Chapter 8
Chest Pain (Case 4)
Arzhang Fallahi MD and Michael Kim MD
Case: A 54-year-old man with a history of smoking, hypertension, and hyperlipidemia comes to the emergency department complaining of chest pain. He has had hypertension for 20 years but is poorly compliant with his antihypertensive regimen. He complains of chest pain while exercising and can make it up only two flights of stairs before having to rest. The day of presentation, he was walking up the stairs when he noticed a sudden onset of chest pressure radiating down the left arm associated with diaphoresis, shortness of breath, and nausea. The patient forgot to bring in his medications but says he is not taking what he was given, which was hydrochlorothiazide 25 mg daily, atorvastatin 20 mg daily, and aspirin 81 mg daily. He has a family history of hypertension, and his father died at the age of 45 from a myocardial infarction (MI). On examination he is a diaphoretic man weighing 150 kg, his pulse is 110 beats per minute (bpm), and his blood pressure (BP) is 170/95 mm Hg. His exam is notable for elevated neck veins, coarse breath sounds bilaterally, an S3 gallop, and 1+ edema in his lower extremities.
Differential Diagnosis*
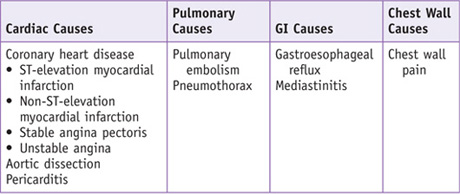
*The differential diagnosis of chest pain is quite broad; this box is limited to the causes elucidated in the Clinical Entities section.
The assessment when approaching a patient with chest pain is as follows:
PATIENT CARE
Clinical Thinking
• Each of the three subtypes of ACS has a different therapeutic management algorithm. If considering an invasive strategy, prompt communication with the cardiology team and catheterization laboratory is in order. Early initial assessment of the patient is critical to provide timely care regardless of an invasive or conservative approach.
History
The history should be focused on risk stratification for cardiac disease and nature of the chest pain to classify as cardiac or noncardiac. In an acute setting, the history must be focused to rule out the most life-threatening conditions.
Physical Examination
• Respiratory exam: Make sure the trachea is in the midline to rule out a tension pneumothorax, listen to breath sounds bilaterally to make sure they are symmetric and to listen for any signs of fluid accumulation either from pulmonary and/or cardiac dysfunction or from other disease. Percussion may help identify areas of consolidation.
• Vascular auscultation (carotid bruits): to assess atherosclerotic status.
Tests for Consideration
| Clinical Entities | Medical Knowledge |
|
ST-Elevation Myocardial Infarction (STEMI) |
|
|
Pφ |
Acute coronary syndrome is often due to rupture of plaques with less than 50% stenosis. Atherosclerotic plaques are typically asymptomatic until exceeding 70% to 80% stenosis. This obstructive lesion can lead to critical reduction in blood flow to the myocardium, which results in typical angina. In patients with STEMI, plaque rupture results in thrombus formation that is typically occlusive as compared to that in NSTEMI, which tends to be nonocclusive. Thrombogenesis is mainly initiated by tissue factor, which is expressed by monocytes, macrophages, endothelial cells, and smooth muscle cells. Tissue factor binds to activated factor VII. This complex then activates factors X and IX, which results in thrombosis. Within the atherosclerotic plaque, apoptotic cell death results in shedding of membrane microparticles that account for nearly all the tissue factor activity within the plaque itself. Instability of plaques is not completely understood, but inflammation and accelerated breakdown of collagen and matrix components are thought to have a role in weakening the fibrous caps of plaques. Inflammation is present at the site of plaque rupture where activated monocytes and macrophages are present. Infiltration by activated neutrophils also has a role in inflammation. Macrophages contribute to plaque instability by releasing metalloproteinases, which further destabilize the fibrous cap. This results in hemorrhage from the vasa vasorum or from the lumen of the artery. Macrophages also release tissue factor, which may initiate thrombus formation. Once the artery is occluded, the myocardium no longer receives blood flow, resulting in hypoxic cell injury resulting in release of cardiac enzymes. |
|
TP |
Classically the patient presents with substernal chest pain or pressure radiating to the neck and left arm, with associated shortness of breath, diaphoresis, and nausea. While this is the classic presentation, there is variability in presentation, particularly in women. |
|
Dx |
STEMI is defined as ST-segment elevation with serum cardiac biomarker elevation. On ECG, acute ST-elevation MI is defined as elevation at the J-point in two contiguous leads with cutoff points: ≥0.2 mV in men or ≥0.15 mV in women in leads V2–V3 and/or ≥0.1 mV in other leads. Initial lab tests should include complete blood count with platelet count, prothrombin time and international normalized ratio (INR), activated partial thromboplastin time (aPTT), chemistry panel, blood glucose, and serum lipid profile. |
|
Management of STEMI includes rapid triage. Oxygen administration and aspirin (162–325 mg) chewed should be given along with sublingual nitroglycerin every 5 minutes for three doses. Morphine can be used to help with pain. Rapid ECG and lab tests should be obtained. If ST elevation or new left bundle branch block is seen, a β-blocker can be given unless the patient has a contraindication, such as bradycardia, or if the patient is thought to be at high risk of cardiogenic shock. IV nitroglycerin can be given for persistent chest pain but should be given cautiously in the setting of hypotension. If patients are going to receive a primary percutaneous intervention, clopidogrel, at a loading dose of 600 mg, should be given. If the patient is going to have thrombolysis and is less than 75 years of age or if no reperfusion therapy is given, he or she can be given a 300-mg loading dose of clopidogrel. A glycoprotein IIb/IIIa inhibitor can be given if the patient is going for percutanous coronary intervention (PCI). The goal door-to-balloon time for primary PCI is less than 90 minutes, and a goal of 30 minutes in patients treated with thrombolysis. A TIMI risk score can help predict 30-day mortality post MI. A score of 0 to 2 is low risk, a score of 3 to 4 is intermediate risk, and a score of 5 to 7 is high risk. See Cecil Essentials 9. |
|
|
Non-ST Elevation Myocardial Infarction (NSTEMI) |
|
|
Pφ |
Pathophysiology is similar to STEMI, but most lesions tend to be nonocclusive. |
|
TP |
Similar presentation to STEMI. |
|
Dx |
NSTEMI often shows ST-segment depression (defined for acute MI as new horizontal or down-sloping ST depression ≥0.05 mV in two contiguous leads; and/or T inversion ≥0.1 mV in two contiguous leads with prominent R-wave or R/S ratio >1. Patients also must exhibit elevation of cardiac enzymes to signify myocardial damage. |
|
Tx |
Treatment strategy is very similar to that in STEMI. Oxygen administration along with aspirin and clopidogrel (dosed by whether patient is going to catheterization and age) should be given. Patients without contraindications, such as hemodynamic compromise or bradycardia, should be given β-blockers. In most patients, anticoagulation with agents such as heparin or fondaparinux can be given but must be weighed against risk of bleeding. See Cecil Essentials 9. |
|
Pφ |
Pathophysiology is similar to STEMI, but most lesions tend to be nonocclusive. |
|
TP |
Unstable angina may present as rest angina, new-onset angina that significantly limits physical activity, or increasing angina that is greater in duration or occurs with less exertion than previous angina. |
|
Dx |
Similar to NSTEMI, with the main difference between NSTEMI and unstable angina being that in unstable angina ischemic symptoms are present but are not severe enough to cause leakage of cardiac enzymes. |
|
Tx |
Treatment strategy is the same as for NSTEMI, since initially cardiac enzymes may not be elevated for several hours. See Cecil Essentials 9. |
|
Aortic Dissection |
|
|
Pφ |
A tear forms in the aortic intima. Blood passes through the tear, forming a false lumen. The tear may propagate, involving branches of the aorta, leading to ischemic injury. The tear is classified as type A if it involves the ascending aorta (regardless of origin), and all others are regarded as type B dissections. |
|
TP |
Patients classically describe an abrupt onset of tearing posterior chest pain. |
|
Dx |
After the patient is stabilized medically, imaging may be used to diagnose the condition. Chest radiography can show widening of the aorta. However, CT angiogram is becoming the study of choice for assessing aortic dissection, as it is most readily available. Transesophageal echocardiogram (TEE) is useful for patients who are clinically unstable. MRI may also be used but is most often not as readily available as CT in an emergency room setting. |
|
Tx |
Patients with an uncomplicated type B dissection can be treated medically with oral β-blockers or a combination antihypertensive therapy to maintain a goal BP of <120/80 mm Hg. Patients with a type A dissection should be treated as a surgical emergency, since they are at high risk for life-threatening complications such as aortic regurgitation, cardiac tamponade, and MI. See Cecil Essentials 13. |
|
Pφ |
Pericarditis involves disease of the pericardium, which can be the result of viral infection, tuberculosis, radiation injury, myocardial infarction, cardiac surgery, trauma, drugs and toxins, uremia, hypothyroidism, malignancy, and collagen vascular diseases. |
|
TP |
Patients typically present with chest pain that is of a fairly sudden onset. Pain is located classically over the anterior chest and is described as sharp and made worse by inspiration. Pain is often decreased when the patient sits up and leans forward. Pain can radiate to the trapezius ridges. |
|
Dx |
Patients may have a pericardial rub on physical exam, which may be because of friction generated by the inflamed layers of pericardium rubbing on one another. However, one may also hear a rub even with large pericardial effusions. ECG shows diffuse new ST elevations and PR depressions. |
|
Tx |
The underlying cause must be addressed. Treatment involves relief of pain and inflammation. Aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) can be administered. In patients refractory to NSAIDs, steroids may be considered. If patients have significant hemodynamic compromise from a pericardial effusion, pericardiocentesis can be done. See Cecil Essentials 11. |
|
Pulmonary Embolism (PE) |
|
|
Pφ |
Most thrombi arise from the deep venous system of the lower extremities but can originate in the pelvic, renal, upper extremity veins, or the right heart. Emboli travel to the lung and may lodge at the bifurcation of the main pulmonary artery or lobar branches causing hemodynamic compromise. |
|
TP |
Symptoms include dyspnea at rest or with exertion (usually occurs acutely), pleuritic chest pain, cough, orthopnea, calf or thigh pain or swelling, and wheezing. |
|
Since many of the symptoms of a pulmonary embolism overlap with those of acute coronary syndromes, similar tests are performed to distinguish the two. ECG can be suggestive of PE; the most common ECG finding is sinus tachycardia. There may also be evidence of right ventricular strain: S wave in lead I, Q wave in lead III, and T-wave inversion in lead III. Chest radiograph is of limited use due to the nonspecific nature of findings. Imaging with ventilation/perfusion (V/Q) scan, which looks at ventilation to perfusion, as well as CT angiography, is typically diagnostic, particularly when patients have a high pretest probability based on risk factors for PE. It should be noted that a V/Q scan is of limited use in patients with severe pulmonary disease. Because of the easy availability of CT scans, this modality is very commonly used. Lower extremity ultrasound may be useful for patients suspected of having PE, since treatment for both conditions is similar. Patients at low probability for a PE may have a D-dimer test, which is very sensitive for PE and is good for ruling it out if negative. However, many comorbid conditions may elevate D-dimer, resulting in a diagnostic and management dilemma, which often results in the need for further imaging. |
|
|
Tx |
The mainstay management of pulmonary embolism is anticoagulation. For those in whom anticoagulation is contraindicated (such as patients with a gastrointestinal hemorrhage), an inferior vena cava (IVC) filter may be used. Patients with significant hemodynamic compromise may be candidates for thrombolysis, which is associated with an increased risk of hemorrhage. See Cecil Essentials 19. |
|
Pneumothorax |
|
|
Pφ |
Air around the pleural space. Causes are classically secondary to trauma or rupture of subpleural blebs. |
|
TP |
Classically patients complain of chest pain on the affected side as well as dyspnea. Patients may also exhibit decreased chest wall excursion on the affected side as well as diminished breath sounds. In a large or tension pneumothorax, patients may have tracheal deviation away from the affected side. |
|
Dx |
Diagnosis is made on clinical grounds a well as imaging showing air around the pleural space. Chest radiograph is often sufficient, but in severe pulmonary disease, chest CT may be needed. |
|
Tx |
Management initially involves removal of air around the pleural space via a chest tube. However, those patients with a small pneumothorax may be monitored. Further management involves treatment of the underlying disorder to prevent recurrence. See Cecil Essentials 21. |
|
Pφ |
Increased gastroesophageal reflux of gastric juice with impaired esophageal clearance. |
|
TP |
Classically described as a retrosternal burning sensation. Burning may often be epigastric with radiation upward along the esophagus. Patients may also complain of cough or a sour taste in their mouth when they wake up in the morning. |
|
Dx |
Diagnosis is most often made on clinical grounds, but in suspected complicated disease, esophageal pH monitoring and esophageal manometry can be used to assess reflux as well as impaired clearance of the regurgitant. |
|
Tx |
Treatment most often is acid-suppressive medication. More complex etiologies require treatment of the underlying disorder such as Helicobacter pylori infection or tumor. See Cecil Essentials 36. |
|
Mediastinitis |
|
|
Pφ |
Often caused by odontogenic infections, esophageal perforation, complications of cardiac surgery, or upper gastrointestinal or airway procedures. |
|
TP |
Typically patients present with chest pain, which may also be accompanied by other symptoms such as vomiting or odynophagia depending on the underlying condition. If infection is present (for instance from a sternotomy wound), there may be redness around the site or possibly purulent exudates. Patients will also have extreme tenderness around the incision site. |
|
Dx |
Diagnosis is often made on clinical grounds depending on the cause, but even if a diagnosis is made, imaging may be needed to help characterize the extent of disease (for instance, the severity of a sternotomy infection). Chest radiography may be helpful as it may show an enlarged mediastinum or free peritoneal air. A CT scan can give a more precise view by showing details such as esophageal wall edema and extraesophageal air. |
|
Tx |
Thoracic rupture typically requires surgical intervention. Infection may be treated with antibiotics but may also require debridement. See Cecil Essentials 21. |
|
Pφ |
May be secondary to minor or major trauma and involves some type of inflammatory response. |
|
TP |
Patients present with chest pain that can often be re-created by palpating the affected area. |
|
Dx |
After more serious causes such as acute coronary syndrome have been ruled out, diagnosis is typically made on clinical grounds. A chest radiograph may be indicated if there is some concern of a more serious process such as a rib fracture. |
|
Tx |
Treatment involves pain management and management of inflammation with NSAIDs. In patients with more severe disease or arthritis, corticosteroid injection may be helpful. In those with muscle spasm, a muscle relaxant may also be helpful. See Cecil Essentials 4. |

Practice-Based Learning and Improvement: Evidence-Based Medicine
Title
Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial
Authors
Chen ZM, Pan HC, Chen YP, et al.
Institution
Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Richard Doll Building, Old Road Campus, Oxford OX3 7LF, UK
Reference
Lancet 2005;366:1622–1632
Problem
Previous randomized trials on the use of early β-blocker therapy in patients with suspected MI in addition to standard interventions such as aspirin and fibrinolytic therapy remain uncertain with respect to risks and benefits.
Intervention
A total of 45,852 patients admitted to 1250 hospitals within 24 hours of suspected acute MI onset were randomly allocated to receiving metoprolol (up to 15 mg intravenously, then 200 mg orally daily; n = 22,929) or matching placebo (n = 22,923). Treatment was to continue until discharge or up to 4 weeks in hospital.
Outcome/effect
Two prespecified co-primary outcomes were (1) composite of death, reinfarction, or cardiac arrest, and (2) death from any cause during the scheduled treatment period. Neither co-primary outcome was significantly reduced by metoprolol. For death, reinfarction, or cardiac arrest, 9.4% of the metoprolol patients had an event compared with 9.9% of those allocated to placebo (P = 0.1). For death, this was 7.7% in the metoprolol group versus 7.8% in the placebo group (P = 0.69). Reinfarction for the metoprolol group was 2.0% versus 2.5% for the placebo group (P = 0.001). There was a reduction in occurrence of ventricular fibrillation, with 2.5% for metoprolol versus 3.0% for placebo (P = 0.001). However, these reductions were associated with 11 per 1000 treated patients in the metoprolol group developing cardiogenic shock (5.0% vs. 3.9%; P < 0.00001). This was mainly during days 0 to 1 after admission. Reductions in reinfarction and ventricular fibrillation emerged more gradually.
Historical significance/comments
This was an important trial, which was cited in the ACC/AHA 2007 STEMI Guidelines. The trial showed the potential harm in early β-blocker use, since it may result in increased risk of cardiogenic shock early despite gradual benefit in reducing ventricular fibrillation and reinfarction. The guidelines now suggest oral β-blockers be used within the first 24 hours in patients who do not have signs of heart failure, evidence of low-output state, risk for cardiogenic shock (age > 70 years, systolic blood pressure < 120 mm Hg, sinus tachycardia > 110 bpm or heart rate < 60 bpm, increased time since onset of symptoms of STEMI), or relative contraindications of β-blockade (PR interval > 0.24 seconds, second- or third-degree heart block, active asthma, or reactive airway disease).
Interpersonal and Communication Skills
Educate Patients about Risk Factor Modification
Patient education is of utmost importance in the management of CAD. Patients should be made aware of risk factors and interventions they can undertake to modify their risk, such as an appropriate diet, weight loss, and smoking cessation. Patients should also be educated on medical management of their disease and the importance of appropriate medication use in preventing adverse outcomes. From a psychosocial perspective, a diagnosis of heart disease and its implications may be a very difficult and scary prospect for many patients. Being aware of these emotions and working together supportively with the patient to arrive at a treatment plan will maximize the likelihood of patient compliance.
Professionalism
Beware of Extraneous Side Conversations
Patients undergoing a procedure such as a cardiac catheterization will be mildly sedated and draped for the procedure. It is important to remember that even under these circumstances, the patient may still be aware of extraneous conversations. Whereas open medical discussion may often be appropriate within earshot of the patient, be mindful of extraneous side conversations. Focus should be on the patient at hand and not how you spent your weekend or with whom you had dinner last night. Being exposed to such conversations may send a message that the patient is not your prime concern.
Door-to-Balloon Time: Advocate for Optimal Patient Care Systems
ACS accounts for about 1.57 million hospitalizations annually. Of those, 1.24 million are for UA or NSTEMI and 0.33 million are for STEMI. The patient in the scenario of this chapter is most likely presenting with an STEMI. Hospitals, as well as emergency medical service (EMS) units, have been trained to ensure prompt management of such patients with quick access to a cardiac catheterization lab where they can be treated. In situations where access to a hospital may be delayed because of distance, EMS workers have now been educated in preliminary management of ACS and trained to communicate findings with the receiving hospital to ensure minimal delay between onset of symptoms and cardiac catheterization. The goal door-to-balloon time should ideally be less than 90 minutes, and if access to a facility with primary coronary intervention is not possible, fibrinolytic therapy should be administered within 30 minutes to patients who do not have contraindications. These standards are part of the US Department of Health and Human Services measures for value-based purchasing, a mechanism through which hospitals will be paid less for failure to achieve these measures and rewarded for better performance. It is of utmost importance for hospitals and EMS units to have systems in place to facilitate prompt treatment.
Suggested Readings
Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction. J Am Coll Cardiol 2007;50:e1–e157.
Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction. J Am Coll Cardiol 2008;51:210–247.
Meisel JL. Diagnostic approach to chest pain in adults. UpToDate (http://www.uptodate.com/contents/diagnostic-approach-to-chest-pain-in-adults). Version 19.2 last updated 18 January 2011.
Reeder GS, Kennedy HL. Criteria for the diagnosis of acute myocardial infarction. UpToDate (http://www.uptodate.com/contents/criteria-for-the-diagnosis-of-acute-myocardial-infarction). Version 19.2 last updated 14 June 2011.
Reeder GS, Kennedy HL, Rosenson RS. Overview of the acute management of acute ST elevation myocardial infarction. UpToDate (http://www.uptodate.com/contents/overview-of-the-acute-management-of-acute-st-elevation-myocardial-infarction). Version 19.2 last updated 17 May 2011.
Ryan TJ, Reeder GS. Management of suspected acute coronary syndrome in the emergency department. UpToDate (http://www.uptodate.com/contents/management-of-suspected-acute-coronary-syndrome-in-the-emergency-department). Version 19.2 last updated 16 February 2011.

