Chapter 14 Chemotherapy of bacterial infections
It also discusses mycobacteria, that infect many sites.
Infection of the blood
Septicaemia
• Septicaemia accompanied by a spreading rash that does not blanch with pressure should be assumed to be meningococcal, and the patient must be referred to hospital urgently (after an immediate parenteral dose of benzylpenicillin): ceftriaxone.
• Community-acquired pneumonia: co-amoxiclav + clarithromycin.
• When septicaemia follows gastrointestinal or genital tract surgery, Escherichia coli (or other coliforms), anaerobic bacteria, e.g. Bacteroides, streptococci or enterococci are likely pathogens: piperacillin-tazobactam or gentamicin plus benzylpenicillin plus metronidazole [meropenem, plus vancomycin if MRSA is a risk].
• Septicaemia related to urinary tract infection usually involves Escherichia coli (or other Gram-negative bacteria), enterococci: gentamicin plus benzylpenicillin or piperacillin-tazobactam alone [meropenem plus vancomycin].
• Neonatal septicaemia is usually due to Lancefield Group B streptococcus or coliforms: benzylpenicillin plus gentamicin [vancomycin + ceftazidime].
• Staphylococcal septicaemia may be suspected where there is an abscess, e.g. of bone or lung, or with acute infective endocarditis or infection of intravenous catheters: high-dose flucloxacillin [vancomycin]. Uncomplicated Staphylococcus aureus bacteraemia should be treated for 14 days to reduce the risk of metastatic infection: patients with prolonged bacteraemia or who fail to settle promptly should be considered for treatment as for staphylococcal endocarditis.
• Severe cellulitis, bites and necrotising fasciitis accompanied by septicaemia should be treated with optimal cover for Lancefield Group A streptococcus, anaerobes and coliforms: piperacillin-tazobactam + clindamycin [meropenem + clindamycin].
• Septicaemia in patients rendered neutropenic by cytotoxic drugs frequently involves coliforms and Pseudomonas spp. translocating to the circulation directly from the bowel, while coagulase-negative staphylococci also commonly arise from central venous catheter infection: piperacillin-tazobactam, sometimes plus vancomycin.
• Staphylococcal toxic shock syndrome occurs in circumstances that include healthy women using vaginal tampons, in abortion or childbirth, and occasionally with skin and soft tissue infection and after packing of body cavities, such as the nose. Flucloxacillin is used, and elimination of the source by removal of the tampon and drainage of abscesses is also important.
Infection of the throat
There is no general agreement as to whether chemotherapy should be employed in mild sporadic sore throat, and expert reviews reflect this diversity of opinion.1,2,3 The disease usually subsides in a few days, septic complications are uncommon and rheumatic fever rarely follows. It is reasonable to withhold penicillin unless streptococci are cultured or the patient develops a high fever: some primary care physicians take a throat swab and give the patient a WASP prescription for penicillin which is only filled if streptococci are isolated. Severe sporadic or epidemic sore throat is likely to be streptococcal and the risk of these complications is limited by phenoxymethylpenicillin by mouth (clarithromycin or an oral cephalosporin in the penicillin-allergic), given, ideally, for 10 days, although compliance is bad once the symptoms have subsided and 5 days should be the minimum objective. Azithromycin (500 mg daily p.o.) for 3 days is effective as long as the streptococci are susceptible, with improved compliance, and 5-day courses of oral cephalosporins are as effective as 10 days of penicillin. Do not use amoxicillin if the circumstances suggest pharyngitis due to infectious mononucleosis, as the patient is very likely to develop a rash (see p. 176). In a closed community, chemoprophylaxis of unaffected people to stop an epidemic may be considered, for instance with oral phenoxymethylpenicillin 125 mg 12-hourly.
Chemoprophylaxis
Chemoprophylaxis of streptococcal (Group A) infection with phenoxymethylpenicillin is necessary for patients who have had one attack of rheumatic fever. Continue for at least 5 years or until aged 20 years, whichever is the longer period (although some hold that it should continue for life). Chemoprophylaxis should be continued for life after a second attack of rheumatic fever. A single attack of acute nephritis is not an indication for chemoprophylaxis. Ideally, chemoprophylaxis should continue throughout the year but, if the patient is unwilling to submit to this, cover at least the colder months (see also footnote p. 167).
Infection of the bronchi, lungs and pleura
Pneumonias
Pneumonia in previously healthy people (community acquired)
Pneumonia in immunocompromised patients
• Until the pathogen is known the patient should receive broad-spectrum antimicrobial treatment, such as an aminoglycoside plus ceftazidime.
• Aerobic Gram-negative bacilli, e.g. Enterobacteriaceae, Klebsiella spp., are pathogens in half of the cases, especially in neutropenic patients, and respond to piperacillin-tazobactam or ceftazidime. These and Pseudomonas aeruginosa may respond better with addition of an aminoglycoside.
• The fungus Pneumocystis carinii is an important respiratory pathogen in patients with deficient cell-mediated immunity; treat with co-trimoxazole 120 mg/kg daily by mouth or i.v. in two to four divided doses for 14 days, as modified by serum assay, or with pentamidine (see p. 236).
Endocarditis
Principles for treatment
• Use high doses of bactericidal drugs because the organisms are difficult to access in avascular vegetations on valves.
• Give drugs parenterally and preferably by i.v. bolus injection to achieve the necessary high peak concentration to penetrate the vegetations.
• Examine the infusion site daily and change it regularly to prevent opportunistic infection, which is usually with coagulase-negative staphylococci or fungi. Alternatively, use a central subclavian venous catheter.
• Continue therapy, usually for 2–4 weeks, and, in the case of infected prosthetic valves, 6 weeks. Prolonged courses may also be indicated for patients infected with enterococci or other strains with penicillin minimum inhibitory concentrations (MICs) above 0.5 mg/L, whose presenting symptoms have been present for over 6 weeks, for those with large vegetations, and those whose clinical symptoms and signs are slow to settle after treatment has started. Highly susceptible streptococcal endocarditis (penicillin MIC of 0.1 mg/L or below) can be treated successfully with 2 week courses.
• Valve replacement may be needed at any time during and after antibiotic therapy if cardiovascular function deteriorates or the infection proves impossible to control.
• Adjust the dose according to the sensitivity of the infecting organism – use the minimum inhibitory concentration test (MIC: see p. 163).
Dose regimens
1. Initial (‘best guess’) treatment should comprise benzylpenicillin (7.2 g i.v. daily in six divided doses), plus gentamicin (1 mg/kg body-weight 8-hourly – synergy allows this dose of gentamicin and minimises risk of adverse effects). Regular serum gentamicin assay is vital: trough concentrations should be below 1 mg/L and peak concentrations 3–5 mg/L; if Staphylococcus aureus is suspected, high-dose flucloxacillin plus rifampicin should be used. Patients allergic to penicillin and those with intracardiac prostheses or suspected MRSA infection should receive vancomycin plus rifampicin plus gentamicin. Patients presenting acutely (suggesting infection with Staphylococcus aureus) should receive flucloxacillin (8–12 g/day in four to six divided doses) plus gentamicin.
2. When an organism is identified and its sensitivity determined:
Meningitis
Drugs must be given i.v. in high dose
The regimens below provide the recommended therapy, with alternatives for patients allergic to first choices, and septic shock requires appropriate management (see p. 191). Intrathecal therapy is now considered unnecessary (except for neurosurgical infections in association with indwelling CSF drains and shunts) and can be dangerous, e.g. encephalopathy with penicillin.
Initial therapy
Initial therapy should be sufficient to kill all pathogens, which are likely to be:
Infection of the intestines
(For Helicobacter pylori, see p. 533)
Both wit and truth are contained in the aphorism that ‘travel broadens the mind but opens the bowels’. Antimicrobial therapy should be reserved for specific conditions with identified pathogens where benefit has been shown; acute diarrhoea can be caused by bacterial toxins in food, dietary indiscretions, anxiety and by drugs as well as by infection. Even if diarrhoea is infective, it may be due to viruses; or, if bacterial, antimicrobial agents may not reduce the duration of symptoms and may aggravate the condition by permitting opportunistic infection and encouraging Clostridium difficile-associated diarrhoea. Maintaining water and electrolyte balance either orally or by i.v. infusion with a glucose–electrolyte solution, and administration of an antimotility drug (except in small children, and those with with bloody, dysenteric stools, and in Clostridium difficile infection), are the mainstays of therapy in such cases (see Oral rehydration therapy, p. 537). Some specific intestinal infections do benefit from chemotherapy:
A carrier state
develops in a few individuals who have no symptoms of disease but who can infect others.4 Organisms reside in the biliary or urinary tracts. Ciprofloxacin in high dose by mouth for 3–6 months may be successful for what can be a very difficult problem, requiring investigation for urinary tract abnormalities or even cholecystectomy.
Escherichia coli
is a normal inhabitant of the bowel but some enterotoxigenic strains are pathogenic and are frequently a cause of travellers’ diarrhoea. A quinolone, e.g. ciprofloxacin, azithromycin or the non-absorbable rifampicin-relative rifaximin are alternatives (see Travellers’ diarrhoea, p. 537). Prophylactic use of an antimicrobial is not usual but, should it be deemed necessary, a quinolone or rifaximin is effective.
Suppression of bowel flora
is thought by some to be useful in hepatic encephalopathy. Here, absorption of products of bacterial breakdown of protein (ammonium, amines) in the intestine leads to cerebral symptoms and even to coma. In acute coma, neomycin 6 g daily should be given by gastric tube; as prophylaxis, 1–4 g/day may be given to patients with protein intolerance who fail to respond to dietary protein restriction (see also Lactulose, p. 551).
Infection of the urinary tract
(Excluding sexually transmitted infections)
Drug treatment of urinary tract infection falls into several categories:
Upper urinary tract infection
Upper or lower tract infection with extended-spectrum β-lactamase (ESBL) coliform strains has become more common in some locales, even in patients with no prior hospital contact (see page 185). Such bacteria are usually resistant also to ciprofloxacin, parenteral cephalosporins and gentamicin. Parenteral meropenem, ertapenem or amikacin, or oral pivmecillinam or fosfomycin may be effective.
Special drugs for urinary tract infections
Nitrofurantoin,
a synthetic antimicrobial, is active against the majority of urinary pathogens except pseudomonads, and has increased in importance recently because it has retained activity against a useful proportion of urinary tract coliforms that have acquired resistance to trimethoprim, oral β-lactams and quinolones. It is well absorbed from the gastrointestinal tract and is concentrated in the urine (t½ 1 h), but plasma concentrations are too low to treat infection of kidney tissue. Excretion is reduced when there is renal insufficiency, rendering the drug both more toxic and less effective. Adverse effects include nausea and vomiting (much reduced with the macrocrystalline preparation) and diarrhoea. Peripheral neuropathy occurs especially in patients with significant renal impairment, in whom the drug is contraindicated. Allergic reactions include rashes, generalised urticaria and pulmonary infiltration with lung consolidation or pleural effusion. Nitrofurantoin is safe in pregnancy, except near to term (because it may cause neonatal haemolysis), and it must be avoided in patients with glucose-6-phosphate dehydrogenase deficiency (see p. 101).
Genital tract infections
Mycobacterial infections
Pulmonary tuberculosis
Principles of antituberculosis therapy
• Kill a large number of actively multiplying bacilli: isoniazid achieves this.
• Treat persisters, i.e. semi-dormant bacilli that metabolise slowly or intermittently: rifampicin and pyrazinamide are the most efficacious.
• Prevent the emergence of drug resistance by multiple therapy to suppress single-drug-resistant mutants that may exist de novo or emerge during therapy: isoniazid and rifampicin are best.
• Combined formulations are used to ensure that poor compliance does not result in monotherapy with consequent drug resistance.
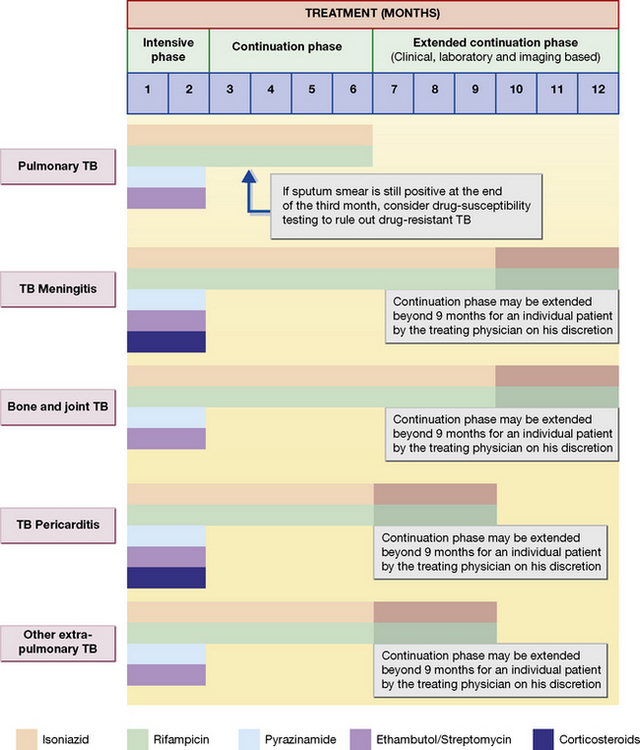
Most contemporary regimens employ an initial intensive phase with rifampicin, isoniazid, pyrazinamide with or without ethambutol, to reduce the bacterial load as rapidly as possible (usually for 2 months), followed by a continuation phase with rifampicin and isoniazid given for at least 4 months (Fig. 14.1).
1. An unsupervised regimen of daily dosing comprising isoniazid and rifampicin for 6 months, plus pyrazinamide for the first 2 months.
2. A supervised (directly observed therapy, DOT) regimen for patients who cannot be relied upon to comply with treatment, comprising thrice-weekly dosing with isoniazid and rifampicin for 6 months, plus pyrazinamide for the first 2 months (isoniazid and pyrazinamide are given in higher dose than in the unsupervised regimen). With both of the above regimens, ethambutol by mouth or streptomycin i.m. should be added for the first 2 months if there is a likelihood of drug-resistant organisms, or if the patient is severely ill with extensive active lesions. Ethambutol should not be administered in small children as they are unable to report visual side-effects.
All of the regimens are highly effective, with relapse rates of 1–2% in those who continue for 6 months; even if patients default after, say, 4 months, tuberculosis can be expected to recur in only 10–15%. Drug resistance seldom develops with any of these regimens.
Special problems
Drug resistant organisms
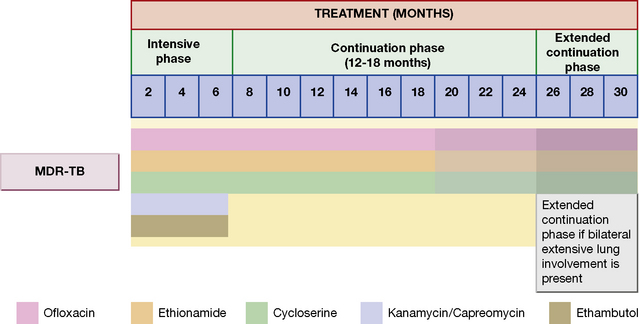
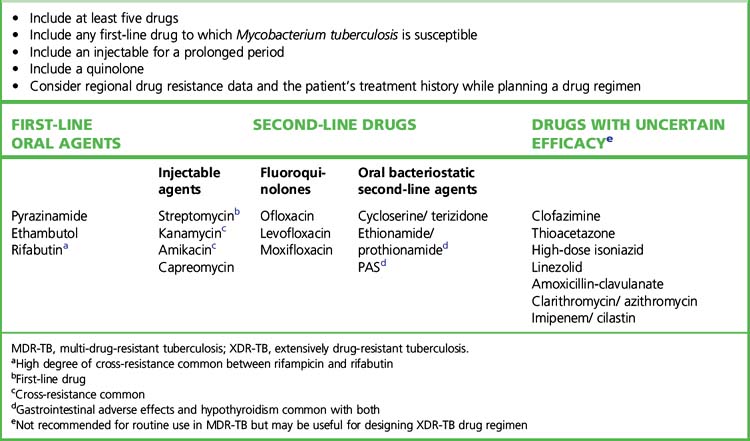
Initial drug resistance occurs in about 4% of isolates in the UK, usually to isoniazid. Patients with multiply drug-resistant tuberculosis, i.e. resistant to rifampicin and isoniazid at least, should be treated with three or four drugs to which the organisms are sensitive, and treatment should extend for 12–24 months after cultures become negative (Table 14.1 and Fig. 14.2). Treatment of such cases requires expert management.
Table 14.1 General principles for MDR-TB and XDR-TB drug regimen according to drug-susceptibility testing
Chemoprophylaxis
• primary, i.e. the giving of antituberculous drugs to uninfected but exposed individuals, which is seldom justified, or
• secondary, which is the treatment of infected but symptom-free individuals, e.g. those known to be in contact with the disease and who develop a positive tuberculin reaction. Secondary chemoprophylaxis may be justified in children under the age of 3 years because they have a high risk of disseminated disease; isoniazid alone for 6–9 months may be used as there is little risk of resistant organisms emerging because the organism load is low. Shorter treatment regimens with an alternative drug (rifampicin for 4 months or 3 months) or drug combinations (rifampicin plus isoniazid for 3 months) have better adherence rates. Combined use of rifampicin and pyrazinamide for 2 months in HIV-negative contacts is not recommended as it produces severe drug induced hepatitis.
Extrapulmonary tuberculosis
The principles of treatment, i.e. multiple therapy and prolonged follow-up, are the same as for pulmonary tuberculosis (see Fig. 14.1). Many chronic tuberculosis lesions may be relatively inaccessible to drugs as a result of avascularity, so treatment frequently has to be prolonged and dosage high, especially if damaged tissue cannot be removed by surgery, e.g. tuberculosis of bones.
Bone and joint tuberculosis
Six to nine months drug regimens containing rifampicin are effective (see Fig. 14.1). Surgery is indicated when chemotherapy fails with evidence of ongoing infection and for relief of cord compression with persistent or recurrent neurological deficits or instability of the spine.
Adrenal steroid and tuberculosis
Corticosteroids are administered in adrenal gland involvement with Addison’s disease, in meningeal and pericardial tuberculosis (see Fig. 14.1).
Antituberculosis drugs
Rifampicin
Interactions
Rifampicin is a powerful enzyme inducer and speeds the metabolism of numerous drugs, including warfarin, steroid contraceptives, narcotic analgesics, oral antidiabetic agents, phenytoin and dapsone. Appropriate increase in dosage, and alternative methods of contraception, are required to compensate for increased drug metabolism (see also paracetamol overdose, p. 246).
Rifaximin
is a semi-synthetic rifamycin that is not absorbed from the gastrointestinal tract (less than 0.4%). Because of the very high faecal concentrations achieved after a 400-mg oral dose (about 8000 micrograms/g faeces), it has broad activity against the common bacterial causes of travellers’ diarrhoea and has proved as effective as an oral quinolone or azithromycin (see p. 537), and adverse effects are rare. Efficacy of rifaximin treatment in acute hepatic encephalopathy is well documented. Its protective effect against breakthrough episodes of hepatic encephalopathy along with lactulose on a long-term basis is being evaluated, as rifaximin has a low risk of inducing bacterial resistance.
Second-line antituberculosis drugs
Leprosy
• For paucibacillary disease: dapsone and rifampicin for 6 months.
• For multibacillary disease: dapsone, rifampicin and clofazimine for 2 years. Follow-up for 4–8 years may be necessary.
Dapsone
is a bacteriostatic sulphone (related to sulphonamides, acting by the same mechanism; see p. 187). It has long been the standard drug for all forms of leprosy. Irregular and inadequate duration of treatment with a single drug has allowed the emergence of primary and secondary resistance to become a major problem. Dapsone is also used to treat dermatitis herpetiformis and Pneumocystis carinii pneumonia, and (with pyrimethamine) for malaria prophylaxis. The t½ is 27 h. Adverse effects range from gastrointestinal symptoms to agranulocytosis, haemolytic anaemia and generalised allergic reactions that include exfoliative dermatitis.
Other bacterial infections
Health-care-associated infections (HCAIs)
comprising ventilator-associated pneumonia, surgical wound infection, intravenous catheter-associated bacteraemia, meningitis following neurosurgery, and infection of prosthetic devices such as joint replacements and heart valves may involve conventional pathogens such as Staphylococcus aureus and Lancefield Group A beta-haemolytic streptococcus, but a number of antibiotic-resistant pathogens are also commonly involved. These isolates can pose difficult therapeutic problems, especially because the infections often present in patients with multiple pre-existing pathologies, including liver and renal impairment. The causative bacteria include MRSA and multiply resistant coagulase-negative staphylococci, ESBL-producing coliforms (see p. 199), and a number of normally weakly pathogenic Gram-negative bacteria such as Stenotrophomonas maltophilia and Acinetobacter spp. These bacteria can be resistant to all conventional antimicrobial agents, and discussion with a microbiologist or infectious diseases physician is recommended before treatment is attempted. A number of unusual combinations of antibiotics have been recommended and previously outdated agents have been resurrected for treatment of infections with these pathogens: for example, colistin.
Algorithm for the early management of suspected bacterial meningitis and meningococcal septicaemia in immunocompetent adults Available online at: http://www.meningitis.org/health-professionals/hospital-protocols-adults (accessed November 2011)
Annane D., Bellissant E., Cavaillon J.M. Septic shock. Lancet. 2005;365:63–76.
Anon. Managing bites from humans and other mammals. Drug Ther. Bull.. 2004;42:67–71.
Anon. Cranberry and urinary tract infection. Drug Ther. Bull. 2005;43:17–19.
Anon. Managing acute sinusitis. Drug Ther. Bull.. 2009;47:26–30.
Avni T., Levcovich A., Ad-El D.D., et al, Prophylactic antibiotics for burns patients: systematic review and meta-analysis. Available online at:. Br. Med. J. 2010;340:c241. http://www.bmj.com/content/340/bmj.c241.full.pdf (accessed November 2011)
Bhan M.K., Bahl R., Bhatnagar S. Typhoid and paratyphoid fever. Lancet. 2005;366:749–762.
Bharti A.R., Nally J.E., Ricaldi J.N., et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis.. 2003;3(12):757–771.
British Association of Sexual Health and HIV, United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease Available online at: http://www.bashh.org/documents/118/118.pdf (accessed November 2011)
recommendations for treatment of infective endocarditis in the UKBritish Society for Antimicrobial Chemotherapy. Available online at:. 2006. http://jac.oxfordjournals.org/cgi/content/short/dkh474v1 (accessed November 2011)
guidelines on management of community-acquired pneumonia in adults and in children updatedBritish Thoracic Society. Available online at:. 2009. http://www.brit-thoracic.org.uk/guidelines/pneumonia-guidelines.aspx (accessed November 2011)
Campion E.W. Liberty and the control of tuberculosis. N. Engl. J. Med.. 1999;340(5):385–386.
Chambers H.F., Moellering R.C., Kamitsuka P. Clinical decisions: management of skin and soft tissue infection. N. Engl. J. Med.. 2008;359(10):1063–1066.
Daum R.S. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N. Engl. J. Med.. 2007;357(4):380–390.
Donovan B. Sexually transmissible infections other than HIV. Lancet. 2004;363:545–556.
DuPont H.L. Bacterial diarrhoea. N. Engl. J. Med.. 2009;361(16):1560–1569.
Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940.
French P. Syphilis. Br. Med. J.. 2007;334:143–147.
Gould F.K., Elliott T.S., Foweraker J., et al. Guidelines for the prevention of endocarditis: report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother.. 2006;57(6):1035–1042.
Habib G., Hoen B., Tornos P., et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis. Eur. Heart J.. 2009;30:2369–2413.
Hasham S., Matteucci P., Stanley P.R.W., Hart N.B. Necrotising fasciitis. Br. Med. J.. 2005;330:830–833.
Health Protection Agency, Clostridium difficile: how to deal with the problem Available online at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1232006607827 (accessed November 2011)
Health Protection Agency. guidance on investigating and treating a wide range of infectious illnesses in primary care. Available online at: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1279888711402 (accessed November 2011)
Hill D.R., Ryan E.T. Management of travellers’ diarrhoea. Br. Med. J.. 2008;337:863–867.
Lazzerini M. Zinc supplements for severe cholera. Br. Med. J.. 2008;336:227–228.
Lew D.P., Waldvogel F.A. Osteomyelitis. Lancet. 2004;364:369–379.
Mathews C.J., Weston V.C., Jones A., et al. Bacterial septic arthritis in adults. Lancet. 2010;375:846–855.
Matthews P.C., Berendt A.R., McNally M.A., Byren I. Diagnosis and management of prosthetic joint infection. Br. Med. J.. 2009;338:1378–1383.
McColl K.E.L. Helicobacter pylori infection. N. Engl. J. Med.. 2010;362:1597–1604.
Morgan M. Staphylococcus aureus, Panton-Valentine leukocidin and necrotizing pneumonia. Br. Med. J.. 2005;331:793–794.
Munoz-Price L.S., Weinstein R.A. Acinetobacter infection. N. Engl. J. Med.. 2008;358:1271–1280.
Newland A., Provan D., Myint S. Preventing severe infection after splenectomy. Br. Med. J.. 2005;331:417–418.
Quagliarello V. Adjunctive steroids for tuberculous meningitis – more evidence, more questions. N. Engl. J. Med.. 2004;351(17):1792–1794.
Rudan I., Campbell H. The deadly toll of S. pneumoniae and H. influenzae type b. Lancet. 2009;374:854–856.
Ryan E.T., Wilson M.E., Kain K.C. Illness after international travel. N. Engl. J. Med.. 2002;347(7):505–516.
Scottish Intercollegiate Guidelines Network, Management of suspected bacterial urinary tract infection in adults: a national clinical guideline. Available online at:. 2006. http://www.sign.ac.uk/pdf/sign88.pdf (accessed November 2011)
Singer A.J., Dagum A.B. Current management of acute cutaneous wounds. N. Engl. J. Med.. 2008;359(10):1037–1046.
Spiro D.M., Tay K.Y., Arnold D.H., et al. Wait-and-see prescription for the treatment of acute otitis media: a randomized controlled trial. J. Am. Med. Assoc.. 2006;296:1235–1241.
Surviving Sepsis Campaign Available online at: http://www.survivingsepsis.org/ (accessed November 2011)
Swartz M.N. Bacterial meningitis – a view of the past 90 years. N. Engl. J. Med.. 2004;351(18):1826–1828.
Theilen U., Wilson L., Wilson G., et al. Management of invasive meningococcal disease in children and young people: summary of SIGN guidelines. Br. Med. J.. 2008;336:1367–1370.
Thwaites G., Fisher M., Hemingway C., et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J. Infect.. 2009;59:167–187.
van de Beek D., de Gans J., Tunkel A.R., Wijdicks E.F. Community-acquired bacterial meningitis in adults. N. Engl. J. Med.. 2006;354:44–53.
Whitty C.J.M. Erasmus, syphilis, and the abuse of stigma. Lancet. 1999;354:2147–2148.
World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva: Emergency updated edition; 2008.
Wormser G.P. Early Lyme disease. N. Engl. J. Med.. 2006;354(26):2794–2801.
1 Cooper R J, Hoffman J R, Bartlett J G et al 2001 Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Annals of Internal Medicine 134:506.
2 Del Mar C B, Glasziou P P, Spinks A B 2008 Antibiotics for sore throat (Cochrane review). Available online at: http://www2.cochrane.org/reviews/en/ab000023.html (accessed November 2011)
3 Thomas M, Del Mar C, Glasziou P 2000 How effective are treatments other than antibiotics for acute sore throat? British Journal of General Practice 50:817.
4 The most famous carrier was Mary Mallon (‘Typhoid Mary’) who worked as a cook in New York City, USA, using various assumed names and moving through several different households. She caused at least 10 outbreaks with 51 cases of typhoid fever and three deaths. To protect the public, she was kept in detention for 23 years.