spinal cord, resulting in a syrinx. Regarding Chiari II, McLone et al.22–28 posited the unified theory that CSF loss at the myelomeningocele site reduces the volume of CSF needed to distend the developing ventricular system, leading to caudal displacement of hindbrain structures. Because Chiari types I through IV represent a spectrum of conditions with various causations, research continues in an effort to understand the exact pathophysiologic processes that lead to these malformations and associated symptoms.
Signs, Symptoms, and Imaging: Pathologic Features
Chiari I
Chiari I malformation involves caudal herniation of the cerebellar tonsils more than 5 mm below the level of the foramen magnum without brainstem descent or hydrocephalus (Table 119-1) and has been associated with numerous conditions (Box 119-1). Patients typically present with nondermatomal pain in the occipital or cervical region that is exacerbated by the Valsalva maneuver.29 Pain in younger children may manifest as irritability, crying, or failure to thrive. Sleep apnea is another common finding in younger patients. Various other symptoms are described in association with Chiari I malformation, including motor and sensory alterations, clumsiness, dysphagia, dysarthria, ataxia, and incontinence (Box 119-2). Signs on examination can include nystagmus, hyperreflexia of lower extremities, diminished upper extremity reflexes, cerebellar signs, and lower cranial nerve dysfunction, including dysarthria, palatal weakness, and decreased gag reflex. Scoliosis also can be seen in some patients, especially in the setting of an underlying spinal cord syrinx.
| Type | Description |
|---|---|
| Chiari I | >5 mm tonsillar herniation below McRae line |
| No hindbrain abnormalities | |
| Syringomyelia possible | |
| Chiari II | Herniation of brainstem, cerebellar vermis, fourth ventricle through foramen magnum |
| Associated with myelomeningocele | |
| Hydrocephalus common | |
| Syringomyelia common | |
| Chiari III | Foramen magnum/high cervical encephalocele |
| Chiari IV | Cerebellar hypoplasia or aplasia |
| Chiari 1.5 | Low brainstem and fourth ventricle |
| Caudal displacement of cerebellar tonsils | |
| No associated myelomeningocele | |
| Chiari 0 | Crowded posterior fossa without hindbrain herniation |
| Syringomyelia |
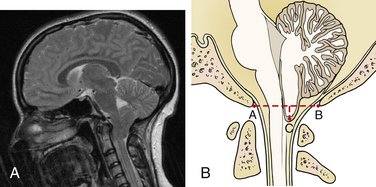
Multiple findings are demonstrated on imaging and at autopsy in patients with Chiari I malformation (Fig. 119-1). Abnormalities of the skull base and craniocervical junction, including a small posterior fossa, empty sella, platybasia, basilar impression, Klippel-Feil syndrome, and atlantoaxial assimilation, are seen in approximately 50% of patients. MRI is used to demonstrate cerebellar tonsils below the level of the foramen magnum, and cine-MRI routinely may show decreased flow posteriorly at the craniocervical junction.19,30 Imaging also may demonstrate scoliosis with a leftward convexity, in contrast to the right convexity curve usually seen in idiopathic scoliosis. The fourth ventricle can be elongated, and hydrocephalus is present in 5% to 10% of cases. The cerebellar tentorium is elevated, but other brain abnormalities common in Chiari II malformation often are absent. A spinal cord syrinx is a common feature, occurring in 50% to 75%, or even more, of patients. Syrinx formation usually is seen in the lower cervical and upper thoracic cord; however, this may vary, and holochord syrinx is possible. Volumetric analysis has demonstrated reduced posterior fossa volumes and upward of 40% CSF volumes with normal brain volume.31–33
Chiari II
Chiari II malformation is characterized by caudal herniation of the cerebellar vermis, the brainstem, and the fourth ventricle in the setting of myelomeningocele. Hydrocephalus is common in patients with this condition, along with multiple skeletal and intracranial abnormalities (Box 119-3). After closure of the myelomeningocele and the shunting that usually is necessary, patients may display symptoms of irritability or apnea as the first sign of a Chiari II malformation. Aspiration can lead to recurrent pneumonia, and problems with dysphagia and dysarthria may be evident. Hindbrain anomalies, usually secondary to respiratory insufficiency, are the leading cause of death in myelodysplastic patients. Findings on physical examination include down-beating nystagmus, quadriparesis with hypotonia, ataxia, ocular motility defects, diminished gag reflex, and stridor (see Box 119-2). Symptom onset in older children usually indicates spinal cord tethering, shunt malfunction, or the development of spinal cord syrinx.
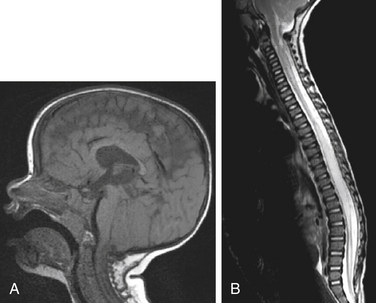
Among the imaging and autopsy findings in patients with Chiari II malformations, the brain may show complete or partial agenesis of the corpus callosum and septum pellucidum, prominent anterior commissure, polygyria, interdigitation of the occipital and parietal lobes, partial or complete agenesis of the olfactory bulb/tracts, enlargement of the massa intermedia, or fusion of the colliculi (i.e., tectal beaking). In addition, the cranial nerve nuclei can be malformed. The cerebellum is reduced in size, there can be dysplasia with absent folia, the medulla can be elongated and flattened with the classic medullary kink, and the cranial and upper cervical nerves can course upward. Luckenschadel, a beaten copper or fenestrated appearance on imaging, can characterize the skull. Scalloping of the petrous bone can occur. In these patients, the posterior fossa is small, and basilar impression/assimilation can be seen. Spine anomalies can include Klippel-Feil deformities and enlargement of the cervical canal. Hydrocephalus is a common feature, seen in upward of 90% of patients with Chiari II malformation, and the ventricles may demonstrate colpocephaly. The tentorium usually is low-lying, creating a more vertical straight sinus and low-lying torcula. In addition to the myelodysplasia, associated spinal cord abnormalities include split cord malformations and syrinx formation (Fig. 119-2).34,35
Chiari 1.5
Patients with Chiari 1.5 malformation have a condition that falls somewhere between Chiari I and Chiari II. Chiari 1.5 malformation is characterized by Chiari I–type tonsillar herniation but with elongation of the brainstem and fourth ventricle.36
Chiari 0
Conversely, Chiari 0 malformation is characterized by syrinx formation without hindbrain herniation. It is thought that dysregulation of CSF equilibrium at the craniocervical junction contributes to the Chiari 0 malformation, and that operative findings such as arachnoid veils and adhesions account for obstruction at the foramen of Magendie or crowding at the foramen magnum.37
Patient Selection and Surgical Management
Surgical Indications
Chiari I
Controversy remains regarding an exact algorithm for patient selection in regard to successful and appropriate Chiari I decompression.38,39 The initial step is to ensure that symptoms are not caused by hydrocephalus or spinal cord tethering. Evidence of hydrocephalus should lead to CSF diversion, followed by reassessment to determine whether the symptoms and imaging findings have resolved after normalization of intracranial pressure. Spinal cord tethering, while uncommon, is sought on spinal MRI. It is defined as termination of the conus below the L2-3 disc space. The filum terminale is considered abnormal if the diameter is greater than 1 mm at L5-S1.40
Once hydrocephalus and spinal cord tethering have been excluded or addressed, our evaluation begins with an assessment of the degree of tonsillar descent and presence or absence of a spinal cord syrinx. Like many of our colleagues, we consider a spinal cord syrinx an indicator of abnormal CSF dynamics consequent to the Chiari malformation. Patients with a spinal cord syrinx are offered surgical decompression unless they are completely asymptomatic and have only a small (<2 mm) syrinx. Patients in this small subset are monitored closely with serial imaging every 6 months and offered surgery if symptoms occur or the syrinx increases in size.41 In several of our asymptomatic patients who were followed conservatively, the syrinx resolved over time.42 Symptomatic patients without a syrinx pose the larger diagnostic and therapeutic challenge. Patients with more than 5 mm of tonsillar descent and clear Valsalva-induced headache or neck pain often benefit from decompression. Additional symptoms also are helpful to support the decision to offer surgery. Patients with headaches unrelated to Valsalva maneuvers who have a minimal Chiari malformation (<10 mm tonsillar descent) are the most challenging to treat and often are the least likely to benefit from operative intervention. A conservative approach often is warranted in these patients, with referral to a headache management specialist and close neurosurgical follow-up with imaging repeated yearly. Symptomatic progression or the development of a syrinx favors surgical intervention. Patients who present with more obscure neurologic findings (e.g., fussiness, head banging, nystagmus, essential hypertension, recurrent aspiration) also are a challenge, and their symptoms ideally should be correlated with radiographic findings. Surgical decompression may have a role in management of these patients, but a conservative approach often is warranted. Progressive scoliosis in the setting of a Chiari malformation is another objective finding that warrants surgical consideration.
Surgical Technique
Anesthetic and Positioning Considerations
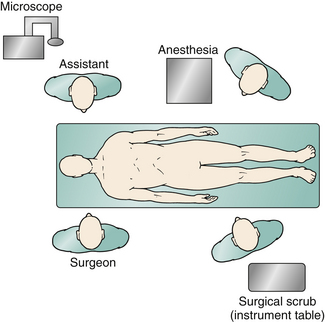
General endotracheal anesthesia is required for all patients. A percutaneous arterial catheter and an indwelling Foley catheter are placed, but central venous catheters usually are not used. Intubation is done either via fiberoptic guidance or with minimal neck extension. Total IV anesthesia typically is used to facilitate neurophysiologic monitoring. Once anesthesia is induced, the patient is connected to neuromonitoring devices to assess brainstem auditory evoked potentials, motor evoked potentials, and somatosensory evoked potentials. Intravenous antibiotics (usually cefazolin) are administered and redosed every 3 hours. Dexamethasone also is given every 4 hours and continued for 48 hours postoperatively. A lumbar drain is not used. Patients older than 5 years of age are put into three-point Mayfield pin fixation and positioned prone on gel rolls that run parallel to the long axis of the body. If the patient is younger than 5 years of age, we typically use a horseshoe headholder but pay considerable attention to avoiding pressure or contact of the eyes with the side of the horseshoe. We use additional foam padding to bolster the padding on the horseshoe. All contact points are meticulously padded, including the iliac crest in thin patients. We ensure that the axilla is free and the shoulders are neutrally positioned. The neck is carefully positioned in a slightly flexed position, ensuring at least two finger-breadths between the neck and chin. Airway pressures are a good indication to ensure that the amount of neck flexion is appropriate. It also is important to ensure that the chin is not touching the bed, because the patient can slide if the bed is in reverse Trendelenburg position. The arms are padded and placed at the patient’s side, and a drawsheet (placed prior to positioning) is then brought over the patient’s torso and secured with towel clips. Strong cloth tape and a security strap are then used to secure the patient to the operating table. After the hair is trimmed with clippers, a standard suboccipital incision is marked, starting at the inion, running along the midline, and terminating at approximately the spinous process of C2 (Fig. 119-3). The senior author (RGE) also prefers to use autologous pericranium for duraplasty, and a separate incision is marked from the inion superiorly for a length of approximately 5 cm to harvest this. Before the incision is made, the field is washed with 4% chlorhexidine soap followed by alcohol and is then dried. Chlorhexidine gluconate antiseptic (ChloraPrep, CareFusion, San Diego, CA) is used to prepare the operative field and the edge of the surrounding hair. During final preoperative positioning, the patient’s head is placed at a 90-degree angle from the anesthesiologist, with surgeons situated on either side of the patient’s head and the scrub technician standing across from the anesthesiologist; a Mayo stand is positioned over the patient’s torso. The operative microscope is positioned for use during the intradural portion of the procedure in a head-to-head configuration with the base behind the assistant (Fig. 119-4).
Exposure
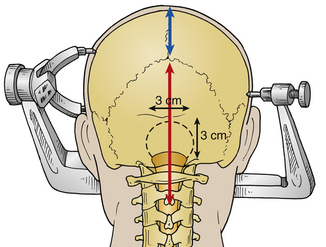
The posterior ring of C1 is then removed using a combination of instruments, including rongeurs, a Kerrison punch, and/or a high-speed drill. Straight and curved curets facilitate the dissection. The lateral extent of the bone removal is the edge of the thecal sac. The suboccipital craniectomy is performed with a high-speed pneumatic or electric drill. The type of drill bit (e.g., M8, Acorn) to be used is selected based on surgeon preference. A craniectomy of approximately 3 × 3 cm is created (see Fig. 119-4). Attention is paid to careful dissection of the dura, especially along the midline keel and along the foramen magnum, because the midline keel can be quite vascular. Any bleeding can be addressed with bone wax. Epidural bleeding is controlled with bipolar cautery or liquid Gelfoam (Pfitzer, New York, NY) mixed with thrombin (Floseal, Baxter, Deerfield, IL). A large craniectomy is to be avoided because of concern over cerebellar sag and its resultant complications in the postoperative period. Pitfalls during the bony removal include low-lying torcula, excessive bone bleeding, ectatic vertebral arteries, and occipitalization of C1. During removal of the dorsal arch of C1, the lateral internal vertebral venous plexus often is encountered. This is an epidural venous plexus located between the dura and the laminae periosteum, consisting of two or more anterior and posterior longitudinal venous channels running along multiple cervical levels. Bipolar cautery is used to control venous plexus bleeding; liquid Gelfoam also can be highly effective for this type of bleeding.
Much discussion also has revolved around the use of craniectomy without duraplasty for decompression of Chiari I malformation.43–45 In patients without a spinal cord syrinx, we will forgo duraplasty if intraoperative ultrasound demonstrates CSF cine flow dorsal to the cerebellar tonsils and the tonsils show adequate pulsatility. We believe that syringomyelia is an indication for expansion duraplasty; however, much debate remains.
Dural and Arachnoid Opening
Before dural opening, we ensure that vascular clips are readily available, because the dura can be quite vascular, especially in children. The wound is irrigated copiously to remove any bone dust, and we change our gloves prior to dural opening. Once meticulous hemostasis is obtained to prevent “drip-down,” we perform a standard Y-shaped dural opening starting at the level of C1. The dura is opened carefully with a no. 15 scalpel and a dental instrument, and the dural edges are coagulated to control venous bleeding. The incision is brought up to the midpoint of the dural exposure and then the edges of the Y are extended. A margin is kept from the bone edge to facilitate dural graft closure. Great care is taken to preserve the arachnoid and prevent blood from entering the subdural space. A combination of bipolar cautery, vascular clips, and cotton patties is used to accomplish this goal. The dura also can be oversewn to control brisk bleeding from the dural edge. The dural edges are then tented up using #4-0 Nurolon sutures (Ethicon, Cornelia, GA). A pledget of Gelfoam is placed in the subdural space over the cervical cord to prevent operative bleeding from escaping down the cervical subdural space (Fig. 119-5A). In cases in which the dura is tight along the cervical cord, we cut the inferior aspect of the dura laterally as described by Pirouzmand and Tucker46 to open the dura more widely.
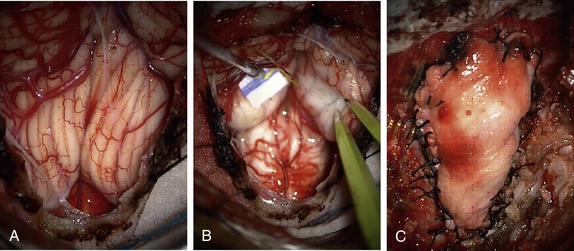
Once the dura is adequately opened and all bleeding is controlled, we irrigate the subdural space to flush away any blood or debris. The operative microscope is then brought in for the remainder of the intradural dissection. Under microsurgical visualization, we first open the arachnoid over the cerebellar tonsils. The cerebellar tonsils can have various configurations, and our goal is to first survey the anatomy. Once the cerebellar tonsils are identified, we begin to coagulate them carefully with bipolar cautery. As the tonsillar tissue begins to shrink, we look for the tip of the tonsil inferiorly. Due diligence must be taken to avoid injury or coagulation of lateral brainstem cranial nerves (especially accessory spinal nerves) and the posterior inferior cerebellar artery. Often, small arachnoid attachments must be sharply divided to allow the cerebellar tonsil to retract and elevate. The process is continued until the tonsil is above the level of the foramen magnum (Fig. 119-5B). The process is repeated for the contralateral cerebellar tonsil. We then inspect the outflow of CSF from the fourth ventricle/obex. Once we confirm adequate flow of CSF, the intradural portion of the procedure is concluded. Irrigation with warm saline is used before dural closure.
Graft Selection and Dural Closure
Many dural graft materials have been used in the long history of Chiari surgery. These include pericranium, autologous fascia lata, cadaveric dura/fascia lata, cadaveric dermis, Gore-Tex, and multiple allograft products constructed from purified bovine collagen. The senior author (RGE) prefers autologous pericranium for several reasons. Autologous tissue avoids any issues with tissue compatibility, allergies, product recalls, sterility, or remote questions of transmission of bovine illness. As previously discussed, the pericranial graft is harvested before dural opening and is soaked in bacitracin irrigation to remove any blood or debris and keep the patch hydrated. The dural closure is performed using interrupted #4-0 Nurolon sutures (Ethicon). The graft is first secured at the three corners of the opening, and then multiple interrupted sutures are placed to create a watertight closure (Fig. 119-5C). It is important not to stretch the graft, to avoid stress that can lead to suture line failure. Before the final few sutures are placed, the subdural space is flooded with saline irrigation to once again remove any debris and evacuate any air trapped under the patch. At this point, the anesthesiologist performs a Valsalva maneuver to 30 mm H2O and then 40 mm H2O to test the integrity of the watertight closure. Any areas that are observed to be leaking are oversewn. Once a watertight closure is obtained, we irrigate with bacitracin, then place a layer of Surgicel (Ethicon 360) over the suture line. Next we obtain 5 to 10 mL of the patient’s sterile peripheral blood from anesthesia and mix it with a small amount of thrombin. As the mixture starts to coagulate, the blood is put over the suture line as a blood patch. A second layer of Surgicel is placed over the patched area. If a large defect is still present, the senior author (RGE) also will use Duraseal (Covidien, Waltham, MA) to fill the defect before closing the wound. A recent multicenter trial has found that this product is safe and effective in this setting.47,48 The deep muscle and fascial layers are reapproximated with #0-0 and #2-0 Vicryl sutures, the dermis is reapproximated with #3-0 Vicryl sutures, and an absorbable suture such as Monocryl (Ethicon) is used in a running fashion for primary skin closure. Skin glue (Dermabond, Ethicon) is used as a sterile dressing directly over the absorbable suture line. We do not routinely place a lumbar drain either before or after the procedure.
Postoperative Care and Complications
Possible complications are listed in Table 119-2. Immediate postoperative complications can include major complications such as posterior fossa hemorrhage requiring emergent operative clot evacuation, stroke, cranial nerve palsy, or bacterial meningitis. Fortunately, these major complications are extremely rare. Delayed complications are more common and can include aseptic meningitis, pseudomeningocele formation, hydrocephalus, and, rarely, swan neck deformities. Aseptic meningitis is treated with 7 to 10 days of high-dose steroids, including a steroid taper. Recurrent aseptic meningitis occasionally responds to serial lumbar puncture in combination with steroid administration. Pseudomeningocele formation is evaluated first by using imaging studies to ensure that hydrocephalus is not the causal factor. Once the diagnosis of hydrocephalus has been excluded, the pseudomeningocele can be treated in one of several fashions. Initially, we manage the condition conservatively as long as the incision is healing well and the pseudomeningocele is not growing. Attempts to drain the pseudomeningocele via needle puncture and use of compression dressing meet with limited success. A trial of lumbar CSF drainage for 3 to 5 days also can be successful. If the incision is in danger of breakdown or the pseudomeningocele is growing despite these other interventions, we often undertake reexploration of the dural graft and repair it primarily.
TABLE 119-2 Prevention and Management of Surgical Complications Associated with Treatment of Chiari Malformation
| Complication | Prevention | Management |
|---|---|---|
| Vertebral artery injury | Avoid wide laminectomy and craniectomy; use gentle dissection; avoid lateral use of cautery; no pulling with rongeurs | Direct arterial repair; tamponade; interventional stenting or occlusion; careful watch for neurologic sequelae |
| Stroke | Avoid coagulation or manipulation of posterior inferior cerebellar and vertebral arteries. | Papaverine if vasospasm noted intraoperatively; postoperative stroke management |
| Hematoma | Meticulous hemostasis; avoid postoperative hypertension; careful graft and wound closure | Immediate postoperative CT, return to surgery if needed for clot evacuation; blood pressure control |
| Dural lake/bleeding | Careful preoperative identification of torcula; open dura slowly | Have hemoclips immediately available; suture dural edge to control bleeding. |
| Aseptic meningitis | Avoid blood in subdural/subarachnoid space | Obtain CSF for culture to rule out bacterial meningitis; high-dose steroid taper; possible serial lumbar punctures |
| Pseudomeningocele | Proper graft selection; meticulous graft suturing | Evaluate for hydrocephalus; tap collection; possible lumbar drain; wound exploration and revision |
| Cerebellar sag | Conservative posterior fossa decompression (3 × 3 cm) | Evaluate for craniovertebral instability, possible ventriculoperitoneal shunt; possible partial cranioplasty |
| Persistent syringomyelia | Repeat initial evaluation. | Reexploration and revision decompression; possible shunting (ventriculoperitoneal or syringosubarachnoid) |
Prognosis
Chiari I
The prognosis in patients with symptomatic Chiari I malformation who undergo a craniovertebral decompression is generally good, with approximately 90% experiencing improvement or stabilization of symptoms.49 Syringomyelia improves in most patients by the third postoperative month, with an approximately 95% likelihood of the syrinx remaining small with stable or improved symptoms. Children with neurologic deficits can recover normal function if operated on in a timely fashion. Recurrent or recalcitrant syrinx is best treated with reexploration as opposed to myelotomy and syringosubarachnoid drainage. Between 5% and 10% of patients continue to experience clinically significant symptoms despite craniovertebral decompression, and alternative treatment options must be sought. These failures often demonstrate poor CSF flow at the craniocervical junction. Regarding the controversial area of duraplasty, recent meta-analysis demonstrated no significant difference between posterior fossa decompression alone and decompression and duraplasty with respect to clinical improvement or decrease in syringomyelia; however, duraplasty was associated with a lower risk of reoperation but a greater risk of CSF-related complications.50–53 Our group still favors duraplasty in the setting of symptomatic Chiari malformation with concurrent spinal cord syrinx.
Chiari II
It is important to reiterate that definitive confirmation of a nominally functioning shunt is paramount in patients with symptomatic Chiari II malformations. Bony decompression for children under 5 years of age with symptomatic Chiari II malformation yields symptomatic improvement in about 50% of cases in our anecdotal experience. Children with profound brainstem dysfunction obviously are the least likely to benefit, but the morbid nature of the deficits makes surgical intervention a worthwhile endeavor in many cases.54–59
Summary
Chiari malformation is one of the most complex and challenging conditions to approach in neurosurgery. Variable signs, symptoms, and imaging findings make appropriate surgical candidate selection critical for successful outcomes. Appropriately selected patients can have dramatic, life-changing benefits from surgery that cannot be achieved with medical therapy or other interventions. The appropriate surgical treatment of Chiari malformations will remain an area of intense debate among neurosurgeons, especially in regard to the value of duraplasty. Importantly, the unprecedented access to digital media and information via the Internet gives patients a knowledge base not seen previously, and the treating surgeon should be prepared to answer a host of questions as families decide on treatment options.60,61
Armonda R.A., Citrin C.M., Foley K.T., Ellenbogen R.G. Quantitative cine-mode magnetic resonance imaging of Chiari I malformations: an analysis of cerebrospinal fluid dynamics. Neurosurgery. 1994;35:214-223. discussion 223–224
Chiari H. Uber Veranderungen des Kleinhirns in Folge von Hydrocephalie des Grosshirns. Dtsch Med Wschr. 1891;17:1172-1175.
Milhorat T.H., Chou M.W., Trinidad E.M., et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017.
Oldfield E.H., Muraszko K., Shawker T.H., Patronas N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
Tubbs R.S., McGirt M.J., Oakes W.J. Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg. 2003;99:291-296.
Tubbs R.S., Oakes W.J. Treatment and management of the Chiari II malformation: an evidence-based review of the literature. Childs Nerv Syst. 2004;20:375-381.
1. Carmel P.W., Markesbery W.R. Early descriptions of the Arnold-Chiari malformation. The contribution of John Cleland. J Neurosurg. 1972;37:543-547.
2. Koehler P.J. Chiari’s description of cerebellar ectopy (1891). With a summary of Cleland’s and Arnold’s contributions and some early observations on neural-tube defects. J Neurosurg. 1991;75:823-826.
3. Pearce J.M. Historical note. Arnold Chiari, or “Cruvilhier Cleland Chiari” malformation. J Neurol Neurosurg Psychiatry. 2000;68:13.
4. Bejjani G.K. Definition of the adult Chiari malformation: a brief historical overview. Neurosurg Focus. 2001;11:E1.
5. Loukas M., Noordeh N., Shoja M.M., et al. Hans Chiari (1851-1916). Childs Nerv Syst. 2008;24:407-409.
6. Schut L., Bruce D.A. The Arnold-Chiari malformation. Orthop Clin North Am. 1978;9:913-921.
7. Wilkins R.H., Brady I.A. The Arnold-Chiari malformations. Arch Neurol. 1971;25:376-379.
8. Chiari H. Uber Veranderungen des Kleinhirns in Folge von Hydrocephalie des Grosshirns. Dtsch Med Wschr. 1891;17:1172-1175.
9. Penfield W. CD: Arnold-Chiari malformation and its operative treatment. Arch Neurol Psychiatry. 1938;40:328-336.
10. Gardner E., O’Rahilly R., Prolo D. The Dandy-Walker and Arnold-Chiari malformations. Clinical, developmental, and teratological considerations. Arch Neurol. 1975;32:393-407.
11. Gardner W.J. Anatomic features common to the Arnold-Chiari and the Dandy-Walker malformations suggest a common origin. Cleve Clin Q. 1959;26:206-222.
12. Gardner W.J. Hydrodynamic factors in Dandy-Walker and Arnold-Chiari malformations. Childs Brain. 1977;3:200-212.
13. Gardner W.J. Letter: The Dandy-Walker and Arnold-Chiari malformations. Arch Neurol. 1976;33:519.
14. Gardner W.J., Abdullah A.F., McCormack C.L. The varying expressions of embryonal atresia of the fourth ventricle in adults: Arnold-Chiari malformation, Dandy-Walker syndrome, arachnoid cyst of the cerebellum, and syringomyelia. J Neurosurg. 1957;14:591-605.
15. Gardner W.J., Goodall R.J. The surgical treatment of Arnold-Chiari malformation in adults; an explanation of its mechanism and importance of encephalography in diagnosis. J Neurosurg. 1950;7:199-206.
16. Williams B. Cerebrospinal fluid pressure-gradients in spina bifida cystica, with special reference to the Arnold-Chiari malformation and aqueductal stenosis. Dev Med Child Neurol Suppl. 1975;35:138-150.
17. Williams B. Chronic herniation of the hindbrain. Ann R Coll Surg Engl. 1981;63:9-17.
18. Williams B. Progress in syringomyelia. Neurol Res. 1986;8:130-145.
19. Boyles A.L., Enterline D.S., Hammock P.H., et al. Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am J Med Genet A. 2006;140:2776-2785.
20. Oldfield E.H. Cerebellar tonsils and syringomyelia. J Neurosurg. 2002;97:1009-1010. discussion 1010
21. Oldfield E.H., Muraszko K., Shawker T.H., Patronas N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
22. McLone D.G., Chiari II. Pediatr Neurosurg. 1998;29:111.
23. McLone D.G. Hydromyelia in a child with Chiari II. Pediatr Neurosurg. 2000;32:328.
24. McLone D.G. Images in pediatric neurosurgery. Chiari I. Pediatr Neurosurg. 1998;29:279.
25. McLone D.G. Images in pediatric neurosurgery. Chiari malformations. Pediatr Neurosurg. 2000;32:164.
26. McLone D.G., Dias M.S. The Chiari II malformation: cause and impact. Childs Nerv Syst. 2003;19:540-550.
27. McLone D.G., Knepper P.A. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989;15:1-12.
28. McLone D.G., Naidich T.P. Developmental morphology of the subarachnoid space, brain vasculature, and contiguous structures, and the cause of the Chiari II malformation. AJNR Am J Neuroradiol. 1992;13:463-482.
29. Nohria V., Oakes W.J. Chiari headaches. Neurology. 1993;43:1272.
30. Armonda R.A., Citrin C.M., Foley K.T., Ellenbogen R.G. Quantitative cine-mode magnetic resonance imaging of Chiari I malformations: an analysis of cerebrospinal fluid dynamics. Neurosurgery. 1994;35:214-223. discussion 223–224
31. Ball W.S.Jr., Crone K.R. Chiari I malformation: from Dr Chiari to MR imaging. Radiology. 1995;195:602-604.
32. Milhorat T.H., Chou M.W., Trinidad E.M., et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017.
33. Tubbs R.S., Hill M., Loukas M., et al. Volumetric analysis of the posterior cranial fossa in a family with four generations of the Chiari malformation Type I. J Neurosurg Pediatr. 2008;1:21-24.
34. Jindal A., Mahapatra A.K. Split cord malformations—a clinical study of 48 cases. Indian Pediatr. 2000;37:603-607.
35. Tortori-Donati P., Rossi A., Biancheri R., Cama A. Magnetic resonance imaging of spinal dysraphism. Top Magn Reson Imaging. 2001;12:375-409.
36. Tubbs R.S., Iskandar B.J., Bartolucci A.A., Oakes W.J. A critical analysis of the Chiari 1.5 malformation. J Neurosurg. 2004;101:179-183.
37. Iskandar B.J., Hedlund G.L., Grabb P.A., Oakes W.J. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg. 1998;89:212-216.
38. Ellenbogen R.G., Armonda R.A., Shaw D.W., Winn H.R. Toward a rational treatment of Chiari I malformation and syringomyelia. Neurosurg Focus. 2000;8:E6.
39. Muraszko K.M., Ellenbogen R.G., Mapstone T.B. Controversies in the surgical management of Chiari I malformations: what is the surgical procedure of choice? To open dura or not to open dura? Clin Neurosurg. 2004;51:241-247.
40. Barkovick A.J. Pediatric neuroimaging, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
41. Lipson A.C., Ellenbogen R.G., Avellino A.M. Radiographic formation and progression of cervical syringomyelia in a child with untreated Chiari I malformation. Pediatr Neurosurg. 2008;44:221-223.
42. Avellino A.M., Britz G.W., McDowell J.R., et al. Spontaneous resolution of a cervicothoracic syrinx in a child. Case report and review of the literature. Pediatr Neurosurg. 1999;30:43-46.
43. James H.E., Brant A. Treatment of the Chiari malformation with bone decompression without durotomy in children and young adults. Childs Nerv Syst. 2002;18:202-206.
44. Krieger M.D., McComb J.G., Levy M.L. Toward a simpler surgical management of Chiari I malformation in a pediatric population. Pediatr Neurosurg. 1999;30:113-121.
45. Kunert P., Janowski M., Zakrzewska A., Marchel A. Comparision of results between two different techniques of cranio-cervical decompression in patients with Chiari I malformation. Neurol Neurochir Pol. 2009;43:337-345.
46. Pirouzmand F., Tucker W.S. A modification of the classic technique for expansion duraplasty of the posterior fossa. Neurosurgery. 2007;60:ONS60-ONS62. discussion ONS62
47. Kim K.D., Wright N.M. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine (Phila Pa 1976). 2011;36(23):1906-1912.
48. Weinstein J.S., Liu K.C., Delashaw J.B.Jr., et al. The safety and effectiveness of a dural sealant system for use with nonautologous duraplasty materials. J Neurosurg. 2010;112(2):428-433.
49. Tubbs R.S., McGirt M.J., Oakes W.J. Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg. 2003;99:291-296.
50. Chauvet D., Carpentier A., George B. Dura splitting decompression in Chiari type 1 malformation: clinical experience and radiological findings. Neurosurg Rev. 2009;32:465-470.
51. Durham S.R., Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr. 2008;2:42-49.
52. Prat R., Galeano I. Pain improvement in patients with syringomyelia and Chiari I malformation treated with suboccipital decompression and tonsillar coagulation. J Clin Neurosci. 2009;16:531-534.
53. Zamel K., Galloway G., Kosnik E.J., et al. Intraoperative neurophysiologic monitoring in 80 patients with Chiari I malformation: role of duraplasty. J Clin Neurophysiol. 2009;26:70-75.
54. Charney E.B., Rorke L.B., Sutton L.N., Schut L. Management of Chiari II complications in infants with myelomeningocele. J Pediatr. 1987;111:364-371.
55. La Marca F., Herman M., Grant J.A., McLone D.G. Presentation and management of hydromyelia in children with Chiari type-II malformation. Pediatr Neurosurg. 1997;26:57-67.
56. Narayan P., Mapstone T.B., Tubbs R.S., et al. Clinical significance of cervicomedullary deformity in Chiari II malformation. Pediatr Neurosurg. 2001;35:140-144.
57. Teo C., Parker E.C., Aureli S., Boop F.A. The Chiari II malformation: a surgical series. Pediatr Neurosurg. 1997;27:223-229.
58. Tubbs R.S., Oakes W.J. Treatment and management of the Chiari II malformation: an evidence-based review of the literature. Childs Nerv Syst. 2004;20:375-381.
59. Yumer M.H., Nachev S.S., Dzhendov T.Y., Kalev O.K. Chiari type II malformation: a case report and review of literature. Folia Med (Plovdiv). 2006;48:55-59.
60. Aldana P.R., Steinbok P. Prioritizing neurosurgical education for pediatricians: results of a survey of pediatric neurosurgeons. J Neurosurg Pediatr. 2009;4:309-316.
61. Garland E.M., Robertson D. Chiari I malformation as a cause of orthostatic intolerance symptoms: a media myth? Am J Med. 2001;111(7):546-552.