25 Cervical StenosisMyelopathy
KEY POINTS
Introduction
Cervical spondylotic stenosis is the number one cause of myelopathy in patients over the age of 55.1 For this reason, the spine surgeon focusing on the care of the aging population must be adept in understanding the clinical presentation of cervical myelopathy and correlating these changes to the radiographic studies available. After this careful review, the appropriate management can be discussed with the patient as it relates to his or her overall medical health.
Degenerative changes that begin in the cervical disk lead to a cascade of changes in the bony and soft tissue anatomy of the cervical spine. The disk height is diminished, and intrinsic changes in the disk prevent the natural distribution of axial and rotational forces.2 These unnatural increased forces at the vertebral endplates, uncovertebral joints, and facet complexes lead to the formation of peripheral osteophytes that can diminish the diameter of the canal. Through repeated cycles of flexion and extension, the spinal cord can be stressed against these osteophytes. Posterior to the spinal cord, degenerative changes can lead to reactive hypertrophy of the ligamentum flavum as well as facet arthropathy.2 Extension of the cervical spine can lead to buckling of the posterior ligaments and further compression on the spinal cord.3 The situation is further exacerbated in individuals with a congenitally narrowed spinal canal.3 The average cervical spinal canal diameter on plain x-ray is 17 mm.4 The diameter of the spinal cord itself, on average, is measured at 9 mm, while the CSF space and ligaments that surround the spinal cord are averaged at 4 mm. Patients are often thought to become symptomatic from cervical stenosis with diameters less than 13 mm.5
The pathophysiology of cervical myelopathy is still under investigation. Pathologic analysis of the injured cervical spinal cord reveals a flattening and indentation of the cord. These changes are most directly seen in the dorsal and lateral columns.1 There is evidence to suggest that ischemic changes are the direct cause of the injury seen in the cervical spinal cord.3 In severe cases of myelopathy, necrosis and cavitations are seen in the central spinal gray matter as well. Demyelination changes are also evident in the lateral columns at the level of compression, with further demyelination changes in the caudal corticospinal tract.1 Furthermore, the cervical spinal cord between C5 and C7 is a watershed region and is acutely sensitive to any changes in vascular flow. Compressive forces that affect the delicate radicular arteries, anterior spinal artery, and dorsal spinal artery at these levels can lead to the central necrosis of the gray matter that is seen with myelopathy.1
The symptoms of cervical spondylotic myelopathy can be varied and subtle. A thorough history and a sensitive physical examination are as essential as the radiographic data. Initial signs of spinal cord injury often manifest in the lower extremities.4 These symptoms include fatigue and subjective weakness in the legs as well as a worsening sense of imbalance and gait difficulties. Symptoms often progress to the upper extremities and manifest as difficulty manipulating objects with the fingers and loss of manual dexterity. Bowel and bladder dysfunction is a variable symptom, and its prognostic indication remains unclear. Pathologic reflexes and other symptoms of upper motor neuron injury are common, including increased tone in the extremities with associated hyperreflexia, clonus, and positive Hoffman and Babinski signs. In severe cases, abnormal sensory changes can also be appreciated.
Cervical spondylosis is considered the most common progressive degenerative disease of the cervical spine. It is seen in 10% of patients by the age of 25 years and 95% of patients by the age of 65.1 Cervical spondylosis remains the most common cause of myelopathy in the aging individual, but there are numerous other causes that must be considered. Other causes of myelopathy include: ossification of the posterior longitudinal ligament, ossification of the ligamentum flavum, trauma, intrinsic/extrinsic tumors, compressive abscesses, or inflammatory disease processes including rheumatoid arthritis.3
Anterior and posterior decompressive procedures have been shown to be effective treatments in halting the progressive course of cervical myelopathy.2 A prospective multicenter nonrandomized comparison of operative and nonoperative treatment for cervical myelopathy with a mean follow up of 11 months revealed that patients treated with operative management had significant improvement in functional status and neurologic symptoms.6 Even with this information, patient selection for operative treatment of myelopathy remains key, as the natural history of the disease remains unpredictable.
Anterior approaches are often successful in treating focal anterior pathology such as a discrete cervical disk herniation. Although the anterior approach is safe and effective, there exist many potential risks that must be carefully understood. These risks include possible injury to the vascular structures (carotid and vertebral artery, jugular vein), neurologic structures (recurrent laryngeal nerve), and soft tissue structures (esophagus, trachea, lymphatic duct).4 Furthermore, the fusion that is performed after an anterior cervical discectomy/corpectomy will limit cervical mobility and can lead to accelerated degenerative changes at the end of the fusion construct.
Posterior cervical decompression for cervical stenosis is a safe and effective treatment option, but can also have increased risks in the elderly population. The procedure requires a significant degree of muscle division and reflection off the cervical spine. These muscles can have the propensity to become denervated or devascularized when retracted for the long procedure. This can lead to postoperative pain and spasm that can be persistently disabling in 18% to 60% of patients.7 The complete removal of the laminae, spinous process, interspinous ligaments, and a portion of the facets can have long-term negative effects on cervical sagittal balance.2 Iatrogenic destabilization of the cervical spine and postoperative kyphosis can be limited by lateral mass fusion of the cervical segments, but this procedure leads to extended operative time, increased blood loss, and restrictive motion.2 Furthermore, as discussed earlier, fusion can lead to adjacent segment degenerative changes.
By utilizing techniques developed for the posterior decompression of the lumbar spine, minimal access surgery can be performed in the cervical spine. The cervical microendoscopic decompression of stenosis (CMEDS) is a procedure that allows for bilateral posterior decompression of one to three cervical levels through a single 1.8 cm incision.8 By utilizing a unilateral approach, the contralateral musculature and ligaments are preserved, leading to less perioperative pain and increased stability of the posterior “tension band.” The limited studies in the use of this procedure have shown the potential for decreased blood loss, decreased length of stay, decreased use of pain medications, and preservation of normal cervical lordosis.7
Clinical Presentation and Evaluation
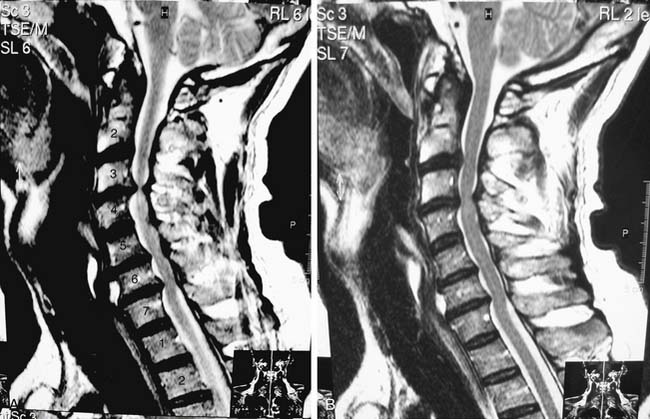
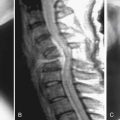
The patient is a 60-year-old man who has complained of worsening neck pain for the past 2 years. He states that the pain is worsened with any flexion or extension of his neck. He also states that at times with these movements he will experience a “shocklike” pain down his spine. He notes a new numbness in his feet and has had frequent falls in the last two years. He is hyperreflexic in his lower extremities bilaterally and has six beats of clonus on testing of his feet. His MRIs in Figures 25-1A and B eg reveal a mild loss of cervical lordosis with spondylotic changes throughout his cervical spine. There is loss of disk height, disk bulging, and anterior compression of the spinal cord, which is worse at the C3-4 disk space. There is severe ligamentum flavum hypertrophy and resulting cervical stenosis at this level as well. Preoperative flexion and extension x-rays were obtained, which revealed no abnormal subluxation. As this patient had clear symptoms of cervical myelopathy, surgical treatment was recommended.
Description of the Devices
Operative Techniques
After the airway and lines are secured, the patient is rotated 180 degrees away from the anesthesiologist. The patient is placed in a Mayfield fixation device and is positioned in the seated position. The head is slightly flexed to straighten the cervical spine, with the ultimate goal of having the spine directly perpendicular to the floor. We have found this positioning to be very effective in decreasing epidural bleeding and minimizing pooling of blood in the operative field. In patients with large shoulders or thin necks, the ability to visualize the entire cervical spine with fluoroscopy is improved in this position as well. All pressure points are padded, and the arms are draped over the patient’s lap. The neck is checked to prevent any hyperflexion, which can lead to obstruction of the airway or compromised venous drainage. In general, there should be at least two fingerbreadths distance from the chin to the chest. The C-arm is then positioned to achieve a true lateral image, with the arc swung either under the table or directly in front of the patient. The base of the C-arm is often placed ipsilateral to the side of the incision. The operative levels are identified and the skin incision is marked. The endoscope and monitor are checked to be in working condition. The monitor is placed above the patient or next to the patient’s head contralateral to the incision site. The monitor is placed directly in the line of sight for the surgeon so that he or she can comfortably manipulate the instruments while observing the monitor.
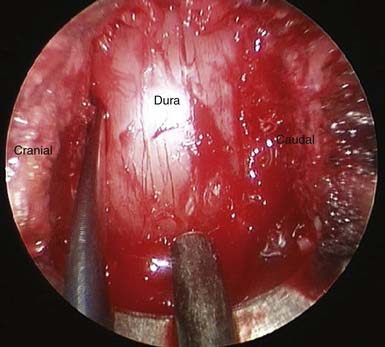
After the bony decompression is completed, attention is then turned to carefully dissecting a plane between the hypertrophied ligamentum flavum and the dura. Angled curettes are used to carefully dissect away the ligament from the dura, and 2-mm Kerrison punches are used to dissect off the ligament. The decompression continues until a pulsatile decompressed dura is visualized (Figure 25-2 ). Any further bony compression from facet hypertrophy or osteophytes off the adjacent lamina are carefully drilled or resected with the Kerrison rongeurs. A fine probe is used to assess the contralateral foramen to reassure that there is no compression of the exiting nerve root. The tube is then returned to its original position. Once again, through the use of angled curettes and Kerrison rongeurs, the hypertrophied ligament is carefully dissected away from the ipsilateral field. Any further bony compression from osteophytes or facets that are visible after removal of the ligamentum flavum is drilled away. The ipsilateral foramen is also tested to assure that no foraminal stenosis remains. The cleared dura should be pulsatile and fully decompressed. The field is then irrigated with antibiotic saline and hemostasis is achieved. The tubular retractor is detached from the retractor arm and is slowly removed. The removal is carefully observed on the monitor to confirm no arterial bleeding in the musculature that must be addressed while removing the retractor system. If a second rostral or caudal level is to be decompressed, the smallest tubular dilator is reinserted through the same cervicodorsal fascial incision and similarly docked to the level of interest, and the decompression is repeated.
Complications and Avoidance
ADVANTAGES / DISADVANTAGES
Conclusion/Discussion
Cervical microendoscopic decompression (CMEDS) was developed by the modification of minimal-access techniques that were pioneered in the lumbar spine. Through the use of tubular retractors, the cervical musculature and ligaments are spared the dissection that is required in open posterior cervical approaches. The long-term effects of this muscle-sparing technique may provide multiple benefits over open decompressions while providing excellent clinical outcomes. These benefits include: decreased blood loss, decreased length of stay, decreased postoperative pain and muscle spasm, decreased disruption of the posterior tension band and risk of sagittal deformity, and decreased risk for infection.7,9
1. Shedid D.B.E., Benzel E.C. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery. 2007;60(S1):7-13.
2. Edwards C.C., Riew K.D., Anderson P.A., Hilbrand A.S., Vaccaro A.F. Cervical myelopathy: current diagnostic and treatment strategies. Spine J.. 2003;3(1):68-81.
3. Baptiste D.C., Fehlings M.G. Pathophysiology of cervical myelopathy. Spine J.. 2006;6:190S-197S.
4. Geck M., Eismont F.J. Surgical options for the treatment of cervical spondylotic myelopathy. Orthop. Clin. N. Am.. 2002;33:329-348.
5. Wolf B.S., Khilnani M., Malis L. The sagittal diameter of the bony cervical spinal canal and its significance in cervical spondylosis. J. Mt. Sinai Hosp. NY.. 1956;23(3):283-292.
6. Sampath P., Bendebba M., Davis J.D., Ducker T.B. Outcome of patients treated for cervical myelopathy: a prospective, multicenter study with independent clinical review. Spine. 2000;25:670-676.
7. O’Toole J.E., Eichholz K.M., Fessler R.G. Posterior cervical foraminotomy and laminectomy. In: In A practical guide to the anatomy and techniques for minimally-invasive spine surgery. Springer; 2007.
8. Perez-Cruet M.J., Samartzis D., Fessler R.G. Microendoscopic cervical laminectomy and laminoplasty. In: In An anatomic approach to minimally invasive spine surgery. St. Louis: Quality Medical Publishing, Inc; 2006.
9. Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(5 Suppl.):S37-45.