Chapter 112 Cervicothoracic Junction Tumors
Regional Challenges
Incidence and Statistics
The upper thoracic vertebrae account for 15% of patients with tumors of the spine. The T1-4 region accounts for 10% of all spinal metastases.1 Subaxial cervical spine tumors also are uncommon and constitute less than 10% of spinal metastases.2 Due to the smaller canal size, tenuous blood supply at the cervicothoracic junction, along with unique biomechanical stresses that can lead to instability, the chance of neurologic involvement can be as high as 80%.3
The cervicothoracic junction is a transition zone between the more mobile cervical spine, with its lordotic alignment, and the much stiffer, kyphotic thoracic spine, stabilized ventrally by the rib cage. A variety of pathologic entities that can result in kyphotic deformity can be seen at the cervicothoracic junction, including tumor, infection, spondylosis, traumatic injury, and iatrogenic destabilization. Following surgery, the kyphotic deformity may worsen further as a result of failing to account for the unique biomechanics of the cervicothoracic junction.4 Cervicothoracic pathology can include either primary or metastatic oncologic disease. Several studies have looked specifically at tumors in this anatomic region.5–7 One series of 19 patients with cervicothoracic tumors had 6 patients with primary pathology, including angiosarcoma,1 chordoma,1 lymphoma,2 plasmacytoma,1 and schwannoma.1 The other 13 patients (68%) had metastatic disease, with metastases to the lung5 and prostate2 being the most common. Other tumors seen were breast cancers, melanoma, and renal, ovarian, and colon cancers. Another series with 32 patients described 19 who had lung cancer and vertebral body invasion (Pancoast tumors), 11 with metastasis, 1 with chondrosarcoma, and 1 with myeloma.6 An and Vaccaro7 reported a group of 15 patients with cervicothoracic pathology, including 8 metastatic lesions (2 breast, 2 lung, 2 prostate, 1 thyroid, and 1 adenocarcinoma of unknown origin) and the remainder primary benign (aneurysmal bone cyst, giant cell tumor) and malignant tumors (myeloma, lymphoma, neurofibrosarcoma, and hemangioendothelioma).
Nonsurgical and Surgical Treatment
Spinal metastases, especially at the cervicothoracic junction, present unique treatment considerations. The goal of treatment is to improve or maintain neurologic function, achieve spinal stability, and attain local tumor control. The traditional methods of dealing with this pathology include radiation therapy, chemotherapy, and hormonal treatment, as well as surgery. Bilsky et al.8 expanded on the NOMS pneumonic (neurologic, oncologic considerations, mechanical instability, and systemic disease and comorbidities), developing an algorithmic approach to decision making.
Radiation, Chemotherapy, and Stereotactic Radiosurgery Preoperatively and Postoperatively
The ultimate goal of treatment in patients with cervicothoracic tumors is preservation or recovery of neurologic and functional status and treatment of pain. Although radiation is less invasive, has fewer complications, and traditionally has been found to be at least equivalent to surgery for the treatment of metastases, patients with epidural spinal cord compression have been shown to respond well to surgical intervention.9 Initially, basic decompressive procedures, such as laminectomy without instrumentation, were used to resect epidural disease, although these procedures did create iatrogenic instability. Patients receiving radiation therapy as a primary treatment had 79% maintenance of ambulatory status and a 42% rate of neurologic recovery. By comparison, patients undergoing surgical intervention as mentioned above had worse odds of improving their preoperative neurologic status, with 33% chance of recovery and up to 50% of the patients worse off neurologically.10,11
In 1978, Gilbert and colleagues compared radiation therapy directly with laminectomy alone. They showed that patients with radiosensitive tumors, including breast cancers, myeloma, and lymphoma, had significantly better functional outcomes when compared with less radiosensitive tumors, including lung, colon, and renal cell carcinoma, regardless of the tumor treatment. Gilbert et al. helped steer primary treatment in certain metastatic diseases to radiation therapy.12
In general, surgical resection of epidural metastases, if tolerated well from a medical standpoint, should be followed by radiation therapy, or possibly systemic chemotherapy or hormonal therapy. This combined approach can be used to secure local tumor control.9 Newer techniques, including image-guided intensity-modulated radiation therapy (IGIMRT) and the cyberknife, improve the chances for tumor control in the cases of radiation therapy–resistant tumors, especially postoperatively, as opposed to conventional external-beam radiation therapy (EBRT).13,14 At Memorial Sloan-Kettering Cancer Center, the typical tumor board protocol currently dictates that a patient with a radiosensitive tumor, regardless of the degree of spinal cord compression or myelopathy, be irradiated with standard EBRT or with single-fraction IGIMRT or chemotherapy for noncompressive masses. Patients with radioresistant tumors and a low degree of compression can undergo EBRT or IGIMRT 24-Gy single fractions, whereas the patients with higher-grade spinal compression are offered surgical decompression and fixation followed by IGIMRT.8 Despite diligent treatment, the chance of recurrence in some of the surgical series remains high, approaching 96% at 4 years.15
Superior sulcus non–small-cell lung carcinomas, which are extremely difficult to treat because of their aggressiveness and invasion of adjacent spine, brachial plexus, and subclavian vessels, have been treated traditionally with radiation and surgery, yielding a 30% 5-year survival rate. More recent data suggest that induction chemoradiation (two cycles of cisplatin and etoposide with radiation) and surgical resection (thoracotomy) followed by two more cycles of chemotherapy can yield higher survival rates, of 44% at 5 years.16
Surgical Treatment
Surgical treatment of tumors of the cervicothoracic junction often is challenging due to the presence of major vascular and soft tissue structures requiring exquisite preoperative planning and approach. The cervicothoracic junction is unique biomechanically. This uniqueness must be accounted for when considering surgery in this region. Preoperative imaging helps define the neurovascular involvement of the tumor, guiding the direction of the surgical treatment. In the cervical spine, for example, MRI can help identify epidural spinal cord compression and vertebral artery involvement. Preoperative angiography and balloon occlusion tests may be necessary if tumor dissection or occlusion of a single vertebral artery is likely or necessary.17 For ventral surgical approaches to the subaxial cervical spine and cervicothoracic junction, the recurrent and superior laryngeal nerve functions must be evaluated. Preoperative laryngoscopy can be used to assess bilateral vocal cord dysfunction; if present, the approach should be from the ipsilateral side. Superficial laryngeal nerve function must be evaluated by swallowing studies.
Although radiation therapy remains the treatment of choice for many patients with metastatic disease, 17 primary malignant tumors often are treated surgically for oncologic considerations, because they can be resistant to chemotherapy and radiation therapy. En bloc resection, which offers a higher probability of cure and local disease control, is preferred, but it typically is more technically demanding than an intralesional resection, especially if it extends beyond the vertebral body or dorsal elements. It may occasionally entail sacrificing the adjoining vertebral artery or an exiting nerve root.
Ideally, most hypervascular spinal tumors should undergo arterial embolization prior to surgical resection to minimize the risk of intraoperative and postoperative bleeding and enhance the surgeon’s ability to safely decompress the spinal cord and maximize tumor removal.18–20 The need for preoperative embolization is based on tumor histology, as seen in renal cell carcinoma, sarcomas (e.g., angiosarcoma and leiomyosarcoma), follicular and papillary thyroid carcinoma, hepatocellular carcinoma, germ cell tumors, and neuroendocrine tumors such as paragangliomas. The arterial blood supply must be conducive to such an attempt, because the feeder vessels must be large and accessible for microcatheterization. Some other hypervascular tumors, such as melanoma and multiple myeloma, are fed by a finer capillary network, which may not be as accessible.18 On MRI, tumor hypervascularity may be identified as bright contrast enhancement and flow voids representing blood vessels. Hyperintensity also may be present on both T1- and T2-weighted images due to extracellular methemoglobin or hypointensity from the breakdown products of methemoglobin. Prabhu et al.18 have recommended preoperative angiography for the following circumstances: if the tumors are of known hypervascularity, regardless of MRI findings; if the MRI suggests hypervascularity regardless of histology; or if the primary tumor origin is unknown. Cervical tumors need special consideration, because there is an increased risk of cerebral or brainstem infarction with preoperative embolization.21 Two percent of patients experience neurologic complications, and 4% to 10% can experience local or systemic complications following embolization.18 Therefore, only in special circumstances is embolization used for cervical malignancies.
Historical Surgical Approaches
Ventral surgical approaches to the thoracic spine initially were developed to treat Pott disease, and similar principles are used today to treat primary and metastatic tumors of the spine, with the cervicothoracic junction being the most challenging. Menard22 was the first to describe an approach to this pathology; he resected a portion of the rib and transverse process to gain access to a relatively small portion of the vertebral body. Capener23 took this approach a step further by removing a longer segment of the rib, resulting in a more generous exposure and allowing ventrolateral decompression of the spinal cord.
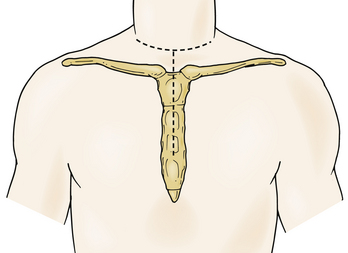
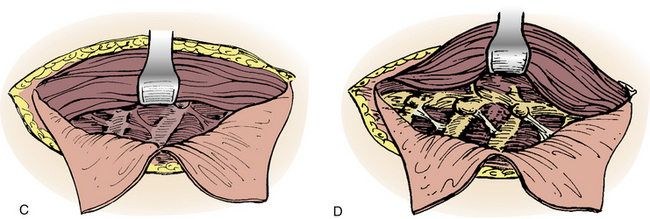
In 1957, Nanson24 described an oblique approach at the cervicothoracic junction that used a supraclavicular incision to gain access to the upper thoracic sympathetic ganglia. The exposure of the vertebral bodies, however, was very limited. Cauchoix and Binet25 described a more aggressive approach to the cervicothoracic junction that involved performing a median sternotomy. This approach was essentially abandoned due to the 40% mortality rate in patients with Pott disease26 and a 33% mortality rate in patients with cervicothoracic pathology.3 In 1984, Sundarsan et al.27 described a ventral approach, which involves a T-shaped incision with the transverse portion just proximal to the clavicles and a vertical component in the midline over the sternum (Fig. 112-1). Flaps are raised superiorly and inferiorly, and the external jugular vein and medial supraclavicular nerve are divided. The medial one third of the clavicle is excised, as well as a rectangular block of bone from the sternum (Fig. 112-2). Under the manubrium sterni the subclavian vein is dissected free, and the thymus may be removed for further exposure. The laryngeal nerve must be identified as the dissection continues deep between the carotid sheath laterally and the esophagus medially. The resected clavicle can be used as structural bone graft at the corpectomy site without further instrumentation.27
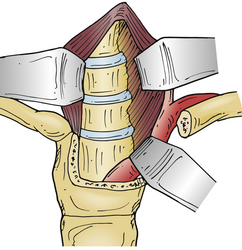
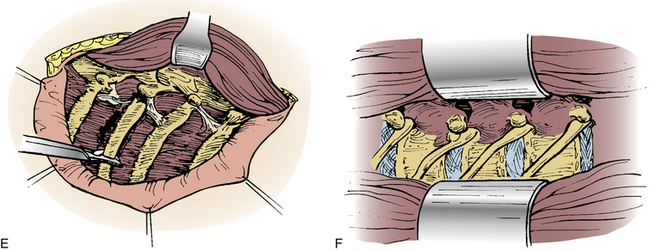
This approach was slightly modified by Birch et al.,28 who described an osseomuscular flap containing the medial one third of the clavicle and manubrium in one piece attached to the sternocleidomastoid muscle and later reattached with wires and plate-screw fixation. There were four clavicle nonunions and one transient recurrent laryngeal nerve palsy in the 17 patients in this study. By leaving the sternocleidomastoid muscle attached, the risk of pulmonary compromise was lowered in patients with respiratory problems. Kurz and Herkowitz29 modified this approach by only removing the medial clavicle without resecting the manubrium. Midline-crossing vascular structures limit exposure caudally to approximately T3 (Fig. 112-3). The lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine has been described for approaches as high as T3 but has limited utility with cervicothoracic junction tumors.30
Ventral “Trapdoor” Approach
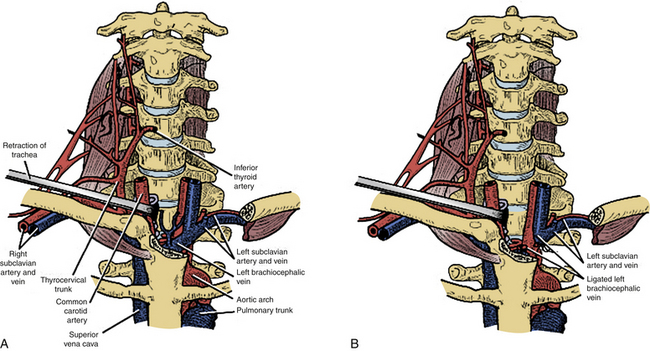
The “trapdoor” exposure to the cervicothoracic junction has the advantage of leaving the sternoclavicular junction as well as the clavicle itself intact. Originally described by Nazzaro et al.,31 it is a combination of the ventrolateral cervical approach, median sternotomy, and ventrolateral thoracotomy. This approach is ideal for patients with ventral pathology at the cervicothoracic junction down to the T3-4 level who need decompression and subsequent reconstruction. Patients undergoing the procedure must have their neurologic status closely monitored with motor and somatosensory-evoked potentials and intravascular volume status with the help of a central line or Swan-Ganz catheter. Lines should not be placed in the right internal jugular or subclavian vein, which will be in the field,32 assuming a right-sided approach is utilized.
The incision is made along the ventral border of the sternocleidomastoid muscle on the right side, to the sternal notch and down to the level of the fourth intercostal space, lying over the midline of the sternum and then curving laterally over the fourth rib. Proximally, the dissection is taken deep in the usual Smith-Robinson technique33 by incising the platysma muscle and staying in the avascular plane between the carotid sheath laterally and the tracheoesophageal viscera medially. It may be necessary to transect or ligate the omohyoid muscle and the middle thyroid vessels in order to obtain a wider exposure. Once the prevertebral fascia is reached overlying the ventral cervical spine, the longus colli muscles are visualized. Distally, a plane is identified between the manubrium and the underlying vascular structures. Once the right lung is deflated, the sternum is transected longitudinally to the level of the fourth rib, and the incision is connected to the laterally made thoracotomy dissection. A chest spreader is used to obtain deep access. The mammary artery is identified; this artery typically requires ligation.
Lateral Parascapular Extrapleural Approach
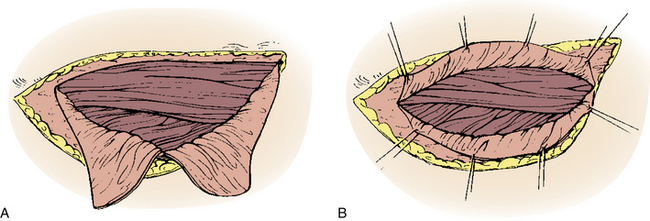
A straight ventral approach occasionally should be supplemented by a dorsal approach to allow for supplemental instrumentation or decompression. In 1991, Fessler et al.34 described a lateral parascapular approach to the cervicothoracic junction that allowed access to the anterior column of the spine and simultaneous placement of dorsal instrumentation. The patient is intubated with a double-lumen endotracheal tube and placed prone on chest rolls. (We often do not use a double-lumen tube.) The incision is begun midline over the spinous processes of the subaxial cervical spine and directed distally in a curved fashion to the ipsilateral scapula. The trapezius and rhomboid muscles are identified under the deep fascia and elevated subperiosteally toward the medial border of the scapula as a musculocutaneous flap, with the interspinous ligaments and a cuff of muscles left intact for approximation later. The intact paraspinal muscles (the spinalis thoracis and longissimus thoracis) are elevated and retracted dorsally and medially. The ileocostalis group of the erector spinae musculature can be elevated and retracted either medially or laterally. These steps provide the surgeon with an excellent exposure of the upper dorsal rib cage and dorsal vertebral elements (Fig. 112-4).
Dorsal Approach
Cervical laminectomy alone as a decompressive procedure is no longer the standard of care. In biomechanical studies, torsional stiffness decreased dramatically, a 2.5% increase in dorsal strain was noted when more than 50% of the facet had been removed at the C5-6 level, and an increase of more than 25% in dorsal strain was seen with a 75% to 100% facetectomy when compared with an intact specimen.35 The decision to use a postoperative orthosis is complex and based on a number of factors, including patient comorbidities such as smoking, diabetes, poor nutritional preoperative status, bone density and, consequently, screw fixation, and, finally, the amount of deformity correction and the iatrogenic stresses placed on the construct.
Cervicothoracic Junction Challenges: Biomechanical Considerations
Five biomechanical studies have looked specifically at fixation at the cervicothoracic junction.36–40 Albert et al.36 compared a lateral mass plating system, a dorsal rod-hook system, and a ventral plate system, showing that all three systems stabilize a dorsal two-column injury similarly. The dorsal fixation systems were found to be stiffer than the others. Chapman37 found that a lateral mass screw fixation system combined with thoracic laminar hooks can provide adequate stabilization at the cervicothoracic junction.
The degree of instability, specifically the number of columns involved, plays an important role in the surgical treatment algorithm for pathology in this region. A biomechanical cadaveric study looking specifically at dorsal stabilization at the cervicothoracic junction offered a comparison of three different fixation devices for stability with dorsal two- and three-column injuries. Kreshak et al.38 evaluated three frequently used dorsal cervical fixation devices, including dorsal rod-screw systems and a dorsal plate-screw system. In extension, there was a significant reduction in construct stiffness with three-column injuries when compared with the fixation of the two-column injuries and the intact spine. Similar results were found with flexion testing. In lateral testing, all the fixation systems were found to be significantly stiffer than the intact spine. The dorsal plate-screw system was found to be weaker in maintaining stability than the screw-rod systems. There are limitations to the plate-screw system, including the need for more surgical precision when placing the screws, difficulty in accounting for variability of the interfacet distances, and difficulty in lining up the predrilled holes in the dorsal plates with the lateral masses. It has been postulated that with a dorsal two-column injury the anterior longitudinal ligament as well as the ventral aspect of the intervertebral disc continue to provide resistance to extension, explaining the findings in this model. Dorsal fixation alone, although it provides a tension band effect in flexion, was not enough to provide stability in the three-column injury model.38
The data also suggest that for a two-column injury at the cervicothoracic junction, there is a trend toward increasing stiffness with prolonged dorsal thoracic fixation, with no differences recorded between the intact state and the dorsal fixation model from C5-T1, with the authors recommending fusion from C6-T2 in order to save cervical fixation points.39 With three-column injuries, dorsal fixation alone results in excessive flexibility in flexion/extension even with instrumentation to T3. Supplementation of such fixation with anterior column instrumentation leads to increased strength of the construct, without any differences in flexion-extension and lateral bending when compared with the intact model, even if the dorsal fixation was stopped at T1. In order to reduce flexibility with axial rotation, however, the instrumentation would have to be extended to T3. For a three-column injury with corpectomy, similar recommendations were made to those for the three-column model with ventral and dorsal instrumentation.39
Another recent study looked at four different dorsal cervicothoracic rod-and-screw constructs, specifically at varying rod diameters and rod connector types, and tested them in flexion bending and axial rotation. There were no significant differences between a dual-diameter rod (3.5 and 5.5 mm) and a solid domino connector extending between two separate rods of the same diameters. A flexible domino connector was found to have similar stiffness but lower ultimate and yield force.40
The length of dorsal spinal instrumentation following decompression is a very important consideration that must be based on the patient’s age, the underlying pathology, the length of the laminectomy, and the quality of the bone stock.41 General indications for spinal fusion, as postulated by White and Panjabi, include (1) restoring clinical stability to a spine with compromised structural integrity, (2) maintaining alignment after deformity correction, (3) preventing formation of a new deformity, and (4) alleviation of pain.42 The location of the decompression, especially at the cervicothoracic junction, must be carefully considered, because there is a higher incidence of postoperative kyphotic deformity. The normal sagittal weight-bearing axis lies dorsal to the C2-7 vertebral bodies, lowering the demand on the cervical paraspinal muscles to maintain alignment. Because laminectomy disrupts the posterior column and impairs the tension band construct dorsally, there is a tendency for the weight-bearing axis to shift ventrally. The ventral shift can lead to a kyphotic deformity, which puts the dorsal muscle groups at a significant mechanical disadvantage, requiring extra work to maintain an upright posture and leading to muscle fatigue, pain, and more kyphosis. This is even harder to achieve following dorsal cervical surgery. The use of a postoperative cervical orthosis, while it can help maintain cervical lordosis and alignment, also may lead to further paraspinal muscular atrophy. Pal and Sherk43 have looked at each column individually at C6 and measured the axial loads. They found that 36% of the total load applied rostrally is transmitted through the two anterior columns, and most of the load is transmitted through the articular processes dorsally, again emphasizing the importance of integrity of the posterior column in load sharing.
Inoue et al.44 had a series of 36 patients who had surgical decompression of their spinal cord tumors via laminectomy, laminoplasty, or hemilaminectomy. Those patients who underwent C7 laminectomy were found to develop kyphosis at the cervicothoracic junction with a compensatory lordosis of the cervical spine. Those who underwent laminoplasty experienced significantly less deformity.44 Steinmetz et al.,45 who looked at a large series of 593 total cases, found that laminectomy alone across the cervicothoracic junction led to failure in 38% of the cases. Development of postlaminectomy cervical kyphosis also has been documented in children who have undergone multilevel laminectomy.46 De Jonge et al.47 retrospectively looked at 76 children who had undergone noninstrumented laminectomy or laminoplasty and/or radiation therapy for malignant spine tumors. Of those, 88% developed postlaminectomy/postradiation spinal deformity. The growing spine also can be adversely affected by radiation therapy, possibly leading to asymmetrical vertebral growth and kyphosis or scoliosis. The use of instrumentation should be strongly considered in pediatric cases requiring destabilization of the dorsal elements at the cervicothoracic junction. In adults, although laminectomy is indicated in patients with cervical lordosis or straightening, it is absolutely contraindicated in the presence of preoperative kyphosis.
Cervicothoracic Junction Tumors: Instrumentation
Dorsal spinal instrumentation added to any decompressive procedure at the cervicothoracic junction should be expected to provide postoperative stability, protecting neurovascular structures from trauma and offering an optimal bed for bony fusion, allowing only micromotion at the fusion sites. These expectations are also the reason why postoperative cervical orthoses are largely unnecessary. Increased fusion rates as a result of instrumentation have been shown in single-level and multilevel discectomies with ventral plating.48,49 There are numerous disadvantages to instrumentation, such as hardware failure, malpositioning, and stress shielding. Imaging artifact from instrumentation should be considered in oncologic cases. The latter factor can inhibit fusion and promote pseudarthrosis, which is why newer dynamically designed plates allow some settling and sharing of the load with the spacers, leading to enhanced fusion as predicted by Wolff’s law, which implies that bone will remodel itself and become stronger when subjected to an external compressive load.
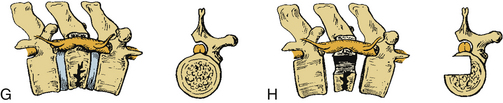
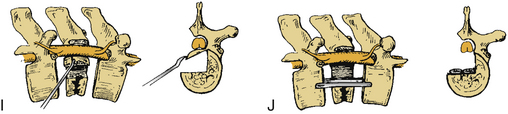
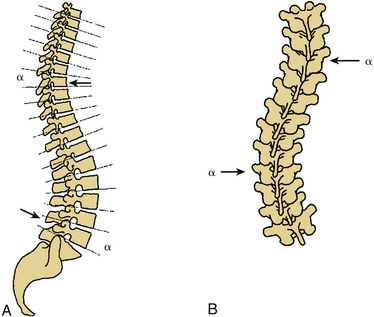
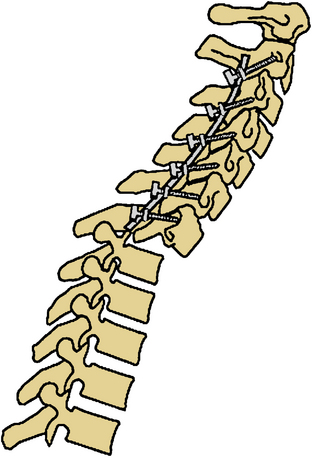
Certain principles must be respected when considering instrumented spinal fusion at the cervicothoracic junction. In general, focal and gradual curves as well as the apical and neutral vertebrae in two orthogonal planes, sagittal and coronal, must be identified. The neutral vertebra usually is the one least angulated and typically is located between the curves. The apical vertebra has the highest degree of segmental angulation at its rostral and caudal ends.41 A long construct should not terminate at or near an apical vertebra4 in either vertical plane, because loads experienced at that point would be highest due to the long moment arm of the axial load in relation to the construct. The apical vertebra is the one associated with the greatest angle (α) between adjacent vertebrae of all vertebrae, in the curve (Fig. 112-5). The second principle that must be respected is that a construct should not end at a junctional level—in this case, at the cervicothoracic junction (Fig. 112-6). It is prone to angular and translational deformation due to the difference in rigidity of the ventrally stabilized thoracic spine by the rib cage and the flexible cervical spine. Other considerations include loss of motion segments, complications related to placement of extra hardware, and cost. The quality of bone and its density also play a role and should be a consideration.50
Historically, dorsal hooks-rods-plate cervical compact Cotrel-Dubousset instrumentation has been described in the context of treatment of cervicothoracic junction pathologies. However, certain limitations with the instrumentation have impeded its continued use.51 As a hook and rod system, the instrumentation required has to be longer than the levels involved with pathology because of the “claw” configuration, which requires fixation two levels above and two levels below the pathology. It obviously also must be above the levels of the laminectomy, and the hooks have to avoid levels with spinal canal stenosis. CCD hook intrusion into the spinal canal has been shown in a cadaveric study to be, on average, 27% of the diameter of the spinal canal.52
In the subaxial cervical spine, lateral mass screw fixation has been used often. C7 is a transitional vertebra that has characteristics common to both the cervical and the thoracic spine, with the lateral masses transitioning to the size and orientation of the thoracic transverse processes, thus offering a poorer fixation point. Panjabi et al.53 have measured the transverse pedicle thickness and found that it increases from 5.1 mm at C3 to 6.6 mm at C7, which would make it amenable to transpedicular fixation. An et al.54 measured the mediolateral and superoinferior outer pedicle diameter as 6.9 mm and 7.5 mm, respectively. The average inner diameter was 5.18 mm. The length of the pedicle was found to be 9.1 mm, with a medial angulation of 34 degrees. The recommended pedicle entry point for the C7 pedicle was 1 mm inferior to the mid-portion of the facet joint with 25 to 30 degrees of medial angulation and perpendicular to the dorsal arch.
Pedicle screw fixation, while very popular in the thoracic and lumbar spine with superior fixation characteristics, has only recently become more widely used in the cervical spine. Anatomic studies have shown the pedicle width of C3 to average 4.9 mm in men and 4.5 mm in women, with the minimum reported width of 3 mm. The C4 pedicle averages 4.7 mm in men and 4.6 mm in women, with a minimum reported width of 3.1 mm.55 Because of this variability, measurements should be made on a case-by-case basis using CT imaging.
Several studies have specifically examined the use of distal subaxial cervical pedicle screw fixation and compared it with lateral mass fixation. The lateral mass anatomy and its particular relationship to nearby neurovascular structures have been precisely established by anatomic studies. Xu et al.56 found that for C3-7, the average distance from the superficial, dorsal center of the lateral mass to the nerve root superiorly was 5.7 mm ± 1.5 mm. Inferiorly, the average distance was found to be 5.5 mm ± 0.8 mm. The average distance to the spinal sac from that same point was found to be 9.2 mm ± 1.4 mm. The average medial angle of the nerve root was 76.3 degrees. Closer to the cervicothoracic junction, at the C6 level, the transverse foramen was found to be located directly ventral to the dorsal midpoint of the lateral mass. These findings suggested that this point would be safe for initiation of screw insertion in the lateral masses.56
Pedicle screw fixation in the subaxial spine has been found to be safer with direct palpation of the medial pedicle wall. Albert et al.57 retrospectively looked at a group of 21 patients who had pedicle screws placed at C7 with the help of a small laminoforaminotomy at C7 and by palpating the pedicle with a right-angle nerve hook. There were no neurologic complications related to screw placement and no failure of fixation related to the use of the pedicles at 1-year follow-up.57
In the thoracic spine, the transverse processes do not provide adequate fixation. The upper thoracic spine pedicles at T1 and T2 are larger than those found in the midthoracic spine.37 On average, the width of the pedicles decreases caudally from 7.8 mm to 4.4 mm. The pedicle axis projects about 7 to 8 mm medial from the lateral edge of the superior facet and about 3 to 4 mm superior to the midaxis of the transverse process.6 An et al.54 recommended an entry point for the T1 and T2 thoracic pedicles at the midpoint 1 mm below the facet joint, whereas Mazel et al.6 preferred 10 to 20 degrees of caudal angulation and 5 to 10 degrees of medial angulation. Mazel’s entry point for screw insertion at the upper thoracic level is located at the intersection of the transverse process axis and the midline of the inferior facet. The more caudal the fixation, the less caudal and medial is the angulation of the trajectories of the pedicle screws. Some have recommended upper thoracic pedicle screw fixation with the help of laminotomy and by directly palpating the external boundaries of the pedicle to reduce pedicle wall violation.58 However, most authors avoid using this technique due to the added time and risks; instead, they use instruments at the level of the thoracic spinal canal.6,59 Kim et al.59 performed a retrospective analysis of 394 patients who underwent dorsal instrumentation with a total of 3204 transpedicular screws placed using a freehand technique by two surgeons over a 10-year period. Their review showed a 6.2% violation of the pedicle cortex, with 1.7% violating the medial wall.59 In this study, there were no neurologic, vascular, or any other soft tissue complications. The low rate of misplacement of transpedicular screws by freehand technique in the thoracic spine, however, should not give an inexperienced surgeon a false sense of security. It is still recommended to lower that risk by closely examining preoperative imaging studies and looking for any anatomic abnormalities such as unusually narrow pedicles.
Abumi et al.60 experienced a 7% misplacement rate out of 669 cervical pedicle screws with conventional screw insertion techniques. Due to the high risks associated with transpedicular fixation at the cervicothoracic junction, including iatrogenic damage to the spinal cord, nerve roots, or vertebral artery,55,60 computer-assisted systems were developed to minimize those risks. Richter et al.61 dorsally instrumented 19 patients with computer assistance, placing 31 cervical pedicle screws and 10 high-thoracic pedicle screws. Postoperative CT scanning with multiplanar reconstruction for each screw did not demonstrate any misplacement greater than 2 mm or any screw-related injury to vascular, neurogenic, or bony structures. They strongly recommend computer-assisted instrumentation, especially at the C3-6 level. Certain disadvantages must be considered when using a computer-assisted instrumentation system at the cervicothoracic junction, however. Computerized systems are prone to errors and system crashes due to hardware, software, or human failure, and the surgeon must be well versed in the more traditional fixation methods. Difficulties can be encountered with registration of instrumented vertebrae where surface matching can be thrown off by small or indistinguishable bony landmarks, because most computer systems have been developed for thoracic and lumbar systems. According to Richter, “the use of a computerized assisted system for dorsal cervical spine surgery will make a good spine surgeon even better, but it can never make a bad spine surgeon a good one.”61
Other methods of dorsal fixation at the cervicothoracic junction that have been closely examined, in addition to screws, include traditional wires and hooks. With tumors of the dorsal elements at the junction it may be difficult to use spinous process wiring or laminar hooks, which can lead to spinal canal stenosis or iatrogenic spinal cord injury.62 Rhee et al.62 found no significant increase in normalized stiffness in any of the lateral mass constructs with wire augmentation that were examined. The C7 pedicle screw fixation constructs demonstrated significantly higher normalized stiffness than any of the other constructs, which included lateral mass screw fixation at C7, C6 and C7 lateral mass screws, and these constructs augmented with triple wiring. There was no predictable difference in stiffness between any of the constructs while in extension. Flexion, right and left lateral bending, and axial torsion showed superior results with the C7 pedicle construct when compared with the lateral mass screws. The addition of another lateral mass screw level at C6 did show increased stiffness comparable to the C7 pedicle screws.62 In another study, Ulrich et al.63 showed that sublaminar wiring is inadequate, even with supplemental ventral plate fixation, in stabilizing torsional and translational loads. In an animal study, C7-T1 pedicle screw fixation was as effective as a ventral plate and dorsal wiring in stabilizing a three-column injury.64 Dorsal wiring is adequate only in offering stability in flexion testing,65 functioning as a supplemental tension band construct dorsally.
Outcomes and Complications Following Surgical Treatment
Ventral and dorsal approaches to the cervicothoracic junction carry specific postoperative complications, which have been discussed with the individual approaches. Recurrent laryngeal nerve injuries may be seen with low ventral cervical or cervicothoracic junction approaches, leading to unilateral vocal cord palsy with possible mild dysphagia and dysphonia. If persistent, treatment may be with medialization thyroplasty. When both recurrent largyngeal nerves are injured, vocal cord paralysis can occur, requiring permanent gastrostomy and tracheostomy.17 Higher approaches to the ventral cervical spine carry the risks of superior laryngeal and hypoglossal nerve injury because of their approximation to the superior thyroid artery.66
Ventral cervicothoracic junction surgery and its complications also have been documented by Boockvar et al.67 in a group of 14 patients, who were followed for a period of 21 months. Five patients (36%) had graft/plate failure, which needed revision, and one patient had a recurrent laryngeal nerve palsy. Surgical complications were associated with male sex, multiple levels of involvement, the use of allograft compared with autograft, and previous surgery. The authors concluded that supplemental fixation is needed when dealing with cervicothoracic junction pathology.
In An et al.’s3 series of 15 patients with tumors, 12 presented with neurologic involvement, with 8 patients having incomplete cord syndromes and 4 having nerve root symptoms.3 Most of the patients improved neurologically by at least 1 or 2 Frankel grades. One patient with giant cell tumor had a recurrence that required a repeat ventral procedure. Three patients died because of tumor progression: breast metastasis at T1-2, myeloma at T1, and lung tumor at T2-3.
Bilksy et al.17 reported a series of 41 patients with subaxial cervical tumors who underwent spinal reconstruction: 12 patients from a ventral approach, 13 patients from a dorsal approach, and 16 patients from a combined approach. Thirty-three of the 41 tumors were metastatic, and 8 were primary (2 chordomas and 6 sarcomas). Twenty-five percent of the patients had surgery-related complications, including bilateral vocal cord paralysis after a right-sided trapdoor approach at the C6-7 level with the left side presumed present preoperatively, a unilateral recurrent laryngeal nerve palsy after thyroplasty, and one CSF leak. In this study, three patients experienced complications with the cervicothoracic junction instrumentation: two patients experienced pull-out of the dorsal plate screw instrumentation (with no ventral fixation performed), and the third experienced failure of the ventral graft following corpectomy of C6 and fusion with plate. No failures were noted in the patients who underwent cervicothoracic instrumentation with screws and rods. No wound issues arose following the preoperative irradiation, even though dissection sometimes was more problematic. Similar instrumentation failures were described by Lenoir et al.,68 who looked at 30 patients who underwent surgery for destabilizing injuries at the cervicothoracic junction. One patient had mobilization of the cervical portion of a plate and another had a thoracic screw loosen. There was no direct fixation of the anterior column in these two cases.
Mazel et al.6 retrospectively reviewed 32 patients treated for cervicothoracic junction tumors who underwent dorsal fixation with cervical lateral mass fixation and thoracic transpedicular screw fixation. Nineteen of the patients with Pancoast tumors had en bloc resections and, therefore, total and partial vertebrectomies with destabilization of the ventral two columns. Thirteen of the patients had only dorsal palliative laminectomy and cervicothoracic fixation. Two of the 32 cases failed, with both of those patients having partial or total vertebrectomies, due to the limitation of fusion length. Mazel et al. concluded that in cases of total or partial vertebrectomies, dorsal instrumentation must extend three levels above and three levels below the site of pathology. Their group had positive results with the use of autologous fibula strut graft for multilevel corpectomies and femoral head bank bone for single-level vertebrectomy.
Steinmetz et al.45 looked at outcomes following cervicothoracic region surgical fixation by reviewing 593 patients treated over a 5-year period at the Department of Neurosurgery at the Cleveland Clinic Foundation who were followed for a mean of 20 months. Treatment failed in 14 patients, with the failures statistically associated with laminectomy and multilevel ventral corpectomies, not supplemented by dorsal fixation, as well as histories of prior cervical surgery, surgery for correction of deformity (P = .033), and tobacco use (P = .019). Three patients underwent decompression and lateral mass fixation ending at, but not crossing, the cervicothoracic junction. Two patients underwent decompression and ventral plate fixation to, but not crossing, the junction. More specifically, three out of eight patients (37.5% of patients, P = .001) who underwent laminectomy that crossed the junction without supplemental fixation and/or fusion failed. Three of 18 patients (16.7%) who underwent two- or three-level corpectomy with fixation/fusion to T1 only also failed. Finally, three of the patients experienced treatment failure after dorsal instrumentation across the cervicothoracic junction. One of those patients, who had osteoporosis, had pedicle screw fixation ending at T1. Another patient, with Ehlers-Danlos syndrome, had undergone occiput-to-T4 instrumented fixation for correction of severe swan-neck deformity, and the tapered rods fractured. Finally, a patient who underwent dorsal fusion from C4-T2 for breast metastasis also experienced failure. Interestingly, no failures occurred in association with a combined ventral-dorsal fixation ending either at C7 or below. However, with the recommendation being made to consider extension of fixation to T1 or T2, a trend toward failure was noted in cases in which the dorsal fixation was terminated at C7. Similar findings were noted by Boockvar et al.,67 who recognized that prior cervical surgery was associated with the failure of ventral surgery at the cervicothoracic junction.
Summary
Cervicothoracic junction tumors present a unique set of challenges from a biomechanical and anatomic standpoint that need to be carefully considered prior to choosing a surgical approach. These recent techniques and considerations have significantly improved surgical outcomes in this set of patients.
An H.S., Vaccaro A., Cotler J.M., et al. Spinal disorders at the cervicothoracic junction. Spine (Phila Pa 1976). 1994;19:2557-2564.
Benzel E.C. Biomechanics of spine stabilization, ed 2. New York: Thieme; 2001.
Chapman J.R., Anderson P.A., Pepin C., et al. Posterior instrumentation of unstable cervicothoracic spine. J Neurosurg. 1996;84:552-558.
Tatsumi R.L., Yoo J.U., Liu Q., et al. Mechanical comparison of posterior instrumentation constructs for spinal fixation across the cervicothoracic junction. Spine (Phila Pa 1976). 2007;32:1072-1076.
White A.A., Panjabi M.M. Clinical biomechanics of the spine, ed 2. Philadelphia: Lippincott Williams & Wilkins, p; 1990. 45
1. Kim D.H., Beck C.E., Dietze D.D.Jr., et al. Surgical approaches to the cervicothoracic junction. In: Schmidek H.H., editor. Schmidek & Sweet operative neurosurgical techniques: indications, methods, and results. ed 4. Philadelphia: WB Saunders; 2000:2107-2121.
2. Joshi A.P., Pedlow F.X., Hornicek F.J., et al. Surgical management of cervical spine metastatic disease. Curr Op Orthop. 2002;13:224-231.
3. An H.S., Vaccaro A., Cotler J.M., et al. Spinal disorders at the cervicothoracic junction. Spine (Phila Pa 1976). 1994;19:2557-2564.
4. Benzel E.C. Biomechanics of spine stabilization, ed 2. New York: Thieme; 2001.
5. Le H., Balabhadra R., Park J., Kim D. Surgical treatment of tumors involving the cervicothoracic junction. Neurosurg Focus. 2003;15(5):1-7.
6. Mazel C., Hoffman E., Antonietti P., et al. Posterior cervicothoracic instrumentation in spine tumors. Spine (Phila Pa 1976). 2004;29:1246-1253.
7. An H.S., Vaccaro A., Cotler J.M., et al. Spinal disorders at the cervicothoracic junction. Spine (Phila Pa 1976). 1994;19:2557-2564.
8. Bilsky M., Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1307-1317.
9. Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;366:643-648.
10. Hall A., Mackay N. The results of laminectomy for compression of the cord or cauda equina by extradural malignant tumour. J Bone Joint Surg [Br]. 1973;55:497-505.
11. Livingston K.E., Perrin R.G. The neurosurgical management of spinal metastases causing cord and cauda equina compression. J Neurosurg. 1978;49:839-843.
12. Gilbert R.W., Kim J.H., Posner J.B. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1973;3:40-51.
13. Rock J.P., Ryu S., Shukairy M.S., et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891-898.
14. Bilsky MH, Yamada Y, Yenice KM, et al: Intensity-modulated stereotactic radiotherapy of paraspinal tumors: a preliminary report. Neurosurgery 54: 823–830.
15. Klekamp J., Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien). 1998;140:957-967.
16. Rusch V.W., Giroux D.J., Kraut M.J., et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol. 2007;25:313-318.
17. Bilsky M.H., Boakye M., Collignon F., et al. Operative management of metastatic and malignant primary subaxial cervical tumors. J Neurosurg Spine. 2005;2:256-264.
18. Prabhu V.C., Bilsky M.H., Jambhekar K., et al. Results of preoperative embolization for metastatic spinal neoplasms. J Neurosurg. 2003;98(Suppl 2):156-164.
19. Berkefeld J., Scale D., Kirchner J., et al. Hypervascular spinal tumors: influence of the embolization technique on perioperative hemorrhage. AJNR Am J Neuroradiol. 1999;20:757-763.
20. Breslau J., Eskridge J.M. Preoperative embolization of spinal tumors. J Vasc Interv Radiol. 1995;6:871-875.
21. Vetter S.C., Strecker E.P., Ackermann L.W., et al. Preoperative embolization of cervical spine tumors. Cardiovasc Intervent Radiol. 1997;20:343-347.
22. Menard V. Causes de la paraplegie dans le mal de Pott. Rev Orthop. 1894:47-64.
23. Capener N. The evolution of lateral rhacotomy. J Bone Joint Surg [Br]. 1954;36:173-179.
24. Nanson E.M. The anterior approach to upper dorsal sympathectomy. Surg Gynecol Obstet. 1957;104:118-120.
25. Cauchoix J., Binet J.P. Anterior surgical approaches to the spine. Ann R Coll Surg Engl. 1957;21:237-243.
26. Hodgson A.R., Stock F.E., Fang H.S.Y., et al. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172-178.
27. Sundaresan N., Shah J., Foley K.M., et al. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg. 1984;61:686-690.
28. Birch R., Bonney G., Marshall R.W. A surgical approach to the cervico-thoracic spine. J Bone Joint Surg [Br]. 1990;72:904-907.
29. Kurz L.T., Pursel S.E., Herkowitz H.H. Modified anterior approach to the cervico-thoracic junction. Spine (Phila Pa 1976). 1991;16(Suppl 10)::S542-S547.
30. Larson S.J., Holst R.A., Hemmy D.C., et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. Surg Neurol. 1976;26:253-256.
32. Wolfla C.E., Resnick D.K. Neurosurgical operative atlas: spine and peripheral nerves, ed 2. New York: Thieme; 2007.
33. Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224. (Abstract.)
34. Fessler R.G., Dietze D.D.Jr., Millan M.M., et al. Lateral parascapular extrapleural approach to the upper thoracic spine. J Neurosurg. 1991;75:349-355.
35. Zdeblick T.A., Zou D., Warden K.E., et al. Cervical stability after foraminotomy: a biomechanical in vitro analysis. J Bone Joint Surg [Am]. 1992;74:22-27.
36. Albert T., Klein G.R., Jaffe D.B.S., et al. Use of cervicothoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine (Phila Pa 1976). 1998;23(14):1596-1599.
37. Chapman J.R., Anderson P.A., Pepin C., et al. Posterior instrumentation of unstable cervicothoracic spine. J Neurosurg. 1996;84:552-558.
38. Kreshak J.L., Kim D.H., Lindsey D.P., et al. Posterior stabilization at the cervicothoracic junction: a biomechanical study. Spine (Phila Pa 1976). 2002;15(24):2763-2770.
39. Prybis B.G., Tortolani P.J., Hu N., et al. A comparative biomechanical analysis of spinal instability and instrumentation of the cervicothoracic junction: an in vitro human cadaveric model. J Spinal Disord Tech. 2007;20(3):233-238.
40. Tatsumi R.L., Yoo J.U., Liu Q., et al. Mechanical comparison of posterior instrumentation constructs for spinal fixation across the cervicothoracic junction. Spine (Phila Pa 1976). 2007;32:1072-1076.
41. Lapsiwala S., Benzel E. Surgical management of cervical myelopathy dealing with the cervical-thoracic junction. Spine J. 2006;6:268S-273S.
42. White A.A., Panjabi M.M. Clinical biomechanics of the spine, ed 2. Philadelphia: Lippincott Williams & Wilkins; 1990. 45
43. Pal G.P., Sherk H.H. The vertical stability of the cervical spine. Spine (Phila Pa 1976). 1988;13:447-449.
44. Inoue A., Ikata T., Katoh S. Spinal deformity following surgery for spinal cord tumors and tumorous lesions: analysis based on an assessment of the spinal functional curve. Spinal Cord. 1996;34:536-542.
45. Steinmetz M.P., Miller J., Warbel A., et al. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4:278-284.
46. Tachdjian M.O., Matson D.D. Orthopaedic aspects of intraspinal tumors in infants and children. J Bone Joint Surg [Am]. 1965;47:223-248.
47. De Jonge T., Slullitel H., Dubousset J., et al. Late-onset spinal deformities in children treated by laminectomy and radiation therapy for malignant tumors. Eur Spine J. 2005;14:765-771.
48. Wang J.C., McDonough P.W., Kanim L.E., et al. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2001;26:643-646.
49. Wang J.C., McDonough P.W., Kanim Endow K., et al. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467-471.
50. Yamagata M., Kitahara H., Minami S., et al. Mechanical stability of the pedicle screw fixation systems for the lumbar spine. Spine (Phila Pa 1976). 1992;17(Suppl 3):S51-S54.
51. Korovessis P., Katonis P., Aligizakis A., et al. Posterior compact Cotrel-Dubousset instrumentation for occipitocervical, cervical, and cervicothoracic fusion. Eur Spine J. 2001;10:385-394.
52. Fagerstroem T., Hedlund R., Bancel P., et al. Cotrel Dubousset instrumentation in the cervical spine. An experimental study on the relation of hooks to the spinal cord. New Orleans: The 65th Annual Meeting of the AAOS; March 12-22, 1998.
53. Panjabi M.M., Duranceau J., Goel V., et al. Cervical human vertebrae: quantitative three-dimensional anatomy of the middle and lower regions. Spine (Phila Pa 1976). 1991;16:861-869.
54. An H.S., Gordin R., Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine (Phila Pa 1976). 1991;16(Suppl):548-551.
55. Ebraheim N.A., Xu R., Knight T., et al. Morphometric evaluation of lower cervical pedicle and its projection. Spine (Phila Pa 1976). 1997;22:1-6.
56. Xu R., Ebraheim N., Nadaud M., et al. The location of the cervical nerve roots on the posterior aspect of the cervical spine. Spine (Phila Pa 1976). 1995;20:2267-2271.
57. Albert T., Klein G.R., Jaffe D.B.S., et al. Use of cervicothoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine (Phila Pa 1976). 1998;23(14):1596-1599.
58. Xu R., Ebraheim N., Ou Y., et al. Anatomic considerations of pedicle screw placement in thoracic spine. Spine (Phila Pa 1976). 1998;23:1065-1068.
59. Kim Y.J., Lenke L.G., Bridwell K.H., et al. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine (Phila Pa 1976). 2004;29(3):333-342.
60. Abumi K., Shono Y., Ito M., et al. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine (Phila Pa 1976). 2000;25:962-969.
61. Richter M., Mattes T., Cakir B. Computer-assisted posterior instrumentation of the cervical and cervico-thoracic spine. Eur Spine J. 2004;13:50-59.
62. Rhee J.M., Kraiwattanapong C., Hutton W.C. A comparison of pedicle and lateral mass screw construct stiffnesses at the cervicothoracic junction: a biomechanical study. Spine (Phila Pa 1976). 2005;30(21):E636-E640.
63. Ulrich C., Woersdoerfer O., Kalff R., et al. Biomechanics of fixation systems to the cervical spine. Spine (Phila Pa 1976). 1991;16;Suppl 3:S4-S9.
65. Pelker R.R., Duranceau J.S., Panjabi M.M. Cervical spine stabilization. A three-dimensional, biomechanical evaluation of rotational stability, strength, and failure mechanisms. Spine (Phila Pa 1976). 1991;16:117-122.
66. Monfared A., Kim D., Jaikumar S., et al. Microsurgical anatomy of the superior and recurrent laryngeal nerves. Neurosurgery. 2001;49:925-933.
67. Boockvar J.A., Philips M.F., Telfeian A.E., et al. Results and risk factors for anterior cervicothoracic junction surgery. J Neurosurg. 2001;94(Suppl 1):12-17.
68. Lenoir T., Hoffmann E., Thevenin-Lemoine C. Neurological and functional outcome after unstable cervicothoracic junction injury treated by posterior reduction and synthesis. Spine J. 2006;6:507-513.