22 Chemical Peels
Chemical peeling is a skin resurfacing procedure that utilizes topical agents to remove the outermost layers of the skin.1 This controlled method of wounding stimulates a reparative healing response with regeneration of a healthier epidermis and dermis. Chemical peeling, also called chemexfoliation, is commonly used for skin rejuvenation to improve photodamage, reduce hyperpigmentation and acne, and smooth rough skin texture and superficial scarring.2
As with all skin resurfacing procedures, deeper penetration into the skin is associated with greater potential benefits. However, risks and complications also increase with greater depths of penetration into the skin. The depth of penetration achieved with chemical peels ranges from the superficial epidermis to deep dermis. Standard terminology for skin resurfacing depths is illustrated in Figure 19-12 in Chapter 19, Aesthetic Principles and Consultation. The focus of this chapter is light chemical peels, which include very superficial chemical peels that remove the stratum corneum, and superficial chemical peels that remove the entire epidermis and may extend to the upper papillary dermis.3
Chemical peels are one of the most common cosmetic procedures performed in the United States, ranking closely behind botulinum toxin, laser hair removal, and dermal fillers, according to data from the American Society for Aesthetic Plastic Surgery.4 They are technically straightforward to perform and, with minimal start-up costs, they are often one of the first aesthetic procedures incorporated into office practice.1,5 Chemical peels can be readily combined with other minimally invasive aesthetic modalities such as microdermabrasion, topical products, and nonablative lasers to enhance skin rejuvenation results.
Patient Selection
While almost any patient will derive benefit from light chemical peels, patients with mild to moderate photoaging changes such as solar lentigines, skin dullness, rough texture6–8 (e.g., Glogau types I and II), and acneic conditions9,10 typically derive the most noticeable benefits (see Chapter 19 for a description of Glogau types). Results with light chemical peels are slow and progressive, requiring a series of treatments for improvements to become evident. Light peels may also improve fine lines, coarse pores, and superficial atrophic scarring,7 but results are not comparable to more aggressive forms of skin resurfacing, such as medium-depth peels or laser resurfacing. Assessment of patients’ expectations at the time of consultation and commitment to a series of treatments is essential to ensure success with these treatments.
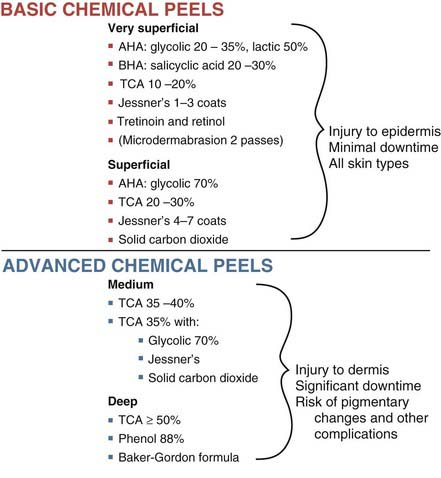
Very superficial chemical peels (e.g., glycolic acid 20%, salicylic acid 20% and retinol) can be used in all skin types (Fitzpatrick types I through VI). Patients with darker skin types (IV through VI) have increased risks with aesthetic procedures, particularly postinflammatory hyperpigmentation, and these gentle peels are one of the treatment options available to manage aesthetic skin conditions in darker skin types such as acne, enlarged pores, and hyperpigmentation (see Chapter 19 for a description of Fitzpatrick skin types).11–14 A conservative approach for providers getting started with superficial chemical peels (e.g., glycolic acid 70%, trichloroacetic acid 20% to 30%, and Jessner’s 4 to 7 layers) is to restrict use of these peels to lighter skin types (Fitzpatrick types I through III) to minimize the risk of complications.
Patients with erythematous conditions such as rosacea, telangiectasias, and poikiloderma of Civatte are also treated conservatively with light chemical peels, as aggressive chemical peels may exacerbate erythema associated with these conditions.15
Indications
Products Currently Available
Numerous chemical peels are available and common agents, along with their typical depth of skin penetration, are summarized in Figure 22-1.15–18 Many factors influence the depth of penetration with chemical peels, and although a given chemical peel may be classified as a superficial peeling agent, in practice, peels may vary in the depth of penetration. Figure 22-1 is, therefore, intended only as a general guide for chemical peel depths.
Chemical Peel Depth of Penetration
The depth achieved with light chemical peels ranges from very superficial resurfacing with removal of the stratum corneum, to superficial resurfacing with penetration to the upper papillary dermis. Although the type and concentration of chemical agent used are the main determinants of the depth of penetration in the skin, other factors also influence the depth of penetration including19:
Alpha Hydroxy Acids
Alpha hydroxy acids (AHAs) are derived from organic fruit acids and include: glycolic (sugar cane), lactic (milk), malic (apples), tartaric (grapes), citric (citrus), mandelic (almonds), and phytic (rice) acids. These products are keratolytic and penetrate through the stratum corneum, causing exfoliation by disrupting corneocyte adhesion. AHAs in more acidic formulations (i.e., lower pHs) and in higher concentrations have stronger biologic effects. Glycolic acid peels typically have a pH of 2.5 to 3. Very low pHs (pH < 2) of glycolic acid, however, are associated with greater risk of necrosis and crusting, and do not offer improved results over less acidic preparations.20 AHAs found in topical products as part of daily skin care regimes typically contain low AHA concentrations (10% or less) in less acidic formulations (pH 3.5 or greater).
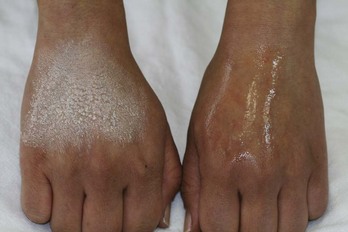
Glycolic acid (GA) is the most commonly used AHA and is an agent frequently selected by providers getting started with chemical peels.12 Glycolic acid is a small, water-soluble molecule that readily penetrates the skin. GA peels are clear colorless solutions that do not change in appearance upon application to the skin, unlike salicylic acid for example. Figure 22-2 shows GA applied to the dorsum of one hand, and salicylic acid on the other hand with its characteristic white precipitate. Because GA chemical peels do not exhibit a reliable clinical endpoint that is visible, application must be carefully monitored and timed, and the acid neutralized at the appropriate point to control the procedure. Clinical response to glycolic acid can be variable. Some patients have a brisk inflammatory response to low-strength (20% to 35%) short-duration applications (1 to 3 minutes), whereas others may tolerate higher strengths (70%) for longer duration (up to 7 minutes). The effects of GA can be terminated after application through neutralization, which raises the pH and renders the acid ineffective. Neutralization of alpha hydroxy acids may be performed with water or sodium bicarbonate solution (5% to 15%). If neutralization is not performed, AHAs will continue to be active and may penetrate deeper than intended.
Beta Hydroxy Acids
The benefits of SA as compared to the AHAs include less stinging, because SA has a mild anesthetic effect; a visible clinical endpoint with a fine white precipitate (“pseudofrost”), which helps ensure even application (see Figure 22-2), and no requirement for neutralization. Once a white precipitate is formed, there is little additional penetration of the acid and water may be used to wash off the precipitate. A disadvantage of SA is more obvious post-treatment desquamation than with GA. SA peels are available as pure or combination products, and instructions for application and endpoints vary based on the product used. For example, La Roche-Posay’s Biomedic SA peel 30% ends with frosting, whereas SkinCeutical’s SA/Mandelic Peel (SA 20% combined with mandelic acid 10%) does not frost.
Trichloroacetic Acid
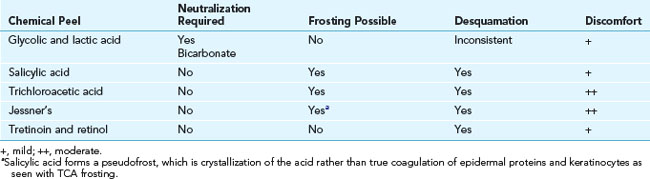
Trichloroacetic acid (TCA) is an agent familiar to many clinicians who have used it as therapy for condylomata acuminata (in highly concentrated preparations of 80% to 90%). For superficial chemical peels, it can be used as pure TCA 10% to 30%, or in combination with other peeling agents, such as TCA 15% combined with lactic acid 10%, or TCA 15% combined with SA 15% and lactic acid 15%. After application, TCA causes skin erythema and a whitish discoloration called frosting, which occurs 30 seconds to 2 minutes after application. Histologically, frosting corresponds to coagulation of epidermal proteins and keratinocytes. The intensity of frosting correlates directly with the depth of penetration (see Table 22-1).21 The desired clinical endpoint for superficial depth peels with TCA is level I frosting, visible as patchy erythema with faint white coloration.
TABLE 22-1 Frosting with Trichloroacetic Acid Chemical Peels and Depth of Penetration
|
Frosting |
Depth of Penetration |
Clinical Findings |
|---|---|---|
| Level I | Superficial | Patchy erythema with faint patchy white color |
| Level II | Medium | Even white color with some erythema visible through the white |
| Level III | Deep | Opaque confluent white |
Jessner’s Solution
Jessner’s solution is a combination of resorcinol 14%, salicylic acid 14%, and lactic acid 14% in ethanol, which was originally developed to lower the concentration of any one agent and, hence, reduce the risk of toxicity.22 Modified Jessner’s formulations are also available that have no resorcinol, such as salicylic acid 14%, lactic acid 14%, and citric acid 8% in ethanol (e.g., PCA Peel®). Once applied, erythema is followed by a powdery whitening of the skin due to precipitation of salicylic acid. Neutralization is not required, but water may be used to remove the white precipitate. Jessner’s peels are frequently applied in multiple layers, with a period of 4 to 7 minutes of observation for clinical endpoints between layers. Figure 22-3 shows a patient with mild erythema and a faint whitish coloration after application of six layers of a Jessner’s peel.
Retinoids
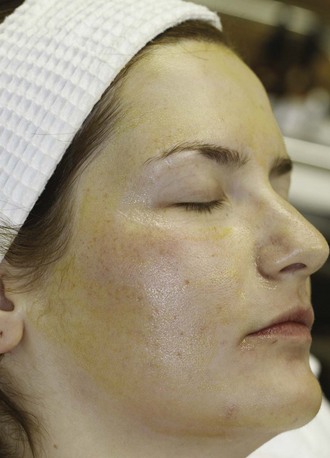
Topical retinoids, such as tretinoin (Retin-A) and tazarotene (Tazorac), have been principal therapies for acne and skin rejuvenation for many years as part of home skin care regimes. More recently, retinoids have been used as superficial peeling agents ranging from tretinoin peels 0.05% to 1%23 to lower strength preparations containing retinol (e.g., retinol 15% combined with lactic acid 15%). Retinoid peels cause a yellowish discoloration after application. They are not neutralized and are typically washed off by the patient 8 hours after application. Retinol peels may be layered over other superficial peeling agents (such as glycolic and salicylic acids) to enhance desquamation. Figure 22-4 shows a patient immediately after application of six layers of Jessner’s peel followed by one layer of retinol 15% peel combined with lactic acid 15% peel with characteristic yellow skin coloration.
Contraindications
Advantages of Superficial Chemical Peels
Anatomy
With photoaging, the stratum corneum thickens while the deeper papillary dermis thins as collagen and elastin become more sparse. Pores dilate and become plugged with desquamated debris. Loss of normal vascularity causes a sallow coloring and slows transportation of nutrients to outer layers of skin. Abnormal vasculature, or telangiectasia, may proliferate and pigmentation increases and becomes irregular, forming lentigines. Atypical cells develop, resulting in preneoplastic lesions such as actinic keratoses, and neoplasia.24
Chemical peels can address many of the skin changes associated with photoaging. They diminish corneocyte cohesion, promoting a more rapid rate of cell turnover, which normalizes epidermal keratinization. Histologic changes in the skin are evident after chemical peels including a compacted stratum corneum, smoother epidermis, and increased dermal thickness with fibroblast production of new collagen, elastin, and glycosaminoglycans.25–27 Chemical peels promote dispersion of melanin accumulated in the basal epidermal layer and transportation of the pigment to the skin surface for exfoliation. They reduce follicular plugging and decrease skin oiliness.
Equipment
Procedure Preparation
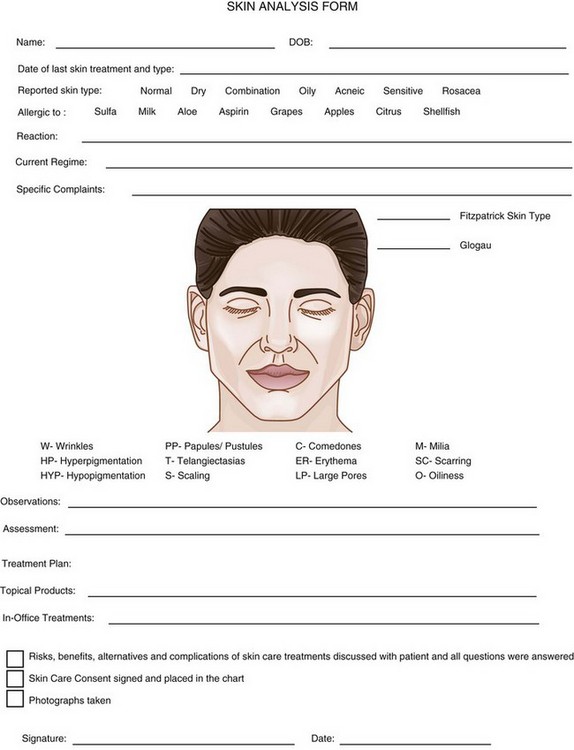
FIGURE 22-5 Skin Analysis Form.
(Copyright Rebecca Small, MD, Monterey Bay Laser Aesthetics, Capitola, CA.)
Preprocedure Skin Care Products
Preparation of the skin prior to chemical peeling enhances chemical peel effects, facilitates postprocedure healing, and may reduce the risks of complications.28 Topical products are typically begun 4 weeks prior to a procedure and consist of a retinoid, alpha hydroxy acid, broad-spectrum zinc or titanium sunscreen with SPF 30 or higher and sun avoidance, and for patients with darker Fitzpatrick skin types or those who are prone to hyperpigmentation, a skin-lightening agent such as hydroquinone (2% to 8%). Retinoids and exfoliants such as alpha hydroxy acids prepare the skin by decreasing stratum corneum thickness and reducing corneocyte cohesion, which allows for enhanced and more uniform penetration of the chemical peel agent. See Chapter 24, Skin Care Products, for a more detailed discussion of preprocedure topical products.
Very Superficial Chemical Peels: Steps and Principles
General Treatment Technique
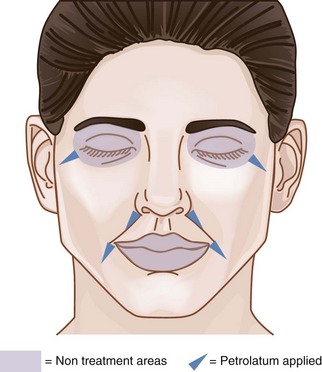
The Chemical Peel Safety Zone, which is the area within which chemical peel treatments can be safely performed on the face, is shown in Figure 22-6. Chemical peels may be applied to the full face apart from the lips and, in the periocular area, peels are applied above the eyebrows and 2 to 3 mm below the inferior eyelash margin.
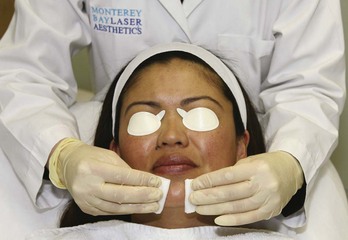
Chemical peels are typically thin liquids and can pool in skinfolds and creases, which intensifies their effects. Common areas of potential pooling include the marionette lines, nasolabial folds, and lateral canthal creases. Petrolatum is applied to these areas as a barrier to protect them from overtreatment (Figures 22-6 and 22-7).
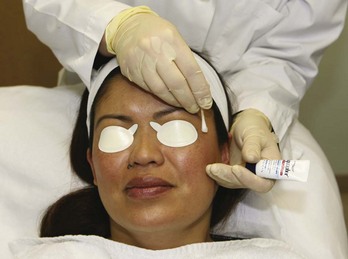
FIGURE 22-7 Application of petrolatum to areas of potential chemical peel pooling.
(Copyright Rebecca Small, MD.)
Most providers develop their own systematic method for applying a chemical peel to the face. In general, the chemical peel is applied to the least reactive areas first, such as the forehead, and to the most sensitive areas, such as the upper lip, last. Figure 22-8 shows one possible sequence for applying a chemical peel to the face.
General Chemical Peel Technique
Clinical endpoints for very superficial and superficial peels depend on the agent used. In most cases mild erythema is the desired endpoint and for those peels that frost, level I frosting with patchy erythema and whitening of the skin is the endpoint. Salicylic acid may form a fine white precipitate, which is also a desired clinical endpoint. Table 22-2 summarizes key characteristics and clinical endpoints for common chemical peel agents.
Performing a Very Superficial Glycolic Acid 20% Chemical Peel
Results
A single superficial or very superficial depth chemical peel typically yields subtle improvements. Multiple peels performed at 2- to 4-week intervals will provide more appreciable benefits, including enhanced skin smoothness, decreased oiliness and comedones, reduced papulopustular lesions, improved fine lines, and fading of hyperpigmentation.2,6,8–10,19,29 In addition, chemical peel results can be enhanced with the use of rejuvenating topical products at home.
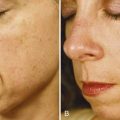
Figure 22-10 shows a patient with melasma on the forehead (A) before and (B) after three modified Jessner’s peels containing lactic acid 14%, salicylic acid 14%, hydroquinone 2%, and kojic acid 3%, pH 1.5 to 1.9 (PCA Peel®). Home care products included a daily arbutin lightening agent, alpha hydroxy acids, and sunscreen.
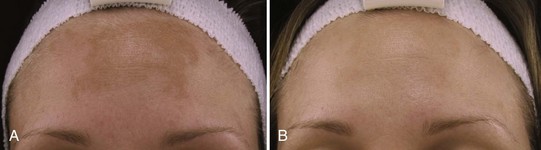
FIGURE 22-10 Melasma (A) before and (B) after three modified Jessner’s chemical peels.
(Courtesy of PCA Skin, Scottsdale, AZ.)
Figure 22-11 shows a patient with papulopustular acne (A) before and (B) after four modified Jessner’s peels (PCA Peel) and four salicylic acid 20% treatments. Home care products included daily benzoyl peroxide 5% cleanser, retinol serum 0.5% with lightening agents of kojic acid, licorice, arbutin, alpha hydroxy acid, and sunscreen.
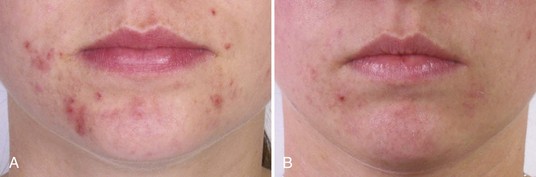
FIGURE 22-11 Papulopustular acne (A) before and (B) after four modified Jessner’s peels and four salicylic acid 20% treatments.
(Courtesy of PCA Skin, Scottsdale, AZ.)
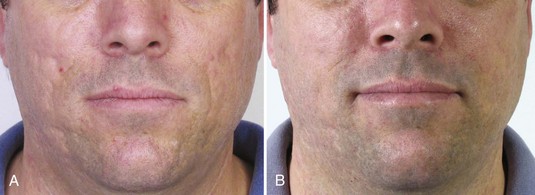
Figure 22-12 shows a patient with acne scarring (A) before and (B) after six combination treatments of microdermabrasion followed by TCA 10% combined with lactic acid 20%, pH 0.62 to 1.02 (PCA Ultra Peel® I). Home care products included daily palmitoyl oligopeptide/tetrapeptide-7 serum (Matrixyl 3000™) for skin texture.
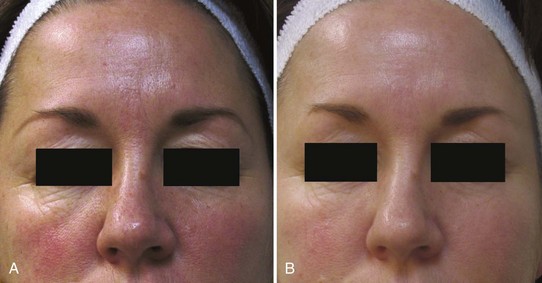
Figure 22-13 shows a patient with wrinkles marked in the periocular areas and erythema (A) before and (B) after one treatment with three layers of TCA 20% combined with lactic acid 10%, pH 0.62 to 1.02 (PCA Ultra Peel® Forte), followed by retinol 10% combined with lactic acid 20%, pH 3 to 3.4 (PCA Esthetique Peel®).
Aftercare
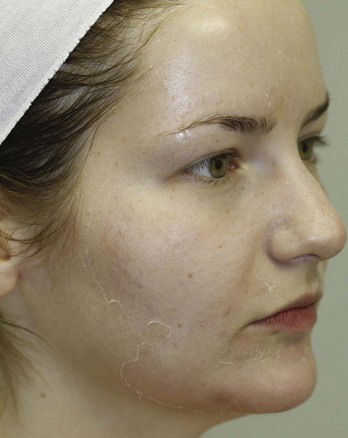
Erythema, dryness, and sensitivity are common in the first 1 to 2 days postprocedure. By day 3 the skin may feel tighter, texture may appear more coarse, wrinkles may be accentuated, and patients may report mild pruritis. Exfoliation usually occurs on day 3 to 5, persists for a few days, and ranges from skin flaking to skin sloughing and peeling. Figure 22-14 shows a patient with peeling on day 3 after treatment with six layers of a Jessner’s chemical peel followed by a combination peel of retinol 15% with lactic acid 15%. In some instances, no flaking or peeling may be evident, particularly when very superficial chemical peels are used in patients whose skin is conditioned (i.e., who regularly exfoliate). Lack of peeling or flaking does not indicate that a peel was histologically or clinically ineffective.
Advanced Chemical Peeling
Microdermabrasion can be used as part of skin preparation prior to the application of chemical peels to allow for more even acid penetration, and increase the depth of penetration to enhance results.30 Providers are advised to follow established manufacturer protocols when combining these two exfoliation modalities because acid penetration can be significantly altered by the lack of the stratum corneum barrier that is removed by microdermabrasion.
Complications
Hyperpigmentation can be treated with lightening agents such as hydroquinone cream (2% to 8%) or cosmeceutical agents such as kojic acid, arbutin, and licorice (see Chapter 24, Skin Care Products). Figure 22-15 shows a patient with hyperpigmentation in the marionette lines 1 month after receiving a Jessner’s chemical peel (six layers) who did not have petrolatum applied to marionette lines, an area of potential chemical peel pooling. Hyperpigmentation usually resolves, but in rare instances, can be permanent. In darker Fitzpatrick skin types, resolution of hyperpigmentation may take several months. The risk of hyperpigmentation is greater with the use of exogenous hormones, photosensitizing medications, and sun exposure. Hypopigmentation is rare and occurs more commonly in darker Fitzpatrick skin types (IV through VI). Hypopigmentation usually resolves, but may be permanent.
Blistering or vesiculation is rare and signifies epidermolysis. This can occur as a result of increased depth of chemical peel penetration if the epidermis is not fully intact at the time of treatment. The epidermis may not be fully intact with retinoid use the week prior to peeling, aggressive exfoliation such as scrubbing with exfoliants or microdermabrasion prior to peel application, active acne, and seborrheic or other dermatoses. Glycolic acids with lower pHs cause more desquamation, have a greater risk of epidermolysis and do not offer improved clinical effects.20 Vesicles typically form into crusts, which are kept moist with Bacitracin and dressed with a bandage until healed.
Systemic toxicity with salicylic acid applied to large areas is reported and extremely rare.31
Financial Considerations
Chemical peeling agents can be purchased for approximately $50 to $80 for a 4-oz bottle, which typically yields 20 peels and can be purchased from skin care product or medical suppliers (see the Resources list below). Charges for chemical peels vary by geographic region, and range from $65 to $450 based on the type of peel. The more aggressive higher strength superficial peels tend to have higher fees. Some insurance companies cover chemical peels for indications such as acne (see Box 22-1), however, most do not and patients pay for these procedures out of pocket. Chemical peels are often performed and charged as a series of six treatments. Committing to a series of treatments helps patients achieve the best possible results and helps to ensure high patient and provider satisfaction.
1. Small R. Aesthetic procedures in office practice. Am Fam Physician. 2009;80(11):1231-1237.
2. Kuwahara R, Rasberry R. Chemical peels. EMedicine; 2007.
3. Zakapoulu N, Kontochistopoulous G. Superficial chemical peels. J Cosm Dermatol. 2006;5(3):246-253.
4. Cosmetic Surgery National Data Bank 2010 Statistics. American Society for Aesthetic Plastic Surgery; 2010.
5. Small R. Aesthetic procedures introduction. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams & Wilkins; 2009:195-199.
6. Swinehart JM. Salicylic acid peeling of the hands and forearms. Effective nonsurgical removal of pigmented lesions and actinic damage. J Dermatol Surg Oncol. 1992;18:495-498.
7. Matarasso SL, Salman SM, Glogau RG, et al. The role of chemical peeling in the treatment of photodamaged skin. J Dermatol Surg Oncol. 1990;16:945-954.
8. Bergfeld WF, Tung RC, Vidimos AT. Improving the appearance of photoaged skin with glycolic acid. J Am Acad Dermatol. 1997;36:1011-1013.
9. Kligman D, Kligman A. Salicylic acid as a peeling agent for the treatment of acne. J Cosm Dermatol. 1997;10(9):44-47.
10. Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34(1):45-50.
11. Khunger N, Sarkar R, Jain RK. Tretinoin peels versus glycolic acid peels in the treatment of Melasma in dark-skinned patients. Dermatol Surg. 2004;30(5):756-760.
12. Sarkar R, Kaur C, Bhalla M, et al. The combination of glycolic acid peels with a topical regimen in the treatment of Melasma in dark-skinned patients: A comparative study. Dermatol Surg. 2002;28:928-932.
13. Lawrence N, Cox SE, Brody HJ. Treatment of melasma with Jessner’s solution versus glycolic acid: a comparison of clinical efficacy and evaluation of the predictive ability of Wood’s light examination. Am Acad Dermatol. 1977;36:589-593.
14. Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg. 1999;25:18-22.
15. Tse Y. Choosing the correct peel for the appropriate patient. In: Rubin M, editor. Chemical Peels. Philadelphia: Elsevier; 2006:13-19.
16. Khunger N. Standard guidelines for chemical peels. Indian J Dermatol Venerol Leprol. 2008;74:S5-S12.
17. Perkins SW, Gillum TG. Management of aging skin. In: Cummings C, editor. Otolaryngology: Head and Neck Surgery. Philadelphia: Elsevier, 2005.
18. Cox SE, Butterwick KJ. Chemical peels. In: Robinson J, Hanke W, Sengelmann RD, Siegel D, editors. Surgery of the Skin. Philadelphia: Mosby/Elsevier; 2005:463-482.
19. Briden ME. Alpha-hydroxyacid chemical peeling agents: case studies and rationale for safe and effective use. Cutis. 2004;73(2 Suppl):18-24.
20. Becker FF, Langford FPJ, Rubin MG, et al. A histological comparison of 50% and 70% glycolic acid peels using solutions with various pHs. Dermatol Surg. 1996;22:463-465.
21. Monheit GD. Chemical peels. Skin Therapy Lett. 2004;9:6-11.
22. Fulton J. Jessner’s peel. In: Rubin M, editor. Chemical Peels. Philadelphia: Elsevier; 2006:57-71.
23. Cuce L, Bertino M, Scattone L, et al. Tretinoin peeling. Dermatol Surg. 2001;27:12-14.
24. Zimbler MS, Kokoska MS, Thomas JR. Anatomy and pathophysiology of facial aging. Facial Plast Surg Clin North Am. 2001;9(2):179-187. vii
25. Berardesca E, Distante F, Vignoli GP, et al. Alpha hydroxyacids modulate stratum corneum barrier function. Br J Dermatol. 1997;137(6):934-938.
26. Murad H, Shamban AT, Premo PS. The use of glycolic acid as a peeling agent. Dermatol Clin. 1995;13:285-307.
27. El-Domyati M, Attia S, Saleh F, et al. Trichloroacetic acid versus chemical peeling: a histometric, immunohistochemical and ultrastructural comparison. Dermatol Surg. 2004;30:179-188.
28. Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. Guidelines/Outcomes Committee: American Academy of Dermatology. J Am Acad Dermatol. 1995;33:479-503.
29. Sehgal V, Luthra A, Aggerwal A. Evaluation of graded strength glycolic acid facial peel: an Indian experience. J Dermatol. 2003;30(758):761.
30. Briden ME, Jacobsen E, Johnson C. Combining superficial glycolic acid (alpha-hydroxy acid) peels with microdermabrasion to maximize treatment results and patient satisfaction. Cutis. 2007;79(1 Suppl):13-16.
31. Davies MG, Briffa DV, Greaves MW. Systemic toxicity from topically applied salicylic acid. Br Med J. 1979;1(6164):661.
Small R, Linder J. Chemical peels. In: Small R, Linder J, editors. A Practical Guide to Skin Care Procedures and Products. Philadelphia: Lippincott Williams & Wilkins, 2011.
Uecker C. Chemical peels. In Pfenninger JL, Fowler GC, editors: Pfenninger and Fowler’s Procedures for Primary Care, 3rd ed, Philadelphia: Mosby/Elsevier, 2010.


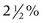 may be applied twice a day to prevent postinflammatory hyperpigmentation. Makeup may be worn 24 hours after the procedure and mineral products are preferable. Strict sun avoidance for 2 weeks postprocedure is important, including the use of a wide-brimmed hat.
may be applied twice a day to prevent postinflammatory hyperpigmentation. Makeup may be worn 24 hours after the procedure and mineral products are preferable. Strict sun avoidance for 2 weeks postprocedure is important, including the use of a wide-brimmed hat.