Chapter 12 Chemotherapy of infections
• Classification of antimicrobial drugs.
• Principles of optimal antimicrobial therapy.
• Use of antimicrobial drugs: choice; combinations; chemoprophylaxis and pre-emptive suppressive therapy; chemoprophylaxis in surgery.
• Special problems with antimicrobial drugs: resistance; superinfection; masking of infections.
History
Many substances that we now know to possess therapeutic efficacy were first used in the distant past. The Ancient Greeks used male fern, and the Aztecs Chenopodium, as intestinal anthelminthics. The Ancient Hindus treated leprosy with Chaulmoogra. For hundreds of years moulds have been applied to wounds, but, despite the introduction of mercury as a treatment for syphilis (16th century), and the use of cinchona bark against malaria (17th century), the history of modern rational chemotherapy did not begin until Ehrlich1 developed the idea from his observation that aniline dyes selectively stained bacteria in tissue microscopic preparations and could selectively kill them. He invented the word ‘chemotherapy’ and in 1906 he wrote:
The antimalarials pamaquin and mepacrine were developed from dyes and in 1935 the first sulfonamide, linked with a dye (Prontosil), was introduced as a result of systematic studies by Domagk.2 The results obtained with sulfonamides in puerperal sepsis, pneumonia and meningitis were dramatic and caused a revolution in scientific and medical thinking.
In 1928, Fleming3 accidentally rediscovered the long-known ability of Penicillium fungi to suppress the growth of bacterial cultures, but put the finding aside as a curiosity. His Nobel lecture in 1945 was prophetic for our current times: ‘It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body.’
In 1939, principally as an academic exercise, Florey4 and Chain5 undertook an investigation of antibiotics, i.e. substances produced by microorganisms that are antagonistic to the growth or life of other microorganisms.6 They prepared penicillin and confirmed its remarkable lack of toxicity.7 When the preparation was administered to a policeman with combined staphylococcal and streptococcal septicaemia there was dramatic improvement; unfortunately the manufacture of penicillin in the local pathology laboratory could not keep pace with the requirements (it was also extracted from the patient’s urine and re-injected); it ran out and the patient later succumbed to infection.
the magic bullets have lost some of their magic. One solution may be to find alternatives to antibiotics when resistance appears, but there is also an urgent need for new antibiotics to be developed. Few pharmaceutical companies are now involved in antibiotic development … The high cost of development, the prolonged safety evaluation, and the probable short duration of field use and the present tendency for any new compound to induce resistance all militate against major investment in new compounds.8
(See the review by Morel and Mossialos9 on how this perverse economic incentive could be turned around.)
Classification of antimicrobial drugs
A few antimicrobials have useful activity across several of these groups. For example, metronidazole inhibits obligate anaerobic bacteria as well as some protozoa that rely on anaerobic metabolic pathways (such as Trichomonas vaginalis).
Antimicrobial drugs have also been classified broadly into:
• Bacteriostatic, i.e. those that act primarily by arresting bacterial multiplication, such as sulfonamides, tetracyclines and chloramphenicol.
• Bactericidal, i.e. those which act primarily by killing bacteria, such as penicillins, aminoglycosides and rifampicin.
The classification is in part arbitrary because most bacteriostatic drugs are bactericidal at high concentrations, under certain incubation conditions in vitro, and against some bacteria. However, there is some clinical evidence for use of conventionally bactericidal drugs for infective endocarditis and meningitis.
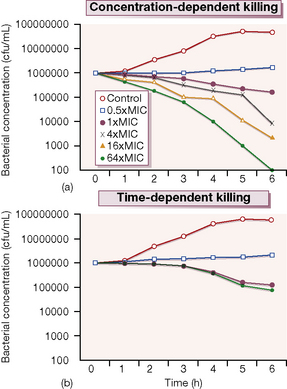
Probably more important is whether its antimicrobial effect is concentration-dependent or time-dependent. Examples of the former include the quinolones and aminoglycosides in which the outcome is related to the peak antibiotic concentration achieved at the site of infection in relation to the minimum concentration necessary to inhibit multiplication of the organism (the minimum inhibitory concentration, or MIC). These antimicrobials produce a prolonged inhibitory effect on bacterial multiplication (the post-antibiotic effect, or PAE) which suppresses growth until the next dose is given. In contrast, agents such as the β-lactams and macrolides have more modest PAEs and exhibit time-dependent killing; their concentrations should be kept above the MIC for a high proportion of the time between each dose (Fig. 12.1).
Figure 12.1 shows the results of an experiment in which a culture broth initially containing 106 bacteria per mL is exposed to various concentrations of two antibiotics, one of which exhibits concentration-dependent and the other time-dependent killing. The ‘control’ series contains no antibiotic, and the other series contain progressively higher antibiotic concentrations from 0.5 × to 64 × the MIC. Over 6 h incubation, the time-dependent antibiotic exhibits killing, but there is no difference between the 1 × MIC and 64 × MIC. The additional cidal effect of rising concentrations of the antibiotic which has concentration-dependent killing can be clearly seen.
Principles of antimicrobial chemotherapy
Make a diagnosis
It is inconsistent that the assessment of new antibiotics for therapeutic use is very much more rigorously controlled than is the introduction of diagnostic tests that direct their use – Gluud and Gluud propose a harmonised approach to the assessment and regulation of new diagnostic procedures in clinical microbiology.10
Remove barriers to cure,
e.g. drain abscesses, remove obstruction in the urinary tract and infected intravenous catheters.
Select the best drug
This involves consideration of the following factors:
• Specificity: indiscriminate use of broad-spectrum drugs promotes antimicrobial resistance and encourages opportunistic infections, e.g. with yeasts (see p. 167). At the beginning of treatment, empirical ‘best guess’ chemotherapy of reasonably broad spectrum must often be given because the susceptibility and identity of the responsible microbe is uncertain. The spectrum may be narrowed once these are microbiologically confirmed.
• Pharmacokinetic factors: to ensure that the chosen drug is capable of reaching the site of infection in adequate amounts, e.g. by crossing the blood–brain barrier.
• The patient: who may previously have exhibited allergy to a group of antimicrobials or whose routes of elimination may be impaired, e.g. by renal disease.
Prophylactic chemotherapy
for surgical and dental procedures should be of very limited duration, often only a single large dose being given (see p. 167).
Use of antimicrobial drugs
Choice
When considering ‘best guess’ therapy, infections may be categorised as those in which:
1. Choice of antimicrobial follows automatically from the clinical diagnosis because the causative organism is always the same, and is virtually always sensitive to the same drug, e.g. meningococcal septicaemia (benzylpenicillin), some haemolytic streptococcal infections, e.g. scarlet fever, erysipelas (benzylpenicillin), typhus (tetracycline), leprosy (dapsone with rifampicin).
2. The infecting organism is identified by the clinical diagnosis, but no safe assumption can be made as to its sensitivity to any one antimicrobial, e.g. tuberculosis.
3. A single infecting organism is not identified by the clinical diagnosis, e.g. in urinary tract infection or abdominal surgical wound infection.
Particularly in the second and third categories, choice of an antimicrobial may be guided by:
Combinations
• To avoid the development of drug resistance, especially in chronic infections where many bacteria are present (hence the chance of a resistant mutant emerging is high), e.g. tuberculosis.
• To broaden the spectrum of antibacterial activity: (1) in a known mixed infection, e.g. peritonitis following gut perforation, or (2) where the infecting organism cannot be predicted but treatment is essential before a diagnosis has been reached, e.g. septicaemia complicating neutropenia or severe community-acquired pneumonia.
• To obtain potentiation (or ‘synergy’), i.e. an effect unobtainable with either drug alone, e.g. penicillin plus gentamicin for enterococcal endocarditis.
• To enable reduction of the dose of one component and hence reduce the risks of adverse drug reactions, e.g. flucytosine plus amphotericin B for Cryptococcus neoformans meningitis.
Chemoprophylaxis and pre-emptive suppressive therapy
• True prevention of primary infection: rheumatic fever,11 recurrent urinary tract infection.
• Prevention of opportunistic infections, e.g. due to commensals getting into the wrong place (coagulase-negative staphylococcal prosthetic joint infection from the patient’s skin during the operation, and peritonitis after bowel surgery). Note that these are both high-risk situations of short duration; prolonged administration of drugs before surgery would result in the areas concerned (mouth and bowel) being colonised by drug-resistant organisms with potentially disastrous results (see below). Immunocompromised patients can benefit from longer-term chemoprophylaxis, e.g. of Gram-negative septicaemia complicating neutropenia with an oral quinolone, or of Pneumocystis carinii pneumonia with co-trimoxazole.
• Suppression of existing infection before it causes overt disease, e.g. tuberculosis, malaria.
• Prevention of acute exacerbations of a chronic infection, e.g. bronchitis, cystic fibrosis.
• Prevention of spread among contacts (in epidemics and/or sporadic cases). Spread of influenza A can be partially prevented by amantadine; rifampicin may be used when there is a case of meningococcal meningitis in a family.
Chemoprophylaxis in surgery
The principles governing use of antimicrobials in this context are as follows.
Chemoprophylaxis is justified:
• When the risk of infection is high because of large numbers of bacteria at the operative site, e.g. in operations on the large bowel.
• When the risk of infection is low but the consequences of infection would be disastrous, e.g. infection of prosthetic joints or prosthetic heart valves.
• When the risks of infection are low but randomised controlled trials have shown the benefits of prophylaxis to outweigh the risks, e.g. single-dose antistaphylococcal prophylaxis for uncomplicated hernia and breast surgery.
Antimicrobials should be given
1. Colorectal surgery: there is a high risk of infection with Escherichia coli, Clostridium spp., streptococci and Bacteroides spp. which inhabit the gut (co-amoxiclav or piperacillin).
2. Gastroduodenal surgery: colonisation of the stomach with gut organisms occurs especially when acid secretion is low, e.g. in gastric malignancy, following use of a histamine H2-receptor antagonist or following previous gastric surgery (co-amoxiclav).
3. Gynaecological surgery: because the vagina contains Bacteroides spp. and other anaerobes, streptococci and coliforms (co-amoxiclav).
4. Leg amputation: because there is a risk of gas gangrene in an ischaemic limb and the mortality is high (benzylpenicillin, or metronidazole for the patient with allergy to penicillin).
5. Insertion of prostheses – joints, heart valves, vessels: chemoprophylaxis is justified because infection (Staphylococcus aureus, coagulase-negative staphylococci and coliforms are commonest) often means that the artificial joint, valve or vessel must be replaced. Single perioperative doses of appropriate antibiotics with plasma elimination half-lives of several hours (e.g. cefotaxime) are adequate, but if short half-life agents are used (e.g. flucloxacillin) several doses should be given during the first 24 h.
6. General surgery: clearance of Staphylococcus aureus from the anterior nares of carriers with mupirocin is known to reduce the incidence of wound infection by about a half, and this treatment has recently been shown in one high-quality trial to be effective when targeted only at staphylococcal nasal carriers who were detected by screening nasal swabs with a rapid real-time PCR assay. This strategy is much more potentially attractive than the alternative of treating all patients preoperatively, which has been demonstrated not to reduce infection rates significantly while maximising unnecessary mupirocin exposure.
Problems with antimicrobial drugs
Resistance
Microbial resistance to antimicrobials is a matter of great importance; if sensitive strains are supplanted by resistant ones, then a valuable drug may become useless. Just as ‘some are born great, some achieve greatness, and some have greatness thrust upon them’,12 so microorganisms may be naturally (‘born’) resistant, ‘achieve’ resistance by mutation or have resistance ‘thrust upon them’ by transfer of plasmids and other mobile genetic elements.
Problems of antimicrobial resistance have burgeoned during the past few decades in most countries of the world, both in and out of hospital, and fortunately a number of international bodies have been established devoted to the reduction of resistance worldwide: ‘Our mission is clear: we must work together to preserve the power of antimicrobials and to return these miracle agents to their rightful position as effective treatments of disease’ (Dr Stuart Levy, http://www.tufts.edu/med/apua/).
Mechanisms of resistance
• Naturally resistant strains. Some bacteria are innately resistant to certain classes of antimicrobial agent, e.g. coliforms and many other Gram-negative bacteria possess outer cell membranes which protect their cell walls from the action of certain penicillins and cephalosporins. Facultatively anaerobic bacteria (such as Escherichia coli) lack the ability to reduce the nitro group of metronidazole which therefore remains in an inactive form.
• Spontaneous mutation brings about organisms with novel antibiotic resistance mechanisms. If these cells are viable, in the presence of the antimicrobial agent selective multiplication of the resistant strain occurs so that it eventually dominates.
• Transmission of genes from other organisms is the commonest and most important mechanism. Genetic material may be transferred, e.g. in the form of plasmids which are circular strands of DNA that lie outwith the chromosomes and contain genes capable of controlling various metabolic processes including formation of β-lactamases (that destroy some penicillins and cephalosporins), and enzymes that inactivate aminoglycosides. Alternatively, genetic transfer may occur through bacteriophages (viruses which infect bacteria), particularly in the case of staphylococci.
Resistance is mediated most commonly by the production of enzymes that modify the drug, e.g. aminoglycosides are phosphorylated, β-lactamases hydrolyse penicillins. Other mechanisms include decreasing the passage into or increasing the efflux of drug from the bacterial cell (e.g. meropenem resistance in Pseudomonas aeruginosa), modification of the target site so that the antimicrobial binds less effectively (e.g. methicillin resistance in staphylococci), and bypassing of inhibited metabolic pathways (e.g. resistance to trimethoprim in many bacteria). More is becoming known of the complex molecular systems which control expression of antimicrobial resistance, and this knowledge should soon lead to novel compounds that inhibit resistance mechanisms at the genetic and phenotypic levels (see Stix13 for an example).
Limitation of resistance
to antimicrobials may be achieved by ‘antibiotic stewardship’ which includes:
• Avoidance of indiscriminate use by ensuring that the indication for, and the dose and duration of treatment are appropriate; studies of hospital and domiciliary prescribing have shown that up to 35% of antimicrobial courses administered in the UK may be inappropriate – either not indicated at all, or administered for too long. Performing ward rounds on areas of the hospital with high rates of antibiotic use (e.g. intensive care units, acute surgical wards) to assess the justification for treatment of individual patients and to educate other doctors in limiting unnecessary courses of antibiotics has recently become an important role for clinical microbiologists.
• Restricting use of antimicrobial combinations to appropriate circumstances, e.g. tuberculosis.
• Constant monitoring of resistance patterns in a hospital or community (changing recommended antibiotics used for empirical treatment when the prevalence of resistance becomes high), and good infection control in hospitals (e.g. isolation of carriers, hand hygiene practices for ward staff) to prevent the spread of resistant bacteria.
• Restricting drug use, e.g. delaying the emergence of resistance by limiting the use of the newest member of a group of antimicrobials so long as the currently used drugs are effective; restricting use of a drug may become necessary where it promotes the proliferation of resistant strains.
• Avoiding transmission of multiply resistant bacteria among patients and staff in hospital, by health-care workers performing careful hand hygiene between each patient contact, and through identification and isolation of carriers.
‘The over-riding principle of medicine is “do no harm”, yet, in the case of antibiotics, harm is inevitable, for use (even appropriate usage) selects for resistance, complicating the treatment of future patients.’14
Doctors are encouraged to avoid use of antimicrobial agents whenever possible, and international efforts are being made to educate the general public not to expect an antibiotic prescription for minor ailments such as coughs and colds (see, for example, http://www.biomedcentral.com/1471-2296/10/20 and http://ecdc.europa.eu/en/EAAD/Pages/Home.aspx/).
Evidence is accumulating that resistance rates do not rise inevitably and irreversibly (see page 169). In both hospital and domiciliary practice, reductions in antibiotic usage are often shown to be followed by reductions in the prevalence of microbial resistance, although there can be a ‘lag’ of months or years. The situation is sometimes complicated by the phenomenon of ‘linked multiple resistance’ whereby the genes coding resistance mechanisms to several antibiotics are carried on the same genetic elements (e.g. plasmid). In this case, use of any of these antibiotics will select for increased resistance via all the mechanisms carried by the plasmid.
Superinfection
Antibiotic-associated (or Clostridium difficile-associated) colitis
is an example of a superinfection. It is caused by alteration of the normal bowel flora, which allows multiplication of Clostridium difficile which releases several toxins that damage the mucosa of the bowel and promote excretion of fluid. Almost any antimicrobial may initiate this condition, but the drugs most commonly reported today are cephalosporins and quinolones (e.g. ciprofloxacin). It takes the form of an acute colitis (pseudomembranous colitis) with diarrhoeal stools containing blood or mucus, abdominal pain, leucocytosis and dehydration. A history of antibiotic use in the previous 3 weeks, even if the drug therapy has been stopped, should alert the physician to the diagnosis which is confirmed by detection of C. difficile toxin in the stools and typical appearances on proctosigmoidoscopy. Mild cases usually respond to discontinuation of the offending antimicrobial, allowing re-establishment of the patient’s normal bowel flora, but more severe cases merit treatment with oral metronidazole. Some strains have been associated with particularly severe disease and have caused large outbreaks in hospitals – combined therapy with oral vancomycin and parenteral metronidazole plus intensive care support is required for the most serious cases. Other therapeutic and preventative measures of unproven efficacy include intracolonic instillation of vancomycin, intravenous immunoglobulin and oral probiotics. A variety of investigational antibiotics is also being assessed, such as rifaximin and fidaxomicin (an RNA polymerase inhibitor), and several compounds taken by mouth that may bind and inactivate faecal toxin. Diarrhoea in some cases can be intractable, and desperate measures have included instillation of microbiologically screened donor faeces in an attempt to restore a normal balance of the gut flora – in some cases with surprisingly good response rates of over 80% in therapeutic trials.15
Drugs of choice
For detailed guidance on the choice of antimicrobial drugs for particular infections the reader is referred to Chapters 13 and 14, and to a variety of contemporary clinical sources, including textbooks of microbiology and infectious diseases.
In previous editions we have referred to the current ‘Medical Letter on Drugs and Therapeutics’ and ‘Treatment Guidelines from the Medical Letter’ (USA) editions from 2000 to 2010 (current version available online at: http://www.medicalletter.org/downloads/t94-1.pdf), and this is still valuable although it is of most relevance to North American practice.
Detailed guidance, intended for use by primary care physicians in England and including evidence-based recommendations on antibiotic choice for particular infections and extensive reference lists, is available via the Health Protection Agency website (http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1279888711402). We also recommend section 5 of the Electronic British National Formulary (http://bnf.org/bnf/bnf/current/2071.htm)
Tables on drugs for viruses, fungi, protozoa and helminths are provided in Chapter 15.
Ada G. Vaccines and vaccination. N. Engl. J. Med.. 2001;345:1042–1053.
Alliance for the Prudent Use of Antibiotics (APUA), The APUA website has a wide range of articles and useful links relating to the control of antimicrobial resistance worldwide. Available at: http://www.tufts.edu/med/apua/ (accessed October 2011)
Anon. Antibacterial prophylaxis for orthopaedic surgery. Drug Ther. Bull. 2001;39:43–46.
Anon. Antibacterial prophylaxis in surgery: 1. Gastrointestinal and biliary surgery. Drug Ther. Bull.. 2003;41:84–86.
Anon. Antibacterial prophylaxis in surgery: 3. Arterial surgery in the abdomen, pelvis and lower limbs. Drug Ther. Bull.. 2004;42:43–47.
Anon. Antibacterial prophylaxis in surgery: 2. Urogenital, obstetric and gynaecological surgery. Drug Ther. Bull.. 2004;42:9–12.
Anon. Major changes in endocarditis prophylaxis for dental, GI and GU procedures. Medical Letter. 2007;49:99–100.
Arias C.A., Murray B.E. Antibiotic-resistant bugs in the 21st century – a clinical super-challenge. N. Engl. J. Med.. 2009;360:439–443.
Aymes S. Treatment of staphylococcal infection. Prescriptions must be part of a package that includes infection control. Br. Med. J.. 2005;330:976–977.
Beynon R.P., Bahl V.K., Prendergast B.D. Infective endocarditis. Br. Med. J.. 2006;333:334–339. Available at: http://www.bmj.com/content/333/7563/334.full.pdf+html (accessed 30.10.2011)
Bode L.G., Kluytmans J.A., Wertheim H.F., et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med.. 2010;362:9–17.
Boudma L., Luyt C.E., Tubach F., et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomized controlled trial. Lancet. 2010;375:463–474.
Colebrook I., Kenny M. Treatment with prontosil for puerperal infections. Lancet. 1939;2:1319. (a classic paper)
Connaughton M., Commentary: controversies in NICE guidance on infective endocarditis. 2008. Available at http://www.bmj.com/content/336/7647/771.full.pdf (accessed October 2011)
Corwin P., Toop L., McGeoch G., et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. Br. Med. J.. 2005;330:129–132.
Dancer S.J. How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet Infect. Dis.. 2004;4:611–619.
Deleo F.R., Otto M., Kreiswirth B.N., Chambers H.F. Community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568.
Fishman J.A. Infection in organ-transplant recipients. N. Engl. J. Med.. 2007;357:2601–2614.
Fletcher C. First clinical use of penicillin. Br. Med. J.. 1984;289:1721–1723. (a classic paper)
Gluud C.G., Gluud L.L. Evidence based diagnostics. Br. Med. J.. 2005;330:724–726.
Health Protection Agency. The ‘Antimicrobial Resistance’ section of the website of the UK Health Protection Agency (http://www.hpa.org.uk/infections/topics_az/antimicrobial_resistance/menu.htm (accessed October 2011)) is a valuable resource of contemporary background information on the prevalence and epidemiology of infectious diseases and antimicrobial resistance in the UK. Also on the HPA website, quarterly-updated reports on MRSA bacteraemia and Clostridium difficile diarrhoea rates in England and Wales can be found at: http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1259151891722 (accessed October 2011)
Kluytmans J., Struelens M. Meticillin resistant Staphylococcus aureus in the hospital. Br. Med. J.. 2009;338:532–537.
Kwiatkowski D. Susceptibility to infection. Br. Med. J.. 2000;321:1061–1065.
Leibovici L., Shraga B., Andreassen S., et al. How do you choose antibiotic treatment? Br. Med. J.. 1999;318:1614–1616.
Livermore D.M. Minimising antibiotic resistance. Lancet Infect. Dis.. 2005;5:450–459.
Loudon I. Puerperal fever, the streptococcus, and the sulphonamides, 1911–1945. Br. Med. J.. 1987;295:485–490.
Morel C., Mossailos. Stoking the antibiotic pipeline. Br. Med. J.. 2010;340:1115–1118.
Pitout J.D. The latest threat in the war on antimicrobial resistance (NMD beta-lactamases). Lancet Infect. Dis.. 2010;10:578–579.
Pitout J.D. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70:313–333.
Queenan A.M., Bush K. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev.. 2007;20:440–458.
Richie R., Wray D., Stoken T., Prophylaxis against infective endocarditis: summary of NICE guidance. Available online:. 2008. http://www.bmj.com/content/336/7647/770.full.pdf (accessed October 2011)
Ryan E.T., Wilson M.E., Kain K.C. Illness after international travel. N. Engl. J. Med.. 2002;347:505–516.
Safdar N., Handelsman J., Maki D.G. Does combination antimicrobial chemotherapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect. Dis.. 2004;4:519–527.
Shannon-Lowe J., Matheson N.J., Cooke F.J., Aliyu S.H. Prevention and medical management of Clostridium difficile infection. Br. Med. J.. 2010;340:641–646.
Stix G. An antibiotic resistance fighter. New Sci.. 2006:80–83. April
1 Paul Ehrlich (1854–1915), the German scientist who was the pioneer of chemotherapy and discovered the first cure for syphilis (Salvarsan).
2 Gerhard Domagk (1895–1964), bacteriologist and pathologist, who made his discovery while working in Germany. Awarded the 1939 Nobel prize for Physiology or Medicine, he had to wait until 1947 to receive the gold medal because of Nazi policy at the time.
3 Alexander Fleming (1881–1955). He researched for years on antibacterial substances that would not be harmful to humans. His findings on penicillin were made at St Mary’s Hospital, London. See http://nobelprize.org/nobel_prizes/medicine/laureates/1945/fleming-lecture.pdf (accessed October 2011).
4 Howard Walter Florey (1898–1969), Professor of Pathology at Oxford University.
5 Ernest Boris Chain (1906–1979), biochemist. Fleming, Florey and Chain shared the 1945 Nobel prize for Physiology or Medicine.
6 Strictly, the definition should refer to substances that are antagonistic in dilute solution because it is necessary to exclude various common metabolic products such as alcohols and hydrogen peroxide. The term ‘antibiotic’ is now commonly used for antimicrobial drugs in general, and it would be pedantic to object to this. Today, many commonly used antibiotics are either fully synthetic or are produced by major chemical modification of naturally produced molecules: hence, ‘antimicrobial agent’ is perhaps a more accurate term, but ‘antibiotic’ is much the commoner usage.
7 The importance of this discovery for a nation at war was obvious to these workers but the time, July 1940, was unpropitious, for invasion was feared. The mood of the time is shown by the decision to ensure that, by the time invaders reached Oxford, the essential records and apparatus for making penicillin would have been deliberately destroyed; the productive strain of Penicillium mould was to be secretly preserved by several of the principal workers smearing the spores of the mould into the linings of their ordinary clothes where it could remain dormant but alive for years; any member of the team who escaped (wearing the right clothes) could use it to start the work again (Macfarlane G 1979 Howard Florey, Oxford).
8 Lord Soulsby of Swaffham Prior 2005 Resistance to antimicrobials in humans and animals. British Medical Journal 331:1219.
9 Morel C M, Mossialos E 2010 Stoking the antibiotic pipeline. British Medical Journal 340:1115–1118
10 Gluud C G, Gluud L L 2005 Evidence based diagnostics. British Medical Journal 330:724–726
11 Rheumatic fever is caused by a large number of types of Group A streptococci and immunity is type-specific. Recurrent attacks are commonly due to infection with different strains of these, all of which are sensitive to penicillin and so chemoprophylaxis is effective. Acute glomerulonephritis is also due to Group A streptococci but only a few types cause it, so that natural immunity is more likely to protect and second attacks are rare. Therefore, chemoprophylaxis is not used (see also p. 168).
12 Malvolio in Twelfth Night, act 2 scene 5, by William Shakespeare (1564–1616).
13 Stix G 2006 An antibiotic resistance fighter. New Scientist April: 80–83
14 Livermore D M 2006 Minimising antibiotic resistance. Lancet Infectious Diseases 5:450–459.
15 Garborg K, Waagsbø B, Stallemo A et al 2010 Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scandinavian Journal of Infectious Diseases 42:857–861.