Chapter 7 Chemical Ablation
Historical Background
Historical data suggest that chemical ablation of the great saphenous vein (GSV) using liquid sclerosant delivered percutaneously via syringe and needle will fail (recanalize) in over 50% of cases at 1 year. Sclerotherapy in Europe became less frequently performed in the latter part of the 20th century, at least in part, because of the work of Hobbs.1 His 10-year randomized controlled study showed the clinical recurrence of varices was common in patients with truncal saphenous reflux managed with sclerotherapy.2 Hobbs found that after 10 years, 71% of patients treated with traditional surgery for saphenous incompetence had a good outcome; this compared with only 6% of patients treated by sclerotherapy. Recent scientific evidence has shown that liquid sclerotherapy is not very effective at eliminating truncal saphenous incompetence and failure to eliminate reflux leads to early recurrence of varices. Scientific evidence is limited when comparing sclerotherapy and surgery due to the fact that many of the trials had methodologic flaws such as lack of evaluation by independent observers, high dropout rates, poorly defined outcomes, and the lack of intention-to-treat analyses.3,4 However, despite these defects, most of the studies clearly demonstrated very high recurrence rates of varicose veins after sclerotherapy, ranging from 20% to 70%.
Etiology and Natural History of Disease
The efficacy of sclerosing agents is a function of concentration and vein diameter.5 If the target vein diameter is greater than 3 mm, liquid sclerosants do not properly reach the vein wall secondary to dilution. Sclerosant in the form of foam has clearly improved the results of sclerotherapy. Foam is more efficacious than liquid6–8 and is more readily monitored with ultrasound imaging. Foam is the reason chemical ablation has made a resurgence. Foam will expand and fill a vein of less than 12-mm diameter, offering better contact with the vein wall. Cabrera and colleagues9 published a clinical series of 500 lower limbs treated with foam sclerotherapy and reported that after 3 or more years, 81% of treated great saphenous trunks remained occluded and 97% of superficial varices had disappeared. This required one session of sclerotherapy in 86% of patients, two sessions in 11% of patients, and three sessions in 3% of patients.
Sclerosants
When Elkins Sinn discontinued production of Sotradecol in the United States in 2000, a nationwide shortage ensued. Since no other manufacturer had FDA approval to produce STS, compounding pharmacies were the only source from which physicians could obtain this agent. The shortage of STS and the stopgap role of compounding pharmacies ended in November 2004 when the FDA granted approval to Bioniche Pharma USA, Inc. (Belleville, Ontario, Canada) to manufacture STS in 1% and 3% strengths. Today, FDA-approved Sotradecol is manufactured by Bioniche Pharma in an FDA-approved facility and sold exclusively by AngioDynamics, Inc. (Queensbury, NY). In a study of compounded STS versus pharmaceutical-grade STS, several findings were reported.10 Compounded STS was found to contain measured levels of impurities, the most important of which was carbitol. Analysis of pharmaceutical-grade STS revealed no detectable levels of impurities. Although the level of STS impurity necessary to precipitate a clinical event is unknown, impurities in other drugs have been linked to significant unexpected adverse events. Concentrations of different compounded STS formulations showed significant variation when measured by an independent laboratory. In one sample, the concentration was 20% below the desired 3% concentration level.
Patient Selection
Sclerotherapy is a good choice for the treatment of nonsaphenous varicose veins, residual veins after surgical correction of axial vein reflux, and recurrent varicose veins secondary to neovascularization or incompetent perforating veins. Sclerotherapy is also the treatment of choice in spider telangiectasias, venectasias, and isolated reticular veins.11 The mode of action is induction of irritation of the vein wall followed by inflammation and fibrosis. Different agents are used such as hypertonic glucose that act by dehydrating the endothelium and substances like ethanolamine oleate8 that have a detergent effect in the endothelial layer.
Operative Steps
The three different methods of injecting large veins are:
Imaging
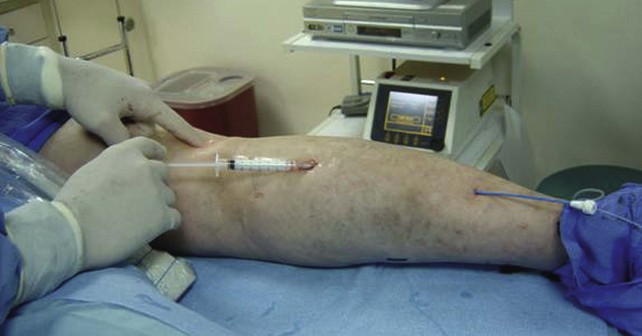
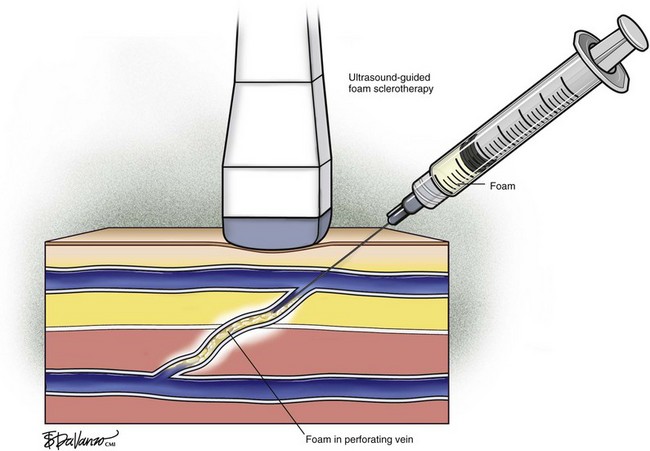
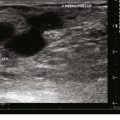
Recent trends have demonstrated a growing use of duplex-guided sclerotherapy, which was first described in 1989.15 It not only minimizes the chances of intraarterial injection and extravasation but also allows the estimation of the degree of spasm, length of vein treated, and position of the deep veins (Figs. 7-6 and 7-7).
Foam Sclerotherapy
A recent revolution in the treatment of venous disease has been the emergence of foam sclerotherapy. Egmont James Orbach in 1944 first proposed the use of foam generated by a simple process of shaking a sclerosant with air. However, interest faded because it could only be used for small veins owing to large bubbles and a high air-to-liquid ratio.16 The renaissance of foam sclerotherapy is credited largely to the work of Cabrera et al.17 and Monfreux et al.18 in the 1990s.
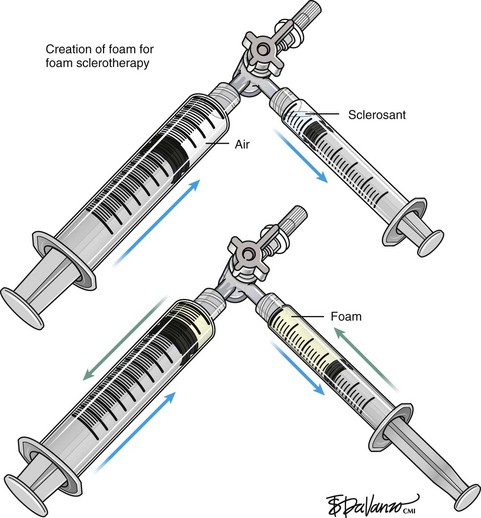
Foam for the purpose of vein wall destruction is a nonequilibrated dispersion of gas bubbles in a sclerosing solution in which the gas fraction is 0.5221 or greater. The foam is composed of tiny bubbles of gas covered by a tensioactive liquid.19 Small bubbles make the foam highly interactive, whereas large bubbles produce ineffective foam. The foam mechanically displaces the blood and comes into contact with the endothelium. Therefore, the same concentration of the sclerosant is suitable for large and small veins. There is sufficient clinical evidence to demonstrate several advantages of foam preparations over conventional liquid sclerotherapy6,20:
Variables like type and concentration of the sclerosing agent, gas, gas-to-liquid ratio, bubble size, and time between preparation and use determine the efficacy of the agent.21 An ideal foam should be durable enough to allow injection before separating into gas and liquid components.22 It is accepted that microfoams with bubble diameters less than 250 µm are commonly used and the ideal effective choice (macrofoams have bubbles larger than 500 µm and minifoams have bubbles ranging from 250 to 500 µm). There are various techniques in the literature that describe effective ways to produce microfoam.
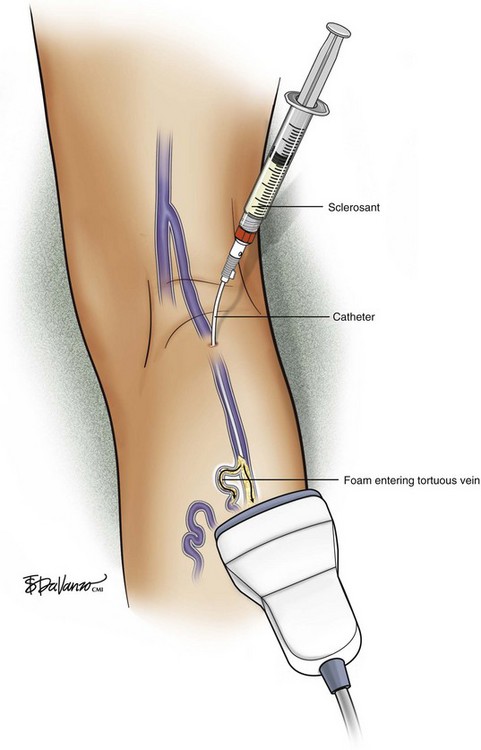
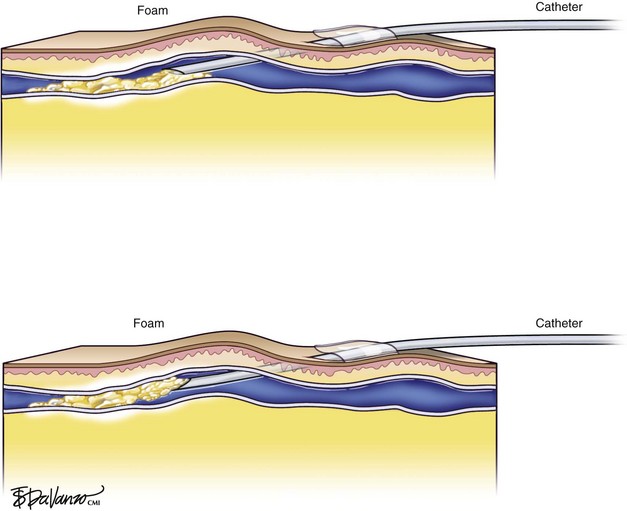
In reference to the biologic behavior of STS, Schneider and Fischer23 showed that endothelial damage is concentration dependent and occurs immediately after injection, with resulting rapid thrombus formation leading to vascular sclerosis. Importantly, 3% STS foam has not yielded 100% GSV closure when injected with a standard needle and syringe; therefore, catheters that can enhance the interaction of drug to vein wall are under investigation.
Foam is an option for controlling saphenous reflux in veins of less than 12 mm and has been shown to be a viable treatment for sclerosis of perforators. Foam is of value in the treatment of varicosed tributaries, tortuous vessels, and venous malformations. Foam also has limitations and carries liability concerns. In veins larger than 12 mm, the sclerosant–blood interface compromises treatment.24 It has been recommended that a 10-mL limit be placed with regard to total foam volume injected because of possible paradoxical embolization via the patent foramen ovale.19 For this reason there has been some interest in maximizing sclerosant contact time with the vein wall through the use of catheters. Future studies will likely focus on maximizing results of liquid or foamed sclerosants through innovations in catheter technology. The known methods of producing foam sclerotherapy include the following:
Cabrera method: Juan Cabrera in 1997 published his 7-year experience of excellent results with special foam that is prepared with a sclerosing agent, CO2, and an unknown tensioactive agent.17
Monfreux method: In this method, foam is produced in a glass syringe with the tip closed by a sterile plug; tension is applied by compressing the piston. While the foam is long lasting, the larger bubbles formed by this method can reduce the effectiveness of the therapy.18
Tessari method: Described in 1999 by Lorenzo Tessari, high-quality foam is produced with two disposable syringes and a three-way tap.24 STS was initially described with this method. The advantages of this method are use of disposable materials, compact foam with small bubble diameter, and the ability to reconstitute the foam if the treatment session takes more time.
Frullini method: Described by Frullini and Cavezzi in 2000, the foam generation uses the same turbulence effect as the Tessari method.18 Foam is generated by a vial and syringe joined by a connector.
Comparative Effectiveness of Existing Treatments
Results of Clinical Trials
A prospective randomized control trial involving more than 800 patients over 10 years compared six treatment options: liquid sclerotherapy, high-dose liquid sclerotherapy, multiple ligations, stab avulsions, foam sclerotherapy, and ligation followed by sclerotherapy. The conclusion was that the results of foam sclerotherapy were superior to those of liquid sclerotherapy and comparable with those of surgery.25
The listed complications ranged from phlebitis, skin necrosis, transient visual disturbance, and, on rare occasions, deep venous thrombosis (DVT). There have been suggestions that foam sclerotherapy may have a slightly higher rate of pigmentation, inflammation, and minimal necrosis when used in small reticular veins and telangiectasias.18 There have been uncertainties regarding the safety of foam preparations as animal studies demonstrated increased pulmonary hypertension. The stress on the human respiratory system is still unclear. The volume used per session depends on the size of the veins. No cases of DVT were reported in a prospective study of large volume foam sclerotherapy for varicose veins.26
Two randomized controlled trials (RCTs) compared foam sclerotherapy with high ligation and stripping (HL/S). The first RCT showed that HL/S is superior to foam sclerotherapy using Varisolve (BTG plc, London, UK) for occlusion and elimination of reflux (86% versus 63%) but that foam sclerotherapy was superior to conventional sclerotherapy (90% versus 76%).26 The second RCT showed that foam sclerotherapy combined with high ligation was less expensive, involved shorter treatment time, and resulted in more rapid recovery compared with HL/S.27
Although foam sclerotherapy is used universally and has become the standard of care, foamed sclerosants can embolize to the arterial circulation via patent foramen ovale. Only three permanent adverse events from paradoxical embolization of foam have been published28,29; however, several transient neurologic events have been reported anecdotally, and therefore foam remains controversial.
Outcomes Using Foam
A detailed review of the outcomes of ultrasound-guided foam sclerotherapy was published in 2007.30 Some important publications and more recent data are discussed here. Cabrera et al.17 published a clinical series of 500 legs treated with foam sclerotherapy. They reported that after 3 or more years, 81% of treated great saphenous trunks remained occluded and 97% of superficial varices had disappeared. This required one session of sclerotherapy in 86% of patients, two sessions in 11% of patients, and three sessions in 3% of patients. No DVT or pulmonary embolism was encountered in this series.
Subsequently, a number of authors have published clinical series based on this technique including Frullini and Cavezzi,18 who reported a series of 453 patients, and Barrett et al.,31 who reported a series of 100 limbs. Cavezzi et al.32 subsequently published a detailed analysis of the efficacy of foam sclerotherapy in 194 patients, reporting a good outcome in 93% of patients. In fact, this technique has become widely used in southern Europe, Australia, New Zealand, South America, and the United States.33 However, few surgeons in the United Kingdom use this method, perhaps because of limited evidence of efficacy. One series has been reported from the United Kingdom involving 60 patients in comparing surgical treatment with foam sclerotherapy combined with saphenofemoral ligation.34
One randomized study of foam sclerotherapy in comparison with surgery has been published. This was a multicenter European study.26 This randomized controlled trial included two separate studies: a surgical part undertaken by surgeons who randomized patients to saphenous stripping or ultrasound-guided foam sclerotherapy. In addition, sclerotherapists randomized patients to ultrasound-guided sclerotherapy conducted using either foam or liquid sclerosant. In all, 654 patients were treated during this study. Up to four sessions of ultrasound-guided foam sclerotherapy were allowed over 3 months to obliterate the saphenous trunks. After 12 months, the surgeons had eliminated truncal saphenous reflux in 130 of 176 patients (74%) by foam sclerotherapy and in 84 of 94 patients (88%) by surgery. In comparison, sclerotherapists had eliminated reflux in 239 of 254 patients (91%) by foam and 104 of 125 patients (83%) by liquid sclerotherapy. Posttreatment pain was assessed by a visual analog scale, which showed that surgery was much more painful during the first week. Normal activities were resumed after a median of 13 days in the surgery group and 2 days in the foam group. DVT was seen in 10 patients treated by foam and one patient treated by liquid sclerotherapy.
The relative efficacy of foam and liquid sclerotherapy has been investigated in a detailed study.35 Patients with truncal saphenous incompetence were injected with either 2 to 2.5 mL of 3% POL liquid or foam into the GSV under ultrasound guidance on only one occasion. Obliteration of saphenous incompetence was obtained in 35% of liquid-treated patients and 85% of foam-treated patients after 3 weeks. At 2 years, 53% of foam-treated and 12% of liquid-treated patients had successful obliteration of the GSV. A further study was performed to assess the relative efficacy of 1% and 3% sclerosant foam.36 Patients with truncal saphenous reflux were randomized to treatment with either 1% or 3% POL foam in a single session. An average of 4.5 mL of foam was injected in both groups. Immediate occlusion rates were 96% (3% concentration) and 86% (1% concentration). After 2 years, saphenous occlusion was seen in 69% of the 3% group and 68% of the 1% group.
In both of these studies, a rather small volume of foam was used; it was well below the maximum of 10 mL recommended in the Tegernsee document37 and the 20 mL suggested earlier in this text. This probably prejudiced the outcome but allowed the authors to demonstrate clearly the advantages of foam and lack of increased efficacy with 3% POL.
A number of papers have addressed particular problems in the management of varicose veins. Recurrent varices managed surgically have a poor outcome, with further recurrence from neovascularization as a common feature.38 The clinical series mentioned found similar outcomes in primary and recurrent varices managed by foam sclerotherapy, with 88% of SFJs and saphenous trunks remaining obliterated at 11-month follow-up.39 Creton and Uhl40 have reported a combined surgical and foam sclerotherapy technique (in one session) for patients with recurrent varices, with obliteration of 93% of varices and saphenous trunks at 40-day follow-up. Perrin and Gillet41 reviewed the available literature on recurrent varices of the popliteal fossa and concluded that unless a grossly incompetent SPJ stump is present, ultrasound-guided foam sclerotherapy is the most appropriate treatment. They acknowledge that this conclusion is supported by reports of clinical series and not randomized clinical trials.
A number of papers have discussed the outcome of foam sclerotherapy in patients with venous ulceration and severe venous disease. One hundred eighty-five truncal veins were treated in 165 patients. A high proportion of veins were complicated (109 CEAP classes 4-6, 76 CEAP 1-3). There was no significant difference in occlusion rates between primary (45/60-75%) and recurrent (23/32-72%) veins; CEAP 2-3 (22/30-73%) and CEAP 4-6 (46/62-74%) veins; and veins with a diameter less than 7 mm (29/38-76%) and veins with a diameter more than or equal to 7 mm (13/23-57%). The authors concluded that foam sclerotherapy was equally effective for complicated and uncomplicated varicose veins.42 The clinical outcome of treatment has been reported in a number of papers.43–45 In general, rapid healing of ulcers is reported following foam sclerotherapy, confirming that this treatment can probably achieve the same outcomes that result from saphenous obliteration in leg ulcer patients.
Pearls and Pitfalls
The major contraindication to the use of sclerotherapy is known allergy to the agent. The minor side effects seen are hyperpigmentation, pruritus, and neovascularization. Other reported side effects include night cramps, swollen foot, thrombophlebitis, and blisters.46,47 Sclerotherapy must be performed with caution in patients with arterial insufficiency, recent or recurrent DVT, hypercoagulability syndromes, and severe systemic diseases. Dangerous complications like intraarterial injection and thromboembolism can be avoided by following a meticulous protocol.
Complications
Systemic complications of foam sclerotherapy have been examined in some detail in view of occasional visual disturbance reported by some patients following both foam and liquid sclerotherapy.48 Visual disturbance has been reported following the injection of a range of liquid sclerosants, although it is clear that it is far more common following foam sclerotherapy. Some serious neurologic adverse events, from which recovery was eventually complete, have also been reported.31 This has led to attention focusing on the effects of a patent foramen ovale. This was the subject of a study by Morrison et al.,49 in which 20 patients with visual or respiratory symptoms following foam sclerotherapy were studied by transthoracic echocardiography during treatment. In this study, 65% of patients were found to have echoes in the left atrium after the injection of foam sclerosant. Five of nine patients who then underwent transcranial Doppler investigation during foam sclerotherapy were found to have high-intensity events in the middle cerebral artery during treatment.
One study has examined the relative efficacy of minimally invasive means of treating incompetent saphenous trunks in comparison with surgical management.50 A meta-analysis of 119 studies including 12,320 limbs was undertaken. The main outcome measure was obliteration of the saphenous trunk assessed by duplex ultrasonography at an average of 30 months following treatment.
Miami Vein Center Experience
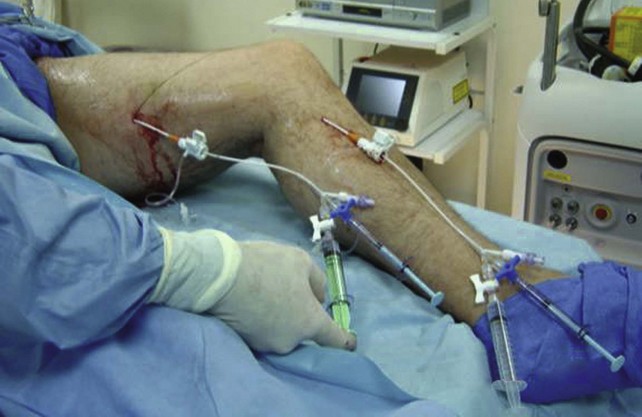
At Miami Vein Center we have worked with two different chemical ablation catheter systems, each quite different in design, which enhance drug interaction with venous endothelium. At the 2006 American Venous Forum, we presented the results of 75 saphenous veins treated with an endovenous catheter with an occlusive balloon at the SFJ (VeinRx, Miami, FL). The idea was to isolate a column of 3% STS foamed sclerosant at the desired target site with a controlled dwell time of 4 minutes. The amount of drug delivered by the catheter was calculated based on vein volume of the target saphenous vein.51 Primary closure was 87% and primary assisted closure was 96% at 6-month follow-up. Three limbs (4%) were treated for asymptomatic DVT. Interestingly, in our series thrombus formed only at sites of decreased flow in duplicated femoral veins. In all three cases of DVT, the foam was forced into the femoral vein via a thigh perforator from the GSV because the balloon blocked the outflow to the common femoral vein.52 Also of interest in this trial was the difference that drug quality played in the role of effective ablation. Pharmaceutical-grade drug was profoundly more effective than drug prepared by a compounding pharmacy.10 In the compounded STS group, 45.7% of veins demonstrated segments of incomplete ablation (SIAs) at some time during follow-up. In the pharmaceutical-grade STS group, SIAs were observed in only in 12.5% of veins. This difference was statistically significant at p = .02.
The second catheter device, presented at the 2008 American Venous Forum, attempts to maximize the effectiveness of 3% liquid STS sclerosant by delivery under pressure, in a pulsed fashion (AngioDynamics, Inc., Queensbury, NY), to the GSV endoluminally. In a pilot study of 10 limbs followed for 1 year, we reported 80% primary occlusion.53 Sixty percent of treated veins demonstrated SIAs at some point during the 1-year follow-up. This catheter does not occlude the SFJ, and one thrombus extension into the common femoral vein was observed postprocedure. The thrombus extension resolved spontaneously without anticoagulation. All treated GSVs were limited to a size of less than 10 mm in diameter.
Sclerotherapy for Superficial Varices and Telangiectases
Most publications mention the injection of 0.25% to 0.5% POL as being the most appropriate. STS 0.2% may also be used with similar outcomes.54 In Europe, chromated-glycerine is commonly used, and a randomized trial has been published comparing this to POL and POL foam.55 A good result can only be expected when the methods used by European phlebologists are followed.56 The essence is to treat the “feeding veins” as well as the telangiectases. The feeding veins comprise the reticular veins that drain the telangiectases plus any incompetent saphenous veins. A region of telangiectases is selected and reticular veins in this area are injected with 0.5% POL liquid, injecting 0.25 to 0.5 mL at each location. Sclerosant will often enter telangiectases, demonstrating that the valves in the reticular veins are incompetent. Then, all telangiectases in the affected area should be injected with small amounts of 0.5% POL using 0.1 to 0.2 mL at each site.
If the reticular varices are not treated, a reasonable outcome will be obtained in about half of patients but may not be sustained. The other half of patients may obtain a disappointing outcome or more telangiectases may develop. The use of compression following sclerotherapy for small veins varies widely. A recent randomized trial showed that it is of value,57 and it is advised that patients wear Class 2 compression stockings for at least 3 days after telangiectases and reticular varices have been injected. Postsclerotherapy compression can be used, although it was shown that the degree and duration of compression made no difference in recurrence rates, symptom improvement, or cosmesis.58
Systemic Complications
Some patients may develop tightness in the chest or coughing after foam. This is probably a direct effect of the foam on the lungs and can also occur following injections of liquid sclerosant. This also resolves in about 30 minutes. Again, lying supine for 30 minutes after treatment may be useful. The incidence of visual disturbances and chest symptoms has been reported to be reduced by using CO2 foams.59 Severe allergic reactions may follow injection of either of the sclerosants mentioned in this text. Appropriate drugs and equipment must be available to manage these potential complications.
1 Hobbs JT. Surgery or sclerotherapy for varicose veins: 10-year results of a random trial. In: Tesi M, Dormandy JA, editors. Superficial and Deep Venous Diseases of the Lower Limbs. Turin, Italy: Panminerva Medica; 1984:243-248.
2 Fegan WG. Injection with compression as a treatment for varicose veins. Proc R Soc Med. 1965;58:874-876.
3 Einarsson E, Eklof B, Neglen P. Sclerotherapy or surgery as treatment for varicose veins: a prospective randomized study. Phlebology. 1993;8:22-26.
4 Neglen P, Einarsson E, Eklof B. The functional long-term value of different types of treatment for saphenous vein incompetence. J Cardiovasc Surg. 1993;34:295-301.
5 Guex JJ. Indications for the sclerosing agent polidocanol. J Dermatol Surg Oncol. 1993;19:959-961.
6 Hamel-Desnos C, Desnos P, Wollmann JC, et al. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg. 2003;29:1170-1175.
7 Goldman MP. Sodium tetradecyl sulfate for sclerotherapy treatment of veins: is compounding pharmacy solution safe? Dermatol Surg. 2004;30:1454-1456.
8 Rao J, Wildemore JK, Goldman MP. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose and telangiectatic leg veins. Dermatol Surg. 2005;31:631-635.
9 Cabrera J, Cabrera JJr, Garcia-Olmedo MA. Sclerosants in microfoam: a new approach in angiology. Int Angiol. 2001;20:322-329.
10 Almeida JI, Raines JK. FDA-Approved sodium tetradecyl sulfate (STS) versus compounded STS for venous sclerotherapy. Dermatol Surg. 2007;33:1037-1044.
11 Guex JJ. Foam sclerotherapy—an overview: use for primary venous insufficiency. Semin Vasc Surg. 2005;18:25-29.
12 Fegan WG. Continuous compression technique for injecting varicose veins. Lancet. 1963;2:109.
13 . Consensus conference on sclerotherapy of varicose veins of the lower limbs, Anonymous. Phlebology. 1997;12:2-160.
14 Sigg K. Treatment of Varicose Veins by Injection Sclerotherapy as Practised in Basle. Treatment of Venous Disorders of the Lower Limb. Lancaster, UK: MTP Press; 1976.
15 Kanter A, Thibault P. Saphenofemoral incompetence treated by ultrasound-guided sclerotherapy. Dermatol Surg. 1996;22:648-652.
16 Orbach EJ. Sclerotherapy of varicose veins: utilization of intravenous airblock. Am J Surg. 1944;66:362-366.
17 Cabrera JJr, Garcia-Olmedo MA. Treatment of varicose long saphenous veins with sclerosant in microfoam: long term outcomes. Phlebology. 2000;15:19-23.
18 Frullini A, Cavezzi AM. Sclerosing foam in the treatment of varicose veins and telangiectases: history and analysis of safety and complications. Dermatol Surg. 2002;28:11-15.
19 Breu FX, Guggenbichler S. European consensus meeting on foam sclerotherapy, April, 4Ð6, 2003, Tegernsee, Germany. Dermatol Surg. 2004;30:709-717.
20 Yamaki T, Nozaki M, Iwasaka S. Comparative study of duplex-guided foam sclerotherapy and duplex-guided liquid sclerotherapy for the treatment of superficial venous insufficiency. Dermatol Surg. 2004;30:718-722.
21 Barrett JM, Allen B, Ockelford A, Goldman MP. Microfoam ultrasound-guided sclerotherapy of varicose veins in 100 legs. Dermatol Surg. 2004;30:6-12.
22 Hsu T-SM, Weiss R. Foam sclerotherapy: a new era. Arch Dermatol. 2003;139:1494-1496.
23 Schneider W, Fischer H. Fixierung and bindegewebige organization artefizieller thrombin bei der varizenuerodung. Dtsch Med Wochenschr. 1964;89:2410.
24 Tessari LM. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27:58-60.
25 Belcaro G. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO* Trial). Angiology. 2003;54:307-315.
26 Wright D, Gobin JP, Bradbury AV, et al. Varisolve polidocanol microfoam compared with surgery or sclerotherapy in the management of varicose veins in the presence of trunk vein incompetence: European randomized controlled trial. Phlebology. 2006;21:180-190.
27 Bountouroglou DG, Azzam M, Kakkos SK, et al. Ultrasound guided foam sclerotherapy combined with saphenofemoral ligation compared to surgical treatment of varicose veins: early results of a randomized controlled trial. Eur J Vasc Endovasc Surg. 2006;31:93-100.
28 Forlee MV, Grouden M, Moore DJ, et al. Stroke after varicose vein foam injection sclerotherapy. J Vasc Surg. 2006;43:162-164.
29 Bush RG, Derrick M, Manjoney D. Major neurological events following foam sclerotherapy. Phlebology. 2008;23:189-192.
30 Jia X, Mowatt G, Burr JM, et al. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;94:925-936.
31 Barrett JM, Allen B, Ockelford A, et al. Microfoam ultrasound-guided sclerotherapy of varicose veins in 100 legs. Dermatol Surg. 2004;30:6-12.
32 Cavezzi A, Frullini A, Ricci S, et al. Treatment of varicose veins by foam sclerotherapy: two clinical series. Phlebology. 2002;17:13-18.
33 Breu FX, Guggenbichler S, Wollmann JC. 2nd European Consensus Meeting on Foam Sclerotherapy, 2006. Tegernsee, Germany. VASA. 2008;3(suppl 1):1-29.
34 Bountouroglou DG, Azzam M, Kakkos SK, et al. Ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: early results of a randomised controlled trial. Eur J Vasc Endovasc Surg. 2006;31:93-100.
35 Ouvry P, Allaert FA, Desnos P, et al. Efficacy of polidocanol foam versus liquid in sclerotherapy of the therapy. Phlebology. 2007;22:34-39.
36 Hamel-Desnos C, Ouvry P, Benigni JP, et al. Comparison of 1% and 3% polidocanol foam in ultrasound guided sclerotherapy of the great saphenous vein: a randomised, double-blind trial with 2 year-follow-up. “The 3/1 Study”. Eur J Vasc Endovasc Surg. 2007;34:723-729.
37 Breu FX, Guggenbichler S, Wollmann JC. Second European Consensus Meeting on Foam Sclerotherapy. Duplex ultrasound and efficacy criteria in foam sclerotherapy from the Second European Consensus Meeting on Foam Sclerotherapy 2006, Tegernsee, Germany. VASA. 2008;37:90-95.
38 Winterborn RJ, Foy C, Heather BP, et al. Randomised trial of flush saphenofemoral ligation for primary great saphenous varicose veins. Eur J Vasc Endovasc Surg. 2008;36:477-484.
39 Coleridge Smith P. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg. 2006;32:577-583.
40 Creton D, Uhl JF. Foam sclerotherapy combined with surgical treatment for recurrent varicose veins: short term results. Eur J Vasc Endovasc Surg. 2007;33:619-624.
41 Perrin M, Gillet JL. Management of recurrent varices at the popliteal fossa after surgical treatment. Phlebology. 2008;23:64-68.
42 O’Hare JL, Parkin D, Vandenbroeck CP, et al. Mid term results of ultrasound guided foam sclerotherapy for complicated and uncomplicated varicose veins. Eur J Vasc Endovasc Surg. 2008;36:109-113.
43 Hertzman PA, Owens R. Rapid healing of chronic venous ulcers following ultrasound-guided foam sclerotherapy. Phlebology. 2007;22:34-39.
44 Ergan J, Pascarella L, Mekenas L. Venous disorders: treatment with sclerosant foam. J Cardiovasc Surg (Torino). 2006;47:9-18.
45 Pascarella L, Bergan JJ, Mekenas LV. Severe chronic venous insufficiency treated by foamed sclerosant. Ann Vasc Surg. 2006;20:83-91.
46 Norris MJ, Carlin MC, Ratz JL. Treatment of essential telangiectasia: effects of increasing concentrations of polidocanol. J Am Acad Dermatol. 1989;20:643-649.
47 Scurr JH, Coleridge-Smith P, Cutting P. Varicose veins: optimum compression following sclerotherapy. Ann R Coll Surg Engl. 1985;67:109-111.
48 Guex JJ, Allaert FA, Gillet JL, et al. Immediate and midterm complications of sclerotherapy: report of a prospective multicenter registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31:123-128.
49 Morrison N, Rogers C, Neuhardt D, et al. Large volume, ultrasound guided polidocanol foam sclerotherapy: a prospective study of toxicity and complications. Presented at UIP World Congress Chapter Meeting, San Diego, California, 27-31 August 2003.
50 van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
51 Raines JK, Garcia de Quevedo W, Jahrmarkt S, et al. Abbreviated method of determining vein volume in balloon-controlled vein ablation. Phlebology. 2007;22:40-44.
52 Almeida JI, Raines JK. Comparison of thermal and chemical saphenous endoablation. Poster: American Venous Forum 18th Annual Meeting, Miami, FL, 2006.
53 Almeida JI, Raines JK. Pulse spray sclerotherapy: a pilot study. Poster presented at the American Venous Forum 20th Annual Meeting, Charleston, SC, 2008.
54 Goldman MP. Treatment of varicose and telangiectatic leg veins: double-blind prospective comparative trial between aethoxyskerol and sotradecol. Dermatol Surg. 2002;28:52-55.
55 Kern P, Ramelet AA, Wutschert R, et al. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30:367-372.
56 Guex JJ. Microsclerotherapy. Semin Dermatol. 1993;12:129-134.
57 Kern P, Ramelet AA, Wutschert R, et al. Compression after sclerotherapy for telangiectasias and reticular leg veins: a randomized controlled study. J Vasc Surg. 2007;45:1212-1216.
58 Fraser IA, Perry EP, Hatton M, et al. Prolonged bandaging is not required following sclerotherapy of varicose veins. Br J Surg. 1985;72:488-490.
59 Morrison N, Neuhardt DL, Rogers CR, et al. Comparisons of side effects using air and carbon dioxide foam for endovenous chemical ablation. J Vasc Surg. 2008;47:830-836.