Challenge
Case 107
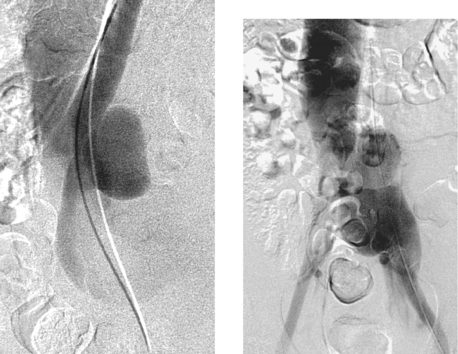 |
1. What vessels are abnormally opacified on this nonselective pelvic angiogram?
2. What vascular abnormality is seen on the oblique image?
3. Is this patient likely to be symptomatic?
4. What treatment options are considered for this type of problem?
ANSWERS: CASE 107
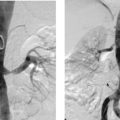
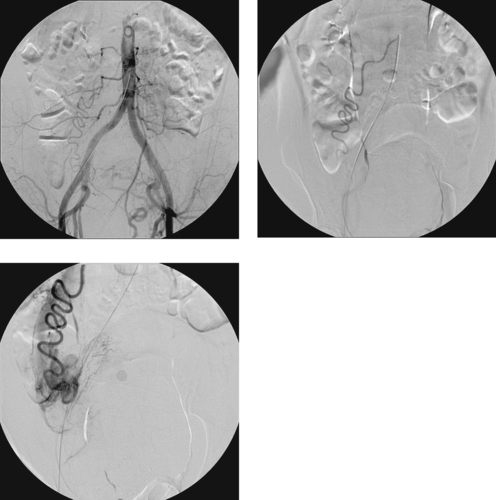
Aortocaval Fistula
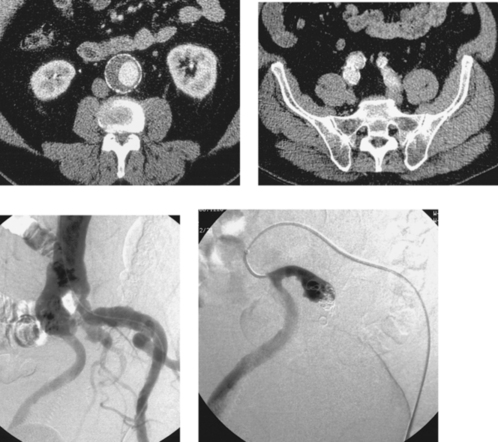
1. The left iliac vein and the inferior vena cava (IVC).
2. Pseudoaneurysm of the aortic bifurcation with a fistula to the IVC.
3. Yes.
4. Surgical ligation of the fistula with arterial repair, insertion of a stent graft, ligation or embolization of the artery distal and proximal to the fistula site with subsequent femorofemoral bypass grafting (not an option in the case above).
Reference
Parodi, J.C.; Schonholz, C.; Ferreira, L.M.; et al., Endovascular surgical treatment of traumatic arterial lesions, Ann Vasc Surg. 13 (2) (1999) 121–129.
Cross-Reference
Comment
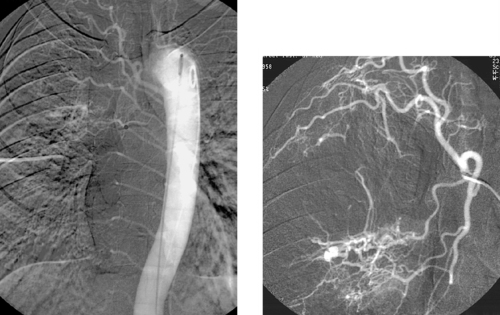
The images demonstrate an aortic bifurcation pseudoaneurysm with formation of a fistulous connection to the common iliac vein confluence.
Arteriovenous fistulas can occur as congenital abnormalities or as rare complications of trauma and surgery. Clinical manifestations can include high-output cardiac failure, ischemia distal to the fistula due to steal, dilated and enlarged peripheral veins, stasis dermatitis and ulceration due to increased venous pressure distal to the fistula, swelling of distal tissues, a palpable mass, an audible bruit, and a palpable thrill.
Angiography is useful to confirm the diagnosis of an arteriovenous fistula and delineate the anatomy, including the site of the fistula, its relationship to the involved vessels, and the presence of nearby branches that would confound endovascular repair. Large-volume injections in multiple projections with rapid filming are often necessary owing to the high flow volumes. Depending upon the location of the fistula and the presence of regional side branches, stent-graft placement can be used to seal the defect in patients who are poor candidates for surgery on anatomic or medical grounds.
Case 108
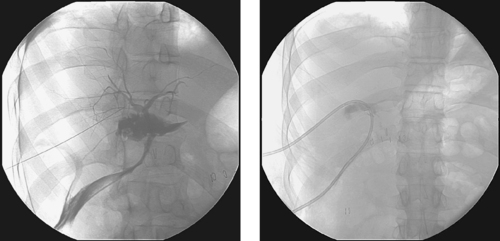 |
1. What problem is visualized in the first image?
2. Name two likely potential etiologies for this problem.
3. Is immediate percutaneous biliary drainage the best initial treatment option?
4. What biliary ductal variant is important to consider in patients presenting with postoperative bile leaks?
ANSWERS: CASE 108
Common Hepatic Duct Transection
1. Common hepatic duct transection with biloma formation.
2. Iatrogenic injury during laparoscopic cholecystectomy, and trauma.
3. Not necessarily. Biloma drainage is often a better and easier initial step.
4. Aberrant right posterior duct.
Reference
Saad, N.; Darcy, M., Iatrogenic bile duct injury during laparoscopic cholecystectomy, Tech Vasc Interv Radiol. 11 (2008) 102–110.
Cross-Reference
Comment
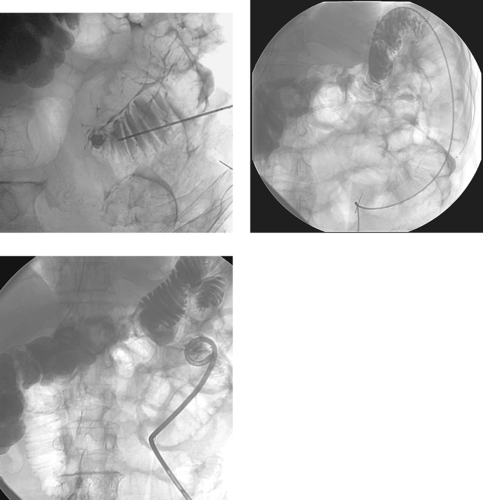
Bile duct injury is a dreaded complication of laparoscopic or open cholecystectomy, because in these situations, patients often require multiple radiologic interventions and/or complex surgical repair. The images here demonstrate contrast extravasating from the common hepatic duct and collecting as a biloma in the right subhepatic space and paracolic gutter. The distal common bile duct is not visualized, indicating a complete ductal transection.
The rate of technical failure of percutaneous biliary drainage is less than 5% in patients with dilated biliary ducts, but it can be as high as 25% in patients without ductal dilation. For this reason, in cases of major ductal injury, a percutaneous drainage catheter may first be placed within the easily accessible biloma to obtain initial control of the leak. After several weeks to a few months, a mature tract might form between the collapsed biloma cavity and the site of ductal injury. At this time, contrast injected into the biloma drain can opacify the biliary system, facilitating biliary drainage. In many cases, passage of a guidewire into the bowel is not possible; in these patients, a snare catheter may be used from the biloma access site to externalize the biliary wire and enable placement of a U-tube (second image), which is more stable than an external biliary drainage catheter. The presence of a drainage catheter assists the surgeon in identifying the duct during dissection, because there may be significant inflammation within the hepatic hilum. Ultimately, definitive surgical repair with hepaticojejunostomy is usually performed.
Case 109
 |
1. Describe the major finding in the first three images.
2. What treatment options exist for this problem?
3. What procedure was performed before the final image and why?
4. What significant clinical sequelae might this procedure produce?
ANSWERS: CASE 109
Internal Iliac Artery Embolization
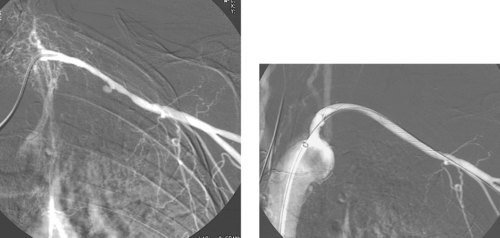
1. Abdominal aortic aneurysm (AAA) extending into the right common iliac artery.
2. Surgical repair of an aneurysm or placement of a stent graft if the aneurysm is large enough to warrant treatment (generally 5cm, although 4-cm AAAs are treated by some).
3. Right internal iliac artery embolization, to prevent endoleak resulting from cross-pelvic collateral flow following stent-graft repair.
4. Ipsilateral buttock claudication and/or pelvic or colonic ischemia.
Reference
Cynamon, J.; Lerer, D.; Veith, F.J.; et al., Hypogastric artery coil embolization prior to endoluminal repair of aneurysms and fistulas: Buttock claudication, a recognized but possibly preventable complication, J Vasc Intervent Radiol. 11 (2000) 573–577.
Cross-Reference
Comment
A variety of anatomic issues must be addressed before placing a stent graft for AAA. First, the iliac arteries must be of suitable caliber (7–8mm) to permit introduction of the stent-graft delivery apparatus. Second, the proximal and distal necks of the aneurysm should be of sufficient length (>15mm) and diameter (device-specific but usually <26-30mm), and should be free of significant angulation or plaque, which can interfere with proper seating of the device. Third, the distance from the origin of the lowest renal artery to the origin of the internal iliac artery must be measured in order to select the proper device length.
When AAA involves the common iliac artery and the distal neck measures less than 15mm, the potential exists for late aneurysm rupture due to endoleak via cross-pelvic collaterals from the contralateral internal iliac artery. For this reason, ipsilateral internal iliac artery coil embolization is performed to prevent this. Two main postprocedural adverse sequelae exist: First, if the contralateral internal iliac artery and inferior mesenteric artery are severely diseased, colonic ischemia can result. Second, about 40% of patients experience buttock claudication after internal iliac artery embolization; although this resolves spontaneously in two thirds of patients within a year, it can be a lifestyle-limiting long-term problem in a minority of patients. The problems can be prevented to some degree by embolizing only the main trunk of the internal iliac artery and not its branches.
Notes
Case 110
 |
1. What study has been performed in the first image in this acutely bleeding patient with metastatic nodal disease in the left axilla?
2. What angiographic finding in the first image would explain the clinical findings?
3. What procedure has been performed before the second image?
4. Is this likely to represent a durable solution to this problem?
ANSWERS: CASE 110
Axillary Artery Stent-Graft Placement
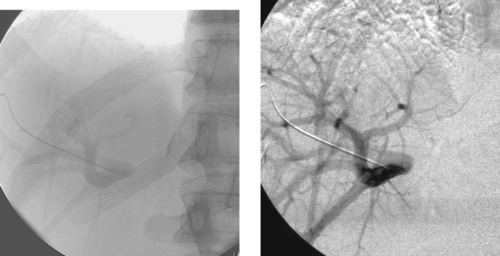
1. Selective left subclavian arteriogram.
2. Pseudoaneurysm from the left axillary artery.
3. Stent-graft placement in the left axillary artery.
4. No. This procedure is palliative. Recurrent bleeding and/or stent-graft occlusion represent potential future problems.
Reference
Hilfiker, P.R.; Razavi, M.K.; Kee, S.T.; et al., Stent-graft therapy for subclavian artery aneurysms and fistulas: Single-center mid-term results, J Vasc Intervent Radiol. 11 (2000) 578–584.
Cross-Reference
Comment
In this case, hypervascularity is observed within the lower neck, consistent with the history of metastatic disease. A small pseudoaneurysm arises from the left axillary artery. Given the patient’s short life expectancy and the presence of tumor in this area, surgical repair would be undesirable and maybe impossible. For this reason, a stent graft was percutaneously inserted across the pseudoaneurysm, providing hemostasis.
The use of nonaortic stent grafts is increasing. Because the long-term durability of these devices has not yet been established, careful patient selection criteria must be used. However, this technology often enables treatment of very difficult surgical problems in a minimally invasive manner.
The stent component of a stent graft enables enlargement of a vascular lumen and facilitates graft fixation. The graft component of the stent graft enables one to reline the interior of a vessel wall. Current applications for these devices include: (1) Atherosclerosis: the graft lining is thought to be associated with lower restenosis potential than bare stents; (2) Peripheral aneurysms; (3) Trauma-related vascular injury in patients with high surgical risk; (4) Arteriovenous fistulas and similar lesions.
Case 111
 |
1. What vessel is being injected in the images above?
2. Why might this examination have been performed?
3. Name two major potential complications of this procedure.
4. How often is the portal vein bifurcation extrahepatic?
ANSWERS: CASE 111
Portal Vein Access for Islet Cell Transplantation
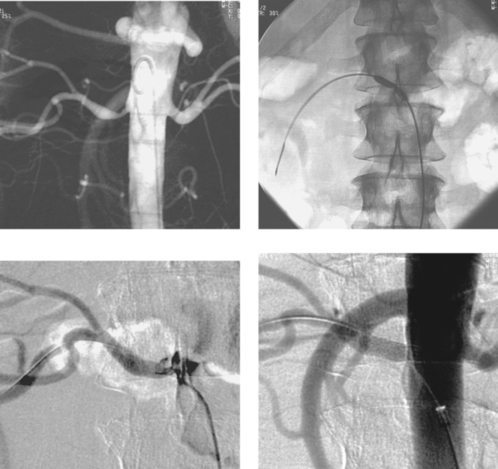
1. Right portal vein.
2. To guide a transjugular intrahepatic portosystemic shunt (TIPS) or to perform islet cell transplantation in diabetic patients.
3. Portal vein thrombosis and intraperitoneal hemorrhage.
4. Approximately 50% of patients.
References
Lin, J.; Zhou, K.R.; Chen, Z.W.; et al., 3D contrast-enhanced MR portography and direct x-ray portography: A correlation study, Eur Radiol. 12 (2003) 1277–1285.
Goss, J.A.; Soltes, G.; Goodpastor, S.E.; et al., Pancreatic islet transplantation: The radiographic approach, Transplantation 76 (2003) 199–203.
Cross-Reference
Comment
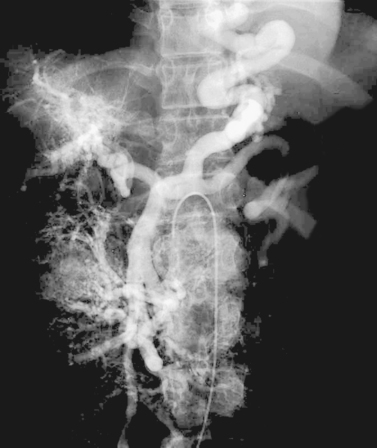
Direct portography via percutaneous catheter placement in the right portal vein for infusion of islet cells in a diabetic patient is depicted. Although this particular procedure is still investigational, direct portal vein access can be useful in a few clinical situations. When estimation of portal pressures is confounded by the presence of sinusoidal liver disease, a small catheter may be placed in the portal vein for direct pressure transduction. When transjugular portal vein access during TIPS is thought to be challenging, some physicians place a catheter within the right portal vein to provide a target for transhepatic puncture. In carefully selected patients, the percutaneous route may be used to clear thrombus from a thrombosed portal vein before placing a TIPS.
Case 112
 |
1. What is the minimum desired diameter for a stent placed in a renal artery?
2. How may stenosis in a small renal artery be treated?
3. If percutaneous and surgical revascularization are not possible owing to the small vessel size, what procedure can be used to ameliorate renovascular hypertension?
4. If it is unclear from angiography and scintigraphy whether a stenosis is causing renovascular hypertension, what procedure may be performed to clarify this?
ANSWERS: CASE 112
Stenosis in a Small Renal Artery
1. Below 6mm the significance of even mild degrees of intimal hyperplasia is great. Stent placement is not likely to lead to durable success in small vessels.
2. Percutaneous transluminal angioplasty, aortorenal bypass.
3. A renal artery supplying only a small amount of parenchyma may be embolized and that parenchyma sacrificed to treat renovascular hypertension.
4. Renal vein renin sampling.
Reference
Martin, L.G.; Cork, R.D.; Wells, J.O., Renal vein renin analysis: Limitations in predicting benefit for percutaneous angioplasty, Cardiovasc Intervent Radiol. 16 (1993) 76–80.
Cross-Reference
Comment
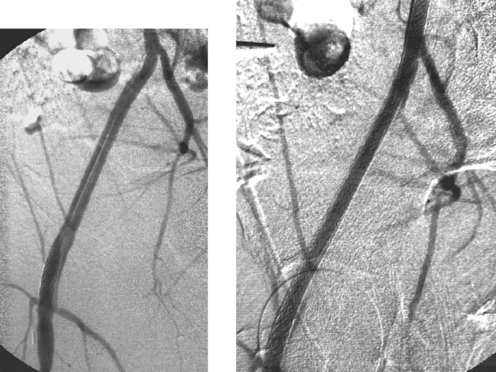
The images depict a stenosis in a fairly small renal artery. A poor technical result was observed after angioplasty (third image), so a stent was placed. The amount of renal parenchyma supplied by this renal artery was too large to sacrifice using embolization.
Essential hypertension is present in many patients with and without renal artery stenosis and is in fact a much more common etiology of hypertension than renovascular disease. For this reason, renovascular hypertension can be challenging to properly diagnose. Given the end-organ consequences of uncontrolled hypertension and the potential complications of renal artery interventions, this distinction is critical to make accurately.
Several adjuncts to anatomic diagnosis can help clarify whether a renal artery intervention should be performed. Direct arterial pressure measurements at the time of angiography determine if a significant (>10mm Hg systolic) pressure gradient is present across the stenosis. Captopril renal scintigraphy can demonstrate whether asymmetric renal perfusion is present, and it might also demonstrate the relative function of each kidney. Selective renal vein renin sampling that lateralizes to a kidney with a visualized renal artery stenosis can indicate the lesion’s clinical significance.
Notes
Case 113
 |
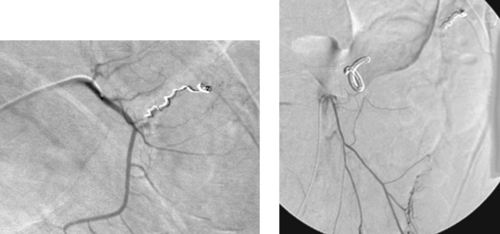
1. What is the diagnosis?
2. What artery most commonly supplies this region?
3. What embolization agent is preferred?
4. If endoscopic examination clearly visualizes a bleeding mucosal tear near the esophagogastric junction but no active extravasation is seen on angiography, what should be done?
ANSWERS: CASE 113
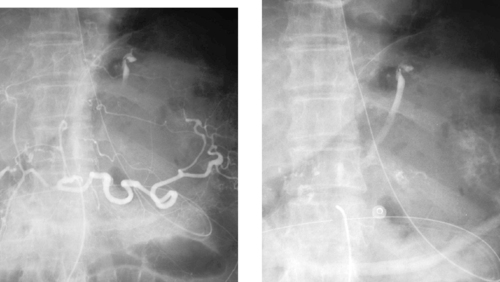
Mallory–Weiss Tear
1. Mallory–Weiss tear.
2. Left gastric artery.
3. Gelfoam.
4. The left gastric artery should be embolized anyway.
Reference
Morales, P.; Baum, A.E., Therapeutic alternatives for the MalloryWeiss tear, Curr Treat Options Gastroenterol. 6 (2003) 75–83.
Cross-Reference
Comment
A Mallory–Weiss tear is the most common nonvariceal source of bleeding at the esophagogastric junction. It most commonly results from a single linear nontransmural tear on the gastric side of the junction. The typical patient is an alcoholic who has suffered repetitive emesis. Therefore, endoscopy is critical to differentiate this disorder from variceal hemorrhage, a diagnosis that is also common in this patient population.
When endoscopic intervention fails to produce cessation of bleeding, arteriography is requested. Although focal accumulations of contrast medium may be seen lining the tear or the adjacent gastric folds (occasionally producing the pseudovein sign seen in the second image above), in many cases active contrast extravasation is not identified. Because of the rich collateral supply to the stomach via the gastroepiploic arteries, short gastric arteries, and right gastric artery, the left gastric artery can be embolized whether active extravasation is seen.
Case 114
 |
1. What vascular problem is pictured in the first image?
2. Name two common causes of this problem.
3. What treatment options are commonly used for this problem?
4. Is vasopressin infusion or embolization more likely to be effective?
ANSWERS: CASE 114
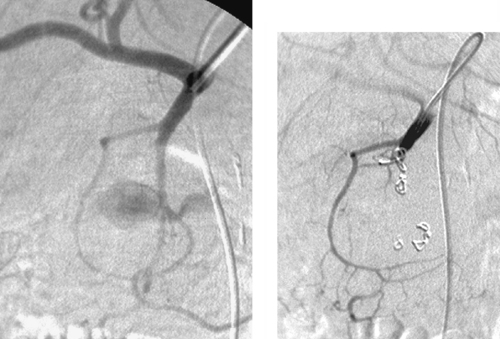
Bleeding Duodenal Ulcer
1. Gastroduodenal artery extravasation with pseudoaneurysm formation.
2. Duodenal ulcer, pancreatitis.
3. Endoscopic therapy, surgical treatment, and percutaneous embolization.
4. Embolization.
Reference
Levkovitz, Z.; Cappell, M.S.; Lookstein, R.; et al., Radiologic diagnosis and treatment of gastrointestinal hemorrhage and ischemia, Med Clin North Am. 86 (2002) 1357–1399.
Cross-Reference
Comment
Upper gastrointestinal (GI) bleeding is typically initially evaluated and managed with endoscopy. However, when endoscopic therapy fails, angiography may be indicated. The gastroduodenal artery runs immediately behind the first part of the duodenum. Consequently, ulcers penetrating its posterior wall can cause life-threatening arterial bleeding into the GI tract.
Depending upon the endoscopically determined location of bleeding, patients with upper GI bleeding are evaluated with selective arteriography of either the celiac artery (most of the time) or superior mesenteric artery. The parent catheter or a microcatheter can then be used to subselect the bleeding artery (in this case the gastroduodenal artery). If contrast extravasation is observed, embolization is performed using either Gelfoam or coils (second image). When using coils, it is important to be sure to embolize from distal to proximal to prevent backfilling of the ulcer from the gastroepiploic system (via the splenic artery) or the pancreaticoduodenal arcade (via the superior mesenteric artery). Given the intermittent nature of GI bleeding, if contrast extravasation cannot be confirmed, embolization of the gastroduodenal artery should still be performed if the endoscopic findings were clear or if the patient is unstable. Embolization therapy, while highly effective in producing initial cessation of bleeding and in stabilizing the patient, is less likely to provide a durable solution for large ulcers, and surgical therapy might ultimately be needed.
Case 115
 |
1. What procedure has been performed?
2. Is this procedure associated with less operative morbidity than open repair?
3. Name two procedure-related complications of this procedure.
4. Name three potential late complications of this procedure.
ANSWERS: CASE 115
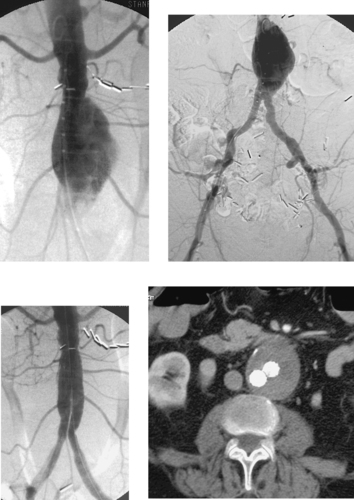
Endoluminal Graft Repair of Abdominal Aortic Aneurysm
1. Endoluminal graft repair of abdominal aortic aneurysm (AAA).
2. Yes.
3. Iliac artery rupture, branch vessel occlusion.
4. Endoleak, graft limb occlusion, device migration.
Reference
Zarins, C.K.; White, R.A.; Schwarten, D.; et al., AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: Multicenter prospective clinical trial, J Vasc Surg. 29 (1999) 292–308.
Cross-Reference
Comment
Endovascular repair (stent-graft placement) of AAA has been increasingly used since 1991. Endovascular repair of AAA is associated with reduced hospital stay, periprocedural morbidity, and need for blood transfusions compared with open repair. By enabling treatment of patients with significant comorbidities, this procedure has expanded the range of patients who can be offered aneurysm repair. However, strict anatomic criteria must be used to select appropriate candidates for this procedure.
Advances in device technology have made it technically feasible to perform the procedure through a percutaneous approach without the need for femoral artery cutdown. Fluoroscopic guidance is used throughout the procedure. An aortogram is performed. Over a stiff wire, a large delivery sheath is carefully advanced into the aorta and, following repeat angiography, the trunk component is deployed with its upper end just below the lowest renal artery origin and expanded by angioplasty. The contralateral limb is catheterized from the other femoral artery, and repeat angiography is performed to define the distal neck of the contralateral side. The contralateral component is then deployed overlapping with the trunk component and expanded by angioplasty. Final angiography is performed to ensure that the graft limbs are patent, no endoleak is present, and all previously noted branch vessels are still patent (third image). The groins are then closed surgically if femoral cutdown was performed, or they are closed by percutaneous arterial closure devices if the procedure was performed in a completely percutaneous manner. After the procedure, patients are followed diligently with CT angiography (final image) and abdominal x-rays to evaluate for endoleak and late endograft migration.
Notes
Case 116
 |
 |
1. What problem is seen in this man with lower gastrointestinal bleeding (LGIB)?
2. What mesenteric branch vessels were successively catheterized?
3. Could vasopressin infusion have been performed to treat this abnormality?
4. How often is symptomatic ischemia observed following superselective colonic artery embolization?
ANSWERS: CASE 116
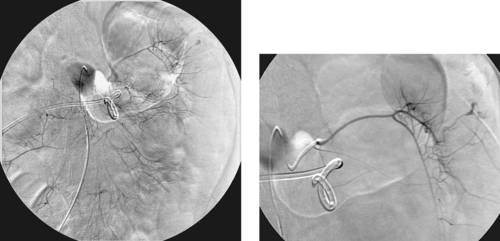
Embolization of Lower Gastrointestinal Bleeding
1. Focal contrast extravasation from a left colon diverticulum.
2. Superior mesenteric artery (SMA), middle colic artery, marginal artery.
3. Yes.
4. From 1% to 4% of cases.
Reference
Darcy, M.D., Treatment of lower gastrointestinal bleeding: Vasopressin infusion versus embolization, J Vasc Intervent Radiol. 14 (2003) 535–543.
Cross-Reference
Comment
Contraindications to vasopressin therapy for LGIB include coronary artery and cerebrovascular disease, arrhythmia, and severe hypertension. Vasopressin infusion has largely been replaced by coil embolization for treating LGIB. The main reason underlying this change is the development of microcatheter technology, which has enabled rapid catheterization of very small arterial branches and fairly easy traversal of tortuous vascular segments. Because colonic branches are often easy to catheterize rapidly, embolotherapy can be a fast way of obtaining hemostasis. Generally, coils are used for embolization of LGIB, although a variety of materials including Gelfoam and polyvinyl alcohol particles have also been used. Since the advent of superselective embolization using microcatheters, symptomatic ischemia following embolization has been extremely uncommon.
It is very important to account for the collateral supply in the bowel when treating GI bleeding. For example, the splenic flexure is usually supplied by a marginal artery that connects the SMA and inferior mesenteric (IMA) arterial circulations (third image). For this reason, in the case presented here, coils were placed in a branch of the marginal artery distal to the point of connection between the SMA and IMA circulations. Following embolization, the SMA and IMA (final image) were injected to confirm that hemostasis was achieved.
Notes
Case 117
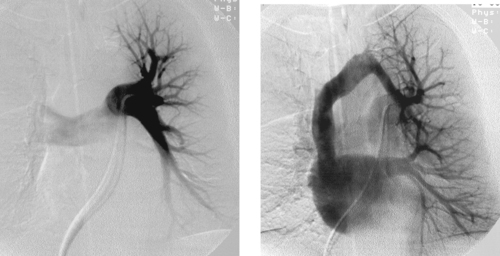 |
1. What vessels are opacified on the second image from this selective left pulmonary arteriogram?
2. What is the diagnosis?
3. Would this abnormality cause cyanosis?
4. Is this abnormality associated with congenital heart disease?
ANSWERS: CASE 117
Partial Anomalous Pulmonary Venous Return
1. Left upper-lobe pulmonary vein, left brachiocephalic vein, superior vena cava.
2. Partial anomalous pulmonary venous return (PAPVR).
3. No.
4. Yes.
Reference
Hong, Y.K.; Park, Y.W.; Ryu, S.J.; et al., Efficacy of MRI in complicated congenital heart disease with visceral heterotaxy syndrome, J Comput Assist Tomogr. 24 (2000) 671–682.
Cross-Reference
Comment
The true incidence of partial anomalous PAPVR is difficult to determine because many patients are asymptomatic. Symptoms typically arise from associated congenital heart defects or from increased pulmonary flow and right heart volume overload resulting from left-to-right shunting. Symptoms are rarely seen when less than 50% of pulmonary venous blood returns to the right atrium.
A number of congenital cardiac defects have been identified in association with PAPVR. The most common is an atrial septal defect, particularly when the PAPVR drains the right lung to the superior vena cava (SVC). Other associated cardiac defects include ventricular septal defect (VSD), tetralogy of Fallot, pulmonary valve atresia with VSD, aortic coarctation, common atrium, and single ventricle.
There are multiple forms of PAPVR, and one or several veins may be involved. The right lung is more commonly affected, and, rarely, both lungs are involved. The most common is an anomalous connection between the right upper-lobe pulmonary vein and the SVC. PAPVR from the right lower-lobe pulmonary vein to the inferior vena cava is called scimitar syndrome because of its classic plain film appearance.
Case 118
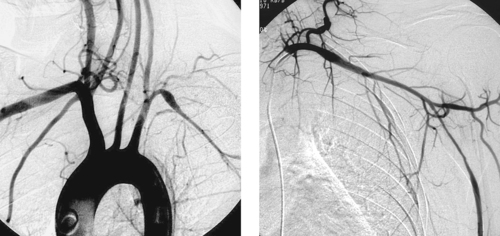 |
1. What is the probable diagnosis in this young patient?
2. Is this disorder more common in males or females?
3. In what situations can angioplasty be performed?
4. Can the pulmonary arteries be involved with this disease?
ANSWERS: CASE 118
Takayasu’s Arteritis
1. Takayasu’s arteritis.
2. Females more than males (3:1 ratio).
3. When flow-limiting stenosis is present and the patient’s inflammatory disease is not in an active phase.
4. Yes.
Reference
Weyand, C.M.; Goronzy, J.J., Medium- and large-vessel vasculitis, N Engl J Med. 349 (2003) 160–169.
Cross-Reference
Comment
Takayasu’s arteritis is a granulomatous vasculitis that primarily involves the thoracic and abdominal aorta and its large branch vessels. Because of infiltration of the adventitia with inflammatory cells, the luminal caliber of the vessel is compromised and fibrotic stenoses eventually develop. It is more common in women younger than 50 years. Patients characteristically have an elevated erythrocyte sedimentation rate, and approximately half exhibit constitutional symptoms in the acute inflammatory stage including myalgias, fatigue, low-grade fever, tachycardia, and pain adjacent to the inflamed arteries. Interestingly, there may be a 5- to 20-year interval between the onset of acute inflammatory symptoms and the development of symptomatic arterial occlusive disease. Patients typically present with neurologic symptoms, history of stroke, or asymmetric arm blood pressure measurements and/or pulses.
Aortography typically shows smooth concentric narrowing of the aorta and/or branch vessel stenoses or occlusions. In 75% of cases the aortic arch and branch vessels are affected. The left subclavian artery is commonly affected (55%), followed in decreasing order of frequency by the right subclavian artery, left common carotid artery, and right common carotid artery. In the case presented here, there is a smooth, elongated stenosis of the left axillosubclavian artery, a fairly characteristic appearance.
Case 119
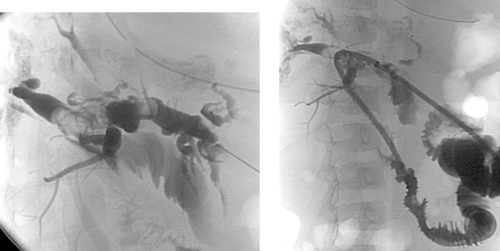 |
1. What surgical procedure has been performed?
2. What symptoms are probably present?
3. What abnormality is seen?
4. What is the probable etiology?
ANSWERS: CASE 119
Postoperative Biliary Calculi
1. Hepaticojejunostomy.
2. Right upper quadrant pain, jaundice, itching.
3. Globular filling defects within the right and left hepatic ducts.
4. Calculi or blood clots.
Reference
De Moor, V.; El Nakadi, I.; Jeanmart, J.; et al., Cholangitis caused by Roux-en-Y hepaticojejunostomy obstruction by a biliary stone after liver transplantation, Transplantation 75 (2003) 416–418.
Cross-Reference
Comment
The images demonstrate a surgical hepaticojejunostomy, in which the common hepatic duct was anastomosed to a loop of proximal small bowel. Several large, globular filling defects are present within the ducts, representing biliary calculi.
Patients with biliary–enteric anastomoses can develop several types of complications. The most significant early complication is anastomotic leakage. Also, early on, poor drainage of bile may be present during the first week due to edema at the biliary–enteric anastomosis.
Several potential late complications exist. The development of an anastomotic stricture can lead to episodes of cholangitis. Such strictures can be treated with prolonged biliary drainage and balloon dilation. The late complication pictured in the images here is the development of biliary calculi, which occurs in a small percentage of patients following this surgical procedure. Because the common presence of a Roux-en-Y limb can render endoscopic intervention impossible in many cases, percutaneous methods of removing or dislodging calculi are desirable in many such patients. With Roux-en-Y limbs, obstruction of the afferent loop also occurs in a small percentage of patients.
Notes
Case 120
 |
1. What percentage of patients do not exhibit clinical response following uterine fibroid embolization?
2. How is clinical success monitored after the procedure?
3. Name two potential causes of failure of uterine fibroid embolization.
4. Why did this patient fail uterine artery embolization and what can be done?
ANSWERS: CASE 120
Ovarian Artery Embolization
1. About 10% to 15%.
2. Patient history (to evaluate symptoms) and MRI (to evaluate fibroid and uterus size).
3. Uterine artery spasm, aberrant arterial supply to the fibroid uterus.
4. Supply to the fibroids via the right ovarian artery. This artery was embolized, resulting in clinical treatment success.
Reference
Worthington-Kirsch, R.L.; Andrews, R.T.; Siskin, G.P.; et al., Uterine fibroid embolization: Technical aspects, Tech Vasc Intervent Radiol. 5 (1) (2002) 17–34.
Cross-Reference
Comment
Uterine artery embolization has been shown to improve menorrhagia symptoms and pelvic pain in 85% to 90% of patients. There are several potential reasons why a minority of patients do not demonstrate clinical response after embolization. One potential reason is incomplete bilateral embolization. The most common reason this might occur is when arterial spasm-related slow flow in the uterine artery deceives the angiographer into believing that embolization has been achieved. Another common reason is the presence of additional blood supply to the fibroid uterus. This can result from congenital variations in uterine artery anatomy or to pelvic collateral supply.
The images here demonstrate the most common source of extrauterine supply to the uterus: an enlarged ovarian artery. In the first image, the right ovarian artery is clearly visualized on the nonselective aortic injection, confirming that it is abnormally enlarged. The final two images demonstrate the characteristic serpiginous appearance of the ovarian artery and its eventual supply to the right part of the uterine fundus.
Fortunately, the ovarian artery can also be embolized. Most interventionalists embolize the main ovarian artery using Gelfoam, although some advance a microcatheter beyond the ovary and attempt subselective embolization of the uterine branches.
Notes
Case 121
 |
1. Which vessel is abnormal in these two patients?
2. What is the anatomic reason for this vessel’s vulnerability?
3. What anatomic factor is an important determinant of symptom severity?
4. Does this abnormality tend to produce distal embolization?
ANSWERS: CASE 121
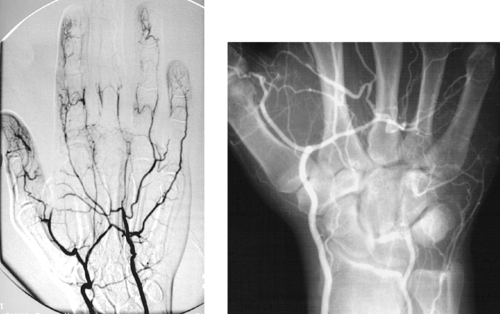
Hypothenar Hammer Syndrome
1. Ulnar artery.
2. It can be compressed against the hook of the hamate.
3. The degree of completeness of the palmar arch.
4. Yes (see the first image above).
Reference
Anderson, S.E.; De Monaco, D.; Buechler, U.; et al., Imaging features of pseudoaneurysms of the hand in children and adults, Am J Roentgenol. 180 (2003) 659–664.
Cross-Reference
Comment
Hypothenar hammer syndrome is an uncommon cause of digital ischemia that occurs as a result of repetitive blunt trauma or mechanical vibration or pressure to the wrist or palm. Typically it is the result of occupational trauma (e.g., jackhammer operators), and it can be seen in persons who practice martial arts. Lesser forms of trauma, including repetitive microtrauma in typists or pianists, can also lead to digital ischemia.
The chronic trauma produces spasm and intimal injury resulting in thrombosis (second image) and/or formation of a pseudoaneurysm (first image). A pseudoaneurysm might serve as a source of emboli to the digital arteries (first image). Although other vessels can be affected, the ulnar artery is particularly vulnerable where it crosses the hamate bone and can be compressed.
The degree of symptomatology depends upon vessel patency, the presence of emboli, and the degree of completeness of the palmar arch. Symptoms include evidence of digital ischemia, Raynaud’s phenomenon, and/or a pulsatile mass.
Case 122
 |
1. What endovascular procedure has been performed?
2. How are patients usually monitored following this procedure?
3. What abnormality is present on this angiogram?
4. Why is this significant?
ANSWERS: CASE 122
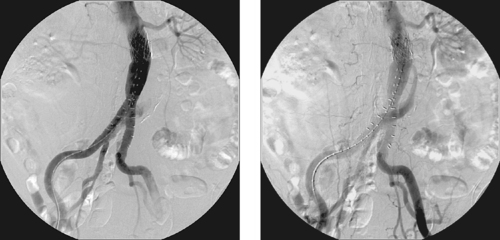
Type I Endoleak
1. Endoluminal graft insertion.
2. Periodic cross-sectional imaging with CT angiography using noncontrast, arterial phase contrast-enhanced, and delayed postcontrast imaging.
3. Type I endoleak from the right distal attachment site.
4. Potential for aneurysm rupture.
Reference
Tummala, S.; Powell, A., Imaging of endoleaks, Tech Vasc Intervent Radiol. 4 (4) (2001) 208–212.
Cross-Reference
Comment
An endoleak is defined as the persistence of blood flow outside the graft within the aneurysm sac following endoluminal repair. An aneurysm sac that is completely excluded from flow typically thromboses and often shrinks in diameter. The presence of perigraft flow leaves the aneurysm at risk for enlargement and/or rupture. The images here clearly depict a type I leak, with filling of the aneurysm sac on the second image.
Endoleaks are classified by type. Type I endoleaks result from flow around the ends of the endograft and are subdivided into type IA (proximal attachment site leak) and IB (distal attachment site leak). These endoleaks require urgent therapy. Type II endoleaks are the most common and result from retrograde arterial flow into the aneurysm sac from patent aortic side-branches (typically lumbar, sacral, gonadal, accessory renal, or inferior mesenteric artery branches). The criteria upon which to base treatment of type II endoleaks are controversial. Some physicians treat all type II leaks via side-branch embolization or glue embolization of the aneurysm sac, and others believe that watchful waiting is sufficient provided the aneurysm is not enlarging. Type III endoleaks are rare and result from tears in the graft fabric or separation of graft components. Type IV endoleaks represent leakage of contrast due to porosity of the graft fabric material.
Case 123
 |
1. What abnormality is seen on the CT scan of this young woman with acute left flank pain?
2. What is the cause of this patient’s pain?
3. What is the most common cause of this problem when it occurs spontaneously?
4. What is the most common overall cause of this problem?
ANSWERS: CASE 123
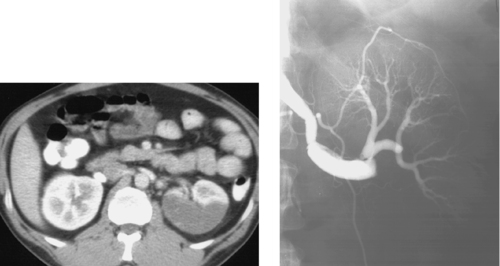
Spontaneous Renal Artery Dissection
1. Severe hypoperfusion of the posterior part of the left kidney.
2. Left renal artery dissection.
3. Fibromuscular dysplasia.
4. Iatrogenic renal artery injury during angioplasty.
Reference
Muller, B.T.; Reiher, L.; Pfeiffer, T.; et al., Surgical treatment of renal artery dissection in 25 patients: Indications and results, J Vasc Surg. 37 (2003) 761–768.
Cross-Reference
Comment
The second image demonstrates apparent enlargement and double-density of the left renal artery with a proximal focal indentation consistent with a renal artery dissection. The flap extends to the bifurcation of the main left renal artery, and its posterior division is occluded. This explains the marked left kidney hypoperfusion on CT.
Renal artery aneurysm and/or renal artery dissection occur in a minority of patients with fibromuscular dysplasia. Given the rarity of other etiologies of these lesions, however, a patient with an unexplained renal artery aneurysm or dissection is highly likely to have underlying fibromuscular dysplasia.
Patients with spontaneous renal artery dissection are nearly always managed with surgical aortorenal arterial bypass. In patients who are poor candidates for surgery, stents can be used to treat focal dissections limited to the main renal artery.
Case 124
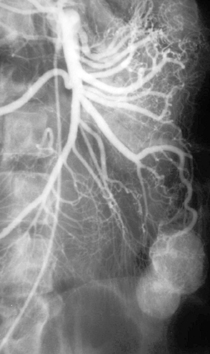 |
1. What vessel is selectively opacified?
2. What abnormality is present?
3. Where is this abnormality most likely located?
4. What is the most likely reason this angiogram was performed?
ANSWERS: CASE 124
Small Bowel Leiomyoma
1. Superior mesenteric artery.
2. A well-circumscribed, bilobed enhancing mass.
3. Small bowel.
4. Evaluation of chronic lower gastrointestinal bleeding.
Reference
Lefkovitz, Z.; Cappel, M.S.; Kaplan, M.; et al., Radiology in the diagnosis and therapy of gastrointestinal bleeding, Gastroenterol Clin North Am. 29 (2000) 489–512.
Cross-Reference
Comment
Many patients develop chronic GI blood loss but have negative workups, including upper and lower endoscopies, small bowel follow-through, and tagged RBC scintigraphy. In such cases, angiography can sometimes help to identify an occult vascular lesion such as angiodysplasia or a small bowel tumor, with the goal of providing guidance for surgical resection. Owing to the intermittent low-grade bleeding associated with these lesions, extravasation of contrast is not typically seen during angiography.
Although small bowel tumors are rarely seen at arteriography, an understanding of their basic imaging features is useful to have. Leiomyomas like the one depicted here have a characteristic appearance with well-circumscribed margins, prominent feeding arteries and draining veins, and irregular corkscrew tumor vessels with a dense tumor stain. Most are benign, although malignancy cannot be excluded on imaging. The most common primary tumor of small bowel is carcinoid. Owing to its prominent desmoplastic reaction within the mesentery, characteristic angiographic findings include retraction, displacement, and occlusions of multiple superior mesenteric artery branches. Hamartomas and adenocarcinomas typically appear hypovascular. Lymphomas have no characteristic findings. They can demonstrate vascular displacement or encasement but are often undetectable despite widespread involvement.
Case 125
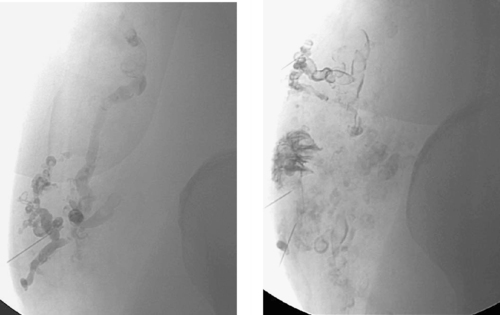 |
1. Why might this patient have presented for evaluation and treatment?
2. What is the most likely diagnosis?
3. What is the first-line imaging method of choice for these lesions?
4. How can this be treated percutaneously?
ANSWERS: CASE 125
Venous Malformation
1. Local pain and swelling; in this 21-year-old police officer, his gunbelt was very uncomfortable.
2. Venous malformation.
3. Magnetic resonance imaging.
4. Direct injection of a sclerosant such as 100% ethanol.
Reference
Lee, B.B.; Do, Y.S.; Byun, H.S.; et al., Advanced management of venous malformation with ethanol sclerotherapy: Mid-term results, J Vasc Surg. 37 (2003) 533–538.
Cross-Reference
Comment
Vascular malformations are congenital lesions that grow periodically, often in relation to trauma, surgery, or hormonal stimulation (via puberty, pregnancy, or hormonal therapy). Vascular malformations are typically categorized into arterial, capillary, venous, lymphatic, and mixed subtypes. MRI is useful to determine the depth and extent of these lesions as well as their proximity to normal structures. MRI is also useful in the imaging follow-up after treatment because multiple ablation sessions are often necessary.
Owing to the presence of an intervening capillary network, the inflow arteries to venous malformations, like the one depicted, are normal in size. Flow within these dilated abnormal veins tends to be slow or stagnant, and calcified phleboliths may be identified.
Small, asymptomatic venous malformations in the extremities are treated conservatively with compressive stockings and follow-up. Surgical resection is not indicated owing to the difficulty in extricating these delicate vascular structures from surrounding normal tissues. Sclerotherapy is favored, and absolute ethanol is the agent preferred by many. After the lesion is directly punctured with a needle, contrast is injected to confirm needle placement and determine the volume necessary to fill the lesion. A similar volume of ethanol is then injected and allowed to dwell in the lesion for about 10 minutes. Follow-up venography is performed to confirm thrombosis of the lesion.
Case 126
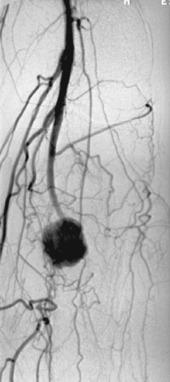 |
1. What findings are present on this magnified arteriogram at the knee level?
2. What is the most likely cause based solely upon the angiographic findings?
3. What might this lesion represent if the patient is an intravenous drug abuser?
4. What symptoms might be present if that was the case?
ANSWERS: CASE 126
Mycotic Aneurysm
1. Irregular distal popliteal artery aneurysm with occlusion of the tibial arteries.
2. Post-traumatic pseudoaneurysm.
3. Mycotic aneurysm.
4. Fever, local tenderness, positive blood cultures, and/or expanding mass.
Reference
Patra, P.; Ricco, J.; Costargent, A.; et al., Infected aneurysms of neck and limb arteries: A retrospective multicenter study, Ann Vasc Surg. 15 (2001) 197–205.
Cross-Reference
Comment
Infectious (mycotic) aneurysms can occur anywhere in the arterial system. The usual cultured infectious organisms are Salmonella, Streptococcus, or Staphylococcus species, and the latter is commonly found in intravenous drug abusers.
Mycotic aneurysms represent an infection of the artery wall itself either caused by seeding of a preexisting aneurysm or by primary infection of a normal artery with subsequent dilation. The infection can be introduced from infected blood in the lumen or vasa vasorum, by spread from a neighboring soft tissue infection, or from penetrating trauma. Therefore, mycotic aneurysms can be true or false aneurysms and may be single or multiple depending upon the underlying condition of the vessel and the method of infection. The symptomatology and angiographic appearance can differentiate these from degenerative aneurysms. The mycotic aneurysm sac is typically irregular, saccular, and eccentric, and the arterial system is less likely to show chronic atherosclerosis.
Treatment consists of antibiotics, surgical resection of the aneurysm, débridement of infected tissues, and drainage of the infected region. In some cases the artery can be ligated, but when revascularization is necessary, an extra-anatomic bypass can often be performed to avoid the infected region. In some cases, an anatomic bypass can successfully be performed with autogenous materials (native veins) or cryopreserved allografts if success in clearing of infection with antimicrobial agents is anticipated. Prosthetic material should not be inserted into a known infected space.
Case 127
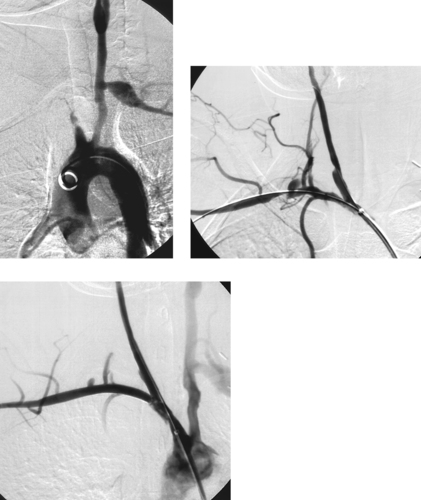 |
1. What is the underlying diagnosis?
2. What procedure has been performed in the second and third images?
3. Is this the first-line treatment for this problem?
4. What is the expected 5-year patency of surgical carotid–subclavian bypass in patients with atherosclerosis?
ANSWERS: CASE 127
Brachiocephalic Artery Stent Placement
1. Takayasu’s arteritis.
2. Stent placement in the innominate, right common carotid, and right subclavian arteries.
3. No. Carotid–subclavian bypass is the first-line treatment.
4. More than 95%.
Reference
Sharma, B.K.; Jain, S.; Bali, H.K.; et al., A follow-up study of balloon angioplasty and de-novo stenting in Takayasu arteritis, Int J Cardiol. 75 (Suppl) (2000) S147–S152.
Cross-Reference
Comment
Patients with Takayasu’s arteritis can present complex management problems. Typically they are first managed with anti-inflammatory therapy until arterial symptoms develop. At this point, surgical bypass is the usual therapy employed, although angioplasty and/or stent placement can also be used for focal, short-segment lesions that are in a chronic phase. Surgery or interventional therapy in the acute inflammatory stages should be avoided owing to lower rates of long-term success.
If the brachiocephalic artery or proximal subclavian artery is occluded and the ipsilateral common carotid artery is normal, reimplantation of the subclavian artery into the carotid or carotid–subclavian bypass is the preferred operation. If the carotid artery is diseased, it is replaced with graft material, and the subclavian artery is implanted into the graft or grafted to the aorta or carotid graft.
In the case presented here, the patient had been operated on multiple times for the brachiocephalic artery occlusion and had multiple occluded bypass grafts. Because she was thought to be a poor risk for further intervention in the same operative bed, an endovascular stent was placed.
Notes
Case 128
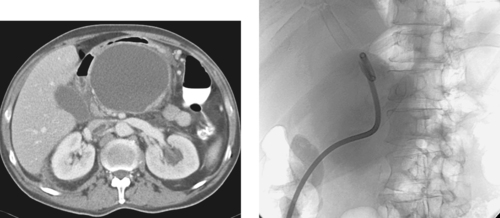 |
1. This patient presented with constant abdominal pain 6 weeks after an episode of acute pancreatitis. What is the diagnosis?
2. Do all patients with this diagnosis need interventional treatment?
3. What approaches can be used for draining these lesions?
4. Will this tube be ready to remove within 2 weeks?
ANSWERS: CASE 128
Transgastric Pancreatic Pseudocyst Drainage
1. Pancreatic pseudocyst.
2. No.
3. Percutaneous transabdominal if a suitable window is present, percutaneous transgastric, endoscopic internal drainage, surgical drainage.
4. No. Pseudocyst drains are often in for months.
Reference
Neff, R., Pancreatic pseudocysts and fluid collections: Percutaneous approaches, Surg Clin North Am. 81 (2001) 399–403.
Cross-Reference
Comment
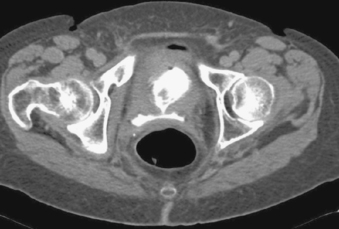
Patients with fluid collections that appear after an episode of acute pancreatitis are typically initially treated with supportive care and observation. The two primary clinical situations in which pseudocyst drainage is indicated are when there is reason to suspect that a particular fluid collection is infected and when the patient has persistent pain at least 6 weeks following the initial pancreatitis episode.
The CT scan shown here demonstrates a large pancreatic pseudocyst immediately posterior to the stomach. A suitable window directly into this collection could not be identified, so transgastric drainage was performed. Given the large size of this pseudocyst, the procedure was performed using fluoroscopic guidance alone, using the indentation upon the gastric air pattern as a landmark for the location of the pseudocyst. Because pancreatic collections typically contain semisolid debris, a large-bore catheter was placed. Because transgastric pseudocyst drainage catheters are prone to displacement back into the stomach due to gastric peristalsis, a locking loop catheter was used.
Pancreatic pseudocysts typically require several months of drainage before they can be removed. In general, the criteria for removing the catheter include minimal output (<10mL/day for 2 days), absence of fistula to the pancreatic duct, and good clinical status (the patient is afebrile and generally improved).
Case 129
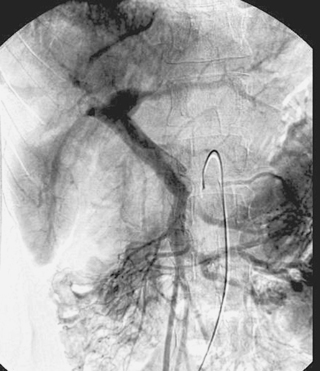 |
1. What procedure was performed?
2. What abnormality is present?
3. Could a celiac arteriogram diagnose this abnormality?
4. Can the underlying problem in this patient be treated with embolization therapy?
ANSWERS: CASE 129
Right Portal Vein Obstruction
1. Superior mesenteric arteriogram.
2. Right portal vein obstruction.
3. Yes.
4. No. Hepatic arterial chemoembolization would be risky owing to the presence of right portal vein occlusion: Hepatic ischemia might result.
Reference
Uflacker, R., Applications of percutaneous mechanical thrombectomy in transjugular intrahepatic portosystemic shunt and portal vein thrombosis, Tech Vasc Interv Radiol. 6 (1) (2003) 59–69.
Cross-Reference
Comment
It is always important to evaluate the arterial, parenchymal, and venous phases of any arteriogram. The image here represents the venous phase of a superior mesenteric arteriogram. The superior mesenteric vein, main portal vein, and left portal vein are clearly patent. There is occlusion of the right portal vein, which in this patient was due to malignancy. The angiogram was being performed to evaluate the feasibility of chemoembolization therapy for this patient’s hepatocellular carcinoma.
Portal vein thrombosis can be caused by primary hepatic or metastatic tumor, dehydration, sepsis, transplantation, and other hypercoagulable states. Its presence is important to the interventionalist in several clinical situations, including patients with cancer in whom hepatic chemoembolization is planned. The presence of portal vein obstruction would render such patients susceptible to developing severe hepatic ischemia were the hepatic artery to be embolized. Portal vein thrombosis is also important in patients with portal hypertension in whom transjugular intrahepatic portosystemic shunt (TIPS) is planned. In these patients, the presence of portal vein thrombosis would render portal vein access more difficult to obtain. However, TIPS can still be performed, either by accessing the left portal system or by recanalizing the occluded segment of the portal vein.
Case 130
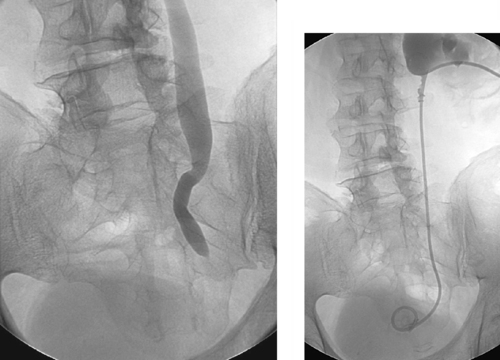 |
1. Name four benign causes of ureteral stricture.
2. Can benign ureteral strictures be balloon dilated with success?
3. How often does a ureteral stent need to be replaced?
4. In what patients are double-J ureteral stents relatively contraindicated?
ANSWERS: CASE 130
Benign Ureteral Stricture
1. Scarring after ureteral calculus, after radiation therapy, iatrogenic injury during surgery, anastomosis following ileal loop diversion.
2. Yes, but the results are usually not durable.
3. Every 2 to 3 months.
4. Patients with incontinence or otherwise poor bladder function, those with urinary fistulas, those who experience refractory bladder spasms following ureteral stent placement, those who are prone to stent obstruction.
Reference
DiMarco, D.S.; LeRoy, A.J.; Thieling, S.; et al., Long-term results of treatment for ureteroenteric strictures, Urology 58 (2001) 909–913.
Cross-Reference
Comment
These images demonstrate a tight stricture of the left ureter at the ureterovesical junction, with consequent hydroureteronephrosis. In this patient, the stricture was due to prior radiation therapy. The benign nature of this stricture is favored by its focal, smooth appearance without evidence of mass effect. That said, the radiographic appearance is fairly nonspecific, and malignancy cannot be excluded on this basis alone. The stricture was successfully crossed, and a nephroureteral stent was placed (second image).
Balloon dilation of the ureter can be effective in treating benign strictures but is associated with high recurrence rates. Recently, cutting balloons have been introduced and are used from a ureteroscopic approach with greater success. Following intervention, a stent is typically left across the stenosis to provide a scaffold for healing while preventing collapse to an unacceptable diameter.
Case 131
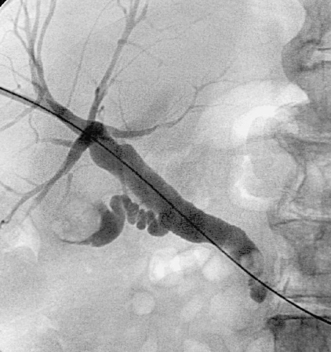 |
1. What is the diagnosis?
2. What is the first-choice treatment in most cases?
3. What percutaneous methods can be used to treat this problem?
4. If this patient has a fever, does this need to be treated urgently?
ANSWERS: CASE 131
Choledocholithiasis
1. Choledocholithiasis.
2. Endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy and stone removal.
3. Balloon sphincterotomy with propulsion of the calculus into the bowel, or the calculus can be removed by a variety of grasping devices.
4. Yes.
Reference
Taylor, A.C.; Little, A.F.; Hennessy, O.F.; et al., Prospective assessment of magnetic resonance cholangiopancreatography for noninvasive imaging of the biliary tree, Gastrointest Endosc. 55 (1) (2002) 17–22.
Cross-Reference
Comment
The image demonstrates a large filling defect within the gallbladder, consistent with calculi and/or sludge (or, less likely, tumor). The cystic duct is patent. An ovoid filling defect is present within the distal common bile duct, consistent with calculus.
Patients with choledocholithiasis can present with acute severe symptomatology (due to impaction of a mid-sized or large calculus within the common bile duct) or with chronic intermittent symptoms (due to passage of small calculi through the duct). Patients with symptoms suggesting the presence of cholangitis must be treated urgently with either stone removal or biliary drainage to rapidly relieve the obstruction. Afebrile patients with acute symptoms are usually managed with ERCP as the first-choice option. If this fails, then the patient may be referred for percutaneous biliary drainage and percutaneous stone extraction.
Case 132
 |
1. What diagnosis would you suspect in this patient with painful, persistent priapism following perineal trauma?
2. What pelvic artery supplies the penis?
3. What vessel is commonly injured in these patients?
4. Can this be treated using endovascular methods?
ANSWERS: CASE 132
High-Flow Priapism
1. High-flow priapism.
2. Internal pudendal artery.
3. Cavernous artery.
4. Yes, by selective embolization.
Reference
Ciampalini, S.; Savoca, G.; Buttazzi, L.; et al., High-flow priapism: Treatment and long-term follow-up, Urology 59 (2002) 110–113.
Cross-Reference
Comment
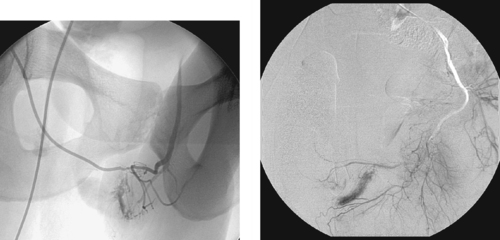
High-flow priapism is a rare arterial abnormality that results from direct trauma to the perineum or penis. Laceration of the cavernous artery results in development of an angiographically visible arteriolacunar fistula (first image) with direct, constant entry of arterial blood into the vascular lacuna of the erectile tissue. It helps to be familiar with the normal minor arterial blush at the base of the corpus cavernosum to be able to clearly distinguish this normal finding from the more-robust enhancement associated with a true arteriolacunar fistula (second image).
The most commonly used treatment is percutaneous embolization of the distal internal pudendal artery. Autologous blood clot and Gelfoam, both temporary agents, have been used most extensively but have a significant rate of recurrence due to subsequent recanalization. Permanent agents, such as microcoils and bucrylate glue, have also been used. Surgical ligation of the cavernous artery is also an effective treatment, but because it produces permanent occlusion it can have damaging effects on the underlying tissues.
The feared complication of both treatment and failure to treat is the development of impotence. Although many patients report a long history of high-flow priapism with normal sexual function, long-standing persistent priapism has been reported to result in fibrosis with associated erectile dysfunction. Because it is difficult to determine which patients will develop fibrosis and impotence, the timing of intervention remains controversial.
Case 133
 |
 |
1. What is the abnormality seen on the CT cystogram in the first image?
2. What procedure has been performed in the second image?
3. Is this a temporary or permanent measure?
4. What are the indications for performing this procedure?
ANSWERS: CASE 133
Vaginovesical Fistula
1. Vaginovesical fistula.
2. Bilateral transrenal ureteral occlusion.
3. Permanent.
4. Lower urinary tract fistulas, urinary incontinence, and intractable cystitis.
Reference
Farrell, T.A.; Wallace, M.; Hicks, M.E., Long-term results of transrenal ureteral occlusion with use of Gianturco coils and gelatin sponge pledgets, J Vasc Interv Radiol. 8 (1997) 449–452.
Cross-Reference
Comment
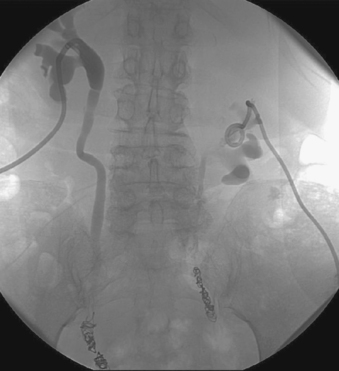
Lower urinary tract fistulas are abnormal communications between the urinary bladder or the distal ureters with adjacent structures (vagina, rectum, sigmoid colon, and perineum). They can develop as the result of trauma, pelvic malignancy, and radiation. Incontinence of urine is the most common presentation and has a significant impact on quality of life. Surgical repair has a high failure rate of 35% in fistulas caused by trauma and an even higher rate in those related to malignancy. Surgical diversion with ileal loop formation has been performed in such cases; however, this might not be feasible in some patients owing to poor general health or local contraindications such as pelvic malignancy or prior radiation.
Percutaneous nephrostomy drainage may be successful in allowing small fistulas to heal. Large fistulas can be managed by ureteral occlusion with permanent external urinary drainage through nephrostomy drains. A number of embolic materials have been used with varying success rates. A combination of coil and Gelfoam has been shown to have favorable technical and clinical efficacy.
Case 134
 |
1. Which hepatic tumors demonstrate contrast pooling?
2. Which hepatic tumors demonstrate neovascularity?
3. Which hepatic tumors commonly exhibit arteriovenous shunting?
4. Which hepatic tumor is most likely in this case?
ANSWERS: CASE 134
Cavernous Hemangioma of the Liver
1. Hemangioma, hepatocellular carcinoma, hepatoblastoma, angiosarcoma, and hypervascular metastases.
2. Adenoma, hepatocellular carcinoma, hepatoblastoma, cholangiocarcinoma, metastases, and occasionally focal nodular hyperplasia.
3. Hepatocellular carcinoma and giant hemangioma of infancy or hemangioendothelioma.
4. Hemangioma.
Reference
Giavraglou, C.; Economou, H.; Oannidis, I., Arterial embolization of giant hepatic hemangiomas, Cardiovasc Intervent Radiol. 26 (2003) 92–96.
Cross-Reference
Comment
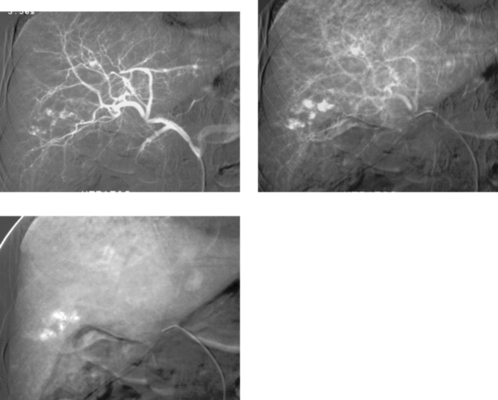
Hemangiomas are the most common benign hepatic tumors and are typically found incidentally during cross-sectional imaging. Pathologically, they comprise endothelium-lined vascular spaces divided by thin septations and suspended in a loose fibroblastic stroma. They typically remain stable in size over time, but occasionally they grow quite large. When a hemangioma is present, symptoms are commonly related to the neoplasm size, but they rarely are due to rupture or platelet sequestration.
The arteriographic appearance is characteristic. The feeding vessels are typically normal in size unless the lesion is very large. Dense nodular opacification of the lesion starts at the periphery (first image) and progresses inward (later images). The lesions are well circumscribed and have dilated, irregular, nodule-like vascular spaces. Contrast opacification persisting well into the venous phase differentiates this lesion from other hepatic neoplasms except for the rare angiosarcoma that can mimic a hemangioma.
Other hepatic lesions that exhibit vascular pooling tend to be malignant tumors, including hepatocellular carcinoma and metastases. Similar to cross-sectional imaging characteristics, these can typically be differentiated by the shorter period of pooling, less-uniform enhancement, enlargement of the feeding artery, and neovascularity.
Notes
Case 135
 |
1. What procedure has been performed?
2. Name one early and one late potential complication of this procedure.
3. Can this be performed through a prior surgical jejunostomy tract?
4. What alternative methods of providing enteral nutrition are considered preferential options for most patients?
ANSWERS: CASE 135
Direct Percutaneous Jejunostomy
1. Direct percutaneous jejunostomy.
2. Early: peritonitis; Late: catheter occlusion.
3. Yes.
4. Surgical jejunostomy and percutaneous gastrojejunostomy.
Reference
Cope, C.; Davis, A.G.; Baum, R.A.; et al., Direct percutaneous jejunostomy: Techniques and applicationsten years experience, Radiology 209 (1998) 747–754.
Cross-Reference
Comment
The examination demonstrates percutaneous transabdominal placement of a jejunostomy catheter. Although surgical jejunostomy and percutaneous gastrojejunostomy represent the standard approaches for obtaining the ability to feed into the jejunum, there are occasional clinical situations in which these procedures are not possible. One common scenario is the patient who has undergone gastrectomy, esophagogastrectomy, or esophagectomy with gastric pullthrough, and who is also a poor operative risk.
Because the small bowel is usually not fixed within the abdomen and can be quite mobile, direct percutaneous jejunostomy can be technically challenging to perform. Factors that help the interventionalist include: (1) The presence of an identifiable scar from a prior surgical jejunostomy, because it is likely that the adjacent loop of small bowel has been surgically tethered to the anterior abdominal wall, making it less likely to fall away during the procedure; (2) The ability to identify a suitable loop of bowel using ultrasound guidance or using fluoroscopic examination with or without injection of air through a nasogastric catheter. Although techniques vary, many interventionalists first place a T-tack (second image) into the jejunal loop under fluoroscopic guidance and use this to maintain gentle traction on the bowel while the tract is dilated to accommodate the jejunostomy catheter. As with gastrojejunostomy, tube feedings into the small bowel are given continuously rather than in bolus fashion.
Notes
Case 136
 |
1. What artery has been selected in the second image above?
2. What procedure is normally performed before the procedure shown above?
3. What is the underlying etiology of symptoms in this patient?
4. What is a Rasmussen’s aneurysm?
ANSWERS: CASE 136
Cavitary Right Lung Mass
1. Right bronchial artery.
2. Bronchoscopy.
3. Cavitary right lung mass.
4. A pulmonary artery branch aneurysm due to tuberculosis.
Reference
Barben, J.; Robertson, D.; Olinsky, A.; et al., Bronchial artery embolization for hemoptysis in young patients with cystic fibrosis, Radiology 224 (2002) 124–130.
Cross-Reference
Comment
The images demonstrate a selective right bronchial arteriogram. The right bronchial arterial branches are markedly splayed, demonstrating a right upper lung mass that is hypervascular at its periphery and avascular in its central region (suggesting necrosis). The differential diagnosis includes primary lung carcinoma (which was the case in the patient shown), metastatic disease, and infectious lesions such as tuberculosis and fungal disease. In this patient, a focus of contrast extravasation was observed at the inferior margin of the lesion, presumably the source of hemoptysis. This stands in contrast to the vast majority of bronchial arteriograms for hemoptysis, in which contrast extravasation is not visualized. In this patient, palliative treatment was performed using embolization with polyvinyl alcohol particles, with subsequent cessation of bleeding.
Common reasons for severe hemoptysis include cystic fibrosis, chronic obstructive pulmonary disease, bronchiectasis, and vascular tumors such as the one depicted above.
Bronchoscopy is routinely performed before arteriographic evaluation of hemoptysis, in order to determine which bronchial segment contains the bleeding source. This greatly helps the angiographer, because many patients have enlarged bronchial arteries bilaterally with no evidence of contrast extravasation. The angiographer can then direct therapy to the artery thought likely to be causing the bleeding based upon bronchoscopy.
Case 137
 |
1. What abnormal finding is present on the first image above?
2. What procedure did this patient have recently that might have caused this?
3. What percutaneous method of treatment is shown in the second image?
4. Is it advisable to extend stents into the common femoral artery?
ANSWERS: CASE 137
Stenting of Iliac Artery Dissection
1. A dissection flap in the right external iliac artery.
2. Stent insertion into the distal right external iliac artery.
3. Insertion of an additional stent to tack the flap down.
4. No. Stents should not be placed in areas of joint flexion when possible.
Reference
Funovics, M.A.; Lackner, B.; Cejna, M.; et al., Predictors of long-term results after treatment of iliac artery obliteration by transluminal angioplasty and stent deployment, Cardiovasc Intervent Radiol. 25 (2002) 397–402.
Cross-Reference
Comment
Stent placement has become an accepted method of treating a variety of arterial vascular abnormalities, including flow-limiting dissections, short-segment arterial occlusions, arterial stenoses refractory to angioplasty, arterial stenoses that recur following successful angioplasty, and eccentric or extremely calcified atherosclerotic lesions. Currently available vascular stents fall into two main categories.
Balloon-expandable stents (prototype: Palmaz stent) are generally less than 4cm long and are premounted on an angioplasty balloon. Their primary advantage is the pinpoint accuracy with which they can be positioned, because the deployment step simply involves inflating the carrier balloon. Their primary disadvantages are the inability to treat longer lesions with a single stent and the possibility of the stent’s slipping off the balloon during introduction. The latter problem has been largely remedied by stents that are securely mounted upon their carrier balloons by the manufacturer.
Self-expandable stents (prototype: Wallstent) are available in longer lengths, possess greater longitudinal flexibility, and do not require balloon mounting. However, because deployment requires a carefully controlled unsheathing of the restrained stent, allowing it to expand, these stents are slightly more prone to malpositioning during deployment.
Case 138
 |
1. Are the opacified vessels arteries or veins?
2. What abnormal findings are present?
3. Are these acute or chronic findings?
4. Would wedged hepatic venography yield accurate estimates of portal pressures?
ANSWERS: CASE 138
Cavernous Transformation of the Portal Vein
1. Veins.
2. Cavernous transformation of the portal vein and a large gastroesophageal varix.
3. Chronic.
4. No.
Reference
Gallego, C.; Velasco, M.; Marcuello, P.; et al., Congenital and acquired anomalies of the portal venous system, Radiographics 22 (1) (2002) 141–159.
Cross-Reference
Comment
Although the cause of portal vein thrombosis remains undetermined in more than half of adult patients, commonly identifiable causes include hepatocellular carcinoma, cirrhosis, periportal inflammatory processes (e.g., pancreatitis, ascending cholangitis), and sepsis (the most common cause in children). Less-common causes include hypercoagulable states and trauma. Many patients are asymptomatic from the portal vein occlusion and present with symptoms of the underlying disease that caused the thrombosis. Despite the venous occlusion, hepatic failure and/or hepatomegaly are often not present.
Cross-sectional imaging modalities such as duplex ultrasound, helical CT, and MRI are fairly accurate in depicting portal vein thrombosis. In questionable cases, definitive diagnosis of acute or partial portal vein occlusion may be identified by observing a focal filling defect within the portal vein during the venous phase of a celiac or superior mesenteric arteriogram. With subacute or chronic portal venous thrombosis, large enhancing serpiginous collateral veins in the porta hepatis with nonvisualization of the normal expected portal venous branching pattern may be observed as in the image here, a finding known as cavernous transformation of the portal vein. Also important to observe in this image is the massively dilated vein that supplies a large gastroesophageal varix.
Notes
Case 139
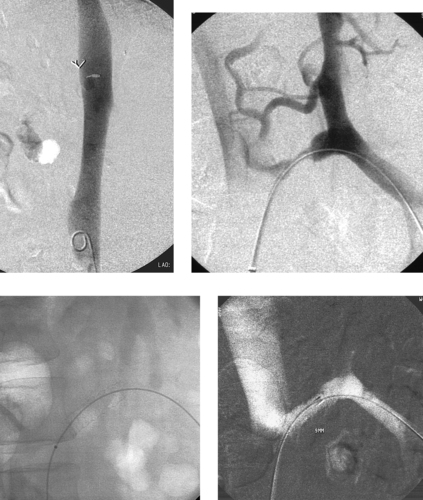 |
1. What surgical bypass procedure has been performed in this patient with hepatic cirrhosis, severe portal hypertension, and a history of variceal bleeding?
2. Why was the diagnostic study depicted in the first two images performed?
3. How was this study performed?
4. Does this study explain the presence of the clinical problems listed in Question 2?
ANSWERS: CASE 139
Stenting of Mesocaval Shunt Stenosis
1. Mesocaval shunt.
2. Recurrent variceal bleeding or refractory ascites.
3. Placement of a catheter in the inferior vena cava (IVC) from a common femoral vein approach, catheterization of the shunt, superior mesenteric venography.
4. Yes. There is a tight stenosis in the mesocaval shunt (second image).
Reference
Zizka, J.; Elias, P.; Krajina, A.; et al., Value of Doppler sonography in revealing transjugular intrahepatic portosystemic shunt malfunction: A 5-year experience in 216 patients, Am J Roentgenol. 175 (2000) 141–148.
Cross-Reference
Comment
Although transjugular intrahepatic portosystemic shunt (TIPS) placement is currently the most commonly used method of providing portal vein decompression in patients with symptomatic portal hypertension, a variety of surgical methods are occasionally used in these patients. Such options include the creation of a portacaval shunt (from main portal vein to IVC), a mesocaval shunt as shown here (from superior mesenteric vein to IVC), or a splenorenal shunt (from splenic vein to left renal vein). However, these conduits are also prone to develop stenosis and occlusion.
The images demonstrate catheterization of the mesocaval shunt from the IVC, with subsequent superior mesenteric venography. In this patient, recurrent variceal bleeding had occurred, and duplex ultrasound was unable to visualize the shunt. A tight stenosis was identified within the shunt. Angioplasty was performed initially but it did not produce a successful result, so a balloon-expandable Palmaz stent was placed within the shunt and dilated to 9mm. An excellent venographic result is noted on the final image; this patient showed significant reduction of the mesocaval pressure gradient and experienced clinical success.
Notes
Case 140
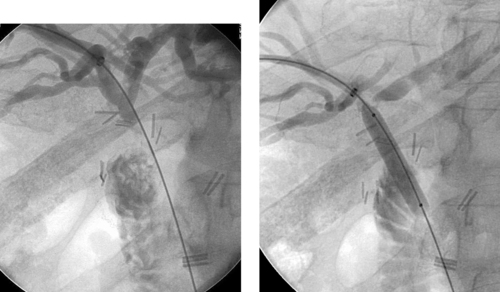 |
 |
1. What surgical procedure has been performed in this patient?
2. What symptoms might currently be present?
3. What abnormality is seen in the first image?
4. Is this typically treated with metallic biliary stent placement?
ANSWERS: CASE 140
Biliary–Enteric Anastomotic Stricture
1. Hepaticojejunostomy.
2. Jaundice, cholangitis, elevated liver function tests.
3. Stenosis of the hepaticojejunostomy anastomosis.
4. No. Metallic stents are reserved for patients with malignant biliary obstruction and short life expectancy.
Reference
Laasch, H.U.; Martin, D.F., Management of benign biliary strictures, Cardiovasc Intervent Radiol. 25 (2002) 457–466.
Cross-Reference
Comment
A primary late complication of biliary surgery may be development of a stricture at the biliary–enteric anastomosis. Although surgical therapy can provide a durable treatment for this problem, the difficulty of performing repeat biliary surgery in the same operative bed is considerable. For this reason, percutaneous biliary drainage is usually performed initially. Balloon dilation of the anastomotic stricture can then be performed (final two images), and an internal–external drainage catheter can be left behind for several months to serve as a scaffold of good caliber around which the anastomosis can heal. Depending upon the results of balloon dilation, definitive surgical anastomotic revision might or might not be required.
Like other biliary interventions, antibiotic prophylaxis is recommended for biliary balloon dilation procedures. If the patient presents with cholangitis, then the balloon dilation procedure should be deferred to a future date when the patient is no longer infected. In this situation, at the initial sitting placement of a biliary drainage catheter should suffice.
Notes
Case 141
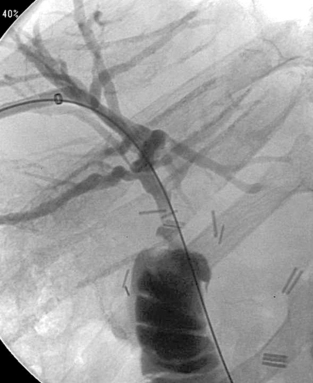
 |
 |
1. Which vessel is being selectively injected in the first image?
2. Which vessel is being selectively injected in the second image?
3. Name the arteries labeled by the arrow and arrowhead in the second image.
4. Which arteries are being filled by the two arteries named in question 3?
ANSWERS: CASE 141
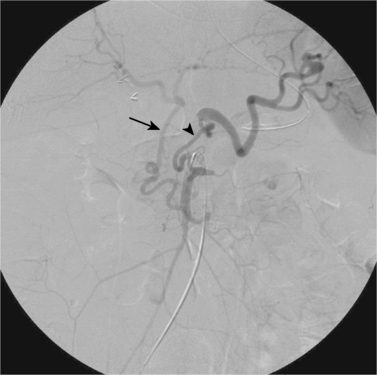
Anatomic Variant: Arc of Buhler
1. Celiac axis.
2. Superior mesenteric artery (SMA).
3. Gastroduodenal artery and the arc of Buhler, respectively.
4. The proper hepatic artery and the celiac axis, respectively.
Reference
Saad, W.E.; Davies, M.G.; Sahler, L.; et al., Arc of Buhler: Incidence and diameter in asymptomatic individuals, Vasc Endovascular Surg. 39 (2005) 347–349.
Cross-Reference
Comment
The celiac axis and the SMA develop from the 10th and 13th ventral segmental arteries off the aorta. The segmental arteries are connected by a ventral anastomosis that usually regresses during fetal development. Failure of regression results in a persistent arterial connection called the arc of Buhler. This acts as a collateral pathway between the celiac axis and the SMA, which can become a critical finding to identify in patients with celiac axis or SMA stenosis and in patients undergoing transarterial chemoembolization, hepatic artery radioembolization, pancreaticoduodenal surgeries, or liver transplantation.
Notes
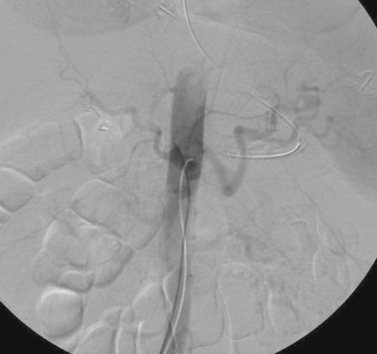
Case 142
 |
1. How was the first image obtained?
2. After placement of a transjugular intrahepatic portosystemic shunt (TIPS), what residual abnormality is seen in the third image?
3. What procedure was performed to treat this abnormality?
4. Can this procedure be expected to be effective in preventing variceal bleeding in the absence of preceding TIPS placement?
ANSWERS: CASE 142
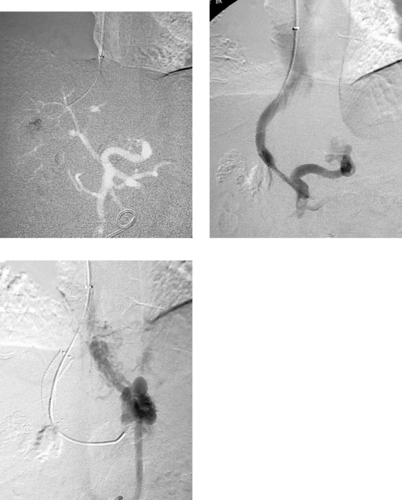
Post-TIPS Variceal Embolization
1. Wedged carbon dioxide portography via a hepatic vein balloon catheter.
2. Large bleeding gastroesophageal varix.
3. Coil embolization of the varix.
4. No.
Reference
Hidajat, N.; Stobbe, H.; Hosten, N.; et al., Transjugular intrahepatic portosystemic shunt and transjugular embolization of bleeding rectal varices in portal hypertension, Am J Roentgenol. 178 (2002) 362–363.
Cross-Reference
Comment
The hemodynamic goal of TIPS is to achieve the optimal degree of reduction in the portosystemic gradient thatwill prevent variceal bleeding and ascites but that will preserve hepatic perfusion (thereby preventing encephalopathy and liver failure). In most patients, reducing the portosystemic gradient to 8 to 12mm Hg produces this effect. Likewise, in most patients, portal venography performed immediately after TIPS demonstrates markedly reduced filling of gastroesophageal varices compared to the pre-TIPS venogram. In a minority of patients, however, persistent variceal filling is observed (second and third images).
When this occurs, the presence of residual stenosis or thrombus within the TIPS tract or portal vein should first be sought and corrected by additional angioplasty or stent placement. If variceal filling is still observed, or in select cases in which the patient is hemodynamically unstable due to variceal bleeding, the gastroesophageal varices can be selected from the portal venous system and percutaneously embolized using coils.
Notes
Case 143
 |
1. What vascular abnormalities are seen?
2. Name four potential causes of these findings.
3. What is the underlying diagnosis in this patient?
4. How can one determine if a significant vasospastic component is present?
ANSWERS: CASE 143
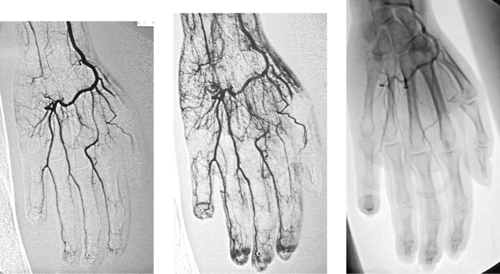
Scleroderma
1. Multiple occlusions and stenoses in the palmar and digital arteries.
2. Emboli, vasculitis, traumatic lesions, vasospasm.
3. Scleroderma.
4. Angiography following infusion of a vasodilator.
Reference
Stucker, M.; Quinna, S.; Memmel, U.; et al., Macroangiopathy of the upper extremities in progressive systemic sclerosis, Eur J Med Res. 5 (7) (2000) 295–302.
Cross-Reference
Comment
Small artery occlusions and stenoses in the palmar and digital arteries can produce severe pain, paresthesias, Raynaud’s phenomenon, fingertip necrosis, and gangrene. The pathophysiology of vascular lesions in these patients often includes embolic, traumatic, vasoreactive, and inflammatory components. Diagnostic evaluation should focus upon identifying contributing factors that are treatable by medical or surgical interventions.
First, the angiogram should be carefully scrutinized for any evidence of emboli. Potential sources of digital emboli include the heart (diagnosed by echocardiography and treated with anticoagulation), atherosclerotic plaques in the subclavian artery (treated with anticoagulation and/or surgical removal of the source lesion), sports-related injury to the axillary artery (subject to surgical repair), and occupational vascular injuries such as hypothenar hammer syndrome (also subject to surgical repair). Second, the presence of a significant vasospastic component to the arterial lesions should be sought during the angiographic evaluation. If the stenotic lesions improve significantly following administration of a vasodilator (such as tolazoline or nitroglycerine), then a sympathectomy may be more likely to provide some degree of symptom relief.
Unfortunately, this patient has irreversible small-vessel occlusions due to scleroderma. The vascular findings mimic those seen with other small-vessel diseases. However, the diagnosis of scleroderma can confidently be made in this patient due to the clear radiographic evidence of acro-osteolysis (final image).
Notes
Case 144
 |
1. What is the diagnosis?
2. For asymptomatic lesions, what diameter mandates surgical treatment?
3. What factors make surgical treatment of this lesion complex?
4. Will embolization alone provide effective therapy?
ANSWERS: CASE 144
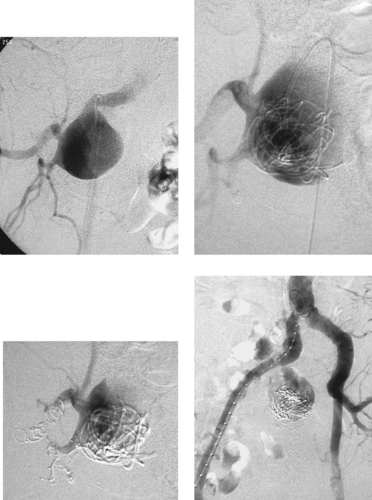
Internal Iliac Artery Aneurysm
1. Right internal iliac artery aneurysm.
2. From 3.0 to 3.5cm in diameter and larger.
3. Location deep within the pelvis, proximity to the ureter, multiple outflow vessels.
4. No. Embolization has been performed to occlude the outflow vessels of the lesion. A stent graft will be placed in the right iliac artery to occlude its inflow.
References
Sanchez, L.A.; Patel, A.V.; Ohki, T.; et al., Midterm experience with the endovascular treatment of isolated iliac aneurysms, J Vasc Surg. 30 (1999) 907–914.
Razavi, M.K.; Dake, M.D.; Semba, C.P.; et al., Percutaneous endoluminal placement of stent-grafts for the treatment of isolated iliac artery aneurysms, Radiology 197 (1995) 801–804.
Cross-Reference
Comment
Isolated iliac artery aneurysms can rupture, embolize, or produce local compression symptoms. The rate of rupture increases significantly between 3 and 4cm, and therefore most surgeons recommend operative repair for iliac aneurysms larger than 3.0 to 3.5cm in diameter. Because retroperitoneal dissection can be difficult when the aneurysm morphology is complex, endovascular methods to repair iliac aneurysms have gained favor in recent years. So far, the primary patencies of stent grafts for iliac artery aneurysms have been excellent (92%-100% at 1-4 years), and the incidence of endoleaks has been very low.
Because of the late potential for cross-pelvic collaterals to reconstitute the aneurysm sac, it is very important to gain control of all distal vessels arising from the aneurysm, either by surgical ligation or by coil embolization (shown in the last three images). Once these outflow vessels are successfully occluded, the inflow to the aneurysm may be occluded by placing a stent graft across the origin of the internal iliac artery. The final image shows a marker catheter in the right iliac artery being used to measure the iliac artery to appropriately size the stent-graft device to be used.
Notes
Case 145
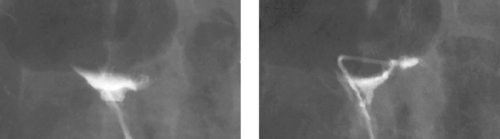 |
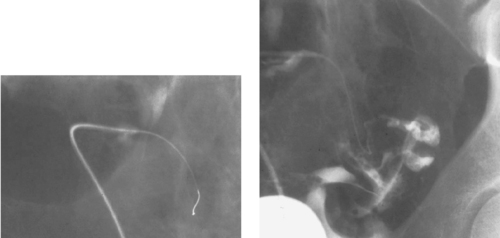 |
1. What examination is depicted in the first image?
2. What examination is depicted in the second image?
3. Why are these examinations being performed?
4. What diagnosis is evident on the first two images?
ANSWERS: CASE 145
Fallopian Tube Recanalization
1. Nonselective hysterosalpingogram (HSG).
2. Selective salpingography.
3. To evaluate and treat female infertility caused by proximal tubal occlusion.
4. Bilateral proximal fallopian tube occlusions.
Reference
Pinto, A.B.; Hovsepian, D.M.; Wattanakumtornkul, S.; et al., Pregnancy outcomes after fallopian tube recanalization: oil-based versus water-soluble contrast agents, J Vasc Interv Radiol. 14 (2003) 69–74.
Cross-Reference
Comment
HSG is commonly performed in the evaluation of female-factor infertility. Several causes of infertility may be detected by HSG, including congenital uterine anomalies (which are also well depicted by MRI), extrinsic uterine cavity distortion by mass lesions (commonly fibroids or adenomyosis), Asherman’s syndrome (filling defects representing intrauterine septations may be visualized on HSG), salpingitis isthmica nodosa (pelvic inflammatory disease–related scarring and tubal occlusion), and proximal tubal occlusion.
When the nonselective HSG depicts proximal tubal occlusion (first image), a small catheter may be used to select the orifice of the fallopian tube (second image). If tubal occlusion is confirmed, as in this case, a guidewire may be gently advanced beyond the occlusion in an attempt to dislodge an occlusive mucus plug (third image). Repeat salpingogram then demonstrates spill of contrast into the peritoneal cavity, consistent with a patent (recanalized) fallopian tube (final image).
Fallopian tube recanalization restores patency to at least one tube in approximately 80% of patients, and it has been shown to significantly improve pregnancy rates.
Notes
Case 146
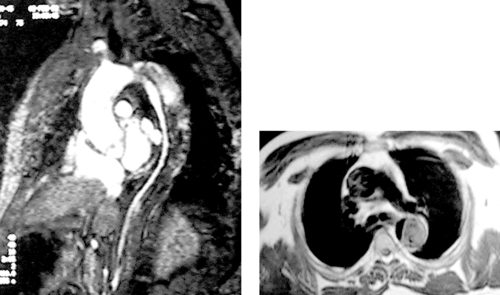 |
1. What is the diagnosis?
2. What complication appears to be present?
3. Is MRI more sensitive and specific than aortography for this diagnosis?
4. What other modalities are equally sensitive and specific for this diagnosis?
ANSWERS: CASE 146
MRI of Aortic Dissection
1. Aortic dissection.
2. True lumen collapse.
3. Yes.
4. Helical CT and multiplanar transesophageal echocardiography (TEE).
Reference
Moore, A.G.; Eagle, K.A.; Bruckman, D.; et al., Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD), Am J Cardiol. 89 (2002) 1235–1238.
Cross-Reference
Comment
The images in this case clearly visualize an aortic dissection flap extending throughout the descending thoracic aorta. The diameter of the true lumen is noted to be extremely small throughout the descending thoracic aorta.
MRI is equal or superior to helical CT and TEE for making the diagnosis of acute and chronic aortic dissection. The longer image-acquisition time and greater difficulty in patient monitoring in the MRI scanner have been considered relative disadvantages of its use in the acute aortic dissection setting. However, MRI and helical CT are better able than TEE to evaluate the entire aorta (including its subdiaphragmatic segment), and MRI has the advantage in not requiring iodinated contrast.
All three cross-sectional modalities are significantly superior to aortography for making the diagnosis of aortic dissection. In current practice, aortography is reserved for evaluating patients with clinical evidence of complications of aortic dissection for whom surgical or interventional therapy is being planned.
Notes
Case 147
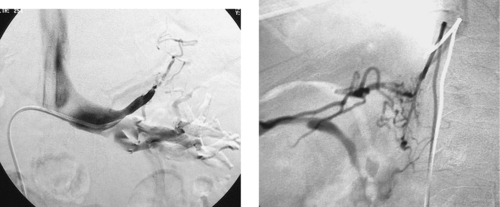 |
1. What procedure is being performed?
2. What endocrine condition might this patient have?
3. What specific information can this procedure provide?
4. What is Conn’s syndrome?
ANSWERS: CASE 147
Selective Adrenal Vein Sampling
1. Bilateral selective adrenal venography.
2. Primary hyperaldosteronism.
3. Measurement of aldosterone level can help to localize an adrenal adenoma.
4. Hypertension due to an aldosterone-producing adrenal adenoma.
Reference
Wheeler, M.H.; Harris, D.A., Diagnosis and management of primary aldosteronism, World J Surg. 27 (2003) 627–631.
Cross-Reference
Comment
Primary aldosteronism is important to identify within the hypertensive population, because most patients with a unilateral source of excess aldosterone production are amenable to surgical cure. Typically, postural hormonal testing is first performed by an endocrinologist. The diagnosis is then confirmed by selective adrenal venous sampling with measurement of aldosterone concentrations (expressed as aldosterone-to-cortisol ratio) in each adrenal vein. Selective adrenal venous sampling has been shown to be more sensitive and specific than cross-sectional imaging or scintigraphy, and endocrine surgeons are often guided by the results of the sampling study. Patients with unilateral disease are ideally treated by laparoscopic adrenalectomy. Patients in whom localization is not achieved usually have bilateral adrenal hyperplasia and are treated medically.
Case 148
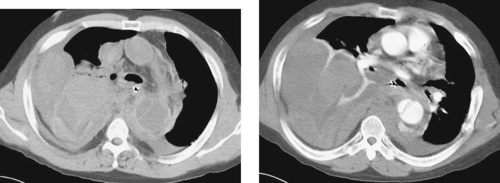 |
1. What is the underlying diagnosis?
2. What complication is seen?
3. What is the appropriate clinical management of this problem?
4. If peripheral ischemic symptoms are present, is this patient a candidate for percutaneous fenestration of the aortic flap?
ANSWERS: CASE 148
Aortic Dissection with Rupture
1. Aortic dissection.
2. Rupture of the thoracic aorta with bilateral hemothorax.
3. Emergency surgical thoracic aortic replacement.
4. No. This patient needs emergency aortic surgery.
Reference
Marty-Ane, C.H.; Berthet, J.P.; Branchereau, P.; et al., Endovascular repair for acute traumatic rupture of the thoracic aorta, Ann Thorac Surg. 75 (2003) 1803–1807.
Cross-Reference
Comment
Aortic rupture is a dreaded complication of aortic dissection. Stanford type A dissections carry a moderate risk of ascending aortic rupture into the pericardial sac, producing pericardial tamponade; to prevent this, these patients are treated with immediate aortic repair. Stanford type B dissections typically are managed medically, and they only occasionally progress to aortic rupture. Clinical signs that can indicate impending or completed aortic rupture are persistent or increasing chest pain, uncontrolled hypertension, the development of a left pleural effusion on chest radiography or CT, and/or hemodynamic instability. In the case shown here, aortic rupture with bilateral hemothorax is clearly depicted on both noncontrast and contrast-enhanced CT.
The standard of care for the treatment of aortic rupture is immediate surgical aortic replacement. Recently, in a small number of centers, stable patients with ruptured aortic dissections and aneurysms have been managed using endovascular stent-graft repair. The main potential advantage of this innovative approach is the ability to avoid thoracotomy and aortic clamping in this subset of patients who tend to have multiple comorbidities. However, aortic stent-graft placement for such high-risk patients has not yet been validated in comparative trials. Early results for aortic stent-graft placement have been encouraging, and it is being used as an alternative to open surgical repair at an increasing rate.
Case 149
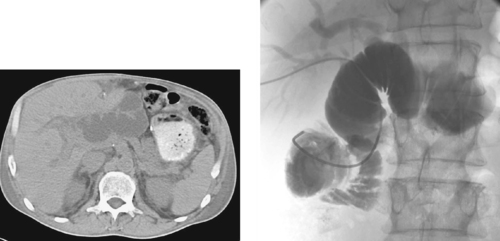 |
1. What is the etiology of biliary dilation in this patient who has previously undergone a Whipple procedure with hepaticojejunosomy and Roux-en-Y?
2. What symptoms might this patient present with?
3. What procedure can be initially performed to palliate these symptoms?
4. Name two anatomic approaches to this procedure.
ANSWERS: CASE 149
Afferent Loop Obstruction
1. Afferent loop syndrome.
2. Abdominal pain, increasing jaundice, possibly cholangitis.
3. Placement of a decompressive drainage catheter within the afferent loop.
4. Transhepatic biliary route, direct transabdominal route into the loop itself.
Reference
Gayer, G.; Barsuk, D.; Hertz, M.; et al., CT diagnosis of afferent loop syndrome, Clin Radiol. 57 (2002) 835–839.
Cross-Reference
Comment
The first image demonstrates bilateral biliary dilation and severe dilation of a loop of bowel that courses into the hepatic hilum. In the setting of abdominal symptoms and a known Whipple procedure, the possibility of an afferent loop obstruction must be considered. Decompression of this loop can be accomplished surgically (usually reserved for patients in whom the hepaticojejunostomy was performed for benign disease) or via percutaneous placement of a drainage catheter within the dilated loop (second image). Once initial decompression has occurred, if a stenosis at the bowel–bowel anastomosis is detected and the patient has short life expectancy, then a metallic stent may be placed within the stenosis to relieve the obstruction in carefully selected patients.
Case 150
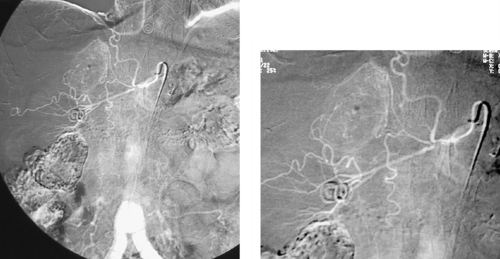 |
1. What procedure has been performed?
2. What abnormality is seen?
3. Is this likely to be malignant?
4. How would this likely be treated?
ANSWERS: CASE 150
Adrenal Adenoma
1. Selective right adrenal arteriogram.
2. Right adrenal mass lesion.
3. No, but one cannot be sure based upon angiography alone.
4. Surgical adrenalectomy with or without preoperative embolization.
Reference
Blake, M.A.; Jhaveri, K.S.; Sweeney, A.T.; et al., State of the art in adrenal imaging, Curr Probl Diagn Radiol. 31 (3) (2002) 67–78.
Cross-Reference
Comment
These images demonstrate a selective right adrenal arteriogram. Although the arterial supply to the adrenal gland can vary, one common arrangement has three main arteries supplying the gland: an inferior adrenal artery derived from the ipsilateral renal artery, a middle adrenal artery derived from the aorta, and a superior adrenal artery derived from the inferior phrenic artery.
In the case presented, a well-defined, enhancing mass is identified in the right adrenal gland. The angiographic appearance favors a benign etiology such as an adenoma (which is what this was). Angiographic features that might suggest malignancy can include more prominent hypervascularity, arteriovenous shunting, neovascularity, recruitment of arterial supply from multiple vessels, and lack of clearly defined borders.
Case 151
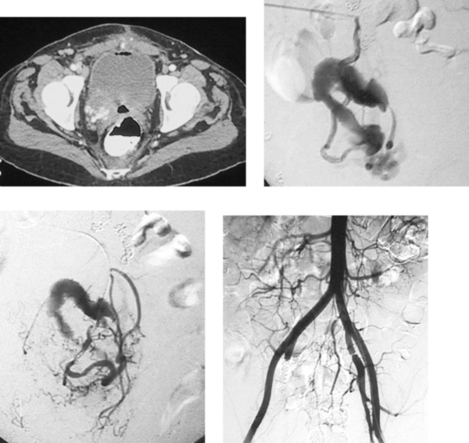 |
1. What abnormality is seen on the CT image above?
2. What artery is catheterized in the second and third images?
3. What is the diagnosis?
4. How might this be treated?
ANSWERS: CASE 151
Uterine Arteriovenous Malformation
1. Multiple enhancing vascular structures in the right hemipelvis.
2. Right uterine artery.
3. Arteriovenous malformation (AVM) of the pelvis.
4. Sequential arterial embolization procedures.
Reference
Gulati, M.S.; Paul, S.B.; Batra, A.; et al., Uterine arteriovenous malformations: The role of intravenous “dual-phase” CT angiography, Clin Imaging. 24 (1) (2000) 10–14.
Cross-Reference
Comment
Life-threatening vaginal bleeding can be caused by a variety of hypervascular masses, including uterine fibroids, gestational trophoblastic disease, cervical carcinoma and other pelvic malignancies, ectopic pregnancies, and AVM. In the case presented here, injection of the uterine artery reveals a tortuous collection of arteries with early opacification of a large draining vein, consistent with a uterine AVM. Embolization was performed, and the final image shows internal iliac artery occlusion.
Uterine AVMs are rare lesions; they can be congenital or acquired. Previous uterine surgery or curettage, uterine trauma, past history of gestational trophoblastic disease, previous pregnancy, genital tract malignancy, and diethylstilbestrol exposure are all predisposing factors associated with acquired uterine AVMs. Although hysterectomy has long been considered the treatment of choice for these lesions, embolization therapy is now used for women wishing to retain their fertility.
Unlike AVMs that are confined to the uterus, curative treatment of extrauterine pelvic AVMs is rarely possible because pelvic organ involvement and complex arterial supply render surgical resection hazardous. Sequential embolization therapy can be used for these patients to palliate symptoms and limit the frequency and severity of recurrent bleeding. Unfortunately, recurrence rates are high despite embolization.
Notes
Case 152
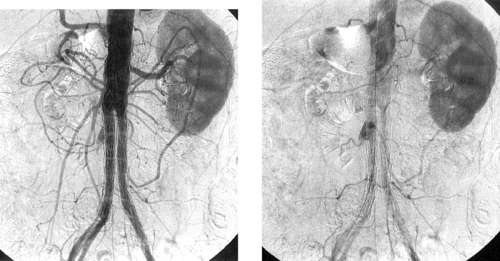 |
1. Is angiography the primary method of follow-up for patients who have undergone endoluminal aortic aneurysm repair?
2. What abnormality is seen in the images above?
3. If this aneurysm is increasing in size, does this abnormality require treatment?
4. Would this be treated by insertion of an additional stent graft?
ANSWERS: CASE 152
Type II Endoleak
1. No. CT scanning is the primary follow-up method.
2. Type II endoleak via retrograde flow in a lumbar artery.
3. Yes.
4. No.
Reference
Faries, P.L.; Cadot, H.; Agarwal, G.; et al., Management of endoleak after endovascular aneurysm repair: Cuffs, coils, and conversion, J Vasc Surg. 37 (1) (2003) 1155–1161.
Cross-Reference
Comment
Patients who undergo endoluminal repair of abdominal aortic aneurysms (AAA) typically receive close surveillance with periodic CT angiography. When extraluminal contrast is seen within the aneurysm sac on CT, the presence of an endoleak is confirmed. Precise characterization of the type and etiology of endoleak is critical in planning treatment and usually requires angiography. These images demonstrate a collection of contrast in the right aspect of the aneurysm sac, which is clearly being fed by retrograde flow in a lumbar artery (second image). Contrast does not enter the aneurysm sac in the early arterial phase (first image), as would typically be seen with a type I (attachment site) endoleak.
Patients with type II endoleaks and evidence of aneurysm enlargment on serial CT scans require treatment due to the risk of aneurysm rupture. The criteria upon which to base treatment of type II endoleaks in the absence of aneurysm enlargement are highly controversial, in part because more than 50% tend to close spontaneously. Hence, some physicians treat all type II leaks, some treat only endoleaks that are first detected several months after the procedure, and others believe that close CT follow-up is sufficient.
Case 153
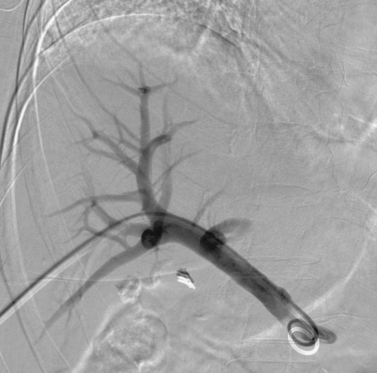 |
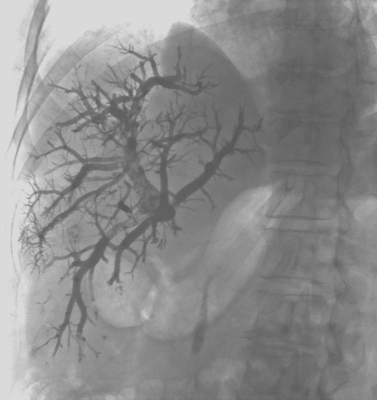 |
1. What examination is being performed in the first image?
2. What intervention has been performed in the second image?
3. What embolic agent has been used?
4. What are the indications for performing this procedure?
ANSWERS: CASE 153
Portal Vein Embolization
1. Percutaneous transhepatic portal venography.
2. Selective right portal vein embolization.
3. N-Butyl cyanoacrylate (NBCA) mixed with ethiodol.
4. To induce hypertrophy of the future liver remnant (FLR) before hepatic resection.
Reference
Madoff, D.C.; Hicks, M.E.; Vauthey, J.N.; et al., Transhepatic portal vein embolization: Anatomy, indications, and technical considerations, Radiographics 22 (2002) 1063–1076.
Cross-Reference
Comment
Major hepatic resection is being performed at an increasing rate in the treatment of primary and secondary hepatobiliary malignancy. One of the potential postoperative complications associated with this type of surgery is fatal liver failure. This can result from the inability of the remaining liver parenchyma after resection to provide adequate function to sustain life. Pre-resection measures to increase the volume of the FLR have been employed to make some patients eligible for this type of surgery. This has been achieved by surgical ligation of the contralateral portal vein in the past, and more recently it is achieved with contralateral portal vein embolization (PVE).
Preprocedural cross-sectional imaging is obtained to evaluate the total liver volume (TLV) and the estimated FLR volume. A FLV/TLV ratio of 20% to 40% is recommended for patients before planned major hepatic resection. An ipsilateral or contralateral approach is used. Various embolic materials have been used for PVE including NBCA, polyvinyl alcohol (PVA) particles, Gelfoam, and coils. Follow-up cross-sectional imaging with calculation of the liver volume is obtained in 2 to 4 weeks to assess the degree of FLR hypertrophy.
Case 154
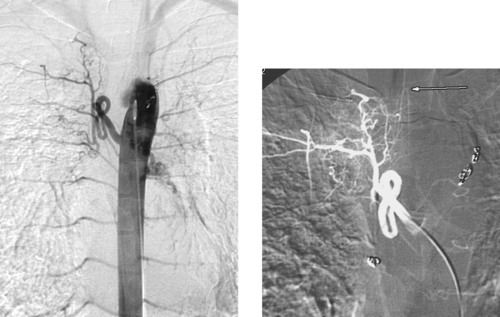 |
1. What examination has been performed in the second image?
2. What artery is marked by the arrow in the second image?
3. From what vessel does this artery arise?
4. Is visualization of this vessel a contraindication to bronchial embolization?
ANSWERS: CASE 154
Spinal Artery
1. Selective right bronchial arteriogram.
2. A spinal artery.
3. From the very proximal right bronchial artery.
4. No. However, the catheter needs to be positioned well distal to the origin of that branch before embolization to prevent reflux of embolic material into that branch.
Reference
Tanaka, N.; Yamakado, K.; Murashima, S.; et al., Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system, J Vasc Interv Radiol. 8 (1 Pt 1) (1997) 65–70.
Cross-Reference
Comment
The first image, a descending thoracic aortogram, demonstrates an enlarged right bronchial artery in a patient being evaluated for hemoptysis. In the second image, a selective right bronchial arteriogram, a tortuous bronchial artery is seen. Importantly, a spinal artery is seen to arise from the proximal aspect of the right bronchial artery. The vessel takes a characteristic hairpin turn before entering the vertebral canal to supply the spinal cord.
Identification of spinal arteries during bronchial arteriography is important in avoiding the complication of paraplegia. Also, whereas in the past embolization was performed from the main bronchial artery through a 4- to 5-F catheter placed in the proximal segment of the bronchial artery, most interventionalists now place a microcatheter distally in the bronchial artery in order to avoid nontarget embolization of any proximal spinal or intercostal branches that may be present. For these reasons, the incidence of neurologic complications following bronchial arteriography is extremely low.
Case 155
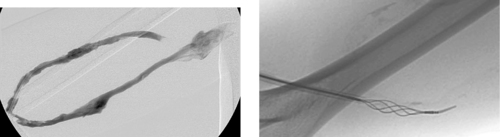 |
1. What study is depicted in the first image?
2. Based upon the appearance of the graft apex region in the first image (which is mid-procedure), what problem may have prompted this examination?
3. What treatment options are available for occluded dialysis grafts?
4. What treatment method is depicted in the second image?
ANSWERS: CASE 155
Thrombosed Dialysis Graft
1. Dialysis fistulogram.
2. Graft thrombosis.
3. Percutaneous declotting of the graft, surgical thrombectomy.
4. A mechanical thrombectomy device (the percutaneous thrombolytic device).
Reference
Vesely, T.M., Mechanical thrombectomy devices to treat thrombosed hemodialysis grafts, Tech Vasc Interv Radiol. 6 (1) (2003) 35–41.
Cross-Reference
Comment
A dialysis graft is a surgically created arteriovenous fistula using prosthetic materials to the bridge the gap between an artery and a vein. There are a variety of configurations, with the most common being loop grafts in the forearm and C grafts in the upper arm.
Because the outflow veins are exposed to “arterial” pressures and flows, and because of the frequent needle punctures needed for dialysis, dialysis grafts are prone to eventual failure. This failure can manifest by high venous pressures during dialysis sessions, excessive bleeding from the puncture sites, arm swelling, high recirculation values, or, ultimately, graft thrombosis.
The goals of treatment are to clear the thrombus from the graft and to treat the underlying cause of the thrombosis. Thrombus may be cleared by percutaneous declotting using pharmacologic thrombolysis or a mechanical thrombectomy device (second image). The underlying cause of graft thrombosis is diagnosed by venography performed during the percutaneous declotting procedure, and it is typically treated using angioplasty and/or stent placement. Unfortunately, a thrombosed graft with completely occluded outflow veins or long-segment stricture of these veins might not be amenable to percutaneous re-establishment of flow. In these cases, surgical revision or creation of new access may be necessary.
Case 156
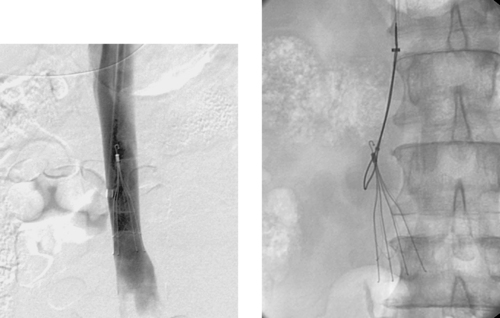 |
1. What type of inferior vena cava (IVC) filter is shown?
2. Why is there a hook at the upper end of the filter?
3. Besides the filter, what device is pictured in the second image?
4. Why is this filter being removed?
ANSWERS: CASE 156
Retrievable Inferior Vena Cava Filter
1. Günther Tulip.
2. To enable percutaneous removal.
3. Amplatz Goose Neck Snare (Microvena).
4. The risk of pulmonary embolism is probably significantly diminished compared with when the filter was placed.
Reference
Kinney, T.B., Update on inferior vena cava filters, J Vasc Intervent Radiol. 14 (2003) 425–440.
Cross-Reference
Comment
The images picture a Günther Tulip (Cook) filter, which is well positioned within the infrarenal IVC. This filter is percutaneously retrievable by snaring the upper hook, provided no thrombus is present within the filter. Particular subsets of patients in whom these filters might be useful include trauma patients and postoperative patients who have a reason to be hypercoagulable for a defined period of time (e.g. hip surgery or abdominal-perineal resection patients).
Currently approved filters in the United States include three types of Greenfield filter (stainless steel, titanium, and over-the-wire), VenaTech filter, bird’s nest filter, Simon nitinol filter, Trap Ease filter, OptEase filter, G2 filter, and the Tulip pictured here. There are few convincing data to demonstrate the superiority of any particular filter.
Notes
Case 157
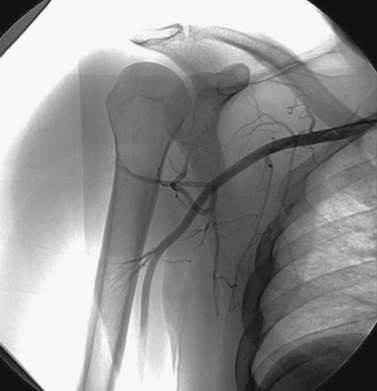 |
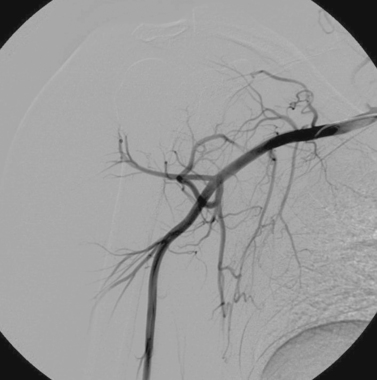 |
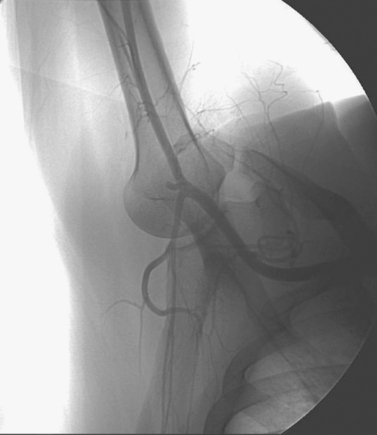 |
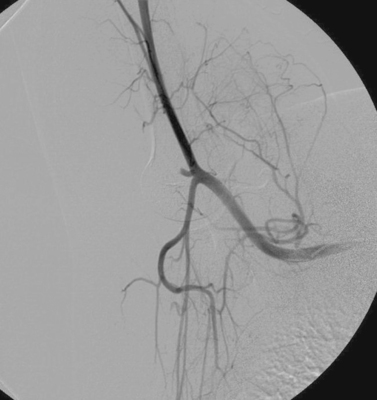 |
1. What procedure is being performed in the first two images?
2. What abnormal finding is seen in the second two images?
3. What provocative maneuver was performed to elicit this finding?
4. What is the diagnosis?
ANSWERS: CASE 157
Quadrilateral Space Syndrome
1. Selective right upper extremity arteriography.
2. Abrupt occlusion of the posterior humeral circumflex artery.
3. Hyperabduction of the shoulder.
4. Quadrilateral space syndrome.
References
Mochizuki, T.; Isoda, H.; Masui, T.; et al., Occlusion of the posterior humeral circumflex artery: Detection with MR angiography in healthy volunteers and in a patient with quadrilateral space syndrome, AJR Am J Roentgenol. 163 (1994) 625–627.
Cormier, P.J.; Matalon, T.A.; Wolin, P.M., Quadrilateral space syndrome: A rare cause of shoulder pain, Radiology 167 (1998) 797–798.
Comment
The quadrilateral space is bounded by the teres minor superiorly, the surgical neck of the humerus laterally, the long head of the triceps medially, and the upper border of the teres major inferiorly. It contains the axillary nerve and the posterior circumflex humeral artery. Quadrilateral space syndrome is a rare condition in which the contents of the quadrilateral space are compressed, leading to vague symptoms of shoulder pain, tenderness over the quadrilateral space on palpation, and teres minor and deltoid denervation.
The most commonly cited cause of compression of quadrilateral space structures is fibrous bands. Other causes include glenoid labral cysts, a ganglion, muscle hypertrophy, and a spike of bone after a scapular fracture.
The prevalence of quadrilateral space syndrome is unknown. On angiography, a position of abduction and external rotation demonstrates significant compression of the contents of the quadrilateral space. Nerve conduction studies and electromyography have also been used to investigate quadrilateral space syndrome. The appearance of denervation changes on MRI affecting the teres minor muscle is considered to confirm the diagnosis of quadrilateral space syndrome. Treatment is centered on surgical decompression of the contents of the quadrilateral space.
Notes
Case 158
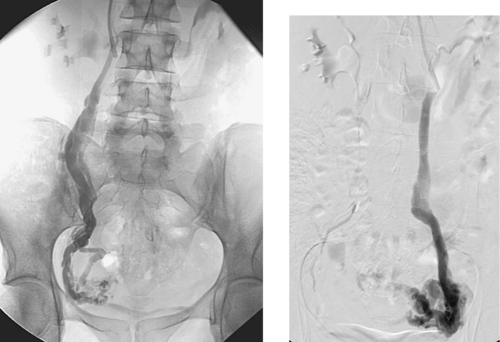 |
1. In order, what veins were catheterized to obtain the first image?
2. In order, what veins were catheterized to obtain the second image?
3. What symptom is this woman likely to present with?
4. What procedure can be performed to treat the symptom?
ANSWERS: CASE 158
Pelvic Congestion Syndrome
1. Inferior vena cava, right ovarian vein.
2. Inferior vena cava, left renal vein, left ovarian vein.
3. Chronic pelvic pain.
4. Ovarian and internal iliac vein embolization.
Reference
Venbrux, A.C.; Chang, A.H.; Kim, H.S.; et al., Pelvic congestion syndrome (pelvic venous incompetence): Impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain, J Vasc Intervent Radiol. 13 (2 Pt 1) (2002) 171–178.
Cross-Reference
Comment
Chronic pelvic pain can result from problems in the reproductive organs or it may be neurologic, musculoskeletal, urologic, or gastrointestinal in nature. However, the association of ovarian and pelvic varices with chronic pelvic pain has been known for many years. Pelvic venous incompetence may be suspected when a woman reports pelvic pain in the upright position, during or after intercourse, or when varicosities are visualized in the thigh, buttocks, or perineum.
Analogous to a male varicocele, ovarian and pelvic varices are amenable to treatment by transcatheter embolization of the refluxing ovarian veins. For this procedure, each ovarian vein is catheterized and examined with venography to determine whether significant reflux is present. If it is, the ovarian vein and internal iliac vein are embolized. Embolization of the internal iliac vein is recommended because of the well-documented communications between the ovarian veins and the internal iliac veins. Embolization therapy may be expected to produce some degree of symptomatic improvement in 70% to 90% of women undergoing the procedure, provided a rigorous clinical and imaging screening process is used to select patients initially.
Notes
Case 159
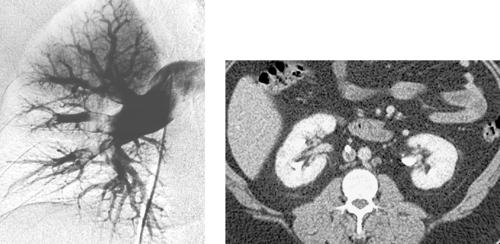 |
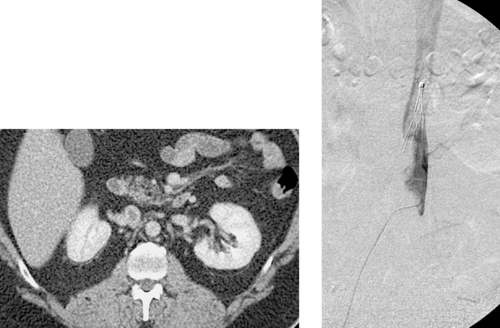 |
1. What is the diagnosis in the first image?
2. Is this finding acute or chronic?
3. What possible explanations exist for pulmonary embolism in the presence of an inferior vena cava (IVC) filter?
4. What is the explanation in this patient?
ANSWERS: CASE 159
Inferior Vena Cava Filter Failure
1. Pulmonary embolism.
2. Acute.
3. IVC filter thrombosis may be present, a collateral pathway around the filter may be present, upper extremity venous thrombosis may have embolized.
4. IVC filter thrombosis (second image) with thrombus extension above the filter (third and fourth images).
Reference
Streiff, M.B., Vena caval filters: A comprehensive review, Blood 95 (2000) 3669–3677.
Cross-Reference
Comment
Inferior vena cava filters are associated with a 2% to 4% annual rate of recurrent pulmonary embolism. There are several reasons this is the case. First, being a metallic intravascular foreign body, the filter itself can predispose to IVC thrombosis in hypercoagulable patients. If this occurs and any thrombus extends above the filter (as in this case), pulmonary embolism can occur. Second, the filter material simply might not prevent small emboli from slipping through. Third, if the filter was not positioned below the lowest renal vein, accessory pathways can provide a route for emboli from the lower-extremity veins to bypass the filter. Fourth, large venous collaterals or a duplicated inferior vena cava (if not identified on initial venography) can rarely provide a similar bypass route for emboli. Fifth, malpositioned filters, excessively angulated filters, and the rare filter that migrates might not be in proper position to catch emboli.
For these reasons, when pulmonary embolism occurs despite IVC filter placement, repeat inferior vena cavography is recommended. If collateral venous pathways are providing a bypass route around the filter or if thrombus is present above the existing filter, then a new suprarenal IVC filter may be placed.
Notes
Case 160
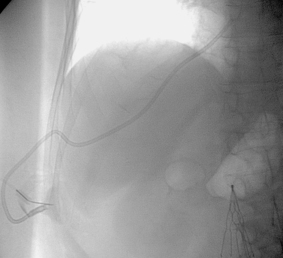 |
1. What type of catheter is visualized?
2. What is the purpose of this procedure?
3. What vein has been accessed for this procedure?
4. If the jugular, subclavian, and femoral veins were occluded, what other route could be used to obtain central venous access?
ANSWERS: CASE 160
Transhepatic Port Catheter Placement
1. Implantable port catheter.
2. To provide venous access.
3. Right hepatic vein.
4. Direct translumbar access into the inferior vena cava.
Reference
Johnson, K.L.; Fellows, K.E.; Murphy, J.D., Transhepatic central venous access for cardiac catheterization and radiologic intervention, Cathet Cardiovasc Diagn. 35 (2) (1995) 168–171.
Cross-Reference
Comment
Adults and children who undergo placement of multiple long-term central venous catheters are prone to develop chronic occlusions of these veins. After the jugular, subclavian, and femoral veins are all exhausted, limited options exist for further central venous access in these patients. When this occurs, a few approaches may be used: (1) Percutaneous recanalization of occluded central veins using angioplasty and/or stents might create enough of a channel through which a new long-term catheter can be placed; (2) Transhepatic venous access may be obtained as depicted here; (3) In extreme cases, translumbar access to the inferior vena cava may be obtained. Unfortunately, the first option may be extremely difficult or impossible, depending upon the chronicity of the occlusion. The last two options can usually be accomplished technically, but the catheter remains at some risk of migrating peripherally due to buckling outside the vein as the patient moves.
Case 161
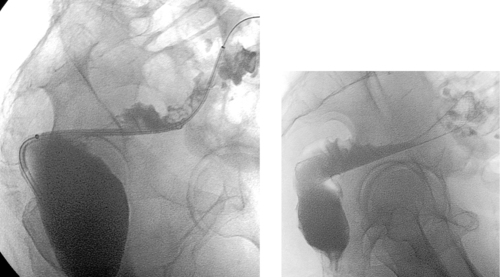 |
1. What is the primary abnormality?
2. What procedure has been performed before the second image?
3. Would this be used in a patient with an anastomotic stricture?
4. Does this device need to be angioplastied during deployment?
ANSWERS: CASE 161
Colonic Stent Placement
1. Stricture at the rectosigmoid junction.
2. Colonic stent placement
3. No.
4. No.
Reference
Mergener, K.; Kozarik, R.A., Stenting of the gastrointestinal tract, Dig Dis. 20 (2) (2002) 173–181.
Cross-Reference
Comment
The images above depict placement of a large self-expanding Wallstent into the rectosigmoid colon, with subsequent relief of bowel obstruction.
Patients with malignant colorectal obstruction have historically required emergency laparotomy and two-stage bowel resection. In recent years, gastrointestinal tract stent placement has been used as a minimally invasive method of relieving large-bowel obstruction in two main subsets of patients. Those who present with acute symptoms may receive stent placement and then undergo treatment planning instead of high-risk emergency surgery. Patients with unresectable and metastatic disease might not require operation at all but might simply be treated palliatively by stent placement. This approach has the potential to spare patients the discomfort and morbidity of surgical bowel resection under inauspicious circumstances when quality of life should be the primary concern.
Case 162
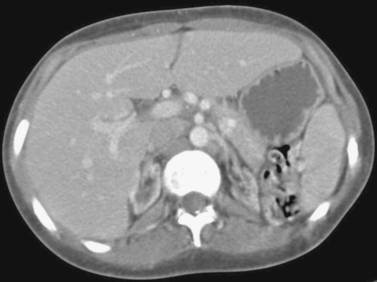 |
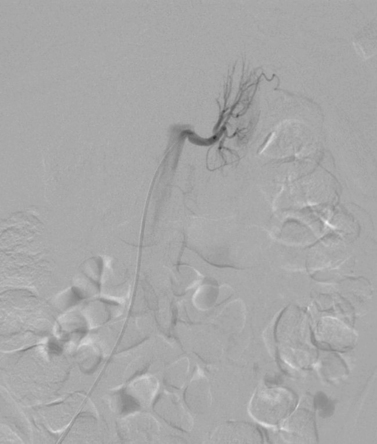 |
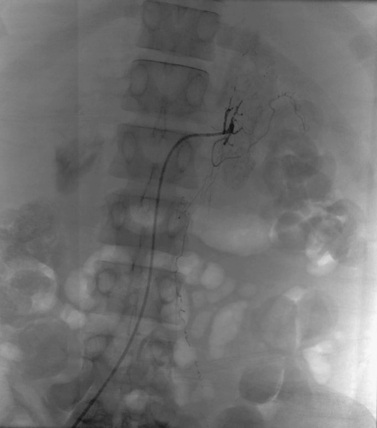 |
1. Describe the findings on the first image.
2. What procedure is being performed in the second image?
3. What procedure is being performed in the third image?
4. What are the indications for performing this procedure?
ANSWERS: CASE 162
Renal Ablation for Hypertension
1. Bilateral atrophic kidneys.
2. Selective left renal arteriography.
3. Transarterial renal ablation.
4. Uncontrolled hypertension, nephritic syndrome, and autosomal dominant polycystic kidney disease.
References
Golwyn Jr, D.H.; Routh, W.D.; Chen, M.Y.; et al., Percutaneous transcatheter renal ablation with absolute ethanol for uncontrolled hypertension or nephrotic syndrome: Results in 11 patients with end–stage renal disease, J Vasc Interv Radiol. 8 (1997) 527–533.
Keller, F.S.; Coyle, M.; Rosch, J.; et al., Percutaneous renal ablation in patients with end–stage renal disease: Alternative to surgical nephrectomy, Radiology 159 (1986) 447–451.
Cross-Reference
Comment
Hypertension affects 80% of patients with end–stage renal disease and can sometimes be refractory to medical treatment. Surgical nephrectomy has been performed in the past in an attempt to treat uncontrolled hypertension in this patient population. Recently, transarterial renal ablation with absolute ethanol has been adopted by some centers as a less–invasive alternative therapy.
Ethanol in its concentrated form delivered selectively into the renal artery is cytotoxic and thrombogenic. Extreme care must be taken while infusing the ethanol to avoid reflux into the aorta. Infusion through a balloon–occlusion catheter may be performed to safeguard against this potentially devastating complication. The dilution effect that occurs once it traverses the renal parenchyma into the venous system renders it harmless, thus avoiding systemic complications.
Case 163
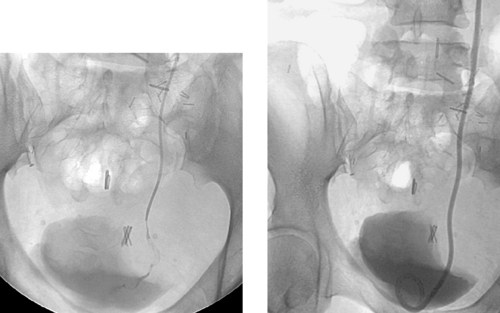 |
1. What study has been performed?
2. Name three imaging methods by which the ureter can be studied.
3. What is the primary finding in the first image?
4. What is the probable etiology of this finding?
ANSWERS: CASE 163
Malignant Ureteral Stricture
1. Anterograde nephrostogram.
2. Anterograde pyelogram, retrograde pyelogram, intravenous pyelogram.
3. Tight irregular stricture of the distal left ureter.
4. Pelvic malignancy.
Reference
Blandino, A.; Gaeta, M.; Minutoli, F.; et al., MR urography of the ureter, Am J Roentgenol. 179 (2002) 1307–1314.
Cross-Reference
Comment
The first image demonstrates a tight, irregular stricture of the left distal ureter. The imaging appearance strongly favors a malignant etiology for several reasons: the irregularity of the stricture; the presence of an irregular filling defect within the strictured segment, presumably representing tumor; and the presence of mass effect upon the ureter in the region of the stenosis and upon the adjacent bladder. However, in most instances pyelography alone will not be sufficient to determine the etiology of a ureteral stricture. In these cases, a knowledge of the patient’s history and correlation with cross-sectional imaging modalities (to visualize a calculus, pelvic mass, or other lesion) may be helpful.
The treatment of malignant ureteral strictures depends largely upon the exact etiology and the overall extent of the disease. In many patients with bladder carcinoma and selected patients with other pelvic malignancies, total cystectomy with ileal conduit formation may be performed, obviating the need for percutaneous intervention. In many other patients, hydroureteronephrosis develops and is not likely to be relieved surgically. In these patients, percutaneous nephrostomy placement is usually performed, often followed by ureteral stent placement if bladder function is adequate. In the second image, the stenosis was successfully crossed and a nephroureteral stent was placed.
Case 164
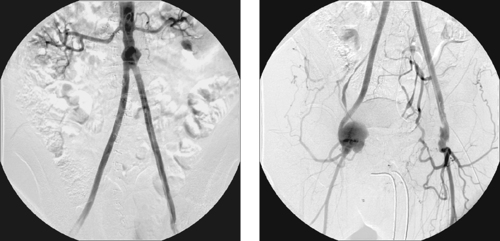 |
1. What surgical procedure has been performed?
2. What abnormality is present on the second image?
3. Name three methods of treating these vascular lesions.
4. Would stent-graft placement be good treatment for this abnormality?
ANSWERS: CASE 164
Femoral Anastomotic Pseudoaneurysm
1. Aortobifemoral bypass graft placement.
2. Right femoral anastomotic pseudoaneurysm.
3. Surgical repair, ultrasound-guided compression, percutaneous thrombin injection.
4. No. Stents and stent grafts should be avoided in the common femoral artery, because hip flexion can predispose to stent fracture.
References
Saad, N.E.; Saad, W.E.; Davies, M.G.; et al., Pseudoaneurysms and the role of minimally invasive techniques in their management, Radiographics 25 (Suppl 1) (2005) S173–S189.
Morgan, R.; Belli, A.M., Current treatment methods for postcatheterization pseudoaneurysms, J Vasc Intervent Radiol. 14 (2003) 697–710.
Cross-Reference
Comment
The development of an anastomotic pseudoaneurysm occurs in approximately 1% of patients per year after aortofemoral grafting. Anastomotic pseudoaneurysms are generally treated with surgical graft revision if the anastomosis is in a region that is readily amenable to surgical access. In carefully selected patients, a stent graft can be placed to address aortic pseudoaneurysms that arise near the proximal graft anastomosis. However, stent grafts are not used for femoral pseudoaneurysms.
Patients with postcatheterization femoral pseudoaneurysms may be treated by two relatively new percutaneous treatments. With ultrasound-guided compression therapy, the sonographer isolates the neck of the aneurysm and applies constant focal pressure to this region for 10 to 30 minutes; this form of therapy is effective in approximately 60% to 80% of cases; however, the recurrence rate after successful treatment may be as high as 20% to 30% in patients receiving anticoagulant therapy. For these reasons, ultrasound-guided compression of the pseudoaneurysm neck has largely been replaced with direct percutaneous injection of thrombin into the sac, which produces instant pseudoaneurysm thrombosis. This technique is faster and more effective (>90% of patients) than ultrasound-guided compression therapy. Surgical treatment is needed for several groups of patients: those with infected or rapidly expanding pseudoaneurysms; those with distal ischemia or neuropathy caused by local pressure by the pseudoaneurysm upon the femoral artery or nerve, respectively; and those who fail percutaneous management.
Case 165
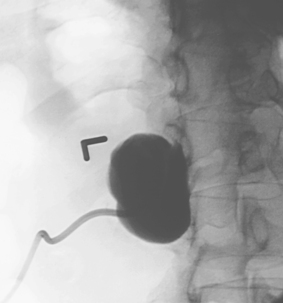 |
1. Name three renal fluid collections for which percutaneous nephrostomy access might be needed.
2. In what situations are uninfected renal cysts drained percutaneously?
3. What therapy can be employed to treat symptomatic renal cysts that do not respond to simple aspiration?
4. Do patients undergoing renal cyst drainage tend to require long-term drainage?
ANSWERS: CASE 165
Renal Cyst Sclerosis
1. Renal abscess (for drainage), stone-containing calyceal diverticulum (to facilitate percutaneous stone removal), and symptomatic renal cyst (for sclerosis).
2. Chronic pain, recurrence after needle aspiration.
3. Ethanol sclerosis.
4. No.
Reference
Paananen, I.; Hellstrom, P.; Leinonen, S.; et al., Treatment of renal cysts with single-session percutaneous drainage and ethanol sclerotherapy: Long-term outcome, Urology 57 (2001) 30–33.
Cross-Reference
Comment
The vast majority of simple renal cysts do not require any form of treatment. However, a small number of patients do complain of significant pain associated with the presence of a large, dominant cyst. In these patients, several percutaneous treatment methods can be used. Renal cyst aspiration via a needle may be performed; many patients respond to this limited form of therapy, although in a large percentage symptoms recur. For this reason, ethanol ablation therapy can be performed either as the first approach or after a trial of aspiration has failed.
Under ultrasound and fluoroscopic guidance, a needle is positioned in the cyst, and a drainage catheter is placed over a guidewire. The cyst is aspirated dry. Contrast is injected into the cyst to ensure that no communication is present with the renal collecting system and to define the volume of the cyst. The contrast is aspirated and is replaced with a smaller volume of absolute ethanol. Protocols vary, but in general the ethanol is allowed to dwell for 15 minutes as the patient periodically changes position. The contrast is then aspirated. This procedure may be repeated twice at the initial sitting. The catheter is then left to gravity drainage.
Case 166
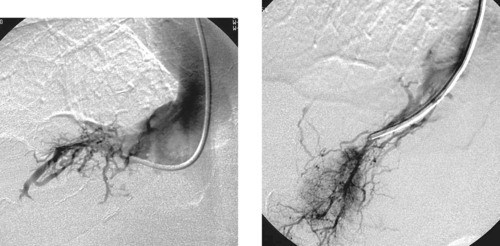 |
1. What veins have been selected in the images shown?
2. What vessels are opacified?
3. What is the diagnosis?
4. What test is the gold standard for diagnosis of this problem?
ANSWERS: CASE 166
Budd–Chiari Syndrome
1. Accessory right hepatic vein and main right hepatic vein.
2. Hepatic venous collaterals.
3. Budd–Chiari syndrome (BCS).
4. Hepatic venography.
Reference
Mancuso, A.; Fung, K.; Mela, M.; et al., TIPS for acute and chronic Budd–Chiari syndrome: A single-centre experience, J Hepatol. 38 (6) (2003) 751–754.
Cross-Reference
Comment
Patients with BCS present with severe ascites (85%-90% of patients), hepatosplenomegaly, abdominal pain, jaundice, vomiting, and/or extremity edema. If untreated, BCS patients develop progressive portal hypertension with esophageal variceal bleeding, encephalopathy, hepatic failure, and death. BCS can be caused by tumor invasion of the inferior vena cava (IVC) or hepatic veins, membranous suprahepatic IVC obstruction, right atrial tumors, polycythemia vera, postpartum state, oral contraceptive use, paroxysmal nocturnal hemoglobinuria, veno-occlusive disease (in patients who have received chemotherapy and radiation), and various hematologic conditions.
CT and MRI findings in BCS include hepatomegaly, ascites, inhomogeneous hepatic enhancement with a fan-shaped area of decreasing peripheral enhancement, and caudate lobe enlargement. Duplex ultrasound can demonstrate hepatic vein flow to be absent, reversed, turbulent, or monophasic. Hepatic venography, the gold standard for diagnosis, can demonstrate webs, stenosis, or thrombus within the suprahepatic IVC or hepatic veins with abundant collaterals (well depicted above) between the main hepatic veins.
Patients with BCS due to IVC or hepatic vein stenosis may be treated effectively with balloon angioplasty or stent placement (when stenosis recurs following angioplasty). Patients with IVC thrombosis may be treated with thrombolytic therapy. Patients with hepatic vein thrombosis have been successfully treated with TIPS, although this may be technically difficult to perform and the long-term results of this approach are unknown.
Case 167
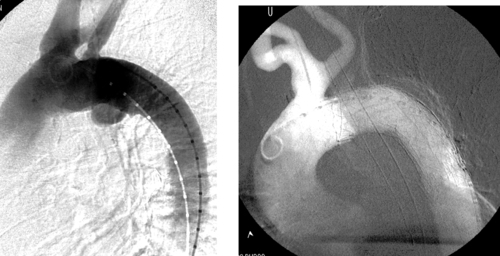 |
1. What abnormality is seen in the first image?
2. How has this been treated?
3. What may have happened to the left subclavian artery?
4. Are thoracic aortic stent grafts also prone to endoleaks and migration over time?
ANSWERS: CASE 167
Thoracic Aortic Stent-Graft Placement
1. Thoracic aortic aneurysm along the underside of the distal part of the aortic arch.
2. Stent-graft placement.
3. It was probably ligated surgically before stent-graft placement.
4. Yes.
Reference
Sakai, T.; Dake, M.D.; Semba, C.P.; et al., Descending thoracic aortic aneurysm: Thoracic CT findings after endovascular stent-graft placement, Radiology 212 (1999) 169–174.
Cross-Reference
Comment
Surgical repair of thoracic aortic aneurysms is associated with the complications of death in 6% to 12%, paraplegia in 3% to 16%, and cardiorespiratory problems in 20% to 30%. Stent-graft repair has hence been viewed as a welcome treatment alternative.
Like abdominal aortic aneurysms, the status of the proximal and distal aneurysm necks (which must be >2cm length and free of significant angulation and plaque) and of the iliac arteries (which must be >7–8mm) are important factors in determining eligibility for endovascular thoracic aortic aneurysm repair. Other considerations specific to the thoracic aorta include: (1) The degree of thoracic aortic curvature; (2) The relationship of the aneurysm to the left subclavian artery: if a 2-cm proximal neck is not present, then the subclavian artery may first be surgically ligated (as in this case); alternatively, it may be covered with the stent graft, provided there is a good seal to prevent a type II endoleak. (Patients with a dominant left vertebral artery, an incomplete vertebrobasilar system, or a left internal mammary artery to coronary artery bypass graft should undergo revascularization before the origin of the left subclavian artery is covered); (3) The location of key intercostal arteries: Although no definite relationship to paraplegia has been established, it is wise to minimize coverage of patent intercostal arteries.
The large initial experience with stent grafts for TAA has mostly used homemade devices, in which a stent was sutured to graft material by the operator. Using these rudimentary devices, initial aneurysm exclusion rates have been 80% to 100%. However, at 4-year follow-up, only about 50% are free of endoleak.
Notes
Case 168
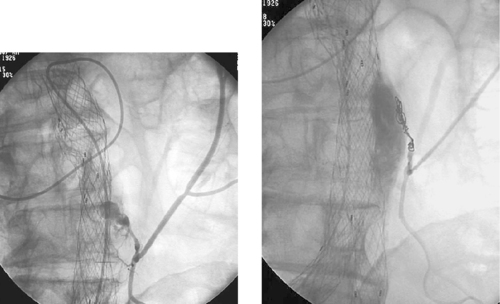 |
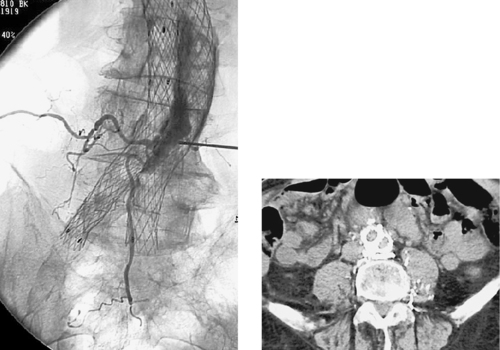 |
1. What diagnosis do Patient A (top two images) and Patient B (bottom two images) have in common?
2. In Patient A, what vascular circuits supply the abnormality?
3. Is this abnormality related to poor initial device positioning?
4. On the CT scan of Patient B (last image), what is the hyperattenuating material posterior to the stent graft?
ANSWERS: CASE 168
Embolization of Type II Endoleak
1. Type II endoleak after endoluminal grafting.
2. Superior mesenteric artery, middle colic artery, marginal artery, inferior mesenteric artery, endoleak.
3. No.
4. Embolization glue that has been injected directly into the aneurysm sac, filling the nearby patent lumbar arteries.
Reference
Kasirajan, K.; Matteson, B.; Marek, J.M.; et al., Technique and results of transfemoral superselective coil embolization of type II lumbar endoleak, J Vasc Surg. 38 (2003) 61–66.
Cross-Reference
Comment
The top left image demonstrates a type II endoleak due to retrograde flow in the inferior mesenteric artery. The top right image demonstrates successful placement of embolization coils in the feeding branch, which resulted in closure of the endoleak. When the feeding vessel can be catheterized, this provides an effective treatment method.
The bottom left image demonstrates a type II endoleak due to retrograde flow in lumbar arteries. The bottom left image depicts filling of the aneurysm sac and the patent lumbar arteries via direct translumbar puncture of the aneurysm sac. The CT scan on the bottom right demonstrates injected N-bucrylate glue posterior to the stent-graft device and within the aneurysm sac and lumbar branches. Early aneurysm thrombosis has been observed following direct injection of glue or thrombin into the aneurysm sac, and this option may be particularly useful in patients in whom catheterization of the feeding artery is difficult. However, the long-term results of this approach (and of coil embolization) in producing durable aneurysm exclusion are unknown.
Notes
Case 169
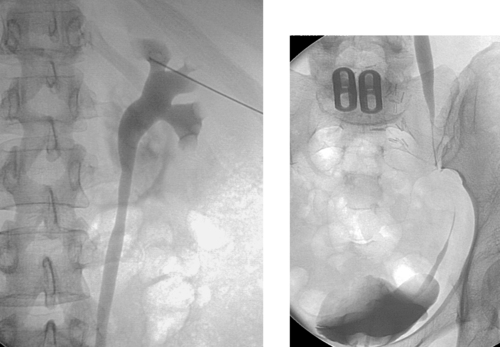 |
1. What study has been performed?
2. What abnormality is seen?
3. Name two noninvasive tests that could be used to determine if the visualized abnormality is causing functional obstruction.
4. Name one invasive test that could be used to determine if the visualized abnormality is causing functional obstruction.
ANSWERS: CASE 169
Whitaker Test
1. Anterograde pyelogram.
2. Ureteral stricture.
3. Intravenous pyelogram, diuretic renal scintigraphy.
4. Whitaker test.
Reference
Tchetgen, M.B.; Bloom, D.A.; Robert, H., Whitaker and the Whitaker test: A pressure-flow study of the upper urinary tract, Urology 61 (2003) 253–256.
Cross-Reference
Comment
The images demonstrate an anterograde pylogram in which a stenosis is present in the distal ureter. In this patient with repeated upper urinary tract infections, diuretic scintigraphy was equivocal as to whether true obstruction was present. For this reason, a Whitaker test was performed and was negative. If the test had been positive, a percutaneous nephrostomy catheter and/or ureteral stent would have been left in place.
To perform a Whitaker test, catheterization of the bladder and needle placement in the renal pelvis is performed. During infusion of dilute contrast at a fixed rate (10mL/min for 9 minutes, then 15mL/min for 9 minutes is one commonly used protocol), manometry of the bladder (to estimate resting intraabdominal pressure) and renal pelvis is performed every 3 minutes. The true renal pelvis pressure is calculated as the difference between these two pressures at each time interval. If the pressures reach 22cm H2O, then the test is clearly positive; if the pressures stay below 15cm H2O, then the test is clearly negative. Reproduction of flank pain symptoms with pressures in the borderline zone is also considered by most physicians to represent a positive test.
Case 170
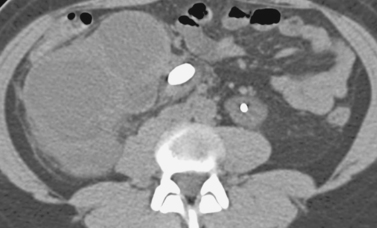 |
1. What is the diagnosis on this noncontrast CT scan?
2. If the patient is afebrile, why would percutaneous nephrostomy be performed?
3. When percutaneous stone removal is being performed, what calyx usually provides the easiest access into the ureter?
4. Name two potential complications that can result from use of this calyx.
ANSWERS: CASE 170
Ureteral Calculus
1. Right hydronephrosis with right ureteral calculus.
2. To preserve renal function, to facilitate percutaneous stone removal.
3. Upper-pole calyx.
4. Traversal of the pleural space can cause pneumothorax or urothorax.
Reference
Landman, J.; Venkatesh, R.; Lee, D.I.; et al., Combined percutaneous and retrograde approach to staghorn calculi with application of the ureteral access sheath to facilitate percutaneous nephrolithotomy, J Urol. 169 (2003) 64–67.
Cross-Reference
Comment
The noncontrast CT images demonstrate a large calculus at the right ureteropelvic junction. The kidney is hydronephrotic and shows marked parenchymal thinning suggesting long-term chronic obstruction. Urgent percutaneous nephrostomy tube placement is indicated if the patient is febrile. If the patient shows no signs of infection, then percutaneous nephrostomy can be electively performed to preserve renal function and/or to facilitate percutaneous stone removal. Evaluation for the presence of significant residual function in the right kidney via renal scintigraphy would be advised before placing a nephrostomy tube in that kidney.
To perform percutaneous stone removal, obtaining optimal access into the renal collecting system is extremely important. Typically the collecting system is accessed and a guidewire is passed into the bladder and snared from below to obtain through-and-through access. A balloon catheter is then used to dilate the subcutaneous tract to accommodate a large (often 30F) sheath. Through this sheath, a nephroscope is passed into the renal collecting system, and under direct visualization the stone is fragmented and removed. A nephrostomy catheter, and sometimes a ureteral stent, is left in place after the procedure.
Case 171
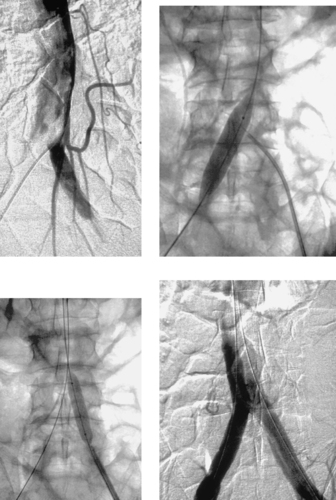 |
1. From where do the left common iliac artery and inferior mesenteric artery derive their blood supply in this patient?
2. What procedure is being performed in the remaining images?
3. Why is this procedure performed?
4. What is the purpose of the angioplasty balloon in the second image?
ANSWERS: CASE 171
Percutaneous Balloon Fenestration of Aortic Dissection
1. From the collapsed true lumen of a dissected aorta.
2. Percutaneous balloon fenestration of the intimal flap.
3. Lower-limb ischemia in the setting of acute aortic dissection.
4. To serve as a fluoroscopically visible target for transseptal needle puncture.
Reference
Slonim, S.M.; Miller, D.C.; Mitchell, R.S.; et al., Percutaneous balloon fenestration and stenting for life-threatening ischemia complications in patients with acute aortic dissection, J Thorac Cardiovasc Surg. 117 (1999) 1118–1127.
Cross-Reference
Comment
The first image demonstrates a pelvic angiogram in a patient with acute type B aortic dissection and left leg ischemia. A collapsed true lumen supplies the inferior mesenteric and left common iliac arteries. The second image demonstrates placement of a target balloon in the aortic false lumen from the right iliac artery, and guided puncture of the balloon using a needle from the left iliac artery true lumen. The third image shows transseptal flap angioplasty, and the final image demonstrates flow from the false lumen crossing the newly created aortic fenestration to supply the left common iliac artery. This patient’s ischemic symptoms resolved instantly and he did not require surgery.
Patients with acute type B dissection complicated by visceral or peripheral arterial ischemia are at high risk for death and paraplegia during surgical aortic repair. For this reason, several percutaneous methods have been used to relieve organ ischemia in these patients. Stents can be placed in branch vessels that are dissected. In addition, patients with true lumen collapse and organ ischemia due to poor inflow to a true lumen-supplied branch artery can be treated with percutaneous balloon fenestration (shown here) or aortic stent placement; in early studies, perfusion was successfully restored to tissue beds that were more than 90% ischemic using these methods. Another endovascular approach involves placement of a stent graft across the primary intimal dissection tear in the thoracic aorta.
Notes