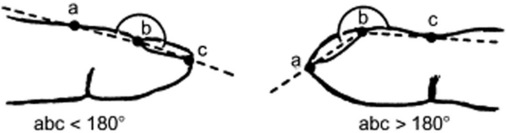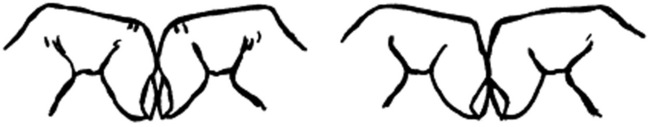Chapter 13 Chest Inspection, Palpation, and Percussion
Generalities
2 What is the usual sequence in a typical pulmonary exam?
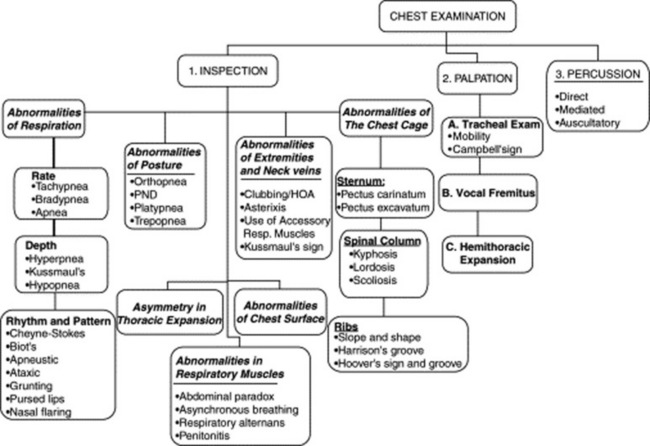
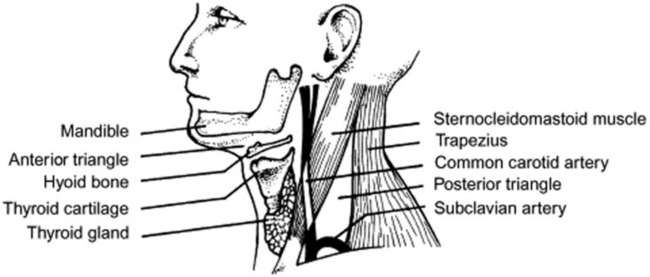
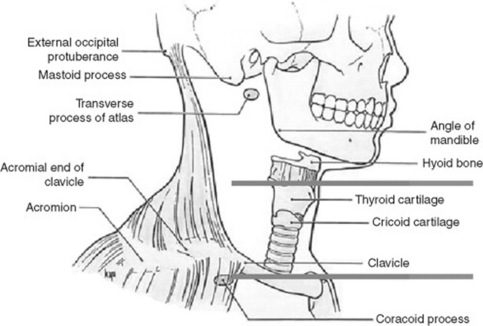
The patient usually remains seated, with the physician moving from front to back and sides. Initial assessment includes an evaluation of effort, rate, and depth of respiration. It also should identify any wheeze, or grunt, or noisy sound that might be audible without the need of a stethoscope. Sequential inspection of the anterior, posterior, and lateral chest should then be carried out. Finally, palpation, percussion, and auscultation complete the exam. The examination should be thorough and go from the chest surface toward the inner structures (Fig. 13-1).
A. Chest Inspection
(1) Abnormalities of Respiration
Abnormalities in the Depth of Respiration
21 Who was Kussmaul?
Adolf Kussmaul (1822–1902) was a graduate of Heidelberg and Würzburg (where he studied under Virchow) and a part-time German Army surgeon. He was the first to describe periarteritis nodosa and progressive bulbar paralysis. He also was the first to attempt gastroesophagoscopy, pleural tapping, and peritoneal lavage. His name is linked to the respiration of patients with metabolic acidosis, the clinical description of pericarditis, pulsus paradoxus, aphasia, and, of course, Kussmaul’s sign, the inspiratory increase in jugular venous pressure (and distention) seen in patients with obstruction to right-sided venous return (see Chapter 10, questions 115–118). A meticulous and precise man famous for complaining that none of his colleagues could write good German, Kussmaul contributed satirical poems to a weekly magazine under the pseudonym of Gottlieb Biedermeier, an imaginary and unsophisticated poet who eventually came to symbolize the values and tastes of the early 19th-century German bourgeois: reliable, hard-working, but boringly unimaginative (like in the Biedermeier style of furniture. “Bieder” is German for “everyday, plain” while “Meier,” or Meyer, is a common German last name).
22 What is hypopnea?
It is shallow respiration, usually indicative of impending respiratory failure or obesity-hypoventilation (Pickwickian syndrome). In this regard, hypopnea is often associated with periods of apnea (see question 17).
Abnormalities in Rhythm and Pattern of Respiration
23 What are the main abnormalities in respiratory rhythm?
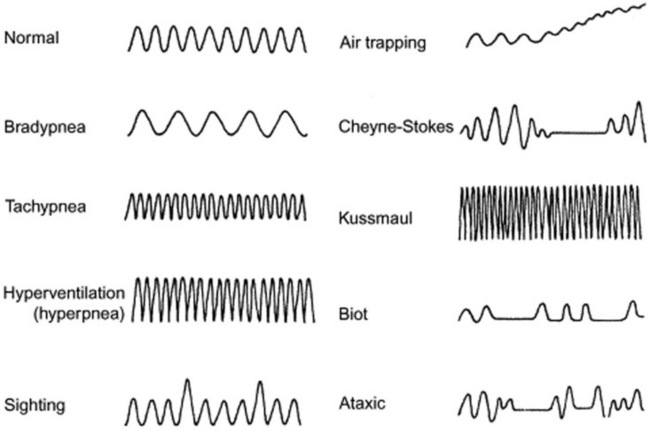
They are many, and usually the result of disruption in the neurogenic control of the respiratory pump. Hence, they are often seen in comatose patients. Thus, they are valuable to recognize because they may help localizing the site of the neurologic lesion (see Fig. 13-2). Moving downward in a rostrocaudal fashion, from the uppermost to the lowermost neurologic center, the most common abnormalities of respiratory rhythm are (1) Cheyne-Stokes respiration, (2) Biot’s respiration, (3) apneustic breathing, (4) central hyperventilation, and (5) ataxic (agonal) respiration.
26 What is the clinical significance of Cheyne-Stokes?
 It may be encountered in normal people as a result of aging or simply sleep.
It may be encountered in normal people as a result of aging or simply sleep.
 It also can be seen in normal individuals who recently moved to high altitudes, where environmental hypoxia leads to a heightened CO2 response of the respiratory centers.
It also can be seen in normal individuals who recently moved to high altitudes, where environmental hypoxia leads to a heightened CO2 response of the respiratory centers.
 The classic association, however, is with congestive heart failure, where Cheyne-Stokes is found in as many as one third of cases, reflecting worse function and prognosis. The reduced cardiac output of these patients leads to a lag between alveolar CO2 and the arterial CO2 delivered to the medulla. Hence, low alveolar CO2 is reflected much later in the blood that bathes the medulla. This asynchrony between alveoli and medulla, coupled to higher sensitivity of the respiratory centers to CO2, eventually leads to the hyperpnea-hypopnea-apnea cycle.
The classic association, however, is with congestive heart failure, where Cheyne-Stokes is found in as many as one third of cases, reflecting worse function and prognosis. The reduced cardiac output of these patients leads to a lag between alveolar CO2 and the arterial CO2 delivered to the medulla. Hence, low alveolar CO2 is reflected much later in the blood that bathes the medulla. This asynchrony between alveoli and medulla, coupled to higher sensitivity of the respiratory centers to CO2, eventually leads to the hyperpnea-hypopnea-apnea cycle.
 Cheyne-Stokes also can be seen in various neurologic disorders (such as meningitis, bilateral or unilateral cerebral infarctions/hemorrhage, and traumatic brain stem or supratentorial damage) in which the underlying mechanism is heightened sensitivity of the respiratory centers to carbon dioxide stimulations. As a result, any increase in CO2 blood levels leads to excessive hyperventilation, until the CO2 bottoms out and respiration ceases completely. Eventually, the apnea-induced increase in CO2 leads to another phase of hyperpnea, and the cycle starts anew.
Cheyne-Stokes also can be seen in various neurologic disorders (such as meningitis, bilateral or unilateral cerebral infarctions/hemorrhage, and traumatic brain stem or supratentorial damage) in which the underlying mechanism is heightened sensitivity of the respiratory centers to carbon dioxide stimulations. As a result, any increase in CO2 blood levels leads to excessive hyperventilation, until the CO2 bottoms out and respiration ceases completely. Eventually, the apnea-induced increase in CO2 leads to another phase of hyperpnea, and the cycle starts anew.
28 Who were Cheyne and Stokes?
William Stokes (1804–1878) was instead a bona-fide Irishman and the son of the anatomy professor who had succeeded John Cheyne at the College of Surgeons School in Ireland. Although lacking in formal education (his father wanted to protect him from a society that did not abide by the scriptures), Stokes eventually went to Edinburgh, where he received his doctor of medicine in 1825. In Scotland, he learned of Laënnec and his recent invention, the “cylinder.” He soon became so enamored of this little tool that he even wrote an introductory book about it, the first of its kind in the English language. In fact, Stokes was such a vocal advocate for the use of stethoscopy that he provoked quite a few reactions (and even some sarcasm) among his colleagues. Still, he was a well-liked physician, who worked among the poor during the Dublin typhus epidemic in 1826 (he even contracted the disease but survived) and then again during the subsequent cholera epidemic. His name is linked not only to the eponymous pattern of respiration but also to Stokes-Adams syncope, which the Irish surgeon Robert Adams had described in 1827 and which Stokes included in his 1854 book, Diseases of the Heart and Aorta. Of course, the Italian Morgagni had preceded them both by describing the condition almost 100 years before (see Chapter 11, question 14).
29 What other abnormalities in rhythm are worthy of recognition? What is their significance?
 Biot’s respiration is a variant of Cheyne-Stokes, insofar as it is a succession of hyperpneas/hyperventilations and apneas, but without the typical crescendo-decrescendo pattern, the abrupt beginning, and the regularity. It is also less common than Cheyne-Stokes. Biot’s is usually observed in patients with either meningitis or medullary compression. Hence, it carries a worse prognosis, usually resulting in complete apnea and cardiac arrest.
Biot’s respiration is a variant of Cheyne-Stokes, insofar as it is a succession of hyperpneas/hyperventilations and apneas, but without the typical crescendo-decrescendo pattern, the abrupt beginning, and the regularity. It is also less common than Cheyne-Stokes. Biot’s is usually observed in patients with either meningitis or medullary compression. Hence, it carries a worse prognosis, usually resulting in complete apnea and cardiac arrest.
 Apneustic breathing is a peculiar pattern of respiration, characterized by a deep inspiratory phase followed by a breath-holding period and a rapid exhalation. It is typical of brain stem (pons) lesions.
Apneustic breathing is a peculiar pattern of respiration, characterized by a deep inspiratory phase followed by a breath-holding period and a rapid exhalation. It is typical of brain stem (pons) lesions.
 Central hyperventilation is often encountered in patients with midbrain/upper pontine lesions. It is an ongoing pattern of hyperpnea and tachypnea (i.e., deep and rapid respirations), which tends to be different from Kussmaul’s respiration, insofar as it is not as fast, but usually deeper.
Central hyperventilation is often encountered in patients with midbrain/upper pontine lesions. It is an ongoing pattern of hyperpnea and tachypnea (i.e., deep and rapid respirations), which tends to be different from Kussmaul’s respiration, insofar as it is not as fast, but usually deeper.
 Ataxic ventilation: From the Greek a- (lack of) and taxis (order), this is a totally anarchic respiratory rhythm—a sort of fibrillation of the respiratory centers, with back-and-forth shifts from hyper- to hypo-ventilation, and from hyperpnea to hypopnea, all intermingled with periods of apnea. It is seen in patients with damage to the medulla, typically preceding death. Hence the term, agonal respiration.
Ataxic ventilation: From the Greek a- (lack of) and taxis (order), this is a totally anarchic respiratory rhythm—a sort of fibrillation of the respiratory centers, with back-and-forth shifts from hyper- to hypo-ventilation, and from hyperpnea to hypopnea, all intermingled with periods of apnea. It is seen in patients with damage to the medulla, typically preceding death. Hence the term, agonal respiration.
31 What is the clinical significance of a grunting respiration?
Very much the same as in Laënnec’s days. It can still be heard in adults with respiratory muscle fatigue (and impending arrest), but nowadays it is much more frequent in children, where it usually presents as a short and low-pitched noise produced by forced expiration against a closed glottis. The “grunt” is due to the sudden opening of the glottis and the loud rush of air from the larynx. Its physiology (and significance) is akin to pursed-lip respiration (see below, questions 32 and 33), insofar as it leads to an increase in expiratory airway pressure, which then acts as a mechanical splint against alveolar collapse, increasing tidal volume and oxygenation while decreasing respiratory rate and CO2. An increased intra-alveolar pressure also has a positive effect against transudation of fluid in patients with pulmonary edema, and thus it is often observed during acute episodes of left ventricular failure.
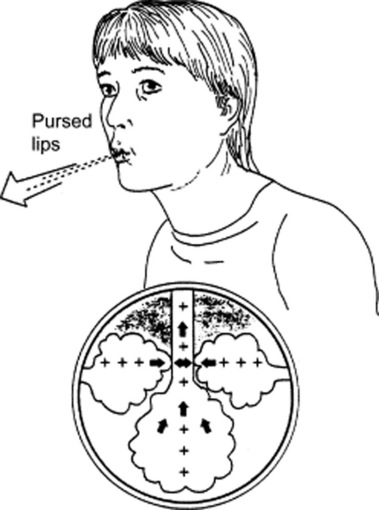
32 What is pursed-lip respiration?
Another respiratory pattern, typically seen in obstructive lung disease—usually emphysema (Fig. 13-3). Given the alveolar hyperinflation (and reduced lung elasticity) of COPD, patients are at risk for expiratory airway closure and air-trapping. Hence, they resort to pursed lip exhalation, as if they were inflating a balloon. This increases intra-airway pressure, thus inducing auto-PEEP (positive end-expiratory pressure). It is often accompanied by an expiratory wheeze or grunt.
39 And what about patients with COPD?
In COPD (which often presents with apical bullae), an upright position improves not only gas exchange but also lung mechanics (because of the increased stretch of accessory respiratory muscles). Hence, COPD patients often tend to prop themselves up, so that they can better use their respiratory muscles. They do so by clasping the side of the bed or pushing with the elbows over the thighs (see question 5, Dahl’s sign).
47 What is the clinical significance of platypnea?
 Multiple recurrent pulmonary emboli (which, because of gravity, tend to involve primarily the bases)
Multiple recurrent pulmonary emboli (which, because of gravity, tend to involve primarily the bases)
 Pleural effusion (which can cause bibasilar atelectasis) or bibasilar pneumonia
Pleural effusion (which can cause bibasilar atelectasis) or bibasilar pneumonia
 Cirrhosis (which often has arteriovenous shunting at the lung bases)
Cirrhosis (which often has arteriovenous shunting at the lung bases)
 Intrapulmonary right-to-left shunt, such as an arteriovenous malformation, often basilar in location
Intrapulmonary right-to-left shunt, such as an arteriovenous malformation, often basilar in location
 Intracardiac right-to-left shunt, usually due to an atrial septal defect. This produces platypnea only when associated with an increase in pulmonary resistances, as in cases of pleurocardial/pericardial effusion or status post lobectomy/pneumonectomy. An upright position will reduce the shunt by redirecting blood toward the atrial septum and by possibly increasing pressure over the right atrium. A supine posture will have instead the opposite effect.
Intracardiac right-to-left shunt, usually due to an atrial septal defect. This produces platypnea only when associated with an increase in pulmonary resistances, as in cases of pleurocardial/pericardial effusion or status post lobectomy/pneumonectomy. An upright position will reduce the shunt by redirecting blood toward the atrial septum and by possibly increasing pressure over the right atrium. A supine posture will have instead the opposite effect.
63 What are the main chest cage abnormalities?
 If the spine is affected, the abnormalities may be on the sagittal or frontal plane.
If the spine is affected, the abnormalities may be on the sagittal or frontal plane.
 If the sternum is involved, the two most common abnormalities are pigeon chest and barrel chest. Both may alter lung mechanics severely enough to reduce lung function.
If the sternum is involved, the two most common abnormalities are pigeon chest and barrel chest. Both may alter lung mechanics severely enough to reduce lung function.
 If ribs are involved, the most common abnormalities concern costal slope and shape.
If ribs are involved, the most common abnormalities concern costal slope and shape.
65 What abnormalities may be seen on the sagittal plane?
These are an increase in spinal convexity (lordosis) or concavity (kyphosis)—often coexisting.
66 What abnormalities may be seen on the frontal plane?
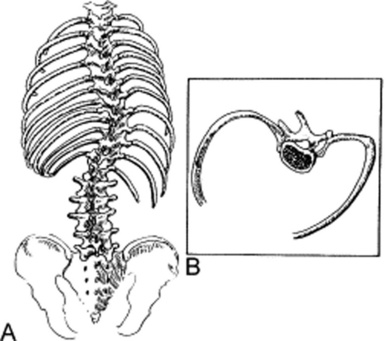
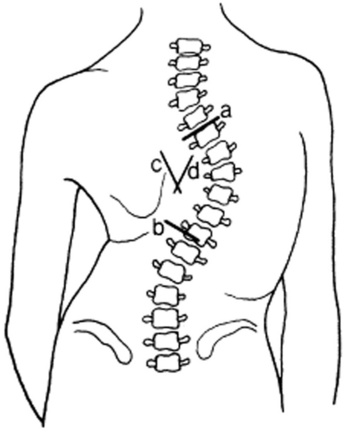
These are lateral spinal curvatures, also called scoliosis (from the Greek word for crookedness). Depending on etiology, a scoliosis may have one curvature, or two curvatures (a primary and a secondary, with the latter providing a compensatory function). Scoliosis also may be fixed (because of muscle and/or bone deformity) or mobile (because of unequal muscle contraction) (see Fig. 13-4).
68 Is physical exam the best way to assess kyphoscoliosis?
No. Chest examination allows detection of the abnormality, but is of little value in quantifying its degree. To do so, you must rely on chest radiographs, and on the determination of the Cobb angle of scoliosis (Fig. 13-5). This can be calculated by drawing two lines parallel to (1) the upper border of the highest and (2) the lower border of the lowest vertebral bodies of the primary curvature, as seen on a spine anteroposterior (AP) film. The angle is then measured at the intersection points of two additional lines, drawn perpendicular to the original lines. As a rule of thumb, a Cobb’s angle of 10 is considered the minimum angulation for a definition of scoliosis. Conversely, a Cobb’s angle >100 degrees represents a severe deformity, one associated with higher risk for pulmonary hypertension and respiratory failure.
73 What is a funnel chest?
It is a deep sternal depression, characterized by backward displacement of the lower half (or two thirds) of the sternum, often more visible after a deep inspiration. The alternative term, pectus excavatum (which is Latin for “hollow chest”), reflects the defining characteristic of this lesion (Fig. 13-6). In fact, even the upper part of the abdomen is often depressed, making it resemble a “potbelly.” Note that a funnel chest may compress not only the lungs (and thus distort pulmonary mechanics), but also the ventricles—especially the right one because the sternal deformity is often asymmetric, displacing the heart toward the left as well as compressing the right chambers.
77 What is a pigeon chest?
It is the opposite of the funnel chest: a sternal protrusion from cartilage overgrowth, with forward sternal projection and secondary flattening of either side of the chest (Fig. 13-7). This makes the sternum resemble the keel of a ship (carina in Latin), and thus the term pectus carinatum. As its counterpart, a funnel chest may be isolated or associated with specific diseases, like rickets, Marfan’s, or Noonan’s. Yet, contrary to the funnel chest, a pectus carinatum is much more of a medical oddity (with prevalence of 0.06% as opposed to 1 in 300–400 births).
79 What can be done to correct these conditions?
Surgery. Both conditions are amenable to surgical correction.
83 What is a barrel chest?
A chest that is almost round, with horizontalized ribs and an equalized thoracic ratio.
85 How are the physiologic consequences of a barrel chest?
Detrimental. A horizontal rib slope may compromise lung function, making ventilation less efficient.
(6) Abnormalities of the Chest Surface
99 Why is it important to observe the characteristics of the chest surface?
Because they can provide important diagnostic clues, such as:
 Abnormalities in color: Skin color helps identify patients with ineffective oxygenation and/or ventilation. Cyanosis is a hallmark of insufficient ventilation (with resulting hypercapnia and a reduced hemoglobin >5 g/100 mL). Conversely, pallor, diaphoresis, and agitation are the hallmarks of ineffective oxygenation. Neurologic repercussions are different, since hypercapnia is a central nervous system (CNS) depressor, whereas hypoxemia is a stressor.
Abnormalities in color: Skin color helps identify patients with ineffective oxygenation and/or ventilation. Cyanosis is a hallmark of insufficient ventilation (with resulting hypercapnia and a reduced hemoglobin >5 g/100 mL). Conversely, pallor, diaphoresis, and agitation are the hallmarks of ineffective oxygenation. Neurologic repercussions are different, since hypercapnia is a central nervous system (CNS) depressor, whereas hypoxemia is a stressor.
 Abnormalities in pigmentation: Patients whose chests are hyperpigmented (especially if the skin is tightly drawn and covered with telangiectasias or vitiligo) also should have their hands and fingers evaluated for tightening. These (and a tight mouth opening) are typical features of scleroderma and important clues to the presence of underlying lung diseases, such as vasculitis, pulmonary hypertension, and cor pulmonale.
Abnormalities in pigmentation: Patients whose chests are hyperpigmented (especially if the skin is tightly drawn and covered with telangiectasias or vitiligo) also should have their hands and fingers evaluated for tightening. These (and a tight mouth opening) are typical features of scleroderma and important clues to the presence of underlying lung diseases, such as vasculitis, pulmonary hypertension, and cor pulmonale.
 Expiratory bulging: Focal expiratory bulging of the intercostal spaces is typical of patients with pneumothorax, whereas diffuse expiratory bulging occurs in patients with obstructive lung disease. Focal inspiratory sinking (tirage) is seen in patients with focal airway obstruction; diffuse tirage is seen in patients with upper airway obstruction. Finally, a localized and paradoxical motion of the thorax is observed in patients with flail chest.
Expiratory bulging: Focal expiratory bulging of the intercostal spaces is typical of patients with pneumothorax, whereas diffuse expiratory bulging occurs in patients with obstructive lung disease. Focal inspiratory sinking (tirage) is seen in patients with focal airway obstruction; diffuse tirage is seen in patients with upper airway obstruction. Finally, a localized and paradoxical motion of the thorax is observed in patients with flail chest.
 Collateral circulations: These may occur on the chest wall of patients with superior or inferior vena cava obstruction, in whom an impeded venous return creates a collateral circulation that flows, respectively, caudad or cephalad.
Collateral circulations: These may occur on the chest wall of patients with superior or inferior vena cava obstruction, in whom an impeded venous return creates a collateral circulation that flows, respectively, caudad or cephalad.
 Dermatomic herpes zoster lesions
Dermatomic herpes zoster lesions
 Chest wall fistulas: These may be seen in patients with empyema necessitatis, a form of pyothorax in which pus burrows to the outside, producing a subcutaneous abscess that finally ruptures. The drainage may actually be beneficial, allowing relief of a closed-space infection and, often, spontaneous recovery.
Chest wall fistulas: These may be seen in patients with empyema necessitatis, a form of pyothorax in which pus burrows to the outside, producing a subcutaneous abscess that finally ruptures. The drainage may actually be beneficial, allowing relief of a closed-space infection and, often, spontaneous recovery.
107 What are the diagnostic features of clubbing?
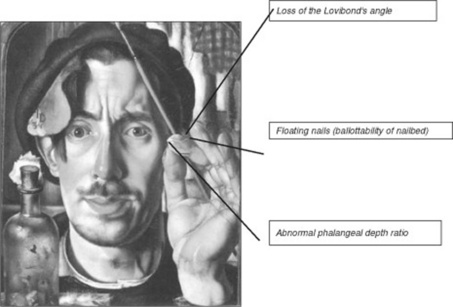
They depend on whether clubbing is present alone or in association with periostosis (see HOA in questions 117–122). Clubbing without periostosis, the time-honored Hippocratic nail, has three diagnostic features (Fig. 13-8):
1. Loss of Lovibond’s angle: This is the angle between the base of the nail and its surrounding skin (hyponychial or unguophalangeal angle). The angle is normally <180 degrees. In clubbing, however, is either obliterated (straight line) or >180 degrees. The loss of the Lovibond’s angle (Fig. 13-9) can be easily visualized by resting a pencil over the nail. Normally, there should be a clear window between the pencil and the nail. In clubbing, however, there will be none. Thus, the pencil will fully rest over the nail.
2. Floating nails (ballotability of the nail bed): This refers to an increased sponginess of the soft tissue at the base of the nail. As a result, the nail plate acquires a “springy” feeling: when the skin just proximal to the nail is compressed, the nail sinks deep toward the bone; upon release, it springs backward and outward (floating fingernail base)—almost like pushing an ice cube down a pot of water. This feeling can be effectively simulated by:
 Pressing your right index finger over the skin just proximal to the nail of your left middle finger. In a normal person, the nail plate feels solidly attached to the underlying bone.
Pressing your right index finger over the skin just proximal to the nail of your left middle finger. In a normal person, the nail plate feels solidly attached to the underlying bone. Repeating the maneuver, but this time applying some tension to the nail by pulling downward its free edge with your left thumb, thereby increasing the natural convexity of the nail plate. After doing so, the nail plate will feel detached from the underlying bone, capable of sinking down during pressure and springing back upon release, almost as if it were floating on a spongy pad.
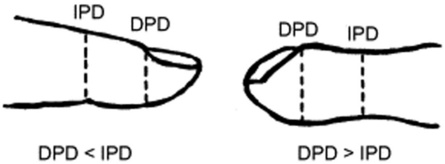
Repeating the maneuver, but this time applying some tension to the nail by pulling downward its free edge with your left thumb, thereby increasing the natural convexity of the nail plate. After doing so, the nail plate will feel detached from the underlying bone, capable of sinking down during pressure and springing back upon release, almost as if it were floating on a spongy pad.3. Abnormal phalangeal depth ratio: This consists of a greater depth of the fingertip when measured at the cuticle (distal phalangeal depth [DPD]) as compared to the interphalangeal joint (interphalangeal depth [IPD]) (see Fig. 13-10). The normal DPD/IPD ratio is, on average, 0.895, which means that the fingertip tends to taper going from the distal interphalangeal joint toward the end. Conversely, in clubbing it bulges, with a DPD/IPD ratio >1.0 (i.e., in excess of the norm by approximately 2.5 standard deviations). The DPD/IPD ratio is an excellent marker for clubbing, with good sensitivity and specificity. For example, a ratio >1.0 is found in 85% of children with cystic fibrosis and fewer than 5% of children with chronic asthma.
110 Is increased curvature of the nail a sign of clubbing?
Not necessarily (see below, question 116). True clubbing requires accumulation of soft tissue at the base of the nail. Hence, it is defined not by an increase in nail convexity but by the three aforementioned characteristics.
111 What is a drumstick finger?
It is one of several terms used to describe the more advanced stages of clubbing (Fig. 13-11). The accumulation of connective tissue extends well beyond the base of the nail and involves the entire digit. Depending on where this accumulation predominates, a few colorful terms have been created. For example, in parrot’s beak clubbing, the swelling is primarily localized to the proximal portion of the distal digit; in the drumstick type, it is circumferential; and in the watchglass form, swelling is mostly at the nail base.
112 What is Schamroth’s sign?
It is the disappearance of the diamond-shaped window normally present when the terminal phalanges of paired digits are juxtaposed (Fig. 13-12). It is just another and more recently described maneuver to confirm the loss of the Lovibond angle. It was first reported in 1976 by the South African cardiologist Leo Schamroth, who noticed it on himself during recurrent clubbing due to bouts of endocarditis.
113 What is the clinical significance of clubbing?
Clubbing is a feature not only of lung disease, but also of several chronic inflammatory conditions not necessarily limited to the lungs. In fact, it has been described in infective endocarditis, lung abscess, bronchiectasis, and even amyloidosis. It also has been reported in chronic inflammatory bowel disease, such as Crohn’s and ulcerative colitis. Although described in hypercapnia (usually from chronic bronchitis), clubbing is not a feature of emphysema. It is, however, encountered in hypoxemia from shunts (either cardiogenic or pulmonary) and in patients with sex hormones imbalance (because of either pregnancy or cirrhosis). Finally, clubbing has been described in various cancers, especially lung (see Table 13-1).
Table 13-1 Disorders Commonly Associated with Digital Clubbing
| Intrathoracic | Cardiovascular |
| Bronchogenic carcinoma* | Congenital cyanotic heart disease |
| Metastatic lung cancer* | Other causes of right-to-left shunting |
| Hodgkin’s disease | Subacute bacterial endocarditis |
| Mesothelioma* | Infected aortic bypass graft* |
| Bronchiectasis* | Hepatic and Gastrointestinal |
| Lung abscess | Hepatic cirrhosis* |
| Empyema | Inflammatory bowel disease |
| Cystic fibrosis | Carcinoma of esophagus or colon |
| Pulmonary interstitial fibrosis | Achalasia |
| Sarcoidosis | Peptic ulceration of the esophagus |
| Pneumoconiosis | Primary biliary cirrhosis |
| Lipid pneumonia | Cirrhosis of the liver |
| Arteriovenous malformations | Malignancies |
| Miscellaneous | Thyroid cancer |
| Pregnancy | Thymus cancer |
| Acromegaly | Hodgkin’s disease |
| Pachydermoperiostosis | Chronic myeloid leukemia |
| Thyroid acropachy |
* Commonly associated with hypertrophic osteoarthropathy.
(Adapted from Hansen-Flaschen J, Nordberg J: Clubbing and hypertrophic osteoarthropathy. Clin Chest Med 8:287–298, 1987.)
129 What are the most important of these accessory muscles?
It depends. For inspiration, the most important are the scaleni and sternocleidomastoids; for expiration, the abdominal obliques are instead predominant. Overall, the scaleni are used before the sternocleidomastoids; both are employed in patients exhibiting retractions (Fig. 13-13).
133 Which neck vein abnormalities may provide clues to the diagnosis of lung disease?
 Distention of neck veins: This is seen in superior vena cava syndrome, often in association with facial, neck, shoulder, and even hand swelling. Neck vein distention also occurs in right-sided or biventricular failure, either at baseline or after abdominal compression (abdominojugular reflux).
Distention of neck veins: This is seen in superior vena cava syndrome, often in association with facial, neck, shoulder, and even hand swelling. Neck vein distention also occurs in right-sided or biventricular failure, either at baseline or after abdominal compression (abdominojugular reflux).
 Kussmaul’s sign: This is an inspiratory bulging of neck veins and an important sign of obstruction to right ventricular filling. As such, it can be observed in many conditions, like superior vena cava obstruction, tricuspid stenosis, constrictive pericarditis, right ventricular infarction, massive pulmonary embolism, or pulmonary hypertension. Conversely, an expiratory bulge in neck veins is a sign of increased intrathoracic pressure and thus a good clue toward a diagnosis of COPD.
Kussmaul’s sign: This is an inspiratory bulging of neck veins and an important sign of obstruction to right ventricular filling. As such, it can be observed in many conditions, like superior vena cava obstruction, tricuspid stenosis, constrictive pericarditis, right ventricular infarction, massive pulmonary embolism, or pulmonary hypertension. Conversely, an expiratory bulge in neck veins is a sign of increased intrathoracic pressure and thus a good clue toward a diagnosis of COPD.
B. Chest Palpation
134 What is the clinical value of palpation?
 To confirm, reject, or supplement information previously gathered through inspection, especially in regard to the position of the trachea and the expansion of the hemithoraces.
To confirm, reject, or supplement information previously gathered through inspection, especially in regard to the position of the trachea and the expansion of the hemithoraces.
 To provide new information, specifically concerning the status of the pleura and lung parenchyma. This is gathered through assessment of the vocal tactile fremitus, detection of pleural friction rubs, search for bronchial fremitus (inspiratory vibration generated by the “rattling” of intra-airway secretions), and identification of various other chest wall abnormalities (like masses and tenderness).
To provide new information, specifically concerning the status of the pleura and lung parenchyma. This is gathered through assessment of the vocal tactile fremitus, detection of pleural friction rubs, search for bronchial fremitus (inspiratory vibration generated by the “rattling” of intra-airway secretions), and identification of various other chest wall abnormalities (like masses and tenderness).
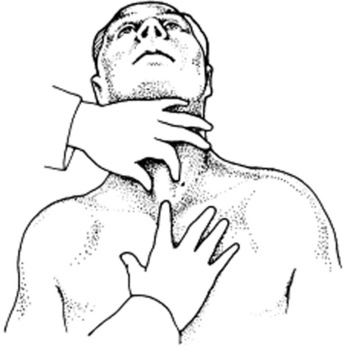
138 How do you detect a tracheal deviation?
First, ask the patient to sit up, lean forward, and keep the head straight. Then place the tip of your small finger in the fossa between the medial end of the sternocleidomastoid and the lateral aspect of the trachea (Fig. 13-14). Compare the depth of this fossa to the contralateral one. They should be symmetric. If not, the patient has a tracheal shift—typically toward the side with the smaller fossa.
140 How can one distinguish tracheal deviation of lung collapse from that of effusion?
 Lung collapse causes a tracheal shift that is ipsilateral to the atelectasis and its findings of dullness, decreased tactile fremitus, and auscultatory silence.
Lung collapse causes a tracheal shift that is ipsilateral to the atelectasis and its findings of dullness, decreased tactile fremitus, and auscultatory silence.
 Massive pleural effusion causes instead a tracheal shift that is contralateral to the findings of dullness, decreased fremitus, and auscultatory silence.
Massive pleural effusion causes instead a tracheal shift that is contralateral to the findings of dullness, decreased fremitus, and auscultatory silence.
141 How do you assess for mobility of the trachea?
 Induced mobility can be detected by pushing the trachea sideward to see whether it is indeed mobile. A rigid and fixed trachea indicates upper mediastinal fibrosis, from mediastinitis or cancer.
Induced mobility can be detected by pushing the trachea sideward to see whether it is indeed mobile. A rigid and fixed trachea indicates upper mediastinal fibrosis, from mediastinitis or cancer.
 Spontaneous mobility can be detected as a rostrocaudal tracheal “tug,” synchronous with each heartbeat. This is often referred to as the Oliver-Cardarelli sign and usually indicates an aneurysmatic aortic arch. It is often referred to as two separate signs: Oliver’s and Cardarelli’s.
Spontaneous mobility can be detected as a rostrocaudal tracheal “tug,” synchronous with each heartbeat. This is often referred to as the Oliver-Cardarelli sign and usually indicates an aneurysmatic aortic arch. It is often referred to as two separate signs: Oliver’s and Cardarelli’s.
145 What are laryngeal height and laryngeal descent?
Laryngeal height is the distance between the top of the thyroid cartilage and the suprasternal notch. Maximum laryngeal height is measured at end of expiration; minimum laryngeal height at end of inspiration. The difference between the two is the laryngeal descent (see Fig. 13-15).
147 What is the value of tracheal auscultation?
It allows for recognition of stridor (i.e., of a “wheeze” that is present only in inspiration). This should not be confused with a true wheeze, which is either expiratory, or both inspiratory and expiratory. Still, even expiratory wheezes should be considered as originating from the upper airways when they are louder over the trachea than the chest. In fact, unless the patient is morbidly obese, these sounds usually reflect expiratory adduction of the vocal cords (vocal cord dysfunction) and thus should prompt laryngoscopy in all patients presenting with “refractory asthma.” Finally, tracheal auscultation also allows for the measurement of the forced expiratory time, a valuable tool for determining the presence and severity of airflow obstruction (see Chapter 14, question 48).
Assessment of the Vocal Tactile Fremitus
148 What is the vocal tactile fremitus (VTF)?
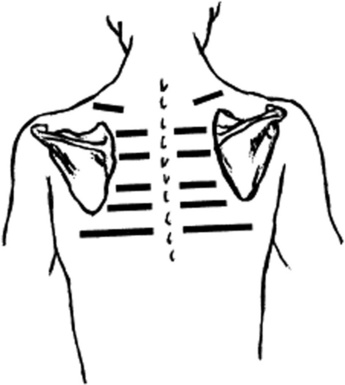
It is the Latin term for a palpable thrill produced by the patient’s voice. This can be detected by placing the hand sequentially over various areas of the chest and by then feeling the thrill that is transmitted whenever the patient is asked to say something (Fig. 13-16). Most commonly, these sounds are “Eeee,” or “1, 2, 3,” or even “99”—all terms that have more historical than clinical values (they go back to the original German neun und neunzig [i.e., “99”]), and really matter very little, since any word or sound would probably do. And yet, it also is true that some sounds, especially the lower-pitched ones, can better pass the alveolar air filter, thus leading to a stronger tactile fremitus. This might explain why men—who have lower-pitched voices than women—have more prominent VTF.
Assessment of Expansion of Hemithoraces
Other Goals of Palpation
154 What are the other goals of palpation?
 It should identify areas of tenderness over the chest, ribs, or sternum. This can be encountered in neoplastic involvement, but also in atypical chest pain due to osteochondritis (Tietze syndrome).
It should identify areas of tenderness over the chest, ribs, or sternum. This can be encountered in neoplastic involvement, but also in atypical chest pain due to osteochondritis (Tietze syndrome).
 It should identify areas of crepitation (indicative of subcutaneous emphysema).
It should identify areas of crepitation (indicative of subcutaneous emphysema).
 It should identify areas of fluctuation or even fistulization (empyema necessitatis, see question 99).
It should identify areas of fluctuation or even fistulization (empyema necessitatis, see question 99).
 It should search for pleural friction rubs or bronchial fremitus (inspiratory vibration generated by the “rattling” of intra-airway secretions).
It should search for pleural friction rubs or bronchial fremitus (inspiratory vibration generated by the “rattling” of intra-airway secretions).
 Finally, it should systematically explore the supraclavicular and axillary fossae for the presence of enlarged lymph nodes (see Chapter 18).
Finally, it should systematically explore the supraclavicular and axillary fossae for the presence of enlarged lymph nodes (see Chapter 18).
C. Chest Percussion
155 How valuable is percussion?
Although less studied than auscultation, percussion remains a key component of the chest exam. Laënnec suggested that it should complement auscultation in the differential diagnosis of pleural effusion, pneumonia, pneumothorax, and emphysema—diseases in which percussion still provides crucial information (see also summary table at the end of Chapter 14).
156 What is the history of percussion?
Listening to sounds of the body (either spontaneous or elicited) was a truly revolutionary advance in physicians’ ability to make a diagnosis. Although succussion had been described by Hippocrates (and so had been percussion of the abdomen), things really took off only in 1754–1761, thanks to Auenbrugger’s work on chest percussion. They got even better 60 years later, when Laënnec, frustrated by percussion, added stethoscopy to the physician’s toolbox (see Chapter 14, question 1), yet it was Giovanni Maria Lancisi (1654–1720), Roman physician to three popes and medical historian in his own right, who first intuited (and reported) the diagnostic value of thoracic percussion.
158 What is the physics behind percussion?
 If the tissue is rich in air and poor in solid/fluid (high air/fluid ratio), the percussion note will have high amplitude and low frequency. This resonant note is loud and typical of the normal lung.
If the tissue is rich in air and poor in solid/fluid (high air/fluid ratio), the percussion note will have high amplitude and low frequency. This resonant note is loud and typical of the normal lung.
 If air is more abundant than normal, the percussion note has greater amplitude and lower frequency than a resonant percussion note. It is also longer. This hyperresonant (or tympanic) note is typical of pneumothorax, emphysema, and big blebs—and also of the gastric bulla or a puffed cheek.
If air is more abundant than normal, the percussion note has greater amplitude and lower frequency than a resonant percussion note. It is also longer. This hyperresonant (or tympanic) note is typical of pneumothorax, emphysema, and big blebs—and also of the gastric bulla or a puffed cheek.
 If the tissue ratio between air and fluid/solid is low (i.e., there is more fluid/solid than air), the percussion note is shorter than a resonant or hyperresonant note. It also is high in frequency and low in amplitude and thus perceived as soft. In fact, flat (or dull) percussion notes are at times so soft that using a very light percussion technique may even fail to elicit anything audible. This technique can be used to better identify the transition between solid and aerated areas, as a change from silence (i.e., barely audible dullness) to noise (resonance). Note that, semantically, “dull” is the percussion note of consolidation and lung collapse, whereas “flat” is the note of a pleural effusion.
If the tissue ratio between air and fluid/solid is low (i.e., there is more fluid/solid than air), the percussion note is shorter than a resonant or hyperresonant note. It also is high in frequency and low in amplitude and thus perceived as soft. In fact, flat (or dull) percussion notes are at times so soft that using a very light percussion technique may even fail to elicit anything audible. This technique can be used to better identify the transition between solid and aerated areas, as a change from silence (i.e., barely audible dullness) to noise (resonance). Note that, semantically, “dull” is the percussion note of consolidation and lung collapse, whereas “flat” is the note of a pleural effusion.
Hence, if the strength of striking and chest wall thickness remain constant, the relative pitch of the percussion note will depend on the ratio between aerated tissue/solid or liquid medium (Table 13-2 and Table 13-3).
| Relative Pitch | Relative Intensity | Percussion Note |
|---|---|---|
| Low | Loud | Resonant |
| Lower | Very loud | Hyperresonant |
| Medium | Medium | Dull |
| High | Soft | Flat |
163 What is the current value of indirect percussion?
Good but limited. The following should be kept in mind:
 A “normal” percussion note can often occur in patients with lung disease. Hence, it should not be used as an exclusion criterion.
A “normal” percussion note can often occur in patients with lung disease. Hence, it should not be used as an exclusion criterion.
 In its “comparative” modality, percussion complements auscultation in the differential diagnosis of consolidation, atelectasis, pneumothorax, and pleural effusion.
In its “comparative” modality, percussion complements auscultation in the differential diagnosis of consolidation, atelectasis, pneumothorax, and pleural effusion.
 In patients with fever and cough, unilateral chest dullness is a strong predictor of pneumonia (but if absent, it cannot be ruled out, given the finding’s excellent specificity but poor sensitivity).
In patients with fever and cough, unilateral chest dullness is a strong predictor of pneumonia (but if absent, it cannot be ruled out, given the finding’s excellent specificity but poor sensitivity).
 In large pleural effusions, percussion has instead outstanding sensitivity, with unilateral dullness occurring in almost all patients (even though specificity is not as good; see auscultatory percussion, questions 166 and 167).
In large pleural effusions, percussion has instead outstanding sensitivity, with unilateral dullness occurring in almost all patients (even though specificity is not as good; see auscultatory percussion, questions 166 and 167).
 Comparative percussion is of little or no value in detecting circumscribed lesions, like nodules.
Comparative percussion is of little or no value in detecting circumscribed lesions, like nodules.
 Finally, while unilateral hyperresonance suggests pneumothorax, a bilateral one is a strong indicator of airflow obstruction, either acute (status asthmaticus) or chronic (COPD). Even in this case, specificity is three times better than sensitivity.
Finally, while unilateral hyperresonance suggests pneumothorax, a bilateral one is a strong indicator of airflow obstruction, either acute (status asthmaticus) or chronic (COPD). Even in this case, specificity is three times better than sensitivity.
164 Are there differences between the percussion note of lung collapse from effusion and consolidation from pneumonia?
167 Is there a role for auscultatory percussion in the detection of pleural effusion?
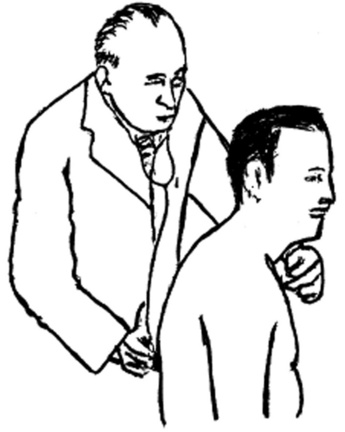
Yes. In fact, in its original form auscultatory percussion was advocated for the detection of pleural effusion. To do so, the patient has to be seated and the stethoscope placed in the back, 3 cm below the 12th rib. The chest is then percussed downward and posteriorly, from the apex to the base. A change in percussion note from dull to loud that is localized over the 12th rib indicates the presence of pleural fluid. In contrast to other more traditional modalities of percussion, this auscultatory variety has the same excellent sensitivity for pleural effusion (>90%), but better specificity (also >90%), being usually negative in pneumonia or atelectasis. Conversely, in its newer and revised modality, auscultatory percussion is practiced a little differently (Fig. 13-17). The physician taps lightly with the distal tip of one finger over the manubrium of the sternum (and thus anteriorly), while at the same time listening with the stethoscope over symmetric locations of the posterior chest wall. The theory is that sound so generated travels unimpeded through the lungs, reaching the opposite chest wall in a symmetric fashion. Thus, any asymmetry in sound intensity is considered a sign of lung disease. By using this technique, Guarino was able to detect pulmonary lesions <2 cm in diameter (which are almost impossible to identify with conventional percussion). Studies to confirm this technique, however, have produced conflicting results.
1 Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care. 1980;25:937-939.
2 Andreas S, Hagenah G, Möller C, et al. Cheyne-Stokes respiration and prognosis in congestive heart failure. Am J Cardiol. 1996;78:1260-1264.
3 Barach AL. Physiologic advantages of grunting, groaning, and pursed-lip breathing: Adaptive symptoms related to the development of continuous positive pressure breathing. Bull NY Acad Med. 1973;49:666-673.
4 Bourke S, Nunes D, Strafford F, et al. Percussion of the chest revisited: A comparison of the diagnostic value of ausculatory and conventional chest percussion. Isr J Med Sci. 1989;158:82-84.
5 Brown HW, Plum F. The neurologic basis of CheyneStokes respiration. Am J Med. 1961;30:849-860.
6 Carroll DG. Curvature of the nails, clubbing of the fingers and hypertrophic pulmonary osteoarthropathy. Trans Am Clin Climat Assoc. 1971;83:198-208.
7 Cohen CA, Zagelbaum G, Gross D, et al. Clinical manifestations of inspiratory muscle fatigue. Am J Med. 1982;73:308-316.
8 Coury C. Hippocratic fingers and hypertrophic osteoarthropathy: A study of 350 cases. Br J Dis Chest. 1960;54:202-209.
9 Garcia-Pachon E. Paradoxical movement of the lateral rib margin (Hoover sign) for detecting obstructive airway disease. Chest. 2002;122:651-655.
10 Godfrey S, Edwards RH, Campbell EJ, et al. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax. 1970;25:285-287.
11 Guarino JR, Guarino JC. Auscultatory percussion. J Gen Intern Med. 1994;9:71-74.
12 Hansen-Flaschen J, Nordberg J. Clubbing and hypertrophic osteoarthropathy. Clin Chest Med. 1987;8:287-298.
13 Kilburn KH, Asmundsson T, et al. Anteroposterior chest diameter in emphysema. Arch Intern Med. 1969;123:379-382.
14 Mahler DA, Snyder PE, Virgulto JA, et al. Positional dyspnea and oxygen desaturation related to carcinoma of the lung: Up with the good lung. Chest. 1983;83:826-827.
15 McAlister FA, Khan NA, Straus S, et al. Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am J Respir Crit Care Med. 2003;167:741-744.
16 McGee S. Percussion and physical diagnosis: Separating myth from science. Disease-a-Month. 1995;41:643-692.
17 Mueller RE, Petty TL, Filley GF, et al. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol. 1970;28:784-789.
18 O’Neill S, McCarthy DS. Postural relief of dyspnea in severe chronic airflow limitation: Relationship to respiratory muscle strength. Thorax. 1983;38:595-600.
19 Remolina C, Khan AU, Santiago TV, et al. Positional hypoxemia in unilateral lung disease. N Engl J Med. 1981;304:523-525.
20 Robin ED, McCauley RF. An analysis of platypnea orthodeoxia syndrome, including a “new” therapeutic approach. Chest. 1997;112:1449-1451.
21 Sakula A. Pierre Adolphe Piorry (1794–1879): Pioneer of percussion. Thorax. 1979;34:575-581.
22 Shamroth L. Personal experience. South Afr Med J. 1976;50:297-300.
23 Straus SE, McAlister FA, Sackett DL, Deeks JJ. The accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. JAMA. 2000;283:1853-1857.
24 Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1966;93:100-106.
25 Yernault JC, Bohadana AB. Chest percussion. Eur Respir J. 1995;8:1756-1760.