Cervical Squamous Cell Carcinoma
Epidemiology and Staging
Epidemiology
Cancer of the cervix is the second most common cancer in women worldwide after cancer of the breast. Each year, approximately 529,828 new cases are diagnosed worldwide.1 In the United States in 2012, cervical cancer was a distant third most common neoplasm of the female genital tract (12,170 cases, or about 14% of all genital cancers), after endometrium (47,130 cases) and ovary (22,280 cases).2 Breast cancer, by comparison, is 22 times more common (229,060 cases in the United States in 2012). Cervical cancer caused about 4220 deaths in 2012 in the United States (2.0 deaths/100,000 women). It is responsible for 1.6% of all deaths from neoplasia and 14% of all deaths from genital tract cancer. This compares with 39,510 deaths annually from breast cancer, 15,500 from ovarian cancer, and 8010 from cancer of the uterine corpus.2
The lifetime risk of a woman developing cervical cancer is 3% worldwide and 1.1% in the United States. The incidence rates for cervical cancer show a wide geographic variation, which is partially explained by differences in healthcare systems, intensity of screening programs, and exposure to major risk factors. A nearly eightfold difference exists between the lowest age-adjusted incidence rate (4.5/100,000 in western Asia) and the highest rate (34.5/100,000 women in eastern Africa).1 The age-adjusted incidence rate in the United States is 10.3/100,000 for African/American women, 11.4/100,000 for Hispanic women, but only 6.7/100,000 for non-Hispanic White women.3 During the past 50 years, both incidence and mortality rates for cervical cancer have declined precipitously in most developed countries. In the United States, the incidence has declined nearly 75% (from 34/100,000 in 1947) while the mortality rate has declined by more than 60%. The single most important factor in this decline is the success of screening with cervical cytology. Studies from both the Nordic countries and British Columbia demonstrate a significant reduction in cervical cancer incidence after only two cervical cytology tests.4 Repeated studies have shown the single most common factor associated with the development of cervical cancer is the history of not having undergone recent cytologic screening.5,6 The marked variations in cervical cancer rates in different regions of the globe relate to environmental, socioeconomic, and cultural factors, but, most importantly, to access to screening programs.
Staging
The most widely adopted staging system for cervical cancers worldwide is that of the International Federation of Gynecologists and Obstetricians (FIGO) (Table 11.1), which was last updated in 2009.7 This is a four-stage system that is in large part clinically determined, as opposed to pathologically determined. Stage I tumors are tumors that are confined to the uterus and are subdivided into two subcategories: those that are not macroscopically visible and invade 5 mm or less into the stroma and tumors that are macroscopically visible and/or invade more than 5 mm into the stroma. Stage II tumors invade beyond the uterus, but not to the pelvic sidewall or the lower third of the vagina. Stage III tumors extend to the pelvic sidewall and/or involve the lower third of the vagina and/or cause hydronephrosis or a nonfunctioning kidney. Stage IV tumors extend beyond the true pelvis or involve the mucosa of the bladder or rectum.
Table 11.1 2009 Modification of FIGO Staging of Carcinoma of the Cervix7
| Stage I | Cervical carcinoma confined to uterus (extension to the corpus should be disregarded) |
| IA | Invasive carcinoma that can be diagnosed only by microscopy with deepest invasion ≤5 mm and largest diameter ≤7.0 mm |
| IA1 | Measured stromal invasion ≤3.0 mm in depth and diameter of ≤7.0 mm |
| IA2 | Measured stromal invasion >3.0 mm but not greater than 5 mm with a diameter of ≤7.0 mm |
| IB | Clinically visible lesion confined to the cervix uteri or preclinical cancers greater than stage IA.* |
| IB1 | Clinically visible lesion ≤4 cm in greatest dimension |
| IB2 | Clinically visible lesion >4 cm in greatest dimension |
| Stage II | Cervical carcinoma invades beyond the uterus but not to the pelvic wall or to lower third of the vagina |
| IIA | Without parametrial invasion |
| IIA1 | Clinically visible lesion ≤4 cm in greatest dimension |
| IIA2 | Clinically visible lesion >4 cm in greatest dimension |
| IIB | With obvious parametrial invasion |
| Stage III | Tumor extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or nonfunctioning kidney |
| IIIA | Tumor involves lower third of the vagina; no extension to pelvic wall |
| IIIB | Extension to pelvic wall and/or causes hydronephrosis or nonfunctioning kidney |
| Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to stage IV |
| IVA | Spread of the growth to adjacent organs |
| IVB | Spread to distant organs |
*All macroscopically visible lesions, even with superficial invasion, are allocated to stage IB. Invasion is limited to a measured stromal invasion with a maximal depth of 5 mm and horizontal extension of not >7 mm. Depth of invasion should not be >5 mm taken from the base of the epithelium of the original tissue, superficial or glandular. The depth of invasion should always be reported in millimeters, even in those cases with ‘early (minimal) stromal invasion’ (<1 mm). The involvement of vascular/lymphatic spaces should not change the stage allotment.
Types of Cervical Malignancies
The World Health Organization (WHO) currently recognizes three general histologic subtypes of cervical cancer: squamous cell carcinoma, adenocarcinoma, and ‘other epithelial carcinomas.’8 While squamous cell carcinoma accounted for upward of 90% of primary neoplasms several decades ago, the overall frequency has now dropped to about 60–70%.9,10 The various histologic variants of adenocarcinoma constitute much of the remainder of cervical cancers. The remaining primary malignancies of the cervix include sarcomas, lymphomas, and melanomas. Endometrial tumors frequently spread to the cervix, but it is unusual to find other tumors metastasizing to the cervix.
Superficially Invasive Squamous Cell Carcinoma
Superficially invasive squamous cell carcinoma (SISCCA), or ‘microinvasive carcinoma,’ a concept introduced over 50 years ago, refers to cancer of the cervix that demonstrates a minimal degree of stromal invasion, and as such has a prognosis much better than that of more invasive cervical carcinomas. In 2012, lower anogenital squamous terminology (LAST) for human papillomavirus (HPV)-associated lesions (LAST consensus recommendations by the College of American Pathologists and American Society of Clinical Pathologists; CAP/ASCP) unified microinvasive lesions under the moniker ‘superficially invasive squamous cell carcinoma,’ further designated by site-specific definitions. Thus, SISCCA of the cervix is defined as invasion ≤3 mm from the basement membrane point of origin, and horizontal spread ≤7mm.92 The critical point with regards to SISCCA is that these tumors, although locally invasive, present a negligible risk for having extended beyond the cervical conization specimen being examined, or of having metastasized to regional lymph nodes. As a result, patients with cervical SISCCA can be treated in a less radical manner without jeopardizing their chance for curative resection than women with more deeply invasive carcinomas. This is important because SISCCA accounts for about 20% of all cervical cancers and the majority of cases occur in women aged 35–46 years.11,12 SISCCA is now most often diagnosed in women presenting with abnormal smears interpreted as high-grade squamous intraepithelial lesion (HSIL).
Depth of Invasion
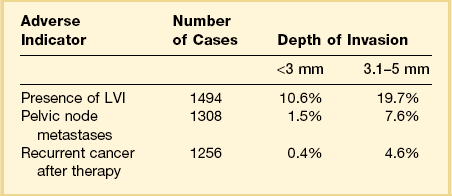
Among the many literature reports on SISCCA, whether presenting the findings of an individual series of patients or meta-analyses of all published articles, there is a general agreement that depth of invasion is a key prognostic indicator. Tumors that invade <1 mm into the underlying stroma are virtually never associated with lymph node metastases, although exemptions have been occasionally reported.13 Moreover, if we take 50–100 sections from a conization specimen performed for HSIL we are much more likely to find small epithelial buds extending <1 mm into the stroma than if we take only 10–15 sections. However, excellent clinical outcomes are achieved in both settings.7 Thus there is uniform agreement that tumors with very superficial stromal invasion (<1 mm) can be safely treated with conservative measures. There is more controversy with respect to the management of tumors with 1–5 mm of invasion. Although most studies have shown that the risk of lymph node metastases and the development of recurrent disease after conservative treatment are relatively low for carcinomas that invade ≤5 mm the risk is clearly not zero. For example, one study examining lesions invading <3 mm found that the recurrence rate was 6% during the 10 year follow-up period.14 Clinicopathologic analyses of carcinomas that invade ≤5 mm have shown a distinct difference between tumors invading ≤3 mm and those with 3–5 mm of invasion (Table 11.2).15–25 Tumors that invade to a depth of 3–5 mm are associated with lymph node metastases in about 8% of cases whereas those that invade ≤3 mm have only a 1–2% risk of lymph node metastases. The threshold of 3 mm invasion has now been formally adopted by the LAST group to define the maximum depth of invasion in SISCCA of the cervix.
Table 11.2 Impact of Depth of Invasion on Nodal Status, LVI, and Recurrence15–25 Modified from sources.
Tumor Volume and Lateral Extent of Spread
Although, as discussed above, it is generally accepted that depth of the invasion correlates with prognosis, other studies have documented that the tumor volume might be an even better prognostic measure. In order to accurately determine tumor volume, an excisional specimen needs to be serially step sectioned. When this is done and the distance between the sections is known, tumor volume can be calculated. This approach was first advocated by Burghardt and Holtzer,26 who reported no pelvic lymph node metastases in patients with <420 mm3 of cancer unless lymphovascular space involvement was identified. However, performing serial step sections to assess tumor volume is not practical for most laboratories. Thus FIGO has recommended that the greatest lateral extent of the tumor be measured as a surrogate for tumor volume. The usefulness of lateral tumor extension as a prognosticator was shown in an analysis of 402 women with squamous cell carcinomas by Takeshima et al.27 In this study, the incidence of nodal metastases was only 1.2% among patients with ≤3 mm of invasion but increased to almost 7% among women with 3–5 mm of invasion. However, in both groups, almost all recurrences occurred in women with >7 mm of horizontal spread. Based on these and other data showing the importance of lateral tumor extension, the FIGO staging system now requires stage IA tumors to be ≤7 mm in greatest diameter. This was reaffirmed by the LAST group, which defines SISCCA of the cervix as having no more than 7 mm in maximal extent.28
Lymphovascular Invasion
The role of lymphovascular invasion (LVI) as a prognostic indicator is more controversial than that of the depth of invasion and lateral extent of tumor. Although it would seem reasonable to assume that a tumor with LVI is more likely to have lymph node metastases than one without, the data are conflicting. In part, this stems from the relatively poor reproducibility of a pathologic diagnosis of LVI, especially when not extensive. It is well documented that the stroma in which foci of invasion lie can retract during preparation of the tissue sections for microscopic examination. A clear space can easily be mistaken for LVI. Another problem with the reporting of LVI is the fact that the finding is contingent on the number of sections that are examined. In one study serial step sections were examined from 30 cervices diagnosed with cervical carcinoma having only 2–5 mm of invasion. Thirty percent of the women had ‘capillary-like space’ involvement based on the first cut of tissue from the blocks, but this increased to 57% when the serial step sections were examined. All of the women in this study had been treated with radical hysterectomy with lymph node dissection. No lymph node metastases were found, regardless of the presence or absence of LVI. This study concluded that the presence of tumor in lymphatic spaces was of no value by itself in predicting which patients are likely to have lymph node metastases.29 However, other studies have reported that the presence of LVI is an important prognostic indicator.30,31 For example, a recent literature review of carcinomas with <3 mm of invasion reported recurrent cancer in 3.1% (3 of 96) of conservatively managed patients with LVI versus only 0.6% (3 of 486) of those without LVI.32 Overall, LVI has been reported in 0–10% of carcinomas invading <1 mm and 3–30% of those invading 1–3 mm.
Morphologic Features
Stromal Invasion
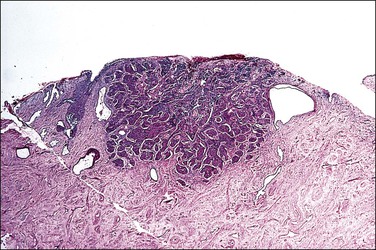
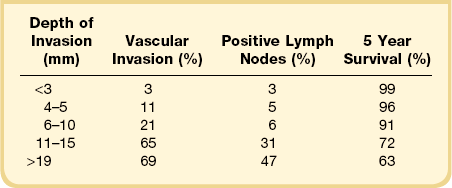
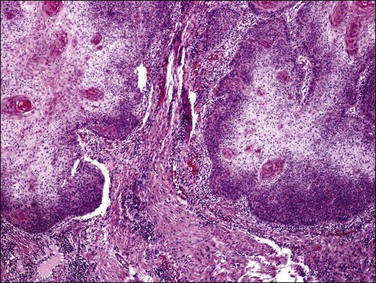
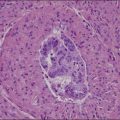
A diagnosis of SISCCA is made based on finding buds or tongues of malignant cells penetrating the basement membrane and extending into the stroma. The earliest stage at which invasion can be recognized is when a well-defined, tiny bud of invasive cells emanates from the base of HSIL on the surface of the cervix or in endocervical glands (Figure 11.1). The bud projects into the stroma, clearly having disrupted the adjacent smooth basement membrane. In one large series a single bud accounted for one-third of all the microinvasive cancers.33 However, in other cases, multiple small buds and sometimes individual tumor cells extend into the stroma.
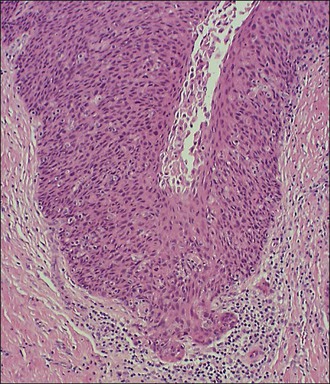
Figure 11.1 Microinvasive carcinoma. A tiny focus of stromal invasion that is barely perceptible arises from an extensive HSIL that fills an endocervical gland.
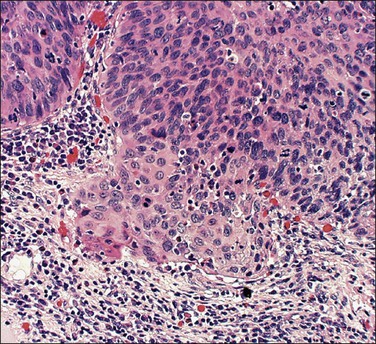
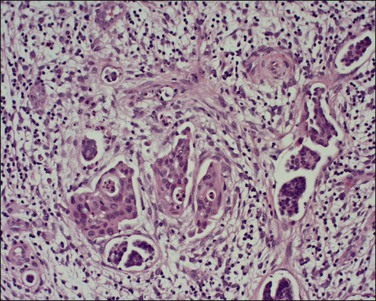
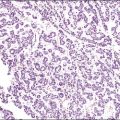
A number of different histologic patterns of SISCCA have been described. In very early foci of invasion the tumor cells are often separated by <1 mm from the nearest involved surface or glandular basement membrane. Cases with <1 mm tumor invasion were previously referred to as ‘early stromal invasion,’ which was the original definition of the FIGO substage IA1 in the 1985 FIGO classification. Another common pattern of growth is when microinvasive cancers appear as tiny nests of cells extending into the stroma that are totally separated from the overlying surface (Figures 11.2 and 11.3). This pattern of invasion has been referred to as a ‘spray bud’ pattern. The spray bud pattern of growth is usually <1–2 mm deep. As the foci of invasion become larger, the growths become broader and longer and eventually, as the tumor becomes more advanced, a ‘confluent’ pattern develops (Figure 11.4). In these larger lesions the invasive foci often form intertwining cords, much like thick tangled roots of a tree. It is important to stress that reproducible definitions of different patterns of invasion such as spray bud and confluent have proven difficult to develop and, not surprisingly, most studies have shown that the pattern of invasion does not influence clinical outcome independently of depth of invasion.30
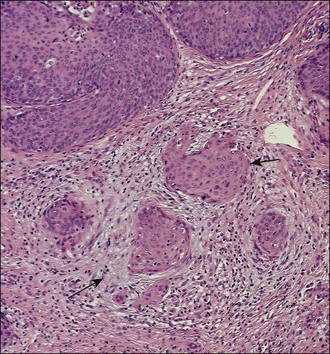
Figure 11.2 Microinvasive carcinoma. A focus of stromal invasion (arrows) that is separated by <1 mm from the overlying HSIL. In this instance multiple small tumor nests are totally separated from the surface epithelium.
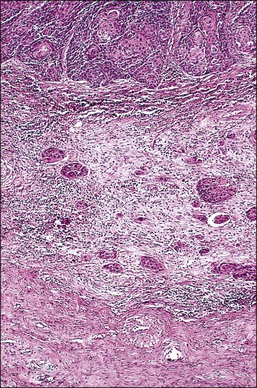
Figure 11.3 Microinvasive carcinoma, spray bud pattern. At high magnification, numerous clusters composed of small numbers of tumor cells are present in a desmoplastic stroma with a mild inflammatory infiltrate. Stromal retraction secondary to processing artifact creates the false impression of lymphovascular space invasion.
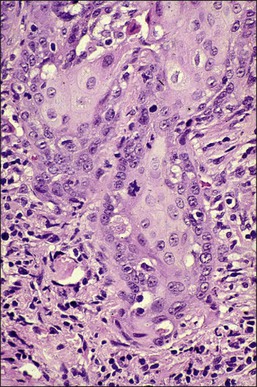
It is frequently difficult to determine whether a given focus represents SISCCA or instead is HSIL that extends into endocervical glands. There are four qualitative histopathologic features that can help with the diagnosis of SISCCA, in addition to measured limits of extent (Table 11.3).28 The first is presence of an epithelial margin that can be described as irregular, ragged, or even scalloped in appearance (Figure 11.5). This irregular margin develops because the adjacent smooth basement membrane has been disrupted. The second histopathologic feature is increased cellular maturation compared to the adjacent HSIL. The cells in the invasive foci often have abundant eosinophilic cytoplasm and are paradoxically keratinized (Figure 11.6). The third feature is that the nuclei of the invasive cells frequently show clearing of the chromatin and develop prominent nucleoli (Figure 11.7). The fourth feature is a stromal reaction to the SISCCA. Invasive foci are often surrounded by a prominent lymphoplasmacytic infiltrate and the stroma frequently has a desmoplastic response (Figure 11.3).
Table 11.3 Histopathologic Features of SISCCA
Qualitative Features:
Irregular, ragged, or even scalloped epithelial margin
Increased cellular maturation compared with adjacent HSIL
Nuclei of invasive cells show clearing of chromatin and prominent nucleoli
Stromal reaction to tumor with desmoplasia and prominent lymphocytic infiltrate
Quantitative Features28
Depth of invasion ≤3 mm from basement membrane of point of origin
Horizontal spread ≤7 mm maximal extent
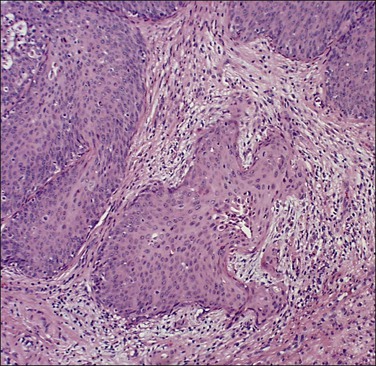
Figure 11.5 Microinvasive carcinoma. The invasive foci have an irregular or ‘scalloped’ margin that clearly distinguishes it from the sharp margin of the adjacent HSIL.
Measuring Depth of Invasion and Lateral Extent of Spread
Of all the variables evaluated during the assessment of SISCCA, depth of invasion is the one that is universally considered the easiest to measure and the most objective. The interobserver variability in assessing depth of invasion is also considered to be the lowest among the various features considered to have prognostic value. In order to accurately determine the maximum depth of invasion, loop electrosurgical excision procedure (LEEP) and conization specimens should be sectioned along the long axis of the cervical canal in a clockwise fashion while trying to avoid tangential sections, which invariably result in inaccurate measurement of the depth of invasion. The current FIGO/LAST staging method recommends that measurement of depth of invasion be made from the base of the epithelium where invasion occurs to the deepest point of carcinoma in a vertical line. In most SISCCAs, invasion either occurs from the base of the surface squamous epithelium or originates from both the surface epithelium and endocervical glands simultaneously. In such cases, measurement should be made from the base of the surface epithelium involved by HSIL. In some cases invasion is limited to the periphery of a few endocervical glands without surface involvement. In these cases, measurement is made from the base of the glands to the deepest point. The deepest measurement should be reported when multiple foci of SISCCA are present.
Assessing Presence of LVI
Even though the 2009 FIGO staging system does not take into account the presence or absence of LVI, most gynecologic oncologists want to know this information so that treatment can be individualized. Therefore the pathologist should always provide this information in the pathology report. Lymphatic space involvement was defined back in the 1960s as endothelial-lined (capillary-like) spaces containing tumor cells that are contiguous with the stroma. Until the recent introduction of the antibody D2-40 that specifically identifies lymphatic vessels, it was impractical to tell the difference between lymphatic and blood vessels under microscopy. Therefore the term lymphovascular space involvement, or invasion, came into widespread use when discussing cervical small vessel invasion by tumor, be it capillary or lymphatic. Identification of LVI, especially when only several foci are present, can be quite challenging. There is general agreement that LVI should be diagnosed whenever tumor is seen within endothelial-lined spaces at the leading edge of the tumor or beyond (Figure 11.8). Although it is preferable to identify tumor cells attached to the endothelium, this is not always possible. It is well documented that the stroma in which foci of invasion lie can retract during preparation of the tissue sections for microscopic examination. A clear space can easily be mistaken for LVI. Various immunohistochemical markers such as CD34, a marker for endothelium, and D2-40, a marker of lymphatic epithelium, can assist in identifying LVI in some instances. However, it should be remembered that there are a variety of markers available and the results obtained with them can be discordant. Moreover, it is unclear whether LVI, as determined by immunohistochemical markers, correlates with clinical outcomes.34 Therefore, the standard for assessing the presence or absence of LVI in the cervix remains H&E staining alone.
Assessing Surgical Margins
Although the 2009 FIGO staging system does not take into account the status of the surgical margins of a conization or LEEP specimen, assessment of the surgical margins of excision is one of the most important contributions of the pathologist to management of women with SISCCA. This is because women with either HSIL or SISCCA on the surgical margin are much more likely to have a residual invasive cervical cancer than are women with clear margins. Inking of LEEP specimens is optional since a thermal artifact is universally present in the resection margin. However, a cold-knife cone requires application of ink at the margins, preferably one color at the endocervical and another on peripheral or deep margins.35
Diagnosis
SISCCA is a diagnosis based almost wholly on microscopic examination and it usually is an incidental finding in either a cold-knife conization or LEEP specimen obtained for the treatment of HSIL. Most experts believe that a diagnosis of SISCCA should only be made on a LEEP or conization specimen for which the margins of excision are free of both HSIL and SISCCA. This is because the depth of invasion seen on a cervical biopsy specimen or a LEEP/conization specimen with a positive margin may not represent the maximal depth of invasion. SISCCA is a condition that is easily misdiagnosed. Almost 50% of cases diagnosed as SISCCA during routine clinical care are either cases of carcinoma in situ that have been overcalled or cases of invasive carcinoma that have been missed. For example, in a series of 265 cases of SISCCA submitted to a reference pathology panel as part of a Gynecologic Oncology Group (GOG) study, 132 (approximately 50%) were reclassified as something other than SISCCA.15 Similarly, in a UK study of 286 cases of SISCCA that underwent pathologic review, 41% were incorrectly diagnosed.36
The utility of immunohistochemistry in the diagnosis of SISCCA has been evaluated for a double-staining method combining collagen IV or laminin antibodies to identify the basement membrane and pan cytokeratin antibodies to identify invasive squamous cells.37 Although in some equivocal cases this approach appears to be useful, since it identified clear-cut invasion in 4 of 10 cases originally reported as ‘suspicious for invasion,’ it does not always clarify whether or not invasion is actually present.
Cytologic Findings
The cytology of microinvasive carcinoma is controversial. Most cytologists believe that the distinction between HSIL and the earliest stages of invasion cannot be made on cell appearances alone, and for this reason SISCCA is not included as a specific entity in the Bethesda System.38
Clinical Management
The treatment for stage IA1 lesions is typically a LEEP, cold-knife conization, or a simple hysterectomy. With these approaches the cure rate approaches 100%.39,40 The presence or absence of LVI as well as the exact depth of invasion will impact the treatment approach for stage IA1 tumors. Tumors with <1 mm of invasion almost never have pelvic lymph node metastasis whereas tumors with 1–3 mm invasion or with LVI have a low, but measurable, rate of nodal involvement. If fertility is not a consideration and a woman has adverse prognostic features such as LVI or involved margins, simple hysterectomy is the standard form of definitive treatment. The management of stage IA2 tumors is more controversial. In the United States, women with stage IA2 tumors and LVI are usually not considered candidates for conservative management and they are typically treated with a modified radical hysterectomy and pelvic lymphadenectomy. There is a growing trend, however, to tailor the management for patients desirous of maintaining fertility who present with stage IA2 tumors without LVI and with negative conization margins.41
Squamous Cell Carcinoma
Squamous Cell Carcinoma is a malignant neoplasm composed of squamous cells. While generally derived from stratified squamous epithelium, it may occur in sites, such as the endocervix, where columnar epithelium is normally present. WHO recognizes nine histologic variants of squamous cell carcinoma (Table 11.4).35
Gross Features
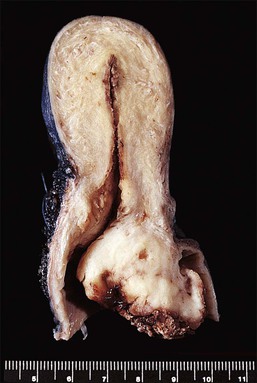
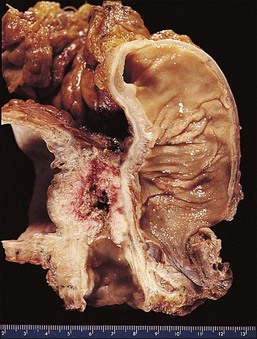
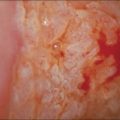
To the unaided eye, early stage invasive squamous cell carcinoma is not easy to diagnose. The tumor may present as a rough, raised, red granular area that bleeds on manipulation. However, it is usually difficult to macroscopically distinguish an early invasive carcinoma from ectropion, which is where endocervical-type epithelium lines the ectocervix. The gross features of a more advanced tumor depend upon its site of origin, the pattern of growth, and the rate of necrosis. Most squamous cell carcinomas, by the time they become clinically apparent, involve the external os and are visible on speculum examination (Figure 11.9). However, there are some patients who show no grossly observable tumor, but clearly have tumor diffusely present in the wall of the cervix (Figures 11.10 and 11.11). A few squamous cell carcinomas remain entirely within the canal so that they are not visible until they expand the endocervix to produce a ‘barrel-shaped’ cervix (defined as a diameter greater than 4 cm). Thus, the growth pattern of a squamous cell carcinoma may be either predominantly exophytic, in which case it grows out from the surface, often as a polypoid excrescence (Figure 11.12), or mainly endophytic so that it infiltrates into the surrounding structures, without much surface growth. Infiltrative lesions that extensively permeate the stroma often result in hard lesions with minimal surface change. If necrosis is marked, ulceration occurs. Ulcerative examples usually involve the ectocervix and sometimes the upper vaginal vault.
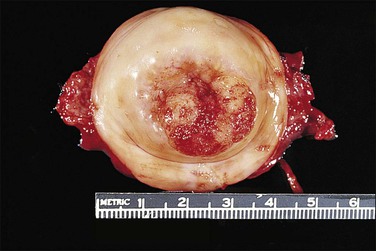
Figure 11.9 Squamous cell carcinoma. Tumor protrudes through the external os and involves the exocervix.
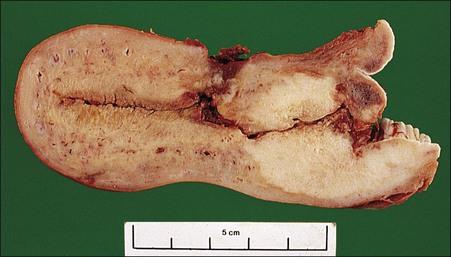
Figure 11.10 Squamous cell carcinoma. The uterus, cut in cross section, discloses an extensive tumor infiltrating throughout the wall of the endocervix (white).
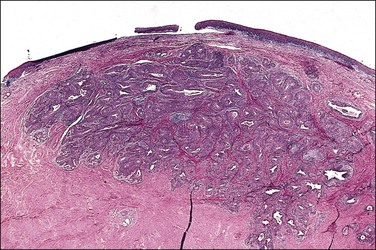
Figure 11.11 Macroscopically invisible ‘occult’ squamous cell carcinoma. The tumor diffusely involves the wall. The overlying epithelium is relatively normal. The tumor in this instance was not obvious to the clinician, who noted at the time of examination only that the cervix might be slightly more firm than normal.
Microscopic Features Used in Classification
Although no histologic classification system currently in use for invasive squamous cell carcinoma provides reliable prognostic correlations, a number of classification systems are in use that emphasize the type and degree of differentiation of the predominant cell. WHO terminology divides pure squamous cell lesions into the following:35
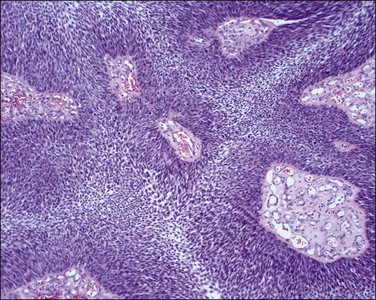
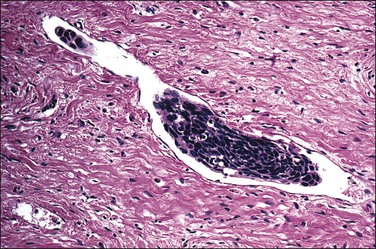
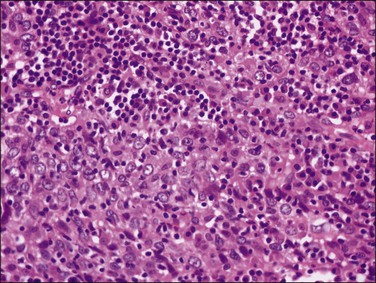
1. Keratinizing carcinomas, which account for one-sixth of cases, by definition require the presence of keratin pearl formation (Figure 11.13). Keratin pearls are circular whorls of squamous epithelium with central nests of acellular keratin. Usually, the tumor cells appear mature and are organized in nests or cords. Individual squamous cells are large and usually show abundant eosinophilic cytoplasm. The cells are tightly adherent and may show prominent intercellular bridges. The nuclei may be enlarged or pyknotic (Figure 11.14). Mitotic activity is relatively sparse compared with the other tumor types. While individual cell keratinization (dyskeratosis) may occur, squamous pearls, or broad expanses of keratinization, are required to establish the diagnosis of the keratinizing subtype.
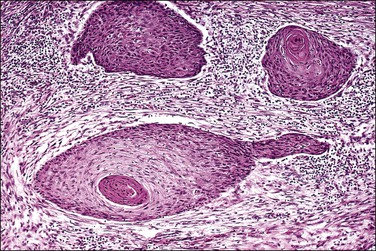
Figure 11.13 Squamous cell carcinoma, keratinizing type. Keratin pearls are present. The irregular angulations that protrude from the tumor nests into the stroma are indicative of invasion.
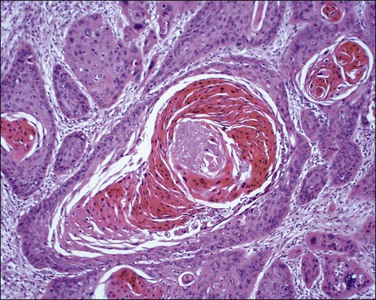
Figure 11.14 Squamous cell carcinoma, keratinizing type. Higher magnification showing keratin pearls. This tumor has many bizarre multinucleated cells.
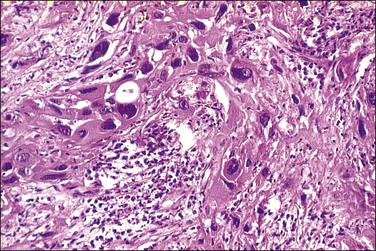
2. Nonkeratinizing carcinomas, which account for two-thirds of cases, contain cells that are generally recognizable as squamous from their polygonal shape. There may be individual cell keratinization, but keratin pearls are not seen. Cellular and nuclear pleomorphism is typically more prominent than in the well-differentiated keratinizing tumors, and mitotic figures may be quite numerous (Figure 11.15). Cell borders may be distinct, sometimes with intercellular bridges. An occasional tumor may focally show differentiation as if it were mimicking the normal squamous lining of the exocervix. The more poorly differentiated cells involve deeper stromal areas of tumor while the more superficial portions show cytoplasmic differentiation with accumulation of extensive intracytoplasmic glycogen (not to be confused with clear cell adenocarcinoma, where the tumor is uniform throughout) (Figure 11.16).
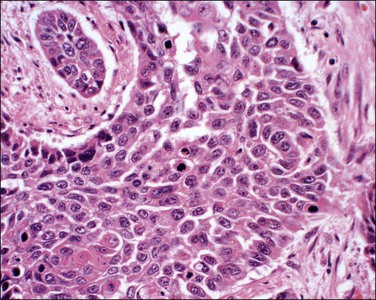
Figure 11.15 Squamous cell carcinoma, nonkeratinizing type. The nuclei are highly pleomorphic and many mitoses are present, suggesting the tumor is poorly differentiated. Although classified as a nonkeratinizing type, individual cells are keratinized.
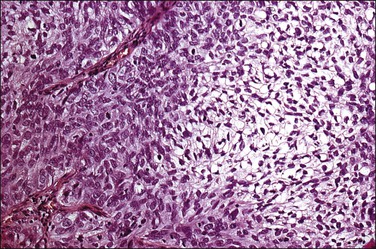
Figure 11.16 Glycogen-rich squamous cell carcinoma, nonkeratinizing type. Much of the tumor shows cellular differentiation with glycogen accumulation, reminiscent of the maturation that occurs in the normal squamous epithelium of the cervix.
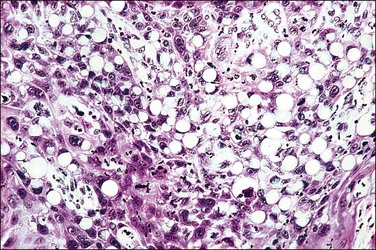
3. Basaloid squamous cell carcinomas account for approximately one-sixth of cases. They consist of small, oval-shaped basaloid cells with scant cytoplasm (resembling the cells commonly seen in HSILs) that grow in masses and nests (Figure 11.17).42 The nuclei are usually fairly uniform, hyperchromatic, small, and display abundant mitotic activity. Necrosis is frequently observed. While foci of squamous differentiation and keratinization may sometimes be present, keratin pearls are rarely present. These tumors resemble the vaginal and vulvar tumors designated as basaloid carcinoma, and, by definition, lack the characteristic argyrophilic, immunohistochemical, and ultrastructural features of endocrine carcinomas, also often called ‘small cell carcinoma.’43 Except for their size and growth pattern, there is little to characterize these latter tumors as squamous cell. This variant of squamous cell carcinoma is under-recognized and is an aggressive tumor. Basaloid squamous cell carcinomas together with adenoid cystic carcinoma form one end of the spectrum of basaloid tumors of the cervix. The other end of the spectrum consists of adenoid basal carcinomas, which are low-grade carcinomas. Therefore to prevent confusion, it is recommended that the basaloid squamous cell carcinomas always be referred to by their complete name and the term ‘basaloid carcinoma’ be avoided.43
Microscopic Grading
The most widely utilized grading system for squamous cell carcinoma is a modification of the Broder system, which was originally introduced in 1920.35 This grading system takes into account the degree of keratinization, cellular atypia, and mitotic activity.
1. Well-differentiated (Grade 1) tumors have individual cell keratinization (dyskeratosis) that is characterized by intense cytoplasmic eosinophilia. Mitotic figures are often present, but are primarily at the edge of tumor nests. Well-differentiated tumors typically have keratin pearls, which are deposits of acellular keratin found within tumor nests.
2. Moderately differentiated (Grade 2) tumor cells are more pleomorphic than in Grade 1 tumors with less cytoplasm and larger irregular nuclei. The cell borders are often indistinct. Although keratin pearls are uncommon, individual tumor cells, especially those in the center of tumor nests, are often keratinized. Mitotic activity is greater than in Grade 1 tumors.
3. Poorly differentiated (Grade 3) tumor cells are primitive appearing with hyperchromatic oval nuclei and scant indistinct cytoplasm that resemble the cells of HSILs. Mitoses are common and there often is extensive necrosis. Evidence of keratinization is difficult to identify. Occasionally, Grade 3 tumors consist of large pleomorphic cells with bizarre nuclei and abnormal mitotic figures. Grade 3 tumors can also present as spindle-shaped cells resembling a sarcoma.
In general, the grade of squamous cell carcinoma has little impact on overall patient survival. A GOG study carefully evaluated the clinical impact of tumor grade determined by a number of different grading systems, including the modified Broder’s system described above in a group of surgically treated stage IB cervical cancers.44 Although there was good reproducibility of tumor grade between different pathologists, none of the grading systems had a significant impact on prognosis. Moreover, when analyzed individually, neither extent of keratinization, nuclear grade, pattern of infiltration nor mitotic activity influenced prognosis.44
Additional Microscopic Features
Tumors classified as squamous cell carcinoma of the cervix often show extensive variation in patterns of growth, cell types, and degrees of cellular differentiation. Most carcinomas infiltrate as networks of anastomosing bands with intervening stroma that on section appear as irregular islands, some rounded and some angular and spiked. Often, particularly in the early tumors, a HSIL may be found on the surface and at the edge of the invasive tumor. Occasionally it may be difficult to distinguish between invasive nests of tumor in the stroma and HSIL in the gland crypts. Useful clues of invasive cancer are an irregular outer perimeter, the presence of a desmoplastic stromal response, or sharp angulations of the tumor suggesting invasive growth through stromal planes (Figure 11.18).
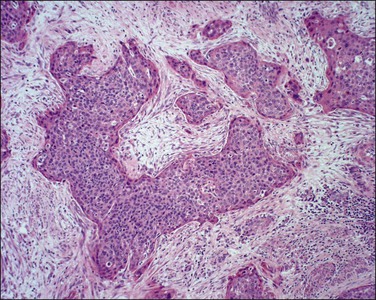
Figure 11.18 Squamous cell carcinoma, invasive. The angulated tumor buds are diagnostic of invasion.
Cytology
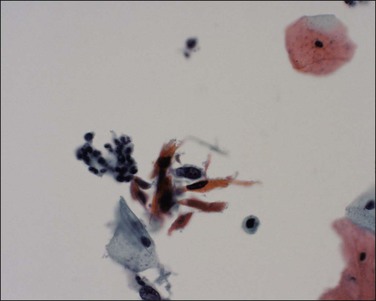
The cytologic features associated with invasive squamous cell carcinoma are well described, although invasive carcinoma is under-recognized cytologically.38 One reason for this is the preconceived impression that many have bizarre keratinizing cells. However, the actual cytologic pattern associated with most cases is that of poorly preserved cells in a background of cell necrosis and inflammatory changes (Figure 11.19). Often the background is as important as the cytology of the cells. Small, highly keratinized cells or cells showing a wide variation in size and shape are important to recognize. Some cells may show hyperchromasia, but others appear pale and insignificant (Figure 11.20). Careful evaluation of all these features is essential.
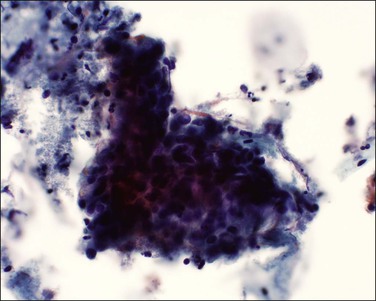
Figure 11.19 Squamous cell carcinoma, invasive. The tumor cells form a sheet of cells and are difficult to visualize. There is necrotic and inflammatory debris present (tumor diathesis) together with pink-staining keratin.
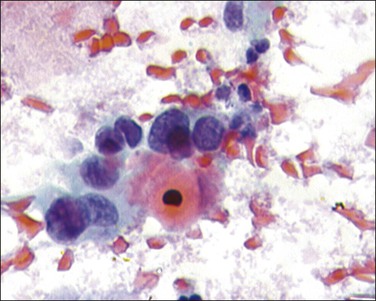
Figure 11.20 Squamous cell carcinoma, nonkeratinizing type. The tumor cells have large, pale pleomorphic nuclei.
Keratinizing carcinoma may show bizarre, elongated, and ‘tadpole’ forms, often associated with excessive keratinization (Figure 11.21). Well-differentiated carcinoma cells may be confused with regenerative tissue cells or squamous metaplasia. When few cells are present, screening becomes more challenging and subtle cytologic detail more important.
Clinicopathologic Correlation
Cervical cancer has several modes of presentation, each of which reflects the extent of tumor spread. Depending upon the level of routine medical care received, the majority of patients present initially with an abnormal cervical cytology. The age in a woman’s life when cervical cancer presents has become lower in recent decades. Currently, the mean age of women with stage I tumors is 47 years, whereas it is 57 years for women with stage III and IV tumors.45 30% of stage I tumors occur in women under 40 years of age; 4.6% occur in women 30 years of age or younger.
About 60% of patients with early stage disease present with intermittent painless vaginal bleeding, which is usually postmenopausal, but is sometimes postcoital. With more advanced disease, bleeding may become continuous and accompanied by a malodorous discharge. As the endopelvic fascia envelops the cervix in an anterior–posterior fashion and therefore serves as a natural barrier, cervical cancer preferentially grows within the parametria and involves the ureters before it infiltrates the bladder or rectum.46 Ureteral obstruction and death from renal failure mark the natural course of untreated cervical cancer. Pain, present in <10% of cases, frequently refers to the flank or leg, indicating that tumor may have invaded the pelvic wall or lumbosacral nerve roots. Edematous lower extremities signify involved lymphatics. During the late stages in the evolution of the cancer, dysuria, hematuria, rectal bleeding, or constipation may herald bladder or rectal involvement.
Clinical Behavior
Squamous cell carcinomas spread primarily by local extension and lymphatic invasion. Local extension includes adjacent vaginal mucosa, parametrial soft tissue and pelvic wall, corpus uteri, bladder, and rectum. Spread into the peritoneum is uncommon, and, when grossly absent, the peritoneal cytologic wash is almost always negative (98.3%).47 Lymphatic spread primarily involves parametrial, paracervical, obturator, hypogastric, external iliac, and sacral lymph nodes and, secondarily, common iliac, inguinal, and para-aortic nodes. A close correlation exists among stage, frequency, and location of lymph node metastasis. Thus, tumor is found in pelvic lymph nodes in 15%, 30%, and 40% of patients with stage I, II, and III/IV disease, respectively. About one-third of patients with para-aortic node involvement have metastases to the scalene nodes. Most recurrences are local and occur within 3 years of the initial diagnosis (Figure 11.22).
Prognostic Features
Numerous prognostic factors have been studied in patients with cervical carcinoma. Stage, which relates to the anatomic extent of the disease and is largely a function of tumor size, is generally considered to be the single most important determinant of outcome (Table 11.5).45 The 5 year survival of stage I patients ranges from 97.5% for those with stage IA1, tumors to 75.7% for those with stage IB2.45 For women with stage III disease, overall 5 year survival is about 40%.
Table 11.5 FIGO Stage and 5 Year Survival45
| FIGO Stage | 5 Year Survival (%) |
| IA1 | 97.9 |
| IA2 | 94.6 |
| IB | 87.5 |
| IIA | 74.8 |
| IIB | 67.4 |
| III | 43.7 |
| IV | 16.3 |
Histopathologic Prognostic Features
Histologic type and tumor grade have been shown to have little impact on prognosis. In women with early stage tumors (stages IB and IIA) that have been surgically treated, tumor size, depth of invasion, parametrial involvement, and nodal invasion and LVI have all been shown to significantly impact survival.44,48,49 Depth of invasion, a feature easily obtained in a surgically removed specimen, is proportional to the volume of tumor present. In stage I tumors, depth of invasion is a reasonably reliable indicator of survival (Table 11.6).
The finding of LVI has long been recognized as an adverse prognostic sign (Figure 11.23).50 However, the critical question is whether this feature is independent, or simply secondary to overall tumor size. Some other factor, such as the quantity of positive LVI, might be a more important predictor.51 While it has been reported that the survival of patients with and without LVI is not significantly different, a correlation exists between the presence of tumor in lymphatic channels and lymph node metastases, even when corrected for size of clinical tumor.52
It is generally agreed that the presence and number of lymph node metastases, the number of nodal groups involved, and the size of the metastases themselves are of prognostic significance.53 While these features may reflect the size of the primary cervical tumor, some report that the presence of nodal metastases is in itself an independent prognostic factor.54 Survival rates decrease with increasing numbers of involved nodes. One series of low-stage tumors reported a 90% survival rate with no involved nodes, 70% with one to three involved nodes, and 38% with over four involved nodes.55 For patients with positive nodes, survival was better if the cervical tumor was small rather than large (82% vs 48%). Lymph node metastases smaller than 2 mm were associated with much higher survival rates than if the metastases exceeded 2 cm (85% vs 38%, respectively).55
Tumor Size
In large part tumor size determines tumor stage, at least for stage I and II tumors, and, as expected, tumor size correlates with prognosis. In most studies, size has been defined using the diameter of the tumor, which is based on two-dimensional measurements.56 When used as a continuous variable, it is a good predictor of survival.57 In some studies, size has also been measured in terms of volume and this has been found to be a key prognostic index.55,58 Tumors with a volume <2 cm3 had 5 year survival rates of about 90% in contrast to those with volumes >30 cm3 (~65% survival). Volumes of 2 and 30 cm3 roughly equate to tumor diameters of 1.6 and 3.8 cm, respectively. With the availability of magnetic resonance imaging (MRI) techniques, precise volumetric measurement has now become routine in recent years, regardless of whether the patient is treated with radical surgery or radiotherapy.
Peritumoral Lymphatic Vessel Density
Peritumoral lymphatic density as evaluated using immunohistochemistry with D2-40 antibodies (selective marker for lymphatic endothelium) or antibodies against lymphatic vessel hyaluronan receptor 1 appears to be a prognostic marker in early stage cervical cancer. High peritumoral lymphatic vessel density is associated with increased lymph node metastases and poor survival.59,60 Similarly, increased amounts of the vascular endothelial growth factor (VEGF), which promotes angiogenesis, have been associated with higher stage and an increase in lymph node metastases, as well as a shorter survival in some but not other studies.61–63 A recent GOG study reported that elevated microvessel density as measured using CD31 immunohistochemical staining, but not VEGF levels, was an independent prognostic factor in high-risk, early stage cervical cancers.63
Biomarkers
Recent studies have evaluated a number of biochemical markers as prognostic factors including specific high-risk HPV genotype. Even though any of the 14 high-risk HPV genotypes can cause squamous cell carcinomas of the cervix, just two, HPV 16 and 18, are found in two-thirds of cases.64 Several studies have evaluated the impact of specific HPV genotype on survival in cervical cancer and, in general, HPV 18 is associated with a worse prognosis than the other genotypes. For example, a recent study of 1067 patients with stage IA–IIA disease identified HPV 18 in 16.5% of the cases. In patients with stage II disease, deep stromal invasion, parametrial extension, and HPV 18 were all significant predictors for death.65 Similarly, a study of 296 stage IB or greater cervical cancers found that HPV 18 was associated with an approximately threefold increase in death for women with stage IB cancers.66 In another study, the 5 year disease-free survival rate was reported as 58% for patients with HPV 16-positive tumors but only 38% for patients with HPV 18-positive tumors. Patients with HPV 18-associated tumors had a relative risk of death 2.4 times greater than that for patients with HPV 16, and 4.4 times greater than that for patients with tumors associated with a viral type different from HPV 16/18.67
Other investigations have examined oncogene and tumor suppressor gene expression, cell cycle protein expression, cell adhesion molecules, loss of heterogeneity, and microsatellite instability.59,68–71 Some of these studies have attempted to discern the changes important in pathogenesis from those that may reflect progression of the cancer. Some of the most interesting potential biomarkers include cyclooxygenase 2 (COX-2) expression, epidermal growth factor receptor (EGFR) expression, and expression of the transmembrane protein, leucine-rich repeats, and immunoglobin-like domains 1 (LRIG1), which has been shown to restrict growth factor signaling by degradation of EGFR. EGFR is expressed in approximately three-fourths of cervical cancers and correlates with a poor prognosis.72 High COX-2 expression has been associated with poor survival in several studies, as has synchronous co-expression of COX-2 and EGFR, which is reported in only one-third of cancers.69 However, there currently is insufficient data to warrant the use of specific biomarkers in assessing the prognosis or response to treatment of individual patients.
Differential Diagnosis
The differential diagnosis for invasive squamous cell carcinoma includes squamous metaplasia, condyloma acuminatum, HSIL with extensive involvement of endocervical glands, gestational decidual reaction of the cervical stroma, trophoblastic lesions such as epithelioid trophoblastic tumor, and granulomatous diseases such as lymphogranuloma venereum with associated reparative reactions. Now that p16 immunostaining is widely available for the identification of HPV-associated squamous intraepithelial lesion (SIL) and invasive cancers, it is much easier to differentiate between squamous metaplasia involving endocervical glands, which stains negatively with p16, and squamous cell carcinoma, which stains diffusely positive with p16. Similarly, condyloma acuminatum, which is usually associated with low-risk HPV genotypes (HPV 6 or 11), typically stains negatively for p16, as do gestational decidual reactions and granulomatous diseases. Histologic features that allow HSIL extending into endocervical glands from invasive squamous cell carcinoma are discussed in the section on SISCCA.
The cells of squamous cell carcinoma may diffusely contain extensive intracytoplasmic glycogen (Figure 11.16). Unlike clear cell adenocarcinomas where the change is uniform throughout, the glycogenated elements in squamous cell carcinoma are confined only to superficial portions of the epithelium. Clear cell adenocarcinoma, in addition to solid areas, usually has a papillary portion or tubulocystic areas with hobnail cells.
Epithelioid trophoblastic tumor frequently involves the endocervix and lower uterine segment.73 This tumor displays a nodular proliferation of a monomorphic population of intermediate-sized epithelioid trophoblasts with eosinophilic or clear cytoplasm, forming nests and cords. The center of tumor nests often displays an area of hyalinization or eosinophilic debris, resembling keratinous material in a squamous cell carcinoma. Occasionally, epithelioid trophoblast tumor shows focal replacement of the surface and/or glandular epithelium with stratified neoplastic cells, simulating HSIL. A high index of suspicion, a clinical presentation in a young woman having a relatively low, but definitely elevated level of serum human chorionic gonadotropin (hCG; <2500 mIU/ml), and/or an intrauterine mass identified by ultrasound help to make a correct diagnosis. Histologically, the absence of a definite HSIL, the presence of decidualized stromal cells in the neighborhood, reactivity for a-inhibin, human placental lactogen (hPL), and cytokeratin 18 (CK18) help to confirm the diagnosis of epithelioid trophoblast tumor.
When utilizing immunohistochemical staining to distinguish between squamous cell carcinomas and other tumors it must be remembered that, although strong diffuse p16 positivity is a marker for high-risk HPV-associated neoplasia, positive immunostaining for p16 can be seen in many non-HPV-related neoplasms. Immunostaining for p63 (marker for squamous differentiation) can also be quite helpful in difficult cases. One study of 250 invasive cervical tumors found strong diffuse staining for p63 in 97% of the squamous cell carcinomas.74 Neuroendocrine carcinomas, melanomas, and lymphomas all stained negatively for p63. Similarly, in situ hybridization for high-risk HPV can be a useful marker when trying to confirm that a given lesion is cervical in origin. Again it should be cautioned that, because many cervical cancers have a low number of HPV copies, a negative high-risk HPV in situ hybridization does not rule out that a given lesion is HPV associated.
Special Considerations
Cervical Stump Carcinoma
Recently supracervical or subtotal hysterectomies have come back into favor, and in the future there will be a considerable number women with a cervix post hysterectomy. The cancers that occur in the residual cervix have been referred to as ‘cervical stump carcinomas.’ Currently, cervical stump carcinomas account only for about 2% of all cervical cancers and occur on average about 17 years post subtotal hysterectomy.75 The proportion of squamous cell carcinoma to adenocarcinoma, and other clinical pathologic features, resemble those of cervical carcinoma in general. However, carcinomas of the cervical stump show a worse stage profile than do cancer cases with an intact uterus.75
Effects of Radiotherapy on Cervical Carcinoma
Microscopically, the effect on cell division that radiation induces is reflected in reduced numbers of mitotic figures. Those that remain often appear even more abnormal than before radiation. At the same time, the same cells tend to exhibit better differentiation, with more abundant cytoplasm that may appear keratinized. Degenerative changes are superimposed, with hyperchromatic nuclei that are sometimes pyknotic and sometimes enlarged and bizarre in shape (Figure 11.24). Vacuolization is seen both in the cytoplasm and in the nuclei. The stroma shows a variable inflammatory infiltrate composed of both acute and chronic inflammatory cells, with fibrosis. Endothelial proliferation may be prominent and is often seen to progress to complete obliteration of the lumen of many arterioles. Areas of necrosis are almost universal; sometimes the biopsies consist entirely of necrotic material. Assessment should, of course, be made on the least degenerated areas.
Micrometastasis
Immunohistochemical analysis is currently used for the detection of breast cancer micrometastasis in sentinel lymph nodes, and this procedure is being actively investigated as a staging procedure for cervical cancer.76 However, the incidence and, more importantly, the clinical significance of micrometastases detected by immunohistochemical methods in cervical cancer are still being defined.77 The term micrometastasis is used to describe small foci of metastases (0.2–2.0 mm), found only microscopically. The process of sentinel node biopsy combined with multiple sections and immunohistochemical staining with cytokeratins is sometimes referred to as ‘ultrastaging.’ In one recent large study of 645 patients with early stage cervical cancer, macrometastasis (>2 mm), micrometastasis (0.2–2.0 mm), and isolated tumor cells (<0.2 mm) were detected in 14.7%, 10.1%, and 4.5% of patients, respectively.77 The presence of isolated tumor cells did not appear to have an impact on either recurrence-free survival or overall survival. However, the presence of micrometastases conveyed a similar risk as did the presence of macrometastasis. In two other studies, immunohistochemical analyses identified 8–15% of patients with lymph node micrometastasis not initially identified by H&E analysis.78,79 These data were conflicting as to whether the patients with micrometastases did or did not have a statistically significant probability of having other high-risk factors, including LVI.
‘Mucin-Secreting’ Carcinoma
Approximately 25–35% of carcinomas lacking definitive glandular structures have intracellular mucin demonstrable with the use of mucin stains. Some have named this as ‘mucin-secreting squamous cell carcinoma,’ or sometimes ‘mucoepidermoid carcinoma’ (Figure 11.25). It has been suggested that these tumors are slightly more aggressive than the typical squamous cell carcinoma, but this has not been borne out uniformly.80,81 In the absence of any substantial information indicating that these tumors have a significantly different clinical pathologic behavior from the typical squamous carcinoma, these lesions are not classified as a distinct entity. For this reason, we see no value in the routine staining for mucin in invasive squamous cell carcinomas to identify these tumors.
Histologic Variants
Verrucous Squamous Cell Carcinoma
Verrucous squamous cell carcinoma is a rare variant of well-differentiated squamous cell carcinoma that can be confused with the more common papillary squamous cell carcinoma. It occurs preferentially in older women, and is found anywhere throughout the lower genital tract or perivulvar regions.82 Only a small number of cases of verrucous carcinoma of the cervix have been described in the literature. These tumors were previously referred to as giant condyloma acuminatum of Buschke and Lowenstein. Unlike other cervical carcinomas, these tumors can be associated with either low-risk or high-risk types of HPV.83 Clinically they are usually large, bulky, and exophytic but sometimes are warty, fungating, or even ulcerated. Their deceptively benign cytologic features distinguish them from more common forms of invasive squamous cell carcinoma. Verrucous squamous cell carcinoma typically grows slowly in size, encroaching on adjoining structures. On sectioning, the sessile tumor commonly invades into the stroma at its base, but the deep margin characteristically is broad and sharply circumscribed. As the tumor invades along a wide front in a ‘pushing’ fashion, it keeps a well-defined deep margin.
The high degree of differentiation of verrucous carcinoma is striking on both low- and high-power microscopic examination. Other than for its immense bulk and the fact that it can recur, the cells are so well differentiated that they often appear benign, or at most only slightly atypical (Figure 11.26). Not infrequently, it is misdiagnosed initially as a condyloma, hence the name ‘verrucous.’ The atypia is also minimal in the basal layers adjacent to the basement membrane. The tumor base shows pushing nests of tumor extending into the stroma and typically surrounded by a dense stromal inflammatory infiltrate. Laminated keratin whorls are sometimes present within the epithelium. Mitotic activity is low, and if present it is usually confined to the basal cells. An accurate diagnosis can only be made if the biopsy is sufficiently large to include the base of the lesion as well as the more superficial, well-differentiated, keratotic areas. The correct diagnosis is impossible if only the surface layers are examined. While the tumor rarely metastasizes, obviating the need for lymphadenectomy, local recurrences are common and occasionally the tumor can be deeply invasive and extend into the adjacent pelvic tissues. Rarely it pursues a relentless course manifested by uncontrolled local recurrence.
Warty Squamous Cell Carcinoma
Warty carcinoma is a well-differentiated variant of squamous cell carcinoma that has marked condylomatous features.42 These tumors are distinguished from verrucous carcinomas by the fact that the tumor cells in the lower portion of the epithelium resemble typical squamous cell carcinomas. They are typically associated with high-risk HPV genotypes. Koilocytes, which are rarely found on cervical cytology when women have typical squamous cell carcinomas, are often seen in women with warty carcinoma.84
Papillary Squamous Cell Carcinoma
These are another rare variant of squamous cell carcinoma that resemble a transitional cell carcinoma of the bladder.85 The tumor consists of papillae with fibrovascular cores covered by several layers of atypical epithelial cells that in many instances resemble HSIL, but in other instances the cells are oval with their long axis perpendicular to the surface and resemble transitional cells.86 The importance of these lesions is that, like verrucous carcinomas, the invasive nature of the lesion is frequently not recognized with superficial biopsies. Papillary squamous cell carcinomas can be distinguished from warty squamous cell carcinomas by their lack of koilocytosis and minimal keratinization.
Lymphoepithelioma-Like Squamous Cell Carcinoma
Lymphoepithelial carcinoma has a histologic appearance similar to that of nasopharyngeal lymphoepithelioma-like squamous cell carcinomas. They exhibit malignant squamous cells enveloped in an intense stromal chronic inflammatory infiltrate (Figure 11.27). The cells have moderate cytoplasm, and often vesicular nuclei with prominent nucleoli.87 The epithelial nature of the tumor is obvious when the cells are aggregated into nests or examined for cytokeratin filaments (Figure 11.28), but, when scattered in small clusters or even individual cells, differentiation from lymphoma can be difficult. This tumor is rare in Caucasian women, but has been described more commonly in Japanese women. The Epstein–Barr virus, which is common in this tumor when in the nasopharynx, has not been identified in most cases from the cervix and most cervical tumors are associated with HPV.82,88 The frequency of nodal metastasis is low and the prognosis is generally favorable.89
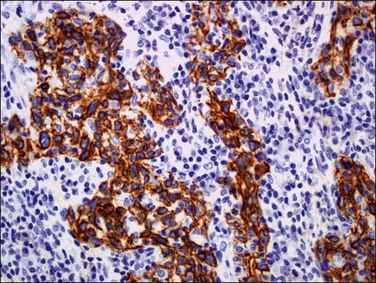
Figure 11.28 Lymphoepithelioma. The squamous component reacts with cytokeratin.
Papillary Squamotransitional Cell Carcinoma
This is another rare form of cervical cancer that is reported to be essentially indistinguishable from similar tumors occurring in the urinary bladder.90 The tumors have a tendency to present at advanced stage and tend to be recurrent and have metastases. The finding of HPV 16 in many tumors, the finding that they may show allelic loss at chromosome 3p, and the fact they are uroplakin III negative suggest that these tumors are cervical, as opposed to urinary bladder, in origin.91 Grossly, the tumor size may range from 0.7 to 6 cm, and the clinical stages may range from intraepithelial to IIIB. Microscopically, the prominent features are a papillary architecture with fibrovascular cores lined by a multilayered, atypical epithelium that is predominantly squamous, mixed squamous, and transitional, or predominantly transitional, and resembles a HSIL of the cervix.92 Nearly all tumors are immunoreactive for CK7 and about 10% are reactive for CK20.90 These potentially aggressive malignant tumors need to be distinguished from the far more common and benign papillary lesions of the cervix. An additional consideration in the differential diagnosis of such neoplasms includes villoglandular carcinomas of the cervix. However, unlike papillary squamotransitional cell carcinomas, villoglandular lesions, as the name implies, are stratified columnar adenocarcinomas without any squamous features.
References
1. Burden of cervical cancer globally. WHO/ICO Information Center on HPV and Cervical Cancer 2011.
2. American Cancer Society. Cancer facts & figures. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf, 2012. [[accessed 24.07.12]].
3. SEER. Cancer statistics. Bethesda, MD: National Cancer Institute. http://seer.cancer.gov/. [[accessed 01.03.11]].
4. Miller, AB, Knight, J, Narod, S. The natural history of cancer of the cervix and the implications for screening policy. In: Miller AB, Chamberlain J, Day NE, et al, eds. Cancer screening. Cambridge, UK: Cambridge University Press; 1991:141–152.
5. Leyden, WA, Manos, MM, Geiger, AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005; 97:675–683.
6. Andrae, B, Kemetli, L, Sparen, P, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008; 100:622–629.
7. Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105:103–104.
8. Tavassoli FA, Devilee P, eds. Pathology and genetics of tumours of the breast and female genital organs. Lyons, France: IARC Press, 2003.
9. Smith, HO, Tiffany, MF, Qualls, CR, Key, CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol Oncol. 2000; 78:97–105.
10. Vizcaino, AP, Moreno, V, Bosch, FX, et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer. 2000; 86:429–435.
11. Cairns, M, Cruickshank, M. A review of women with microinvasive cervical cancer in the Grampian region. J Low Genit Tract Dis. 2007; 11:290–293.
12. Mobius, G. Cytological early detection of cervical carcinoma: possibilities and limitations. Analysis of failures. J Cancer Res Clin Oncol. 1993; 119:513–521.
13. Kohlberger, P, Edwards, L, Hacker, NF. Microinvasive squamous cell carcinoma of the cervix: immunohistochemically detected prognostic factors in a case with poor clinical outcome. Gynecol Oncol. 2003; 90:443–445.
14. Raspagliesi, F, Ditto, A, Quattrone, P, et al. Prognostic factors in microinvasive cervical squamous cell cancer: long-term results. Int J Gynecol Cancer. 2005; 15:88–93.
15. Sedlis, A, Sall, S, Tsukada, Y, et al. Microinvasive carcinoma of the uterine cervix: a clinical-pathologic study. Am J Obstet Gynecol. 1979; 133:64–74.
16. Bohm, JW, Krupp, PJ, Lee, FY, Batson, HW. Lymph node metastasis in microinvasive epidermoid cancer of the cervix. Obstet Gynecol. 1976; 48:65–67.
17. Copeland, LJ, Silva, EG, Gershenson, DM, et al. Superficially invasive squamous cell carcinoma of the cervix. Gynecol Oncol. 1992; 45:307–312.
18. Attanoos, R, Nahar, K, Bigrigg, A, et al. Primary adenocarcinoma of the cervix. A clinicalpathologic study of prognostic variables in 55 cases. Int J Gynecol Cancer. 1995; 5:179–186.
19. Ehrmann, RL, Dwyer, IM, Yavner, D, Hancock, WW. An immunoperoxidase study of laminin and type IV collagen distribution in carcinoma of the cervix and vulva. Obstet Gynecol. 1988; 72:257–262.
20. Hasumi, K, Sakamoto, A, Sugano, H. Microinvasive carcinoma of the uterine cervix. Cancer. 1980; 45:928–931.
21. Maiman, M, Fruchter, RG, DiMaio, TM, Boyce, JG. Superficially invasive squamous cell carcinoma of the cervix. Obstet Gynecol. 1988; 72:399–403.
22. Morgan, PR, Anderson, MC, Buckley, CH, et al. The Royal College of Obstetricians and Gynaecologists micro-invasive carcinoma of the cervix study: preliminary results. Br J Obstet Gynaecol. 1993; 100:664–668.
23. Simon, NL, Gore, H, Shingleton, HM, et al. Study of superficially invasive carcinoma of the cervix. Obstet Gynecol. 1986; 68:19–24.
24. van Nagell, JR, Greenwell, N, Powell, DF, et al. Microinvasive carcinoma of the cervix. Am J Obstet Gynecol. 1983; 145:981–991.
25. Sevin, BU, Nadji, M, Averette, HE, et al. Microinvasive carcinoma of the cervix. Cancer. 1992; 70:2121–2128.
26. Burghardt, E, Holzer, E. Diagnosis and treatment of microinvasive carcinoma of the cervix uteri. Obstet Gynecol. 1977; 49:641–653.
27. Takeshima, N, Yanoh, K, Tabata, T, et al. Assessment of the revised International Federation of Gynecology and obstetrics staging for early invasive squamous cervical cancer. Gynecol Oncol. 1999; 74:165–169.
28. Darragh, TM, Colgan, TJ, Cox, JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012; 136:1266–1297.
29. Roche, WD, Norris, HJ. Microinvasive carcinoma of the cervix. The significance of lymphatic invasion ans confluent patterns of stromal growth. Cancer. 1975; 36:180–186.
30. Benedet, JL, Anderson, GH. Stage IA carcinoma of the cervix revisited. Obstet Gynecol. 1996; 87:1052–1059.
31. Marana, HR, de Andrade, JM, Matthes, AC, et al. Microinvasive carcinoma of the cervix. Analysis of prognostic factors. Eur J Gynaecol Oncol. 2001; 22:64–66.
32. Witkiewicz, AK, Wright, TC, Ferenczy, A, et al, Carcinoma and other tumors of the cervix In: Kurman RJ, Hedrick Ellenson L, Ronnett BM, editors, 6th ed. Springer, New York, 2011.
33. Ostor, AG, Rome, RM. Micro-invasive squamous cell carcinoma of the cervix: A clinico-pathologic study of 200 cases with long-term follow-up. Int J Gynecol Cancer. 1994; 4:257–264.
34. Bean, SM, Kurtycz, DF, Colgan, TJ. Recent developments in defining microinvasive and early invasive carcinoma of the uterine cervix. J Low Genit Tract Dis. 2011; 15:146–157.
35. Wells, M, Ostor, AG, Franceschi, S, et al. Epithelial tumors of the uterine cervix. In: Tavassoli FA, Devilee P, eds. Tumors of the breast and female genital organs. Lyons, France: IARC Press; 2003:221–232.
36. Morgan, PR, Anderson, MC, Buckley, CH, et al. The Royal College of Obstetricians and Gyneecologists micro-invasive carcinoma of the cervix study: preliminary results. Br J Obstet Gynaecol. 1993; 100:664–668.
37. Rush, D, Hyjek, E, Baergen, RN, et al. Detection of microinvasion in vulvar and cervical intraepithelial neoplasia using double immunostaining for cytokeratin and basement membrane components. Arch Pathol Lab Med. 2005; 129:747–753.
38. Solomon, D, Davey, D, Kurman, R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002; 287:2114–2119.
39. Kolstad, P. Follow-up study of 232 patients with stage IA1 and 411 patients with stage IA2 squamous cell carcinoma of the cervix (microinvasive carcinoma). Gynecol Oncol. 1989; 33:265–272.
40. Morris, M, Mitchell, MF, Silva, EG, et al. Cervical conization as definitive therapy for early invasive squamous carcinoma of the cervix. Gynecol Oncol. 1993; 51:193–196.
41. Gadducci, A, Sartori, E, Maggino, T, et al. The clinical outcome of patients with stage Ia1 and Ia2 squamous cell carcinoma of the uterine cervix: a Cooperation Task Force (CTF) study. Eur J Gynaecol Oncol. 2003; 24:513–516.
42. Kurman, RJ, Toki, T, Schiffman, MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993; 17:133–145.
43. Grayson, W, Cooper, K. A reappraisal of “basaloid carcinoma” of the cervix, and the differential diagnosis of basaloid cervical neoplasms. Adv Anat Pathol. 2002; 9:290–300.
44. Zaino, RJ, Ward, S, Delgado, G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. Cancer. 1992; 69:1750–1758.
45. Quinn, MA, Benedet, JL, Odicino, F, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006; 95(Suppl 1):S43–103.
46. Nguyen, HN, Averette, HE. Biology of cervical carcinoma. Semin Surg Oncol. 1999; 16:212–216.
47. Takeshima, N, Katase, K, Hirai, Y, et al. Prognostic value of peritoneal cytology in patients with carcinoma of the uterine cervix. Gynecol Oncol. 1997; 64:136–140.
48. Delgado, G, Bundy, B, Zaino, R, et al. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecolgic Oncology Group study. Gynecol Oncol. 1990; 38:352–357.
49. Kristensen, GB, Abeler, VM, Risberg, B, et al. Tumor size, depth of invasion, and grading of the invasive tumor front are the main prognostic factors in early squamous cell cervical carcinoma. Gynecol Oncol. 1999; 74:245–251.
50. Delgado, G. Lymphovascular space involvement in cervical cancer: an independent risk factor. Gynecol Oncol. 1998; 68:219.
51. Roman, LD, Felix, JC, Muderspach, LI, et al. Influence of quantity of lymph-vascular space invasion on the risk of nodal metastases in women with early-stage squamous cancer of the cervix. Gynecol Oncol. 1998; 68:220–225.
52. Zhang, Y, Yan, M, He, J, et al. Significant effects of lymph and blood vascular invasion on the prognosis of early-stage cervical squamous cell carcinoma. J Obstet Gynaecol Res. 2010; 36:1015–1022.
53. Okazawa, M, Mabuchi, S, Isohashi, F, et al. The prognostic significance of multiple pelvic node metastases in cervical cancer patients treated with radical hysterectomy plus adjuvant chemoradiotherapy. Int J Gynecol Cancer. 2012; 22:490–497.
54. Fyles, AW, Pintilie, M, Kirkbride, P, et al. Prognostic factors in patients with cervix cancer treated by radiation therapy: results of a multiple regression analysis. Radiother Oncol. 1995; 35:107–117.
55. Pickel, H, Haas, J, Lahousen, M. Prognostic factors in cervical cancer. Eur J Obstet Gynecol Reprod Biol. 1997; 71:209–213.
56. Lambin, P, Kramar, A, Haie-Meder, C, et al. Tumour size in cancer of the cervix. Acta Oncol. 1998; 37:729–734.
57. Finan, MA, DeCesare, S, Fiorica, JV, et al. Radical hysterectomy for stage IB1 vs IB2 carcinoma of the cervix: does the new staging system predict morbidity and survival? Gynecol Oncol. 1996; 62:139–147.
58. Kinney, WK, Hodge, DO, Egorshin, EV, et al. Identification of a low-risk subset of patients with stage IB invasive squamous cancer of the cervix possibly suited to less radical surgical treatment. Gynecol Oncol. 1995; 57:3–6.
59. Zhang, SQ, Yu, H, Zhang, LL. Clinical implications of increased lymph vessel density in the lymphatic metastasis of early-stage invasive cervical carcinoma: a clinical immunohistochemical method study. BMC Cancer. 2009; 9:64.
60. Gombos, Z, Xu, X, Chu, CS, et al. Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early-stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res. 2005; 11:8364–8371.
61. Loncaster, JA, Cooper, RA, Logue, JP, et al. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer. 2000; 83:620–625.
62. Cheng, WF, Chen, CA, Lee, CN, et al. Vascular endothelial growth factor and prognosis of cervical carcinoma. Obstet Gynecol. 2000; 96:721–726.
63. Randall, LM, Monk, BJ, Darcy, KM, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2009; 112:583–589.
64. de Sanjose, S, Quint, WG, Alemany, L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010; 11:1048–1056.
65. Lai, CH, Chang, CJ, Huang, HJ, et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J Clin Oncol. 2007; 25:3628–3634.
66. Schwartz, SM, Daling, JR, Shera, KA, et al. Human papillomavirus and prognosis of invasive cervical cancer: a population-based study. J Clin Oncol. 2001; 19:1906–1915.
67. Lombard, I, Vincent-Salomon, A, Validire, P, et al. Human papillomavirus genotype as a major determinant of the course of cervical cancer. J Clin Oncol. 1998; 16:2613–2619.
68. Baykal, C, Ayhan, A, Al, A, Yuce, K. Overexpression of the c-Met/HGF receptor and its prognostic significance in uterine cervix carcinomas. Gynecol Oncol. 2003; 88:123–129.
69. Kim, GE, Kim, YB, Cho, NH, et al. Synchronous coexpression of epidermal growth factor receptor and cyclooxygenase-2 in carcinomas of the uterine cervix: a potential predictor of poor survival. Clin Cancer Res. 2004; 10:1366–1374.
70. Goff, BA, Sallin, J, Garcia, R, et al. Evaluation of p27 in preinvasive and invasive malignancies of the cervix. Gynecol Oncol. 2003; 88:40–44.
71. Skomedal, H, Kristensen, GB, Lie, AK, Holm, R. Aberrant expression of the cell cycle associated proteins TP53, MDM2, p21, p27, cdk4, cyclin D1, RB, and EGFR in cervical carcinomas. Gynecol Oncol. 1999; 73:223–228.
72. Kersemaekers, AM, Fleuren, GJ, Kenter, GG, et al. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clin Cancer Res. 1999; 5:577–586.
73. Fadare, O, Parkash, V, Carcangiu, ML, Hui, P. Epithelioid trophoblastic tumor: clinicopathological features with an emphasis on uterine cervical involvement. Mod Pathol. 2006; 19:75–82.
74. Wang, TY, Chen, BF, Yang, YC, et al. Histologic and immunophenotypic classification of cervical carcinomas by expression of the p53 homologue p63: a study of 250 cases. Hum Pathol. 2001; 32:479–486.
75. Hellstrom, AC, Hellman, K, Pettersson, BF, Andersson, S. Carcinoma of the cervical stump: fifty years of experience. Oncol Rep. 2011; 25:1651–1654.
76. Darlin, L, Persson, J, Bossmar, T, et al. The sentinel node concept in early cervical cancer performs well in tumors smaller than 2 cm. Gynecol Oncol. 2010; 117:266–269.
77. Cibula, D, Abu-Rustum, NR, Dusek, L, et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol Oncol. 2012; 124:496–501.
78. Juretzka, MM, Jensen, KC, Longacre, TA, et al. Detection of pelvic lymph node micrometastasis in stage IA2-IB2 cervical cancer by immunohistochemical analysis. Gynecol Oncol. 2004; 93:107–111.
79. Lentz, SE, Muderspach, LI, Felix, JC, et al. Identification of micrometastases in histologically negative lymph nodes of early-stage cervical cancer patients. Obstet Gynecol. 2004; 103:1204–1210.
80. Samlal, RA, Ten Kate, FJ, Hart, AA, Lammes, FB. Do mucin-secreting squamous cell carcinomas of the uterine cervix metastasize more frequently to pelvic lymph nodes? A case-control study? Int J Gynecol Pathol. 1998; 17:201–204.
81. Husniye Dilek, F, Kucukali, T. Mucin production in carcinomas of the uterine cervix. Eur J Obstet Gynecol Reprod Biol. 1998; 79:149–151.
82. Wong, WS, Ng, CS, Lee, CK. Verrucous carcinoma of the cervix. Arch Gynecol Obstet. 1990; 247:47–51.
83. Frega, A, Lukic, A, Nobili, F, et al. Verrucous carcinoma of the cervix: detection of carcinogenetic human papillomavirus types and their role during follow-up. Anticancer Res. 2007; 27:4491–4494.
84. Ng, WK, Cheung, LK, Li, AS. Warty (condylomatous) carcinoma of the cervix. A review of 3 cases with emphasis on thin-layer cytology and molecular analysis for HPV. Acta Cytol. 2003; 47:159–166.
85. Brinck, U, Jakob, C, Bau, O, Fuzesi, L. Papillary squamous cell carcinoma of the uterine cervix: report of three cases and a review of its classification. Int J Gynecol Pathol. 2000; 19:231–235.
86. Odida, M. Papillary squamous cell carcinoma of the cervix in Uganda: a report of 20 cases. Afr Health Sci. 2005; 5:291–294.
87. Reich, O, Pickel, H, Purstner, P. Exfoliative cytology of a lymphoepithelioma-like carcinoma in a cervical smear. A case report. Acta Cytol. 1999; 43:285–288.
88. Martorell, MA, Julian, JM, Calabuig, C, et al. Lymphoepithelioma-like carcinoma of the uterine cervix. Arch Pathol Lab Med. 2002; 126:1501–1505.
89. Kaul, R, Gupta, N, Sharma, J, Gupta, S. Lymphoepithelioma-like carcinoma of the uterine cervix. J Cancer Res Ther. 2009; 5:300–301.
90. Koenig, C, Turnicky, RP, Kankam, CF, Tavassoli, FA. Papillary squamotransitional cell carcinoma of the cervix: a report of 32 cases. Am J Surg Pathol. 1997; 21:915–921.
91. Maitra, A, Wistuba, II, Gibbons, D, et al. Allelic losses at chromosome 3p are seen in human papilloma virus 16 associated transitional cell carcinoma of the cervix. Gynecol Oncol. 1999; 74:361–368.
92. Kokka, F, Verma, M, Singh, N, et al. Papillary squamotransitional cell carcinoma of the uterine cervix: report of three cases and review of the literature. Pathology. 2006; 38:584–586.