Chapter 157 Cervical Laminoplasty
Indications and Techniques
Cervical laminoplasty is a posterior spinal operation where the positions of the lamina are altered without intersegmental fusion to augment the volume of the spinal canal. Laminoplasty is primarily used to treat cervical spondylotic myelopathy caused by ossification of the posterior longitudinal ligament, resulting in stenosis of the cervical canal.1 Laminoplasty is usually performed in cases of multisegmental stenosis of the spinal canal. In the cervical spine, C3 through C7 are most frequently addressed, but C2, T1, and even C1 have been modified by laminoplasty techniques. Many surgeons choose laminoplasty over anterior fusion techniques when more than two levels require decompression. Laminoplasty can be used in other situations where the volume of the cervical spinal canal needs augmentation, but fusion is not desired. Laminoplasty is effective in treating cervical myelopathy caused by stenosis, and long-term studies indicate that laminoplasty can improve symptoms of myelopathy providing many years of improvement in Japanese Orthopaedic Association (JOA) scores.2 No studies demonstrate that laminoplasty is superior to laminectomy alone, laminectomy with posterior fusion, or anterior decompression with fusion for the treatment of cervical spondylotic myelopathy.3–5 There is no difference in the recovery rates in myelopathic patients treated from a posterior laminoplasty approach versus anterior cervical fusion.
Laminoplasty appears to work by allowing the spinal cord to shift posteriorly into the augmented space.6 Laminoplasty was first described in 1968, and much of the literature regarding laminoplasty comes from Asia, where cervical myelopathy from ossification of the posterior longitudinal ligament is commonly diagnosed.7,8
Theoretical benefits of laminoplasty include preservation of cervical motion, prevention of postlaminectomy kyphosis, lessening of adjacent segment degeneration, and avoidance of development of postlaminectomy membrane formation. Unfortunately, these benefits are not always realized because cervical range of motion diminishes approximately 50% after laminoplasty, and development of kyphotic deformity occurs in 10% to 35% of patients after laminoplasty. Despite the goal of avoiding fusion after laminoplasty, spontaneous intravertebral and intralaminar fusion has been reported frequently after laminoplasty. Long term studies of laminoplasty indicate interlaminar fusion occurs in 53% of patients with the C2–C3 level involved most frequently. This did not appear to influence the clinical outcome, but did diminish range of motion of the cervical spine.9 It is suggested that limiting the use of rigid cervical orthosis from 4 to 8 weeks may lessen fusion and increase cervical range of motion.10
Additionally, there is a 6% to 60% incidence of chronic neck pain after laminoplasty. This may be more common than in cases of anterior fusion, since one study found a 19% incidence of axial neck pain after anterior fusion versus 60% after laminoplasty.11,12
Laminoplasty is thought to decrease the incidence of adjacent level degeneration in the cervical spine. To date, only a few cases of adjacent level degeneration have been reported after laminoplasty. The reported incidence of adjacent level degeneration in patients who had undergone previous anterior cervical fusion, later requiring additional surgery, is estimated to be between 19% and 25% 10 years after the initial surgery. A 10-year study of anterior cervical decompression and fusion (ACDF) patients compared to normal volunteers confirms that ACDF accelerates adjacent level degeneration as measured by MRI scans. The incidence of progression varies according to the spinal level studied (see Table 157-1).13 The rate of adjacent segment degeneration appears to be much less in laminoplasty, but there are no studies presently available to support this claim.14
Table 157-1 Adjacent Level Radiographic Degeneration over 10 Years: ACDF Compared to Normal Controls
| ACDF (%) | Control (%) | |
|---|---|---|
| C2–C3 | 20.0 | 0.0 |
| C3–C4 | 60.0 | 10.9 |
| C4–C5 | 69.4 | 29.4 |
| C5–C6 | 38.9 | 50.2 |
| C6–C7 | 56.8 | 33.8 |
| C7–T1 | 31.6 | 2.5 |
ACDF, anterior cervical decompression and fusion.
Results of Cervical Laminoplasties
Because the majority of laminoplasty operations are performed to treat cervical myelopathy, the preoperative and postoperative conditions are frequently scored using the JOA scoring system for cervical myelopathy (Table 157-2). Several studies comparing cervical corpectomy and several different laminoplasty techniques determined that both techniques are effective in treating cervical myelopathy. The recovery rates for myelopathy were no different statistically between either procedure.15–17
| Motor dysfunction of the upper extremity |
| 0 = Unable to feed oneself |
| 1 = Unable to handle chopsticks; able to eat with a spoon |
| 2 = Handle chopsticks with slight difficulty |
| 3 = None |
| Motor dysfunction of the lower extremity |
| 0 = Unable to walk |
| 1 = Walk on flat floor with walking aid |
| 2 = Up and/or down stairs with hand rail |
| 3 = None |
| Sensory deficit |
| Upper extremity |
| 0 = Severe sensory loss or pain |
| 1 = Mild sensory loss |
| 2 = None |
| Lower extremity |
| 0 = Severe sensory loss or pain |
| 1 = Mild sensory loss |
| 2 = None |
| Trunk |
| 0 = Severe sensory loss or pain |
| 1 = Mild sensory loss |
| 2 = None |
| Sphincter dysfunction |
| 0 = Unable to void |
| 1 = Marked difficulty in micturition |
| 2 = Difficulty in micturition |
| 3 = None |
Comparison of the single-door versus double-door laminoplasty techniques shows no significant difference in the outcome between these two groups.18
Comparison of laminectomy, anterior decompression and fusion, and laminoplasty showed no significant difference in outcome as measured by change in JOA scores.4,19–23 A comparison of subtotal corpectomy and laminoplasty showed identical results from the two procedures at 1- and 5-year follow-up evaluations. There may be a significant difference in outcome in the special case of massive ossification of the posterior longitudinal ligament, where over 50% of the canal is occupied by ossified posterior longitudinal ligament (OPLL). In this condition, the outcome was much better for the anterior approach patients because none of them worsened neurologically, as compared with a 33% neurologic complication rate for the laminoplasty patients.24
Laminoplasties can provide rapid improvement of myelopathic symptoms within the first postoperative year as measured via JOA myelopathy scores (Tables 157-2 and 157-3, and Figs. 157-1 and 157-2). Factors predicting recovery have been noted. Urinary bladder function recovers less than other symptoms. Patients older than 60 years improve less than younger patients. Preoperative JOA score, Pavlov ratio, and compression ratio also affect outcomes. Lower JOA scores, lower Pavlov ratios, and lower compression ratios all correlate with less improvement after open-door laminoplasties.3,25–28 Abnormal spinal cord signal on preoperative magnetic resonance imaging (MRI) scanning has been associated with poor postoperative prognosis.29 Local kyphosis exceeding 13 degrees is a crucial risk factor for poor recovery after laminoplasty. If kyphosis exceeding 13 degrees is detected, either anterior or posterior fusion should be considered30 (Fig. 157-3). Transverse area of the spinal cord at the level of maximum compression is also related to outcome, since areas of 42.6 mm2 correlated with excellent outcomes and areas of 31 mm2 correlated only with good outcomes in postoperative JOA scores.31 It appears that the ideal enlargement of the canal is 4 mm along an anterior posterior line, and that a 3-mm posterior cord shift is required for clinical improvement.6 Reoccurrence of myelopathy postoperatively due to posterior shifting of the cord into the area of split lamina has been reported. This rare event usually occurs in the first few days after laminoplasty and responds to laminectomy of the affected segments.32
| Excellent recovery rate: >75% | |
| Good: 50%-75% | |
| Fair: 25%-50% | |
| Poor: <25% | |
| Nurick Scale | |
| 0 | Signs or symptoms of root involvement, but without evidence of spinal cord disease |
| 1 | Signs of spinal cord disease, but no difficulty walking |
| 2 | Slight difficulty walking that does not prevent full-time employment |
| 3 | Difficulty walking that prevents full-time employment or ability to do all housework, but that is not so severe as to require a person’s help to walk |
| 4 | Able to walk only with a person’s help or with the aid of a frame |
| 5 | Chair bound or bedridden |
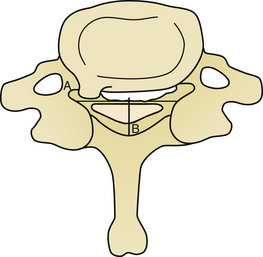
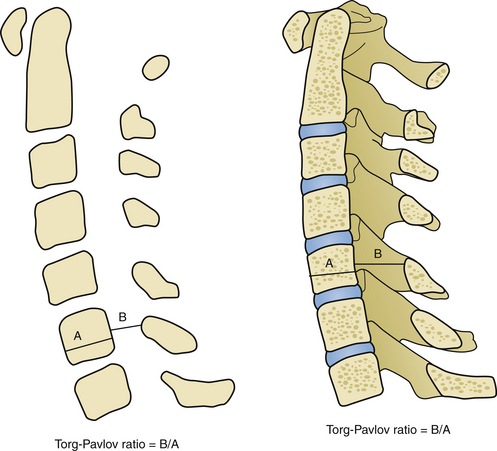
FIGURE 157-1 Compression ratio is the sagittal cord diameter divided by the transverse cord diameter times 100.
Risk factors for failure of adequate decompression with laminoplasty have been determined in cases of OPLL (Fig. 157-4). Maximum thickness of the ossification of more than 7 mm, lordosis of less than 10 degrees, or any kyphosis are significantly correlated with continued cord contact in the postlaminoplasty state. If these risk factors are present, then anterior decompression should be considered.26,33,34 It is recommended that patients be treated surgically as soon as myelopathy is detected. Patients with OPLL usually have improved JOA scores in the postlaminoplasty state, and those last for at least 5 years before myelopathy progresses. Ossification of the posterior longitudinal ligament progresses in 73% of patients after laminoplasty, and younger patients with the mixed or continuous type of OPLL progress most often.33 Progression of OPLL cannot be predicted after laminoplasty, but if symptomatic progression occurs, an anterior approach may be considered. Late neurologic deterioration may be seen in 18% to 60% of cases after cervical laminoplasty, and may be due to instability or development of kyphosis.35
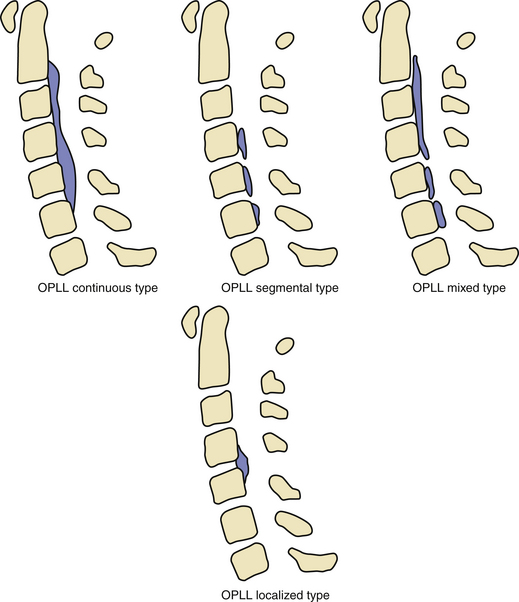
FIGURE 157-4 Ossified posterior longitudinal ligament types: continuous, segmental, mixed, and localized.
Segmental motor paralysis occurs in 7% to 20% of patients after laminoplasty. Radiculopathy most often appears within 2 weeks of surgery, and is evenly split between the hinge and open-door side of the laminoplasty when using the open-door technique. The C5 nerve root appears to be particularly predisposed to postoperative radiculopathy. Performing preoperative electromyogram (EMG) may prevent postoperative C5 palsy. If radiculopathy is found on EMG, selective microforaminotomies should be performed during the laminoplasty procedure.36,37 There is evidence to suggest that segmental motor paralysis may be due to changes in the central gray matter of the spinal cord, rather than in the nerve root. In those cases, there is a definite delay from the end of surgery to the onset of deficit, and MRI scan may show a high-intensity zone in the spinal cord on T2 images.38 The mechanism is unknown, but may be attributable to surgical trauma or possibly due to rapid reperfusion into the ischemic central gray of the cord.39 Other causes of severe postoperative neck pain or motor paralysis may be due to malposition of osteotomized lamina, surgical trauma, or reclosure of opened lamina.2 Some patients may develop kyphosis after laminoplasty, and those who develop kyphosis tended to have a poor recovery. Additionally, patients who had preexisting kyphosis had less reversal of myelopathy.40,41 Some degree of lordosis is lost in 87% of patients after laminoplasty, and this appears to be related to detachment of the semispinalis cervicis muscle on C2, and the nuchal ligament at C6–C7. For this reason, it is recommended that the semispinalis cervicis be left attached whenever possible.42,43 Laminoplasty techniques have been developed to spare the muscular and ligamentous attachments at the spinous processes. Despite this modification, 26.9% of patients continued to complain of axial neck pain postoperatively, and no good data yet exist to indicate that the extra time spent with this technique is beneficial to patient outcome.41 One retrospective study of double-door laminoplasty with reconstruction of the extensor musculature found that there was no influence in either the development or resolution of axial pain symptoms. This suggests that muscle reconstruction techniques may have merit in decreasing axial pain postoperatively after laminoplasty. It appears that preventing denervation of the paraspinal muscles by limiting dissection to the lateral border of the facet complex, reconstructing the extensor musculature, avoiding damage to the facet joint when making the lateral gutter hinges, and providing muscular exercise in the postoperative period all contribute to less axial neck pain.44
Fusion spontaneously occurs in patients after laminoplasty and may involve multiple levels. Intervertebral body fusion has been reported in 18% of patients who have undergone laminoplasty, and interlaminar fusion has been seen in 80% of patients who have undergone laminoplasty.45
Neck pain exists in up to 80% of patients after cervical laminoplasty, and seems to be greater in those who have more operated levels, and in those who have less range of motion postoperatively.46 This may be related to damage to the facet capsules, or the paravertebral muscles. The nuchal muscles may be reduced to 80% of their preoperative size in those patients who have undergone laminoplasty.47 This is likely due to denervation of the musculature from dissecting lateral to the facet joints, and may play a role in persistent postoperative neck pain. Neck pain appears to correlate with an imbalance in the ratio of strength between the flexor and extensor muscles in the postlaminoplasty state.48 Comparing laminoplasty to anterior fusion for the incidence of postoperative chronic neck and shoulder pain reveals the incidence is 60% in the laminoplasty group as compared with 19% in anterior cervical fusion.11
Occupational recovery after cervical laminoplasty for OPLL depends on the severity of preoperative myelopathy, and the degree of postoperative recovery. Return to work rates vary depending on job demands, with 95% of light sedentary laborers, 50% of standing laborers, and 35% of heavy laborers returning.49
Long-term follow-up of open-door laminoplasty indicates that the canal size increases 48% initially, and decreases slightly to 40% after 5 years. The average loss of motion on flexion is 35%, and the average loss of motion on extension is 57%.50 Approximately 8% of patients have preserved neck motion after laminoplasty. Ten years after laminoplasty by the double-door technique, 78% of patients maintain their initial improvement in degree of myelopathy.51 Causes of late deterioration in patients with OPLL who have undergone laminoplasty include thoracic myelopathy from ossification of the yellow ligament, minor trauma, and rarely progression of OPLL at the operative levels.2,45 Adjacent segment degeneration can occur after laminoplasty, but compared with anterior cervical fusion, laminoplasty may present fewer predispositions for development of adjacent segment degeneration.52
Types of Cervical Laminoplasty
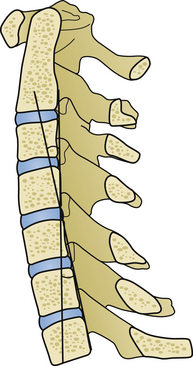
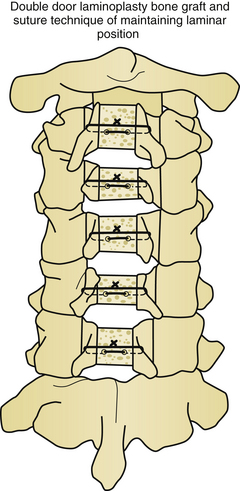
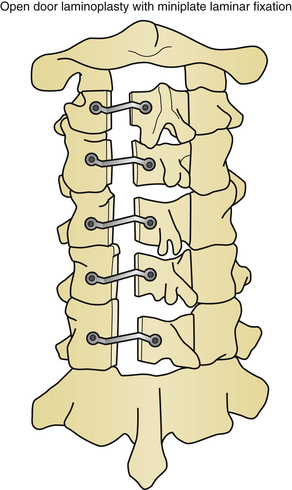
Five basic types of laminoplasty exist, but only three have gained in popularity. The earliest form of laminoplasty was called the Z-plasty type (Fig. 157-5). In this type of laminoplasty the spinous processes are removed, and the lamina are thinned to the inner cortical region. An opening is made along the long axis of the lamina, and two lateral troughs are made. The canal is augmented by then shifting the lamina and securing them to each other to maintain the new alignment. This procedure has been largely abandoned as technically demanding without superior results, as compared with the other techniques. The open-door or Hirabayashi-type laminoplasty utilizes a thinned hinge on one side of the lamina, and a complete cut through the lamina on the opposite side (Fig. 157-6). The double-door or Kurokawa-type laminoplasty uses hinges fashioned bilaterally just medial to the junction of the lamina and facet, and a midline bicortical cut (Fig. 157-7). In both the double-door and open-door types of laminoplasty, the bicortical cut area is opened and may be fixed by a variety of techniques to maintain the augmented position. En bloc laminoplasty is utilized in cases where it is desirable to operate inside the spinal canal. This necessitates the removal of the lamina, but in contrast to conventional techniques, the lamina are reconstructed. En bloc laminoplasty offers several advantages because it lessens postoperative scarring and facilitates reoperation. In en bloc laminoplasty, the lamina are removed as a whole by cutting bicortical slots just medial to the junction of the lamina and facet. The entire complex of spinous process, lamina, and intraspinous ligament are removed as a unit and replaced at the conclusion of the procedure. Foraminotomies have been added to each of these techniques to treat radiculopathy as well as myelopathy. A variety of techniques have been utilized to preserve muscle attachments to the spinous processes to aid in reconstruction of the posterior tension band.53
Techniques and Materials
Preservation Technique of the Posterior Cervical Elements in Laminoplasty
Along one side only, the lateral attachments to the spinous processes are dissected in a subperiosteal plane, exposing the lamina and facet complex. Next, an oscillating saw was used to cut the spinous processes approximately 5 mm from the base of the lamina. At this point, subperiosteal dissection is performed along the opposite lamina and facet complex to completely expose the lamina and facet complexes bilaterally. Care must be taken during this part of the dissection to avoid damaging either facet complex. The spinous processes, with their ligamentous attachments, are retracted to one side. Either a double- or single-door laminoplasty is then performed.54
Bony Dissection for Open-Door Laminoplasty
To perform an open-door laminoplasty, bone is dissected in two locations. Along one side, a unicortical cut is made to construct one hinge. On the other side, the lamina is cut bicortically to make the “door” that opens. The hinge can be fashioned on either side, which mandates that the bicortical cut is made on the opposite side. The location of the bicortical cut is at the junction of the lamina and facet complex. The hinge is made at the same location on the opposite side.55,56
Bony Dissection for Double-Door Laminoplasty
To perform a double-door laminoplasty, bone is dissected in three locations. Two hinges are constructed; they are located bilaterally at the junction of the lamina and facet complex. A central bicortical cut is made to split the spinous processes and lamina in the midline. With this technique, bilateral foraminotomies can be performed at each level if required.54,57,58
Constructing and Opening the “Hinge”
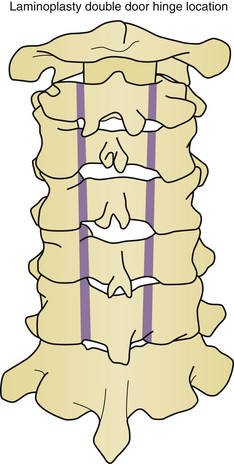
The hinge is key to overall success in any laminoplasty operation. A high-speed air drill, with either a diamond bit or bone-cutting bit, is used. The bit should be around 3 mm in diameter, and an acorn-type bone-cutting bit works well for this task (Fig. 157-8). When making the hinge, care must be taken so that the lamina is not thinned to the point that it fractures, or that it offers no spring-like resistance to deformation. This resistance holds the bone graft material in place. The proper resistance of the hinge is found by making the complete laminar cut first, then drilling the hinge side. The resistance is checked once the lamina is thinned from the outer cortex through the cancellous bone. Once the inner cortex of the lamina is identified, the resistance is checked with gentle finger pressure. The inner cortex of the lamina is thinned until gentle finger pressure opens the laminoplasty segment.12 When performing double-door laminoplasty, an interlaminar spreader may be used to open the hinges. This will probably be required if the spinous process has been resected, as in a posterior element-sparing technique. The ligamentum flavum must be opened, with care taken to avoid lacerating the epidural venous plexus, prior to rotating the lamina and opening the segment. This can be done in a variety of ways-with a dural guide, or simply with a scalpel.59 Bleeding can be lessened by using the bipolar electrocautery to coagulate the epidural veins during section of the ligamentum flavum.
Maintaining the Final Laminar Position
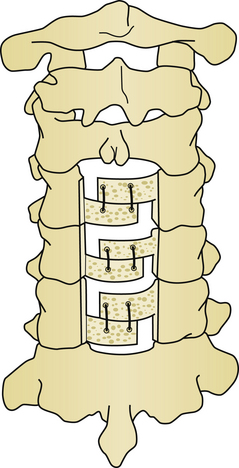
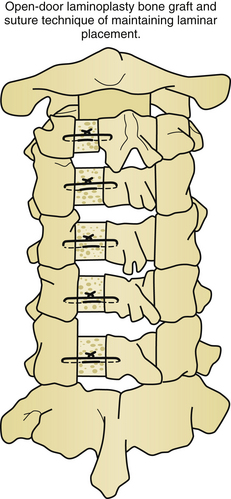
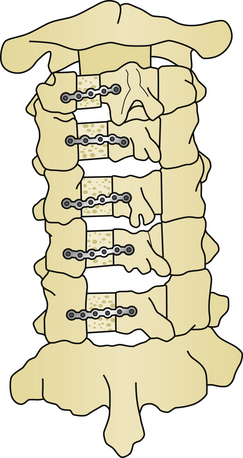
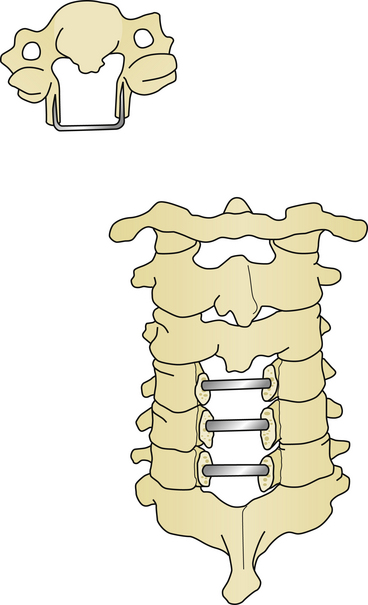
For long-term improvement of myelopathy, the laminae must remain in their altered position. A known complication is a phenomenon called “spring back.” This occurs when the canal augmentation is lost when the opened door closes postoperatively. Kyphosis appears to be the only significant risk factor for laminar closure after laminoplasty.60 Laminar closure appears to be related to operative failure in up to 38% of noninstrumented cases.25 Instrumentation lowers the complication rates in laminoplasty. Historically, there have been a variety of changes in technique and materials to maintain the augmented volume of the spinal canal. Originally, suture and fat graft were used to maintain expansion. Later, placing bone in the area of complete bicortical opening strengthened this region and helped maintain the expansion. Iliac crest, spinous process, and rib from autogenous or allograft sources, as well as custom designed hydroxyapatite spacers have all been utilized. Titanium miniplates from either standard sources or of custom design are useful for maintaining alignment (Figs. 157-9 and 157-10). Miniplates are extremely useful for fixating the bone graft to the opened area of the laminoplasty, thereby preventing the spring-back phenomenon. During the opening of the door in the laminoplasty procedure, the bony hinge can fracture. In the event of this complication, miniplates can be used to repair the fracture.61–63 Artificial titanium laminae have also been utilized for this procedure.64 In cases of open-door laminoplasty where no instrumentation is desired, commercially available suture anchors placed in the center of the lateral mass of the hinge side have been successful in maintaining canal volume augmentation.65 A nonabsorbable suture is passed through a hole drilled in the spinous process, and fixated either to a suture anchor, or to the facet capsule to maintain the altered position of the lamina. In double-door laminoplasty, holes are drilled with a right-angle drill through the apex of the spinous process and through the intervening bone graft. A nonabsorbable suture is passed through the holes and tied to maintain the bone graft between the split lamina. Titanium screws have replaced the role of suture in many cases.66 Suture anchors have been used in double-door laminoplasty where suture anchors are placed bilaterally in the lateral masses. A suture is passed through a hole drilled in the split lamina and through the suture anchor and then tied securing the split lamina in an open position.67,68 Titanium plates are now commercially available for use in double-door laminoplasty. These plates have the appearance of large staples and insert into each side of the cut edge of the opened lamina keeping the lamina apart and open69 (Fig. 157-11).
T-Laminoplasty
A modification of the double-door technique called the T-laminoplasty was reported in 1995, and is not to be confused with T-saw laminoplasty, although a T-saw may be used in the procedure. The name “T-laminoplasty” comes from the T shape of the resected spinous process that is used as a bone graft between the split lamina. In T-laminoplasty, a standard midline incision with bilateral subperiosteal dissection is performed over the affected levels, including the level immediately superior and inferior to the pathologic levels. The spinous processes are removed, leaving a 5-mm projection from the lamina. The lamina is cut in the midline using a small drill or craniotome, and the lateral lamina is thinned to the point of plastic deformation when force is placed laterally through the midline cut. Ligamentum flavum in the interlaminar spaces is removed. A 1-mm hole is drilled from medial to lateral in the lamina on each side. The saved, resected spinous processes are sutured into place utilizing the holes in the lamina.70
T-Saw Laminoplasty
T-saw laminoplasty was described by Tomita in 1998 and uses a threadwire saw to perform the midline cut as a modification to the double-door type of laminoplasty. This allows less bone loss, less operative time, less blood loss, and less chance of dural injury.69 The patient is positioned prone with the head neutral or lordotic to prevent kyphosis. The ligamentum flavum is resected and a protective sleeve is passed under the lamina. The wire saw is then passed under the lamina and using a reciprocating motion the lamina is split (Fig. 157-12). If kyphosis is present, cutting multiple levels in one pass is dangerous as the saw may bowstring into the canal, causing catastrophic neurologic injury. The outer cortex is then cut along the junction of the lamina and facet complex, taking care to preserve the facets. The lamina are then expanded and a spacer of bone or porous hydroxyapatite is tied into place with suture. Root injury has been reported with the T-saw technique, and occurred in the thoracic spine when the saw was threaded underneath a nerve root.71
En Bloc Laminoplasty
Laminoplasty can be used in a nonexpansible method. In this case, the laminae are replaced in their original positions. This approach is especially useful when intradural pathology is addressed through a laminectomy approach, particularly for benign tumor with a propensity for recurrence. The approach will be made easier in the event of reoperation, because there will be much less epidural scarring. This technique also allows for reconstruction of the posterior ligamentous tension band. Bicortical cuts are made at the junction of the lamina and facet processes, and the intraspinous ligament is cut, usually at one end only. A threadwire saw works well for the bilateral bony cuts, and by angling the saw from medial to lateral during the sawing process, a trapezoidal cut can be made. This trapezoidal cut prevents the lamina from sinking into the canal. A high-speed drill can also be used for this task, but it is more difficult to make a trapezoidal cut. The ligamentum flavum is then sectioned, and the laminae are moved from the operative field, allowing access to the spinal canal. To reconstruct the laminae, miniplates or sutures are used to fixate the laminae to their original positions. The intraspinous ligament is then reconstructed by suturing the spinous processes to the levels above and below in a figure-of-eight type of stitch.71
Lift-Up Laminoplasty
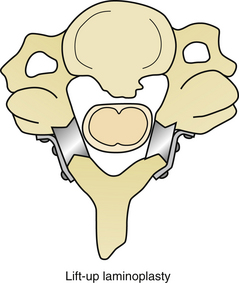
Lift-up laminoplasty is similar to en bloc laminoplasty with the addition of some type of support structure to “lift up” the lamina from their original position. A standard posterior approach with dissection to the medial facet complex is performed followed by an en bloc multilevel laminectomy. The intraspinous ligament is sectioned at the superior and inferior ends of the laminectomy. Soft tissue is removed from the ventral surface of the lamina and the intraspinous ligaments are left intact. Custom-designed spacers, or bone graft, can then be placed between the lamina and facet complexes and secured with miniplate fixation. Some surgeons use miniplate fixation only and omit placing any type of spacer. This type of construct lifts up the lamina augmenting the spinal canal volume. Bilateral foraminotomies can be performed with this technique, as well as any type of intradural procedure72,73 (Fig. 157-13).
Postoperative Care
Early ambulation is encouraged. Patients should be mobilized on the first day of surgery. They should be placed in a rigid cervical collar and maintained for 8 to 12 weeks. At that point, cervical spine radiographs with flexion extension views are performed to assess stability. MRI or computed tomography scans can be used to check canal volume augmentation if surgical outcome does not meet expectations.74
Limiting Complications of Laminoplasty
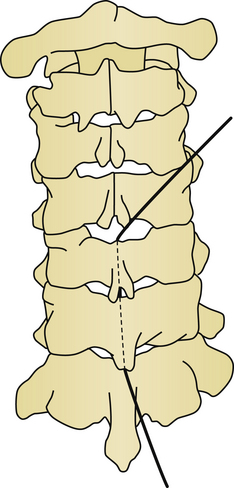
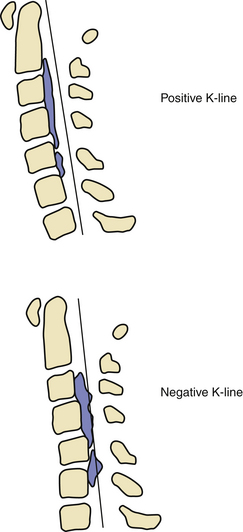
Kyphosis and large posterior projection of osteophytes are known factors for poor improvement of myelopathy after laminoplasty. If osteophyte or OPLL occupies more than 60% of the canal, then an anterior approach would offer a higher statistical chance of patient improvement.75 In cases of myelopathy caused by OPLL, it has been observed that the improvement from laminoplasty is low in cased where the K-line is negative. The K-line for kyphosis is a measurement of these two factors. The K-line is measured by taking the midpoint of the spinal canal at C2 and C7 and connecting these two points with a line. If the K-line contacts the posterior projection of the OPLL, then the K-line is defined to be negative, and 85% of patients in this group do not improve with laminoplasty76 (Fig. 157-14).
Laminoplasty complications are frequently associated with damage of posterior cervical musculature. Electrocautery-based dissection lateral to the facet complex frequently results in denervation of the adjacent muscle, this may lead to increased neck pain and kyphotic deformity of the spine. This complication can be lessened by limiting electrical dissection to the middle of the facet complex. Some surgeons have advocated a muscle splitting technique in open door laminoplasty so that on the hinged side of the construct the cervical musculature is spared.77,78 It is well established that removal of the C2 lamina in laminoplasty worsens postoperative alignment. If the semispinalis Cervicis is removed from C2 during dissection, it should be reattached. If laminoplasty includes C2 then efforts should be undertaken to reattach the semispinalis cervicus and the rectus capitus to the C2 spinous process. If it is impossible to reattach these muscles to the spinous process of C2, then it is recommended that a bilateral repair be performed where the rectus capitus be attached to the contralateral semispinlis cervicus.79,80 Dissection and damage to any of the facet capsules are to be avoided.
Axial neck pain is a frequently reported complication of laminoplasty. It is thought that by limiting the dissection from C3 to C6 instead of C7 that the incidence of neck pain is lessened. This appears to be an acceptable practice as long as more than 1 mm of subarachnoid space surrounds the cord at C7.81–83 One class II study found that performing a C3 laminectomy while sparing the muscular insertion to C2 combined with a C4–C7 laminoplasty greatly reduced the incidence of postoperative axial neck pain as compared to C3–C7 laminoplasty.84–86 A class I study determined that sparing the C7 spinous process and nuchal ligament significantly reduced axial neck pain over a 2-year period as compared to C3–C7 laminoplasty group.87
Patients with hypermobility of the cervical spine and the elderly tend to do poorly with laminoplasty. Other techniques should be considered should be given consideration in these cases.88
Handa Y., Kubota T., Ishii H., et al. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis: a retrospective comparison with younger patients. J Neurosurg (Spine 2). 2002;96:173-179.
Hirabayashi K., Watanabe K., Wakano K., et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983;8:693-699.
Iizuka H., Iizuka Y., Nakagawa Y., et al. Interlaminar bony fusion after cervical laminoplasty: its characteristics and relationship with clinical results. Spine. 2006;31(6):644-647.
Iseda T., Goya T., Nakano S., et al. Serial changes in signal intensities of the adjacent discs on T2-weighted sagittal images after surgical treatment of cervical spondylosis: anterior interbody fusion versus expansive laminoplasty. Acta Neruochir (Wien). 2001;143:710.
Kawaguchi Y., Kanamori M., Ishihara H., et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am. 2001;83:1798-1802.
Kawakami M., Tamaki T., Iwasaki H., et al. A comparative study of surgical approaches for cervical compressive myelopathy. Clin Orthop Rel Res. 2000;381:129-136.
Masaki Y., Yamazaki M., Okawa A., et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20(1):7-13.
Nagata K., Ohashi T., Abe J., et al. Cervical myelopathy in elderly patients: clinical results and MRI findings before and after decompression surgery. Spinal Cord. 1996;34:220-226.
Ono A., Tonosaki Y., Yokoyama T., et al. Surgical anatomy of the nuchal muscles in the posterior cervicothoracic junction: significance of the preservation of the C7 spinous process in cervical laminoplasty. Spine. 2008;33(11):E349-E354.
Ratliff J.K., Cooper P.R. Cervical laminoplasty: a critical review. J Neurosurg (Spine 3). 2004;98:230-238.
Satomi K., Nishu Y., Kohno T., Hirabayashi K. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine. 1994;19(5):507-510.
Takami T., Ohata K., Goto T., et al. Lift-up laminoplasty for myelopathy caused by ossification of the posterior longitudinal ligament of the cervical spine. Neurol India. 2004;52(1):59-63.
Wang M.Y., Green B.A. Open-door cervical expansile laminoplasty. Neurosurgery. 2004;54(1):119-123. discussion 123-4. Review
Yoshida M., Tamaki T., Kawakami M., et al. Does reconstruction of posterior ligamentous complex with extensor musculature decrease axial symptoms after cervical laminoplasty? Spine. 2002;27:1414-1418.
Yue W.M., Tan C.T., Tan S.B., et al. Results of cervical laminoplasty and a comparison between single and double trap-door techniques. J Spinal Disord. 2000;13:329-335.
1. Hirabayashi K., Watanabe K., Wakano K., et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983;8:693-699.
2. Satomi K., Nishu Y., Kohno T., Hirabayashi K. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine. 1994;19(5):507-510.
3. Nagata K., Ohashi T., Abe J., et al. Cervical myelopathy in elderly patients: clinical results and MRI findings before and after decompression surgery. Spinal Cord. 1996;34:220-226.
4. Nakano N., Nakano T., Nakano K. Comparison of the results of laminectomy and open-door laminoplasty for cervical spondylotic myeloradiculopathy and ossification of the posterior longitudinal ligament. Spine. 1988;13(7):792-794.
5. Ratliff J.K., Cooper P.R. Cervical laminoplasty: a critical review. J Neurosurg (Spine 3). 2004;98:230-238.
6. Sodeyama T., Gota S., Mochizsuki M., et al. Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine. 1999;24(15):1527-1532.
7. Hirabayashi K. Expansive open-door laminoplasty for cervical spondylotic myelopathy. Shujutsu. 1978;32:1159-1163.
8. Morimoto T., Yamada T., Okumora Y., et al. Expanding laminoplasty for cervical myelopathy-spinous process roofing technique. Acta Neurochir (Wien). 1996;138:720-725.
9. Iizuka H., Iizuka Y., Nakagawa Y., et al. Interlaminar bony fusion after cervical laminoplasty: its characteristics and relationship with clinical results. Spine. 2006;31(6):644-647.
10. Iizuka H., Nakagawa Y., Shimegi A., et al. Clinical results after cervical laminoplasty: differences due to the duration of wearing a cervical collar. J Spinal Disord Tech. 2005;18(6):489-491.
11. Hosono N., Yonenobu K., Ono K. Neck and shoulder pain after laminoplasty a noticeable complication. Spine. 1996;21:1969-1973.
12. Kuwaguchi Y., Matsui H., Ishihara H., et al. Axial symptoms after en bloc cervical laminoplasty. J Spinal Disord. 12, 1999. 392–365
13. Matsumoto M., Okada E., Ichihara D., et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine. 2010;35(1):36-43.
14. Wang M.Y., Green B.A., Vitarbo E., Levi A. Adjacent segment disease: an uncommon complication after cervical expansive laminoplasty: case report. Neurosurgery. 2003;53(3):770-773.
15. Edwards C.C., Heller J.G., Murakami H. Corpectomy versus laminoplasty for multilevel cervical myelopathy. Spine. 2002;27:1168-1175.
16. Ieasaki M., Ebara S., Miyamoto S., et al. Expansive laminoplasty for cervical radiculomyelopathy due to soft disc herniation: a comparative study of laminoplasty and anterior arthrodesis. Spine. 1996;21:32-38.
17. Kawakami M., Tamaki T., Iwasaki H., et al. A comparative study of surgical approaches for cervical compressive myelopathy. Clin Orthop Rel Res. 2000;381:129-136.
18. Yue W.M., Tan C.T., Tan S.B., et al. Results of cervical laminoplasty and a comparison between single and double trap-door techniques. J Spinal Disord. 2000;13:329-335.
19. Hasegawa K., Homma T., Chiba Y., et al. Effects of surgical treatment for cervical spondylotic myelopathy in patients greater than or equal to 70 years of age: a retrospective comparative study. J Spinal Disord Techniques. 2002;15:458-460.
20. Wada E., Suzuki S., Kanazwa A., et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy a long term follow-up study over 10 years. Spine. 2001;26:1443-1448.
22. White A.A., Panjabi M.M. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine. 1988;13:856-860.
23. Yoshida M., Tamaki T., Kawakami M., et al. Indication and clinical results of laminoplasty for cervical myelopathy caused by disc herniation with developmental canal stenosis. Spine. 1998;23:2391-2997.
24. Tani T., Ushida T., Ishida K., et al. Relative safety of anterior microsurgical decompression versus laminoplasty for cervical myelopathy with a massive ossified posterior longitudinal ligament. Spine. 2002;27:2491-2498.
25. Chung S.S., Lee C.S., Chung K.H. Factors affecting the surgical results of expansive laminoplasty for cervical spondylotic myelopathy. Int Orthop. 2002;26:334-338.
26. Fujimura Y., Nishi Y., Chiba K., et al. Multiple regression analysis of the factors influencing the results of expansive open-door laminoplasty for cervical myelopathy due to ossification of the posterior longitudinal ligament. Arch Orthop Trauma Surg. 1998;117:471-474.
27. Handa Y., Kubota T., Ishii H., et al. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis: a retrospective comparison with younger patients. J Neurosurg (Spine 2). 2002;96:173-179.
28. Lee T.T., Manzano G.R., Green B.A. Modified open door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86:64-68.
29. Mochida J., Nomura T., Chiba M., et al. Modified expansive open-door laminoplasty in cervical myelopathy. J Spinal Disord. 1999;12:386-391.
30. Suda K., Abumi K., Ito M., et al. Local kyphosis reduced surgical outcomes of expansive open-door laminoplasty for cervical spondylotic myelopathy. Spine. 2003;28:1258-1268.
31. Yamazaki T., Yanaka K., Sato H., et al. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52:122-126.
32. Kimura S., Homma T., Uchiyama S., et al. Posterior migration of cervical spinal cord between split lamina as a complication of laminoplasty. Spine. 1995;20:1284-1288.
33. Kawaguchi Y., Kanamori M., Ishihara H., et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am. 2001;83:1798-1802.
34. Yamazaki A., Homma T., Uchiyama S., et al. Morphologic limitations of posterior decompression by midsagittal splitting method for myelopathy caused by ossification of the posterior longitudinal ligament in the cervical spine. Spine. 1999;24:32-34.
35. Hoshi K., Kurokawa T., Nakamura K., et al. Expansive cervical laminoplasties: observations on comparative changes in spinous process lengths following longitudinal lamina divisions using autogenous bone or hydroxyapatite spacers. Spinal Cord. 1996;34:725-728.
36. Sasai K., Saito T., Akagi S., et al. Preventing C5 palsy after laminoplasty. Spine. 2003;28:1972-1977.
37. Uematsu Y., Tokuhashi Y., Matsuzake H. Radiculopathy after laminoplasty of the cervical spine. Spine. 1998;23:2057-2062.
38. Sakaura H., Hosono N., Mukai Y., et al. Segmental motor paralysis after cervical laminoplasty: a prospective study. Spine. 2006;31(23):2684-2688.
39. Chiba K., Toyama Y., Matsumoto M., et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine. 2002;27:2108-2115.
40. Kawaguchi Y., Kanamori M., Ishihara H., et al. Minimum 10-year follow-up after en bloc cervical laminoplasty. Clin Orthop Rel Res. 2003;411:129-139.
41. Kawakami M., Tamaki T., Ando M., et al. Preoperative instability does not influence the clinical outcome in patients with cervical spondylotic myelopathy treated with expansive laminoplasty. J Spinal Disord Techniques. 2002;15:277-283.
42. Iizuka H., Shimizu T., Tateno K., et al. Extensor musculature of the cervical spine after laminoplasty. Spine. 2001;26:2220-2226.
43. Sasai K., Saito T., Adagi S., et al. Cervical curvature after laminoplasty for spondylotic myelopathy—involvement of yellow ligament, semispinalis cervicis muscle, and nuchal ligament. J Spinal Disord. 2000;13:26-30.
44. Yoshida M., Tamaki T., Kawakami M., et al. Does reconstruction of posterior ligamentous complex with extensor musculature decrease axial symptoms after cervical laminoplasty? Spine. 2002;27:1414-1418.
45. Iwasaki M., Kawaguchi Y., Kimura T., Yonenobu K. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow-up. J Neurosurg (Spine 2). 2002;96:180-189.
46. Kawaguchi Y., Matsui H., Ishihara H., et al. Axial symptoms after en bloc cervical laminoplasty. J Spinal Dis. 1995;12:392-395.
47. Fujimura Y., Nishi Y. Atrophy of the nuchal muscle and change in cervical curvature after expansive open-door laminoplasty. Arch Orthop Trauma Surg. 1996;115:203-205.
48. Nakama S., Nitanai K., Oohashi Y., et al. Cervical muscle strength after laminoplasty. J Orthop Sci. 2003;8:36-40.
49. Kamizono J., Matsunaga S., Hayashi K., et al. Occupational recovery after open-door type laminoplasty for patients with ossification of the posterior longitudinal ligament. Spine. 2003;28:1889-1892.
50. Baba H., Maezawa Y., Furusawa N., et al. Flexibility and alignment of the cervical spine after laminoplasty for spondylotic myelopathy. Int Orthop. 1995;19:116-121.
51. Seichi A., Takeshita K., Ohishi I., et al. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine. 2001;26:479-487.
52. Iseda T., Goya T., Nakano S., et al. Serial changes in signal intensities of the adjacent discs on T2-weighted sagittal images after surgical treatment of cervical spondylosis: anterior interbody fusion versus expansive laminoplasty. Acta Neruochir (Wien). 2001;143:710.
53. Nakano N., Nalano T. Clinical results following enlargement of the cervical spinal canal by means of laminoplasty. Jpn Orthop Assoc. 1988;62:1139-1147.
54. Tani S., Isoshima A., Nagashima Y., et al. Laminoplasty with preservation of posterior cervical elements: surgical technique. Neurosurgery. 2002;50:97-102.
55. Morimoto T., Uranishi R., Nakase H., et al. Extensive cervical laminoplasty for patients with long segment OPLL in the cervical spine: an alternative to the anterior approach. J Clin Neurosci. 2000;3:217-222.
56. Nakano N., Nakano T. Clinical results following enlargement of the cervical spinal canal by means of laminoplasty. Jpn Orthop Assoc. 1988;62:1139-1147.
57. Kawakami M., Tamaki T., Ando M., et al. Relationships between sagittal alignment of the cervical spine and morphology of the spinal cord and clinical outcomes in patients with cervical spondylotic myelopathy treated with expansive laminoplasty. J Spinal Disord Techniques. 2002;15:391-397.
58. Koshu K., Tominaga T., Yoshimoto T. Spinous process splitting laminoplasty with an extended foraminotomy for cervical myelopathy. Neurosurgery. 1995;37:430-435.
59. Tsuji H. Laminoplasty for patients with compressive myelopathy due to so-called spinal canal stenosis in the cervical and thoracic regions. Spine. 1982;7:28-34.
60. Matsumoto M., Watanabe K., Tsuji T., et al. Risk factors for closure of lamina after open-door laminoplasty. J Neurosurg Spine. 2008;9(6):530-537.
61. Frank E., Keenan T. A technique for cervical laminoplasty using mini plates. Br J Neurosurg. 1994;8:197-199.
62. O’Brien M.F., Peterson D., Casey A.T., Crockard H.A. A novel technique for laminoplasty augmentation of spinal canal area using titanium miniplate stabilization. Spine. 1996;21:474-484.
63. Shaffrey C.I., Wiggins G.C., Piccirille C.B., et al. Modified open-door laminoplasty for treatment of neurological deficits in younger patients with congenital spinal stenosis: analysis of clinical and radiographic data. J Neurosurg (Spine 2). 1999;90:170-177.
64. Fornari M., Luccarelli G., Giombini S., Chiapparini L. Artificial lamina-assisted laminoplasty performed in seven cases. J Neurosurg (Spine 1). 1999;91:43-49.
65. Wang J.M., Roh K.J., Kim D.J., Kim D.W. A new method of stabilizing the elevated lamina in open-door laminoplasty using an anchor system. J Bone Joint Surg Br. 1998;80:1005-1008.
66. Takayasu M., Takagi T., Nishizawa T., et al. Bilateral open-door cervical expansive laminoplasty with hydroxyapatite spacers and titanium screws. J Neurosurg (Spine 1). 2002;96:22-28.
67. Miyata M., Neo M., Fujibayashi S., et al. Double-door cervical laminoplasty with the use of suture anchors: technical note. J Spinal Disord Tech. 2008;21(8):575-578.
68. Khan S.N., Edmonds E.W., Titelman R.M., et al. Using suture anchors for cervical laminoplasty: a reliable, safe, and simple technique. Am J Orthop. 2008;37(8):400-402.
69. Ono A., Tonosaki Y., Yokoyama T., et al. Artificial laminaassisted laminoplasty performed in seven cases. J Neurosurg (Spine 1). 1999;91:43-49.
70. Hamburger C. T-laminoplasty-A surgical approach for cervical spondylotic myelopathy: technical note. Acta Neurochir (Wien). 1995;132:131-135.
71. Hara M., Takayasu M., Takagi T., Yoshida J. En bloc laminoplasty performed with a threadwire saw: technical note. Neurosurgery. 2001;48:235-239.
72. Takami T., Ohata K., Goto T., et al. Lift-up laminoplasty for myelopathy caused by ossification of the posterior longitudinal ligament of the cervical spine. Neurol India. 2004;52(1):59-63.
73. Casha S., Engelbrecht H.A., DuPlessis S.J., et al. Suspended laminoplasty for wide posterior cervical decompression and intradural access: results, advantages, and complications. J Neurosurg Spine. 2004;1(1):80-86.
74. Edwards C.C., Heller J.G., Wilcox D.H. T-saw laminoplasty for the management of cervical spondylotic myelopathy. Spine. 2000;25:1788-1794.
75. Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 2. Advantages of anterior decompression and fusion over laminoplasty. Spine. 2007;32(6):654-660.
76. Fujiyoshi T., Yamazaki M., Kawabe J., et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine. 2008;33(26):E990-E993.
77. Hosono N., Sakaura H., Mukai Y., et al. En bloc laminoplasty without dissection of paraspinal muscles. J Neurosurg Spine. 2005;3(1):29-33.
78. Kotani Y., Abumi K., Ito M., et al. Minimum 2-year outcome of cervical laminoplasty with deep extensor muscle-preserving approach: impact on cervical spine function and quality of life. Eur Spine J. 2009. $ $[Epub ahead of print]
79. Takeshita K., Seichi A., Akune T., et al. Can laminoplasty maintain the cervical alignment even when the C2 lamina is contained? Spine. 2005;30(11):1294-1298.
80. Wang M.Y., Green B.A. Open-door cervical expansile laminoplasty. Neurosurgery. 2004;54(1):119-123. discussion 123–124
81. Higashino K., Katoh S., Sairyo K., et al. Preservation of C7 spinous process does not influence the long-term outcome after laminoplasty for cervical spondylotic myelopathy. Int Orthop. 2006;30(5):362-365.
82. Takeuchi K., Yokoyama T., Aburakawa S., et al. Postoperative changes at the lower end of cervical laminoplasty: for preservation of the C7 spinous process in laminoplasty. J Spinal Disord Tech. 2006;19(6):402-406.
83. Hosono N., Sakaura H., Mukai Y., et al. C3-6 laminoplasty takes over C3–7 laminoplasty with significantly lower incidence of axial neck pain. Eur Spine J. 2006;15(9):375-379.
84. Takeuchi K., Yokoyama T., Aburakawa S., et al. Axial symptoms after cervical laminoplasty with C3 laminectomy compared with conventional C3–C7 laminoplasty: a modified laminoplasty preserving the semispinalis cervicis inserted into axis. Spine. 2005;30(22):2544-2549.
85. Kato M., Nakamura H., Konishi S., et al. Effect of preserving paraspinal muscles on postoperative axial pain in the selective cervical laminoplasty. Spine. 2008;33(14):E455-E459.
86. Ono A., Tonosaki Y., Yokoyama T., et al. Surgical anatomy of the nuchal muscles in the posterior cervicothoracic junction: significance of the preservation of the C7 spinous process in cervical laminoplasty. Spine. 2008;33(11):E349-E354.
87. Takeuchi T., Shono Y. Importance of preserving the C7 spinous process and attached nuchal ligament in French-door laminoplasty to reduce postoperative axial symptoms. Eur Spine J. 2007;16(9):1417-1422.
88. Masaki Y., Yamazaki M., Okawa A., et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20(1):7-13.