Chemical stability in dosage forms
Andrew R. Barnes
Chapter contents
Chemical degradation reactions
Dimerization and polymerization
Stability of proteins and peptides
Physical stability of proteins
Chemical aspects of protein stability
Key points
Introduction
Chemical degradation of the drug is often the critical factor that limits the shelf-life of a pharmaceutical product. The degradation of other ingredients in the formulation, such as antimicrobial preservatives or antioxidants, may also be a critical factor.
The nature of the degradation products that form in the dosage form may be the factor that limits the shelf-life of a product. This may be because the degradation products are toxic; for instance, the antifungal drug flucytosine degrades to fluorouracil, which is cytotoxic. The degradation products may alternatively give the product an unacceptable appearance. For instance, the oxidation products of epinephrine (adrenaline) are highly coloured.
In general, drug molecules tend not to undergo spontaneous chemical degradation; the cause is usually some other reactive molecule within the dosage form. Often, this is due to the presence of water or molecular oxygen, but the drug may also react with other formulation constituents or react with other molecules of the same drug. Protection of the formulation from chemical degradation is one of the primary aims of dosage form design.
This chapter discusses the common types of chemical degradation that affect ‘small molecule’ drugs and then looks at the specific stability issues affecting proteins and peptides.
Chemical degradation reactions
Hydrolysis
Hydrolysis is the breaking of a molecular bond by reaction with water. Water is common in pharmaceutical products, either as an ingredient or as a contaminant, and hydrolysis reactions are the most common cause of chemical degradation. Most hydrolysis reactions involve derivatives of carboxylic acids, such as esters and amides, which are frequently found in drug molecules.
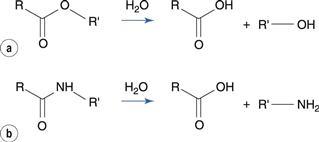
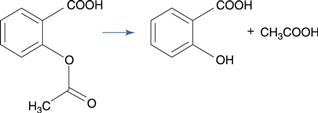
The ester group hydrolyses to produce a carboxylic acid and an alcohol (Fig. 48.1a). The carbon of the ester carboxyl group is relatively electron deficient, due to bond polarization caused by the adjacent oxygen atoms. Nucleophilic attack by water is therefore promoted at this carbon atom. For instance, the degradation of aspirin (acetylsalicylic acid) results in the formation of salicylic acid and acetic acid (Fig. 48.2). Aspirin is too unstable to allow the formulation of an aqueous aspirin product with a suitable shelf-life.
Hydrolysis reactions of esters, amides and related molecules are catalyzed by acid and by base. To use ester hydrolysis as an example, catalysis by base involves nucleophilic attack by hydroxyl ion at the electron-deficient carbon of the carbonyl group to produce a tetrahedral intermediate (Fig. 48.3a,b). This is followed by ejection of the alcohol (Fig. 48.3c). In this scheme, ionization of the carboxylic acid in alkaline solution is ignored for clarity.
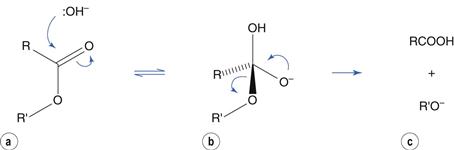
Fig. 48.3 Base-catalyzed ester hydrolysis.
Catalysis by acid involves protonation of the carbonyl group (Fig. 48.4a) to produce resonance structures (Fig. 48.4b, c). The positively-charged carbon atom promotes nucleophilic attack by water (Fig. 48.4c) to produce a tetrahedral intermediate (Fig. 48.4d). Transfer of H+ within the molecule (Fig. 48.4e) results in loss of the alcohol portion (Fig. 48.4f).
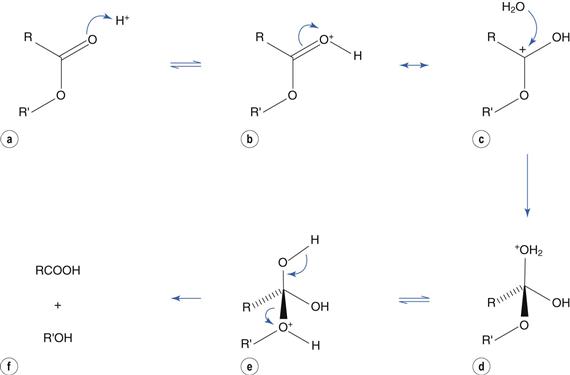
Fig. 48.4 Acid-catalyzed ester hydrolysis.
Amides degrade to a carboxylic acid and an amine (Fig. 48.1b). Amides tend to be more stable to hydrolysis than the corresponding esters because the nitrogen atom is less electronegative than the oxygen atom in the corresponding ester. Examples of amide-containing drugs include lidocaine and paracetamol (acetaminophen). The antimicrobial drug chloramphenicol is an amide-containing drug that is relatively susceptible to hydrolysis compared to many amides (Fig. 48.5). This is due to a high degree of polarization of the amide bond by the highly electronegative chlorine substituents that are adjacent. Eye drop preparations of chloramphenicol therefore require storage in a refrigerator.
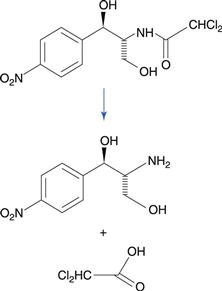
Fig. 48.5 Hydrolysis of chloramphenicol to 2-amino-1-(4-nitrophenyl)propane-1.3-diol and dichloroacetic acid.
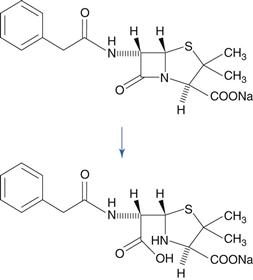
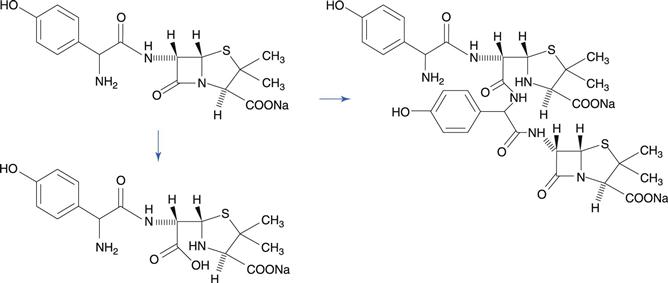
The lactam group, which is a cyclic amide, is important because it is present in penicillin and cephalosporin antibiotics, and this group is very susceptible to hydrolysis. This reactivity of the molecule is due to bond strain in the four-membered β-lactam ring. A variety of hydrolysis products is formed. For benzylpenicillin, a major hydrolysis product is benzylpenicilloic acid (Fig. 48.6). Penicillins have a side chain that possesses an amide group, but this is less susceptible to hydrolysis than the β-lactam ring. Benzylpenicillin cannot be administered by the oral route because it is hydrolysed rapidly by the acidic conditions in the stomach. Orally-active penicillins such as amoxicillin are relatively less susceptible to hydrolysis.
Oxidation
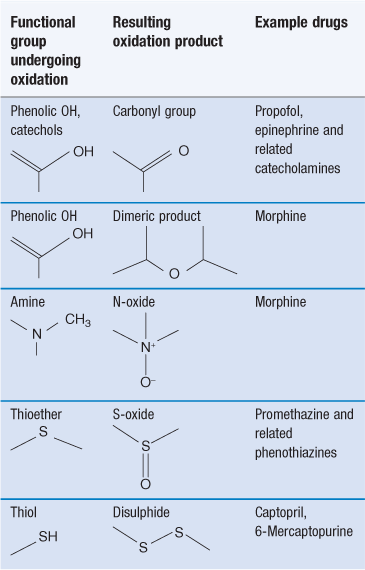
Oxidation reactions involve an increase in the number of carbon-to-oxygen bonds in a molecule or a reduction in the number of carbon-to-hydrogen bonds. These reactions are a common cause of chemical instability of drugs. They are also responsible for the deterioration of vegetable oils, which may be used in pharmaceutical products as a solvent or as an emollient in emulsions and creams. Oxidation reactions tend to be complex, giving a variety of degradation products. Table 48.1 summarizes common examples.
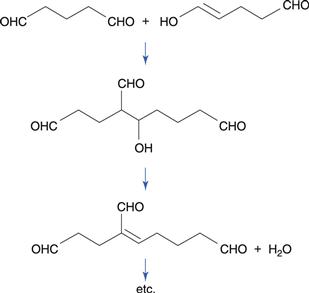
Oxidation that takes place at ambient temperature and involves molecular oxygen is known as autoxidation. Most such reactions involve free radicals, which are chemical species that possess an unpaired electron. Free radical oxidations are often complex but involve three main phases. The following scheme is a representative summary of the oxidation of many drugs and of vegetable oils.
The initiation phase results in the formation of a low concentration of free radicals. For a drug, RH, the generation of free radicals can be represented as:
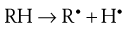
Initiation is promoted by light and the presence of heavy metals, which are inevitably found as trace contaminants of pharmaceutical products.
During the propagation phase, the concentration of free radicals increases greatly:
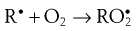
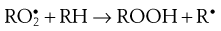
The presence of oxygen results in the formation of hydroperoxides (ROOH), which react further to produce stable oxidation products. In this phase, degradation accelerates, with potentially disastrous results for the product.
In the termination phase, the availability of oxygen or drug diminishes, the rate of reaction slows and free radicals combine to produce unreactive endproducts. The stable reaction products formed in vegetable oils include carboxylic acids, which are responsible for the rancid smell formed when such oils deteriorate.
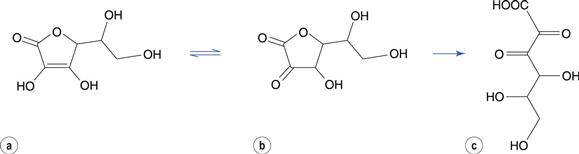
Oxidation of some drugs occurs rapidly in solution at room temperature. Ascorbic acid (vitamin C) undergoes a rapid oxidation in solution to dehydroascorbic acid (Fig. 48.7). This reaction is reversible but dehydroascorbic acid is rapidly and irreversibly hydrolysed at the ester linkage to form diketogulonic acid.
Dimerization and polymerization
Reaction of a drug molecule with another molecule of the same drug may result in the formation of a dimer or polymer. The penicillin antibiotic amoxicillin, besides undergoing hydrolysis of the β-lactam ring, also undergoes dimerization by nucleophilic attack on the β-lactam ring by the amino group (Fig. 48.8), especially in more concentrated solutions. The reaction can continue to produce a trimer and tetramer.
Polymerization is a major mechanism of degradation of the disinfectant glutaraldehyde at alkaline pH (Fig. 48.9). Because glutaraldehyde undergoes ketoenol tautomerism, an aldol condensation reaction between the keto and enol forms of the molecule results in the production of a dimer. Further reaction to produce a polymer then occurs. In order to avoid polymerization on storage, glutaraldehyde solution needs to be formulated at an acidic pH, where polymerization does not occur. Its disinfectant activity is optimal at a slightly alkaline pH, so an alkaline buffer is added immediately before use.
Hofmann elimination
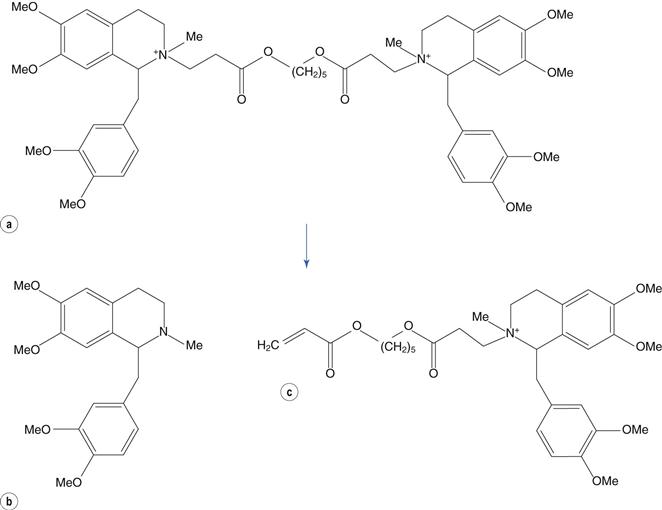
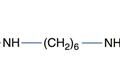
The reaction of a quaternary amine with a base, known as the Hofmann elimination, results in the elimination of a tertiary amine and formation of an alkene. This reaction is not a widespread mode of pharmaceutical degradation; however it is of major interest because of its role in the metabolism of the neuromuscular blocking drug atracurium, and the related drug cisatracurium. In order to avoid the variable duration of action of other classes of neuromuscular blocking drugs, due to variability in liver function and enzymatic metabolism, the atracurium molecule was designed to undergo spontaneous chemical degradation after administration to the patient. Atracurium is relatively stable when formulated as an injection at pH 3–4 and stored under refrigeration. When injected into the patient, the higher pH of around 7.4 (and to some extent the higher temperature) causes rapid chemical degradation via the Hofmann elimination, resulting in reproducible removal of the drug from the patient (Fig. 48.10).
Isomeric change
Different isomers of a drug often have differing pharmacological activity or toxicity.
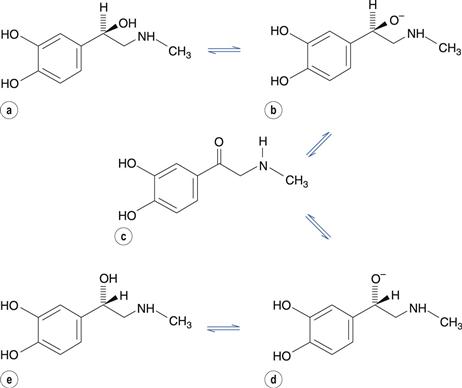
Optically active molecules with one chiral centre are known as enantiomers. The conversion of one enantiomer into its mirror-image is known as racemization. In addition to susceptibility to oxidation, adrenaline (epinephrine) may also undergo racemization in aqueous solution. The reaction is acid- and base-catalysed and involves the formation of an alcoholate anion, which reversibly forms a carbonyl-containing intermediate with no chiral centre (Fig. 48.11). Regeneration of the alcoholate anion in either of its isomeric forms is then possible. This reaction would eventually result in an equilibrium mixture of equal concentrations of each isomer. The R-isomer of adrenaline has much greater pharmacological activity than the S-isomer. The reason for the difference in potency is that in the R-isomer, the amino and hydroxyl substituents are orientated on the same side of the molecule, allowing them both to form hydrogen bonds with the adrenaline receptor in vivo. However, in the S-isomer, only one of these substituents can bond with the receptor because they are on opposite sides of the molecule.
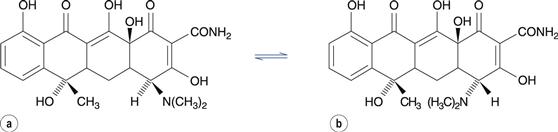
In drug molecules with more than one chiral centre (diasteriomers), racemization at one of the chiral centres is known as epimerization. The antibiotic tetracycline, for instance, forms the 4-epitetracycline epimer (Fig. 48.12), which is not active against microorganisms and is more toxic than tetracycline.
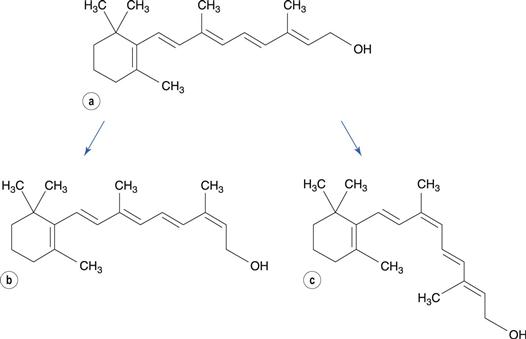
Geometrical isomers differ in the conformation of groups around a carbon-to-carbon double bond or a cyclic group. Retinol and all other related molecules, which together are known as vitamin A, contain an unsaturated hydrocarbon chain. In addition to making the molecule susceptible to oxidation, this also enables the molecule to undergo geometrical isomerization. The double bonds in the chain are all in the trans configuration. On storage of all-trans-retinol or on subjecting it to heat, the molecule changes configuration at the double bond at the 13-position of the molecule, to form 13-cis-retinol (Fig. 48.13), which has no activity as a vitamin.
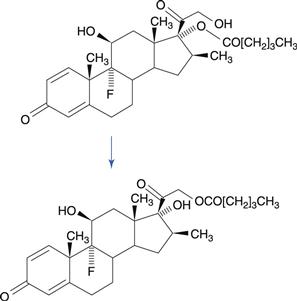
Structural isomers are sometimes formed as a result of drug degradation. The best known example of this is betamethasone-17-valerate, a potent corticosteroid. A major route of degradation of this drug is by migration of the valerate ester substituent to the side chain, forming betamethasone-21-valerate (Fig. 48.14). The mechanism is promoted by the close proximity of the hydroxyl group in the side chain to the ester substituent. This reaction is of concern where topical formulations of betamethasone-17-valerate are diluted with an inappropriate diluent.
Photodegradation
Molecules that absorb the wavelengths of light associated with sunlight or artificial light may be susceptible to light-induced degradation (photolysis). The 300–400 nm wavelengths tend to be most damaging. Shorter wavelengths are also damaging but are not of practical concern because they are not present in sunlight or artificial light.
Carbonyl, nitro, alkene, aryl chloride and phenolic compounds are most susceptible to photodegradation. Many photolysis reactions involve oxidation mechanisms, although other mechanisms may occur. Photodegradation of retinol, as well as promoting oxidative reactions, also results in formation of a cis-isomer of the molecule around the double bond at position 9. In contrast, degradation occurring in the absence of light causes isomerization at position 13 (see Fig. 48.13).
Chemical incompatibilities
Degradation of a drug may be caused by reaction with another drug present in the formulation or with a formulation excipient.
Hydroxybenzoate ester (parabens) antimicrobial preservatives undergo transesterification reactions with sugars and sugar alcohols, which may be present in a formulation as sweetening agents. For instance, methyl hydroxybenzoate undergoes reaction with sorbitol (Fig. 48.15) to produce a variety of sorbitol hydroxybenzoate esters by reaction with sorbitol’s various hydroxyl groups.
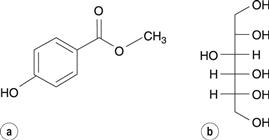
Fig. 48.15 (a) methylhydroxybenzoate. (b) sorbitol.
A related reaction involves the interaction of aminophylline with suppository bases. Aminophylline is a complex, formed between theophylline and ethylenediamine, which has increased aqueous solubility compared to theophylline alone. On storage of aminophylline suppositories, the melting point of the base increases to above physiological temperature, preventing release of drug. The mechanism for this is the formation of amide bonds between ethylenediamine and the carboxyl groups of fatty acids present in the suppository base. The reaction is the reverse of the amide hydrolysis reaction shown in Figure 48.1b.
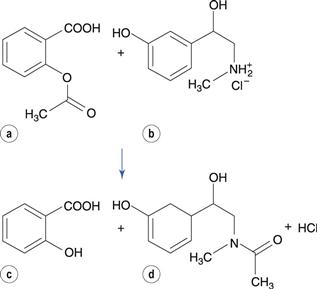
Transacetylation reactions have been reported for some drugs. For instance, in tablet formulations that contain aspirin and phenylephrine hydrochloride (a drug used as a nasal decongestant), the acetyl group is transferred from aspirin to phenylephrine (Fig. 48.16). A similar reaction occurs between aspirin and paracetamol (acetaminophen). Aspirin also reacts with the polyethylene glycol base of suppository formulations, transferring the acetyl group to the polyethylene glycol.
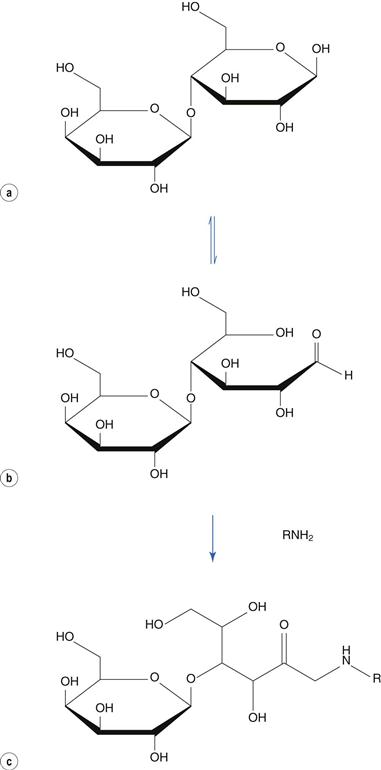
The Maillard reaction involves a reducing sugar and an amine. Reducing sugars tautomerize to an open ring form, containing a reactive aldehyde or keto group. The reaction is responsible for the browning of cooked foods, where the amino group is provided by the protein present in the food. It may also occur between amine-containing drugs and lactose or other sugars employed as a diluent in solid dose formulations. This reaction results in yellowing of white tablets on storage. For example, lactose (Fig. 48.17a) tautomerizes to its aldehydic form (Fig. 48.17b), which reacts with an amine to produce, via several intermediate stages, a coloured l-amino-2-keto sugar (Fig. 48.17c). Other reducing sugars include glucose and fructose. Non-reducing sugars, which do not undergo this reaction, include sucrose and mannitol.
Sodium metabisulphite is commonly added to epinephrine (adrenaline) injection as an antioxidant. However, it reacts with the drug to form epinephrine sulphonate (Fig 48.18), and this is a significant degradation route for epinephrine.
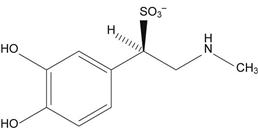
Fig. 48.18 Epinephrine (adrenaline) sulphonate.
Stability of proteins and peptides
Proteins and peptides have a long-established use as therapeutic agents, but are becoming increasingly important (along with other biopharmaceuticals) due to the opportunities offered by the use of recombinant DNA technology. This allows the production of molecules designed with specific properties, for instance monoclonal antibodies. These products are discussed in detail in Chapter 46 while this section discusses specifically the stability issues associated with these complex molecules.
Physical stability of proteins
The biological activity of a protein is governed by its three-dimensional conformation, whereby the specific sequence of amino acid residues in the protein (its primary structure) results in it coiling (secondary structure) and folding (tertiary structure). There is a close inter-relationship between the chemical and physical stability of proteins, so these aspects will be dealt with together.
A protein in its naturally folded (native) state adopts a conformation whereby hydrophobic regions of the molecule are located within its globular structure. This is its most energetically favourable conformation because these regions are hidden from the surrounding aqueous environment. As the protein unfolds (to its non-native state), the hydrophobic regions are exposed. These may then interact with surfaces, causing loss of protein from the solution by adsorption. However a major mode of protein loss is for the hydrophobic regions on adjacent protein molecules to associate, forming aggregates. These aggregates initially remain in solution, however the process can continue until a visible precipitate is formed.
Partially unfolded protein molecules may revert to the native state; however aggregate formation is generally irreversible.
In addition to loss of therapeutic activity, aggregation of protein drugs can also result in an increase in the immunogenicity of the protein, potentially causing safety problems in clinical use.
Aggregation of proteins is caused by several factors:
Chemical aspects of protein stability
On storage, more than one position in a peptide or protein molecule may be subject to chemical change, sometimes by various modes of chemical reaction. The extent to which chemical change alters the protein’s activity is variable and depends on the specific protein. The factors influencing reactivity are:
Amino acid.
Different amino acids vary in their susceptibility to chemical degradation
Position in the amino acid sequence.
Some combinations of amino acids are highly susceptible to degradation
Location within the protein tertiary structure.
Residues that are immobilized due to being buried within the protein are protected from reaction with water, oxygen or other protein chains.
The most common chemical reactions are oxidation, disulphide bridge interchange, hydrolysis, deamidation and racemization.
Oxidation of amino acid residues
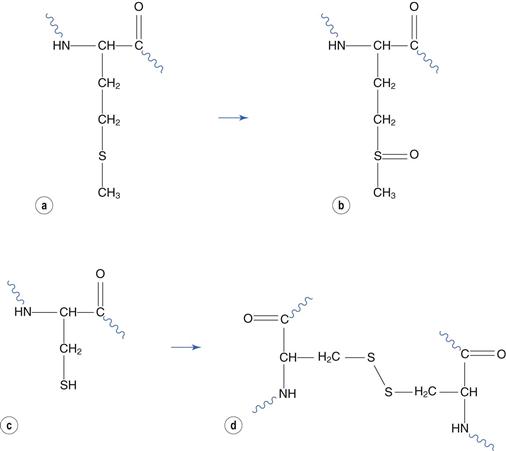
Reaction with atmospheric oxygen is a major route of protein degradation, both in solution and in freeze-dried formulations. Amino acids that are susceptible to oxidation are similarly susceptible to this form of degradation when incorporated into a peptide or protein. Methionine and cysteine residues are most susceptible. Methionine oxidizes to form methionine sulphoxide (Fig. 48.19a and 48.19b). Two cysteine residues react together to form a disulphide bridge (Fig. 48.19c and 48.19d). Bridges between cysteine residues within the same molecule play a part in maintaining the native protein conformation. However reaction between residues on different molecules may lead to aggregation. This reaction is thus more likely to cause change of the protein’s conformation, and therefore aggregation, than oxidation of methionine residues.
Disulphide bond interchange
These bonds may break and reform between different cysteine residues, potentially destabilizing the protein’s conformation.
Hydrolysis of proteins
Scission (division) of the amino acid chain by hydrolysis of the peptide bond between residues is possible. The asparagine-proline bond is most susceptible (Fig. 48.20).
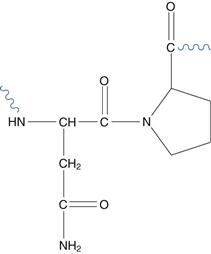
Fig. 48.20 The asparagine-proline sequence.
Deamidation
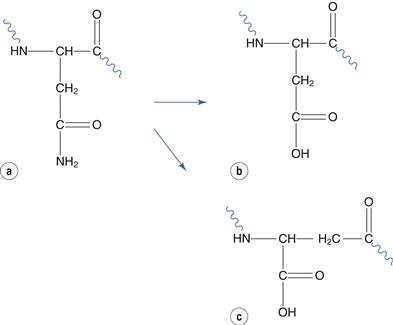
Glutamate and asparagine residues degrade by reaction at their amide side chain. Asparagine-glycine sequences are especially susceptible. Deamidation at asparagine residues forms aspartate and its structural isomer iso-aspartate (Fig. 48.21). The relatively minor change to chemical structure caused by deamidation can result in substantial change to the protein conformation and hence bioactivity.
Six of the fifty one amino acid residues that comprise insulin are susceptible to deamidation, especially the C-terminal asparagine residue.
Racemization of amino acid residues
Conversion of the amino acids within the protein chain from the naturally-occurring L-conformation to the D-conformation may occur; this may change the protein’s activity. Asparagine residues are most susceptible.
Chemical modification of protein stability
Genetic manipulation may be employed in order to improve the stability of a protein pharmaceutical by introducing selective mutations into specific sites in the protein.
Chemical stability may be improved by substituting chemically-reactive amino acid residues with less reactive ones.
Physical stability can also be directly improved. Substitutions that improve intramolecular interactions within the protein molecule, either between different regions of the molecule or within the structure of helical regions, will reduce flexibility of the protein chain, reducing the risk of unfolding.
For instance, selective substitution of cysteine or asparagine residues with the less reactive serine (Fig. 48.22) may increase chemical stability. However, inserting additional cysteine residues may increase protein stability due to its ability to form disulphide bonds within the molecule. The stability of biopharmaceuticals, including proteins and peptides, and formulation strategies to reduce degradation are discussed in detail in Chapter 46.
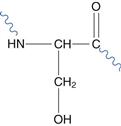
Fig. 48.22 Serine residue.
Bibliography
1. Albini A, Fasani E. Drugs: Photochemistry and Photostability. Cambridge: Royal Society of Chemistry; 1998.
2. Banga AK. Stability of therapeutic peptides and proteins In: Therapeutic Peptides and Proteins: Formulation, Processing and Delivery Systems. 2nd edn Boca Raton: Taylor and Francis; 2006.
3. Connors KA, Amidon GL, Stella VJ. Chemical Stability of Pharmaceuticals: a Handbook for Pharmacists. 2nd edn New York: Wiley; 1986.
4. ICH Guideline. Q5C Stability testing of biotechnological/biological products. Geneva, Switzerland: International Conference on Harmonisation; 1995.