Chapter 13 Chemotherapy for Tumors of the Spine
INTRODUCTION
The goal of this chapter is to discuss the general principles of chemotherapeutic management for metastatic spinal disease. The vast majority of spinal metastases are extradural, originating in the marrow of spinal vertebral bodies.1 These bone lesions can significantly diminish the quality of life of cancer patients, creating pain, medical problems such as hypercalcemia, fractures that may be unstable, and the potential for spinal cord compromise. The most common primary tumors that disseminate to bone in adults are multiple myeloma and common solid tumors such as breast, prostate, lung, and kidney. In children, bony metastases are rare and are caused by Ewing’s sarcoma, neuroblastoma, and sarcomas.2 Spinal metastatic tumors that present in non-acute fashion are treated with systemic chemotherapy. Successful treatment of spinal metastases, especially when they present with acute symptoms, requires the combined efforts of multiple disciplines, including radiation oncology, neurosurgery, orthopedics, anesthesia, and medical oncology.
Only a minority of metastatic lesions to the spine lie within the central nervous system (CNS). Most CNS metastatic tumors are intradural and extramedullary, and they originate from intracranial lesions that disseminate to the spine via cerebrospinal fluid (CSF) pathways. Unfortunately, the prognosis for patients with leptomeningeal disease is poor, and chemotherapy directed into the CSF cisterns is palliative. Intramedullary metastases originate from hematogenous seeding of the spinal cord and comprise only 0.5% of spinal axis metastases.1,3 They are so rare that treatment options are not well-studied.
DIAGNOSIS
More than 90% of patients with metastatic spinal disease present with pain. If the pain is more severe with movement, spinal stability must be evaluated, and consultation by a spine surgeon is necessary if instability is uncovered.4 A general physical examination with a detailed musculoskeletal exam is warranted for all patients with possible metastatic spinal disease. As lesions progress in size, they eventually impinge on neurological structures, resulting in neurological deficits that can progress to paraplegia without appropriate intervention. Symptomatic lesions occur most often in the thoracic rather than the lumbar spine because of the smaller dimension of the thoracic spinal canal relative to the spinal cord.5 Patients presenting with neurological deficit require urgent evaluation, and if deficits are rapidly progressive, urgent local therapy with radiation or surgical intervention may be indicated. Rapid progression and severe deficits indicate a poor prognosis.
Plain radiography is not sensitive for metastatic disease, but it is invaluable for evaluation of spinal stability. Upright and flexion-extension views are used to evaluate spinal alignment. Instability with anterolisthesis, vertebral collapse, kyphosis, or scoliosis requires immobilization, CT for better imaging resolution, and the attention of a surgeon. On plain film, osteolytic tumors create lucencies, osteoblastic lesions have sclerotic margins, and large masses invading paraspinal structures produce soft tissue shadows. Technetium-99 bone scan has good sensitivity but poor specificity for metastatic disease. Radionuclide build-up from osteoid formation6 is a reactive process to insults, including trauma, infection, and degenerative disease, and results in falsely positive studies.7 However, this same reactive process is difficult to generate with aggressive, fast-growing lytic lesions, such as multiple myeloma, renal and lung tumors, and certain sarcomas, resulting in false negatives.8 MRI, the gold standard in neoplasm imaging, allows evaluation of the entire spine in multiple orthogonal views, resulting in sensitivity and specificity in excess of 90%. However, long acquisition time can lead to patient claustrophobia and thoracic motion artifact.8 CT myelography, performed when MRI is not possible, can reveal the location and level of metastases and their anatomical relationship to the dura, spinal cord, and spinal roots. As an invasive procedure, risks of myelography include spinal cord herniation or “coning” in the presence of a complete spinal block from a large tumor mass. In the absence of a previously biopsied primary lesion, or if a diagnosis requires confirmation, a biopsy should be performed before the initiation of treatment.
INITIAL EVALUATION AND TREATMENT
Many patients present in a dehydrated state with acute renal insufficiency related to prerenal azotemia. Hypercalcemia can be seen either as a by-product of metastatic bone lysis or as a paraneoplastic syndrome. If hypercalcemia is present, electrocardiography is indicated. After initial rehydration and normalization of kidney function, hypercalcemia is treated with courses of steroids and intravenous bisphosphonates before the onset of chemotherapy. Steroids benefit patients with cord or root impingement, and severe acute pain may require local therapy with radiation or surgical intervention.9 The presence of neoplastic cells with CSF sampling is an indication for CSF-delivered chemotherapy, and a neurosurgical consultation is necessary for placement of an Ommaya reservoir for intraventricular chemotherapy and treatment monitoring. Evaluation of serum cancer markers is obtained to follow the progression of treatment. These include serum electrophoresis and urine for Bence-Jones protein in multiple myeloma, prostate-specific antigen (PSA), and carcinoembryonic antigen for cancer of the colon, breast, lung, pancreas, and ovaries. Other commonly evaluated markers for metastatic disease and bone lesions include CA15–3 and alkaline phosphatase, respectively.
SYSTEMIC CHEMOTHERAPY
Among skeletal locations, the spinal column is the most common site of metastatic dissemination10 and also is the initial site of spread in 12–20% of patients who present with spinal symptoms.1,11 Most metastatic spinal lesions originate from arterial hematogenous spread, but other primary cancers such as prostate and renal cell cancer disseminate through the venous system in retrograde fashion via the valveless venous plexus described by Batson.12–14 Lesions that disseminate via hematogenous pathways are clearly accessible for treatment by systemic chemotherapy.
BREAST CARCINOMA
Bone is the most common site of metastases for breast cancer. According to the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database, 200,000 women develop breast cancer in the United States each year, with more than one-third of these individuals developing bone metastases, including spinal lesions.15 As with other cancer types that invade bone, control of bone disease affords several years of survival, and patients do poorly once the disease invades soft tissue organ systems, including the brain.16,17 Breast metastases are both osteolytic and osteoblastic, and patients require careful evaluation to ensure that skeletal complications are not evidenced on presentation. The use of bisphosphonates improves survival by increasing the time to skeletal complication.18,19 Receptor-positive disease is a positive predictor for a longer median survival, as is the presence of metastases solely to bone. More than 60% of breast cancers have hormonal receptors and are thus responsive to hormonal therapy.20,21
The drug most commonly used for breast cancer is tamoxifen, a selective estrogen receptor modulator with both agonist and antagonist effects. The use of hormonal systemic agents has drawbacks. Even though tamoxifen has an agonist effect that helps preserve bone density, negative side effects include hot flashes and increased incidence of venous thrombosis and endometrial cancer. Another class of hormonal agent is aromatase inhibitors, which block the peripheral conversion of androgenic precursors to estrogen. Aromatase inhibitors have been demonstrated to increase survival in postmenopausal women with metastatic disease when compared with tamoxifen, and in postmenopausal women with estrogen receptor-positive metastatic disease, they are the agents of choice after tamoxifen failure.22 Unfortunately, this hormonal agent, by decreasing systemic estrogen, leads to osteoporosis, a negative side effect. Other hormonal agents, including progestins, androgens, and estrogen, also have therapeutic effects on breast metastases. Response to therapy may take 2 to 3 months, but once it has been demonstrated, further response often can be generated by second-line agents.
When response to hormonal manipulation has run its course, breast cancer is usually responsive to treatment with chemotherapy. The most effective chemotherapeutic agents in metastatic breast disease are cytotoxic single agents, which have become the mainstays of chemotherapy after hormonal drugs. These agents include the anthracycline drugs doxorubicin and epirubicin, and the antitubulin taxanes including docetaxel and paclitaxel.23 Anthracyclines such as doxorubicin produce cell death by affecting cellular targets located within the nucleus, intercalating with DNA to create DNA topoisomerase inhibition. Taxanes are believed to exert their cytotoxic effect through stabilization of tubulin polymerization and promoting the abnormal aggregation of intracellular microtubules. Another agent with a Food and Drug Administration (FDA) indication for breast metastases is oral capecitabine, which exhibits little myelosuppression. Capecitabine has fewer side effects and is easier to administer than fluorouracil. Its decreased toxicity profile is believed to be a result of its preferential activation in malignant tissues, whereas thymidine phosphorylase, the enzyme that activates the final step in the conversion of the pro-drug capecitabine into fluorouracil, is overexpressed.
Second- and third-line therapies lacking U.S. FDA indications but used in off-label fashion in the treatment of breast cancer include vinorelbine for non-small cell lung cancer, gemcitabine for pancreatic and non-small cell lung cancer, pegylated liposomal doxorubicin for Kaposi’s sarcoma, and mitoxantrone for hormone-refractory prostate cancer and acute nonlymphocytic leukemia. In the last few years, fulvestrant and zoledronic acid also have been U.S. FDA-approved for breast cancer therapy, and there has been increased use of combination therapy for breast metastasis.23 Despite higher response rates and longer time-to-progression with the use of combination therapy, there is controversy regarding whether it improves survival over the sequential use of the same agents. Although no single trial has demonstrated a survival advantage of combination therapy over sequential administration of single agents, in all areas of oncology where cures have been produced, combination chemotherapy has been used. Combinations include capecitabine/docetaxel, gemcitabine/paclitaxel, doxorubicin/paclitaxel, and a broadly adopted class of combination chemotherapy that includes various cytotoxic agents with the drug trastuzumab, a humanized monoclonal antibody to the human epidermal growth factor receptor-2 (HER-2) protein for patients with HER-2 overexpressed metastatic disease. With trastuzumab, combination chemotherapy has been demonstrated to be clearly superior to single-agent trastuzumab therapy in multiple clinical studies.24–26
PROSTATE CARCINOMA
Prostate cancer commonly metastasizes to bone. Despite widespread initiatives to screen men for the early detection of prostate cancer, rates for organ-confined disease on presentation are disappointingly low, ranging from 8–30%. Prostate cancer takes a more aggressive clinical course in African-American men because of the existence of genetic polymorphisms and CAG/GGC microsatellites of the androgen receptor gene.27,28 Organ-confined disease, associated with PSA levels of 10 ng/mL or less, can be treated with radical prostatectomy, resulting in a cure rate of more than 80%.29 Unfortunately most patients do not fall within this category, and more than 37,000 men die each year as a result, making prostate cancer the second-leading cause of cancer-related death after lung cancer in men in the United States.30
Most prostate cancers are initially responsive to hormonal therapy. Androgen deprivation, the mainstay of treatment, is provided by gonadotropin-releasing hormone (GnRH) antagonists or surgical castration. Anti-androgens such as flutamide are used to prevent an initial surge in androgen formation after the start of GnRH treatment. Secondary anti-androgens include bicalutamide31; estrogens such as diethylstilbestrol that target ER-b, an estrogen receptor expressed by prostate cancer cells;32 and agents that inhibit adrenal steroid synthesis such as glucocorticoids, ketoconazole, and aminoglutethimide. Other non-hormonal treatments include external beam radiotherapy and radioisotope treatment with strontium. Prostate cancer metastases typically are osteoblastic, and the patient’s bone becomes dense, brittle, and more prone to fracture. The use of bisphosphonates reduces bone pain and the risk of fracture and improves bone density that otherwise decreases with anti-androgen treatment.33 Unfortunately, prostate cancer becomes refractory to androgen deprivation. Once prostate cancer becomes resistant to treatment with initial hormonal therapy, the likelihood of response to a second agent is much lower than breast cancer, and median survival is less than 1 year.34,35 At this stage of prostate disease, more than 95% of patients have prostatic metastases to the spine.36 Treatment response is followed by serum PSA level.
Prior studies suggesting ineffectiveness of cytotoxic chemotherapy35,37 made anti-androgen therapy the mainstay of treatment by default, but more recent studies using combination chemotherapy demonstrate improved results with an acceptable toxicity profile, offering a previously unavailable option for hormone refractory disease.38 Several Phase II studies show that estramustine, a conjugate of nitrogen mustard and estradiol, produces significant responses in more than 50% of patients in combination with vinblastine, etoposide, or the antitubulin taxanes such as paclitaxel and docetaxel.39–41 Interestingly, rather than working on an estrogen receptor expressed by prostate cancer cells, this estrogen conjugate actually exerts a cytotoxic effect as a microtubule inhibitor and appears to be more effective when paired with other antitubulins.42 When estramustine is paired with paclitaxel or docetaxel, PSA response rates occur in the 50–80% range. Mitoxantrone, an FDA-approved drug for the palliative treatment of hormone-refractory prostate carcinoma, is related to the anthracyclines. In multiple trials, it has been shown to be more effective in improving pain in combination with steroids than steroids alone.43,44 Combination chemotherapy with cytotoxic agents in conjunction with radical prostatectomy now is under investigation for the treatment of aggressive prostate cancers during the earliest stages of therapy.38
LUNG CARCINOMA
Lung cancer is the leading cause of cancer deaths in the United States. At the time of death, up to 40% of lung cancer patients have disseminated disease to the axial skeleton.45 Lung cancers of various histological subtypes are divided into two main groups, small cell and non-small cell, reflecting their behavior properties in response to treatment. Non-small cell lung cancer (NSCLC), which accounts for 80–85% of all lung cancers, is more resistant to treatment, whereas small cell lung cancer (SCLC) is more sensitive to both chemotherapy and radiotherapy. Thirty to 40 years ago, the median survival of patients with disease metastatic to the spine was 6 months, and most patients succumbed to systemic disease before the onset of skeletal complications.46 The only treatments available in the past were surgical resection for early local disease, and for locally inoperable lesions, radiation was offered. Treatment for advanced disease was supportive, and chemotherapy, when offered, was palliative. Until more than two decades ago when cisplatin was tested in end-stage patients in combination with drugs that had shown efficacy with other tumors,47–49 NSCLC was believed to be resistant to chemotherapy, with treatment believed to have a greater toxic than clinical effect.50–52
Since that time, platinum compounds have been tested in combination with other cytotoxic drugs. After multiple trials in the 1980s demonstrating efficacy with combination chemotherapy given for all different stages of metastatic NSCLC disease, cisplatin combination chemotherapy became the standard treatment for good performance patients.53 The toxicity profile of cisplatin, however, led in the last 15 years to the creation of alternative combinations that used the cisplatin-analog carboplatin in combination with other cytotoxic drugs. Carboplatin has shown efficacy in combination with antitubulins, including the vinca alkaloids vinblastine and vinorelbine and the taxanes paclitaxel and docetaxel, nitrosoureas, cyclophosphamide, gemcitabine, vindesine, doxorubicin or epirubicin, mitomycin-C, ifosfamide, irinotecan, tirapazamine, and premetrexed. Finally, when many of these agents alone were found to be effective as first-line therapy, combinations were created based around the taxanes paclitaxel and docetaxel, without platinum-based compounds. These combinations were found to be similar or better in clinical response, with a toxicity profile shifted away from renal and neurotoxicity toward myelotoxicity. With steady but gradual developments in combination chemotherapy during the last 30 years, median survival has shifted from 6 months without chemotherapy to slightly more than 1 year in some trials.54
SCLC makes up 15–20% of all lung cancers. Clinically, SCLC is a biologically aggressive disease with a 3-month median survival without treatment. With treatment, response of the disease to both chemotherapy and radiation therapy is excellent initially. Surgical excision with local radiation is an option if disease is local, with concomitant radiation therapy and chemotherapy for early stage disease. Chemotherapy is the treatment choice for extensive disease. SCLC has been described as chemosensitive because combination chemotherapy treatment was used in the 1970s, yet despite decades of trials, little progress has been made toward maintaining this initial response with long-term remission, and median survival of extensive stage SCLC disease now is similar to that of NSCLC when the latter is treated with combination cytotoxic chemotherapy.55 Cisplatin and other platinum compounds in combination with etoposide have remained the standard of care for SCLC for more than a decade,56,57 with triple-drug therapies and newer agents not showing demonstrable survival improvements. With limited local disease, in conjunction with radiation, a median survival of 20 months has been achieved.58
MULTIPLE MYELOMA
Multiple myeloma is a B-lymphocyte disorder that accounts for 10% of all hemopoietic tumors,59 with 15,000 new patients in the United States each year.60 Osteolytic bone disease is a key hallmark of multiple myeloma, and the existence of the lesions is one of the staging criteria for the disease. Plain radiographs demonstrate skeletal lesions in more than 80% of patients at presentation, and skeletal complications are the main clinical finding, resulting in early presentation with bone pain. Even though early forms of disease may not be lytic, solitary plasmacytomas inevitably metastasize to involve the axial skeleton, and all patients have bone metastases at the time of death. The disease also results in medical problems. More than one-half of all patients have anemia, more than one-third have elevated creatinine, and one-quarter of all patients present with hypercalcemia. Electrophoresis or immunofixation for the serum Bence-Jones or M protein is positive in 80–90% of patients. Diagnosis requires greater-than-10% plasma cells with bone marrow aspiration or a positive biopsy of a plasmacytoma mass. Alleviation of pain is a major factor in treatment, and bisphosphonates, such as pamidronate, play a key role in decreasing pain and reducing skeletal events such as pathological fracture and spinal cord compression, while symptomatically improving quality of life. Bisphosphonates, however, do not change patients’ overall survival, and eventually they succumb to organ failure and infectious complications. Patients with substantial bone loss may benefit from vertebroplasty or kyphoplasty to restore height and decrease pain.
Median survival for multiple myeloma is 3 years, but patients with lower-risk disease may live for more than 10 years. Treatment is often started at the time patients develop symptomatology because there is no evidence that early treatment of asymptomatic multiple myeloma prolongs survival. For decades, standard chemotherapy has consisted of oral melphalan plus prednisone or other glucocorticoids.61 In the last 15 years, autologous stem cell transplantation (ASCT) has been shown to prolong survival, and it now is performed at initial diagnosis or with recurrence for patients with good performance status.62,63 When ASCT is being considered, alkylating agents such as melphalan are avoided to ensure adequate stem cell mobilization. Commonly, dexamethasone is given in conjunction with other agents for induction therapy before ASCT to diminish tumor load. More recently, novel chemotherapy agents for multiple myeloma, including thalidomide, bortezomib, and lenalidomide, also have been given for induction before single or tandem ASCT or in high-dose therapy in conjunction with melphalan and prednisone. Allogeneic bone marrow transplantation also can be performed, with the advantage of a lack of graft contamination with myeloma tumor cells. Unfortunately, the need for immunosuppressive therapy and the lack of human leukocyte antigen-matched sibling donors make this a difficult proposition. Patients with multiple myeloma eventually relapse, and treatment with one drug or regimen is followed by another after toxicity or another relapse occurs.
RENAL CELL CARCINOMA
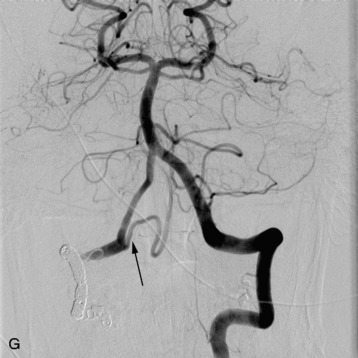
Nearly 85% of renal cancers arise from the epithelium and are classified as a group as renal cell carcinoma (RCC). About 36,000 cases of renal cell cancer are diagnosed yearly, and between 25–50% of patients present with metastatic disease,60 with a 13-month median survival for patients with metastases. One-third of cases with local disease recur after surgical resection. Less than one-half of patients develop skeletal metastases. Radiographically, these lesions are lytic, with large soft tissue components that are easily seen on MRI or CT. Like multiple myeloma, the aggressive nature of this disease sometimes prevents the formation of a bone reaction, and bone scans may be negative. Epidural compression is rare. The metastases are extremely vascular, and angiography with vascular embolization has been used to treat patients if they require surgery or if they are in severe pain.
RCC responds poorly to hormonal therapy and chemotherapy, and also is radioresistant. The poor response rate to chemotherapy, around 5%, is thought to be caused by drug resistance mediated by a multidrug transporter that eliminates cytotoxic agents in the cells of the proximal tubule.64,65 Immunomodulatory therapies, however, have made some impact on this disease, with about 14% of cases responsive to interferon alfa and 21% of patients responsive to interleukin-2 (IL-2).66 High dose IL-2 is the only drug therapy approved by the FDA for RCC treatment. For patients who respond to IL-2 therapy, the improvements may be dramatic, and the median survival is 54 months.67 A subgroup of patients has a complete response. Unfortunately, many patients cannot tolerate high dose IL-2 because it leads to a capillary leak syndrome.
CHEMOTHERAPEUTIC MANAGEMENT PRINCIPLES FOR SPINAL METASTASES
One common treatment that can be used with many metastatic diseases to the spine is radiopharmaceutical treatment with an agent such as strontium. Strontium 89 is deposited in bone tissue during calcification, is effective on solid tumors that are osteoblastic in nature, and has myelosuppression as its major toxicity.68 In breast and prostate cancer, patients who receive this therapy have reported significant improvements in pain. Combination treatments of radiopharmaceutical agents with cytotoxic chemotherapy agents have shown some benefit in a few small studies.35,69
Although anatomical considerations are not as important in the chemotherapeutic treatment of different cancer types, two spinal compartments for metastatic tumor spread deserve separate attention. Intradural extramedullary metastatic disease requires, in addition to systemic chemotherapy, the administration of CSF-delivered chemotherapy. The agents used via CSF delivery to this compartment for different tumor types are not as varied as the agents used via systemic hematogenous delivery. Intramedullary disease, as a result of the small volume of the spinal cord, is rare.70–72 Most centers treat these lesions with local therapy using either radiation or surgical excision, followed by the appropriate systemic chemotherapy for the patient’s tumor diagnosis.
CEREBROSPINAL FLUID CHEMOTHERAPY
Metastatic disease to the intradural portion of the spine occurs much less frequently than vertebral bone disease. The overwhelming majority of intradural lesions are extramedullary, and they are disseminated via CSF pathways. Metastases exist as multiple discrete lesions and also can coat the dura and enmesh the nerve roots with thickened layers of nodular tumor.1,73 This is a disease with many names, known alternatively as leptomeningeal metastasis, drop metastasis, neoplastic meningitis, carcinomatous meningitis, and meningeal carcinomatosis. In adults, these spinal metastases usually spread from intracranial metastases of extraneural primary cancers via CSF. Hemopoietic malignancies such as lymphoma and leukemia have a propensity to seed via CSF, and solid tumors that are known to disseminate via CSF include SCLC, breast adenocarcinoma, gastrointestinal cancers, and melanoma.74–76 In children, intradural extramedullary spinal metastases also disseminate via CSF from the brain, but the brain tumors, such as ependymoma, medulloblastoma, and other primitive neuroectodermal tumors, usually are primary neoplasms of the posterior fossa instead of metastases from extraneural primary cancers.77–79 As patients with cancer live longer and develop more brain metastases, leptomeningeal metastases increase in incidence as a late complication of systemic neoplastic disease. Treatment requires a combination of CSF chemotherapy, systemic chemotherapy, and craniospinal radiotherapy with local radiation boost to solid portions of metastatic disease.
Patients with spinal leptomeningeal metastases may present with pain or focal deficits because of nerve root compromise or mass effect on the spinal cord and cauda equina. The neurological findings, reflecting the disseminated foci of tumor, are often multifocal. An Ommaya reservoir is placed for CSF access for both administration of intraventricular therapy and CSF sampling to monitor treatment response.80 CSF chemotherapy is administered in induction, consolidation, and maintenance phases that differ in dosage. Methotrexate is the most commonly administered CSF agent, with cytarabine and thiotepa also administered as single-agent therapies. Newer agents that are promising but still unproven include monoclonal antibodies such as rituximab 50. Chemotherapy with multiple CSF-delivered agents has not been demonstrated to improve outcome. CSF chemotherapy treatment usually halts neurological deterioration but, like all treatment of this disease, is palliative with a median survival of less than 6 months for most tumor types. The clinical course is progressive, and cures are exceedingly rare. Patients who respond poorly to therapy live for only weeks.
INTRAMEDULLARY METASTASES
Intramedullary secondary metastases are exceptionally rare, and lung cancers comprise one-half of the lesions that have been reported in the literature.81 There is a higher incidence of NSCLC, but the greater rate of hematogenous spread of SCLC to the CNS results in a relatively equal division of intramedullary lesions between small cell and non-small cell lung metastases. Other cancer types that are represented in reported cases include breast, kidney, gastrointestinal, and melanoma. The low number of intramedullary metastases has resulted in a lack of clear guidelines regarding options for treatment. Although the CNS has been considered a protected space for tumor growth in the face of systemic chemotherapeutic treatment, more recent investigations suggest that the blood-brain barrier is disrupted enough in neoplastic conditions to enable many drugs to cross. Steroids are often used to treat mass effect, edema, and the propensity toward early neurological deterioration. Local intervention, consisting of radiation or surgery early in the course of the disease, is believed to result in better neurological outcomes.82
TREATMENT FOLLOW-UP
Of the other imaging modalities, bone scan is the most frequently used to follow chemotherapy progress because of its sensitivity. N-telopeptide is a serum marker of bone loss, and levels decrease when patients respond to chemotherapy or adjuvant treatments such as bisphosphonate therapy.85 Bisphosphonates, such as pamidronate and zoledronic acid, have been shown to be of benefit in clinical trials, decreasing skeletal events and improving pain control in patients, and are FDA approved for use in multiple myeloma and solid tumors with bone metastases.85 Ultimately, improvements in level of pain, metabolic derangements, and serum marker levels will occur as effective chemotherapy decreases tumor burden and bone regrowth improves bone quality. As patients respond to chemotherapy and other adjuvant treatments, their subjective level of pain improves, and they have less anxiety and improved mood and energy.
7 Kamholtz R, Sze G. Current imaging in spinal metastatic disease. Semin Oncol. 1991;18:158-169.
9 Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97:866-873.
15 Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588-1594.
18 Lipton A. Bisphosphonates and metastatic breast carcinoma. Cancer. 2003;97:848-853.
20 Hortobagyi GN. Bone metastases in breast cancer patients. Semin Oncol. 1991;18:11-15.
30 Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8-31., 1
42 Hudes G. Estramustine-based chemotherapy. Semin Urol Oncol. 1997;15:13-19.
55 Sandler AB. Chemotherapy for small cell lung cancer. Semin Oncol. 2003;30:9-25.
59 Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860-1873.
60 Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30.