Chapter 44 Cervical Laminectomy and Laminoforaminotomy
General Principles
Cervical spondylosis, disc herniation, and allied pathologies are a common cause of neurologic compression. Cervical spondylotic myelopathy is the most common cause of spinal cord dysfunction in adults.1
In spite of more than 40 years of evaluation of various techniques, there is no class I or class II evidence to strongly support one procedure over another, be it anterior or posterior, in the overall group of patients.2 The natural history of the compressive myelopathies varies, eroding the efficacy of procedural long-term outcomes.1 The origin of cervical spondylotic myelopathy appears to originate from two principle forces on the spinal cord and associated structures:
1. Reduction in the ventral/dorsal cervical canal volume leading to direct neurologic compression.
2. The dynamic forces (i.e., “stretch”) on the spinal cord during head motion in the presence of such compressive forces.3–5
In essence, the compressed spinal cord is stretched, or distracted, through a compromised canal, leading to damage in the spinal cord. Interestingly, the damage is often in the lateral cord region in early to moderate cases of myelopathy.4 It has long been felt that, given the frequent ventral location of the compressive spur, the procedure must be directed to that location. It appears that it is the combination of compressive and dynamic motion of the spinal cord that leads to compromised function. In light of the static (compressive) and dynamic forces involved in the genesis of myelopathy, all the ventral and dorsal surgical options that address either one, or both, of the involved factors have a role.4,5
Given the preceding two underlying factors (compression and motion) in the genesis of cervical spondylotic myelopathy (CSM), a wide variety of procedures are available to address the problem. As a primary goal, decompression should be achieved (i.e., the spinal canal volume enlarged). This can be accomplished with anterior discectomy-spurectomy and fusion, anterior corpectomy and fusion, cervical laminectomy, cervical laminoplasty, and cervical laminectomy and fusion.2–6 Each procedure has its attendant downside in the form of various complications. All have been shown to be effective and in the overall population of CSM patients, but no one procedure has clearly outclassed the other options.2 Cervical laminectomy for decompression of the spinal cord and/or nerve roots has been shown to be effective in the treatment of CSM.2,7 It addresses the compressive forces in CSM, but does not reduce the dynamic forces. Nevertheless, many patients do well with this option, and in appropriately selected patients the procedure is a safe, relatively simple, and effective option. A number of advantages can be ascribed to cervical laminectomy/decompression:
• A relatively simple technique with a moderate number of technical steps
• An effective means of decompressing an extensive rostral/caudal compression (two to five levels)
• No potential pseudarthrosis, as no fusion is included; similarly, no hardware-related failures or complications
• No hypermotility segmentation stress and delayed adjacent segment concerns because the motion of the spine is preserved (a motion-sparing procedure)
• Usable in elderly patients in whom osteoporotic bone may not favor successful hardware implantation
• Fairly rapid multilevel procedures possible, which may be advantageous in the patient with multiple subsystem issues (e.g., cardiac, renal) that increase perioperative risk
As with all surgical options in the treatment of CSM, there are a number of potential limitations:
• May represent same risk of delayed kyphosis over time secondary to loss of the posterior tension band (lamina and intraspinous ligaments).8 The exact incidence of postlaminectomy kyphosis is not well established, and the published reviews have generally been limited in patient numbers. Clinically relevant, as opposed to radiographically identified, incidental sagittal balance change is probably in the 5% to 10% range.8 This problem, to some degree, can be limited by proper technical performance9 (see section on technique).
• In light of the preceding point, cervical laminectomy should be limited to patients with reasonable lordosis, and not utilized in those with a frank kyphosis. Patients younger than age 20 are at greater risk for delayed cervical kyphosis after laminectomy.7,8
• In patients with advanced CSM, reducing the dynamic component of CSM by adding simultaneous dorsal instrumentation (arthrodesis may be beneficial, but at the time of this writing, the exact subgroup to benefit from this is uncertain).2,5
Patient Selection
Cervical laminectomy addresses the compressive aspects of CSM and associated disorders, but not the dynamic forces.2,5,7 If concerns arise regarding frank stability, or it is felt that the dynamic aspects must be addressed, simultaneous dorsal instrumentation and fusion can be utilized in addition to simple decompression. The decision to select one posterior option or another, and even the consideration of anterior options, is often a combination of science and surgeon preference and experience.2,5,7 Over the years, and over the course of 400 cases, I have used the following general guidelines:
A cervical laminectomy is considered in the following circumstances:
• For patients with multilevel (more than two) canal size reduction and reasonable lordosis
• In cases where decompressive laminectomy alone is used if an early to moderate myelopathy is present, but not dramatic signal change within the spinal cord on MRI (myelomalacia). In advanced cases of CSM, I generally favor simultaneous reduction of motion/dynamic forces by adding dorsal instrumentation (lateral mass screws) and fusion to the procedure. This preference is based on outcome trends, and no strong scientific data exist to bolster any dogmatic decision.
• Cervical laminectomy is a reasonable option in patients with a relatively “clean” canal (i.e., without dramatic ventral spurs, such as can be seen in congenital narrowing).
• A good option in the elderly multilevel CSM patients with poor bone stock and severe subsystem diseases that would increase morbidity in more complex procedures.
Technique
Positioning
Two basic positions are available: prone or sitting. A less common approach is lateral decubitus.
1. Prone—The patient is placed on appropriate padding material. The head is fixed in neutral position. Care must be taken to get a solid purchase in bone with the head holder because the weight of the head and neck in the prone position can lead to slippage with attendant lacerations and head shift. It is important to flex the knees to prevent migration of the patient on the table, which can lead to neck extension if the patient slides down the table during the procedure.
2. Sitting—Over the years, the trend had been to avoid the sitting position as inherently hazardous because of the risk of air embolization.10,11 Recently, a trend back to this option has been noted. Although air ingress can be seen in 5% to 8% of the sitting cases, clinically significant air embolization in sitting cervical cases is unusual.10,11 The risk of air embolization in cranial cases is higher because of the noncollapsible venous structures of the cranium. Over 1500 sitting cases accumulated in the literature attest to the safety, with proper technique, of the sitting position.10,11 The sitting position provides a fairly bloodless field due to dependent flow of blood to the bottom of the field and reduced epidural venous tension. Nerve root decompression via dorsolateral foraminotomy can be facilitated for this reason in the sitting position.
Monitoring
Historically, a panoply of monitoring devices is used for dorsal decompression.12,13 Over the last 10 years, I have gravitated to two peripheral intravenous catheters and a systemic arterial line if subsystem diseases suggest such a line may be beneficial. No Foley catheter is generally used since the procedure is brief (80–110 minutes). The literature supports the use of the sitting position without the use of a central line.10,11 I have not used central venous pressure lines in such cases in more than 10 years.
After many years of using electrophysiologic monitoring, I no longer use “routine” physiologic monitoring in standard decompressive laminectomies. There is extensive literature regarding the option of physiologic monitoring, but no clear scientific data or consensus exists.13 It may have a more credible role in tumor resections, spinal reconstruction, and so on, but even in these cases, there is sufficient variability to consider such monitoring on a case by case basis.
Incision and Dissection
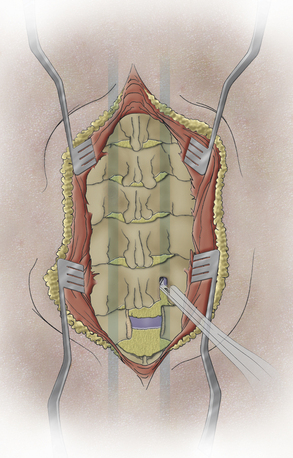
A skin incision adequate for the proposed decompression is utilized. Excessive attempts to limit the incision may jeopardize the ease and safety of deep dissection. A standard subperiosteal dissection is employed to just lateral to the laminar facet groove (Fig. 44-1). There is no need nor benefit to carrying the dissection more laterally into the facet/facet capsule because this only exacerbates the risk of delayed kyphosis. Remember that a small group of patients may have an occult spina bifida, so care must be taken to avoid dissection through such a midline breach.
Laminectomy
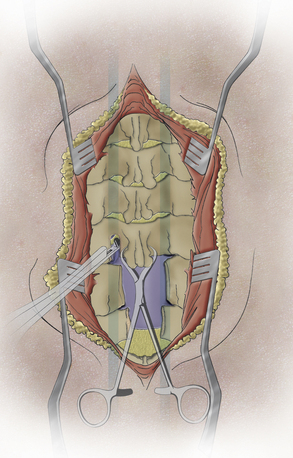
1. The trough is started at the caudal, lateral area of the lamina (Fig. 44-2). Be certain to begin the bone removal medial to the laminar facet groove. A more lateral dissection only leads to more facet damage and does not allow an effective trough to be fashioned through the lamina.
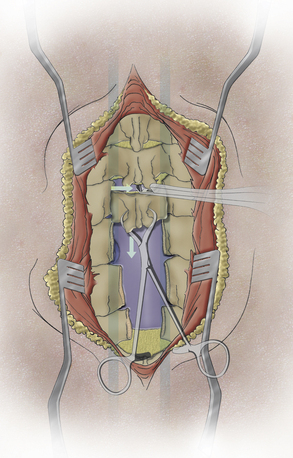
2. The Kerrison rongeur is carefully inserted, slowly and carefully fully approximating the inner cortical surface of the lamina. Note: No “bowling” is allowed, that is, no intrusion into the canal and pulling back to the bone. Whether a drill, Kerrison rongeur, or other instrument is used, pushing into the canal and pulling back to the inner bone surface can cause cord or root compression. The thin foot-plate, cervical Kerrison rongeur “walks” in a rostral direction using a 10 o’clock and 2 o’clock bone punch sequence. This alternating direction of each successive site of bone prevents the instrument from being trapped in the bone trough, which makes smooth movements more difficult. By staying in the lateral lamina, the likelihood of cord compression is reduced (Fig. 44-3).
3. The contralateral bone trough is fashioned, a medium-sized towel clip is inserted near the base of the spinous process, and gentle traction is applied inferior and dorsal to the wound, that is, “down and out” of the wound, which elevates the laminar segment safely off the spinal cord as the second trough completes the freeing of the lamina from its boney attachments. The ligamentum flavum is sectioned with the lamina gently tractioned off the dura. A Kerrison punch (2-mm thin foot plate) can be used to section the lamina and free it from its soft tissue attachments. Each laminar segment, or occasionally two lamina, can be removed en bloc segmentally. If the troughs have been properly placed, an “equator to equator” decompression of the spinal cord results, and the proximal nerve roots are just visualized.
Laminoforaminotomy
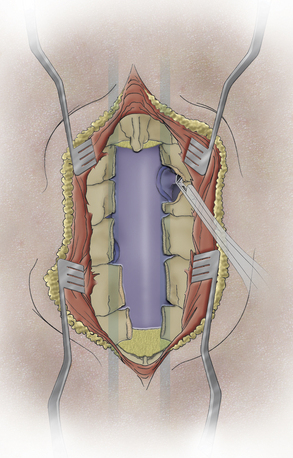
A moderate foraminotomy (3–4 mm) is generally completed or progresses until a microspatula easily passes the foramen (Fig. 44-4). If a specific radiculopathy is a concern, a more extensive laminoforaminotomy can be completed. The nerve root almost always arises within 4 to 5 mm rostrally or caudally to the facet articular line. The 2-mm Kerrison rongeur, (or drill and curette, etc.) can now be directed laterally, forming a series of successive “crescent moon” bone removals at the medial facet. As the proximal nerve is identified, simply tightly “hug” the bone with the foot plate (or curette) and remove bone from the rostral to caudal line of the nerve. (Again, the facet articular line is a good guideline as the center of the crescent moon.) A microspatula can be passed over the dorsal nerve surface to ensure adequate decompression of the nerve root. It is rarely necessary to remove more than 6 to 7 mm of the medial facet to accomplish this task.
Although I used to pursue the ventral root/bone area for spurs, the literature and experience suggest that a thorough foraminotomy alone (exclusive foraminotomy) is successful and limits the morbidity resulting from attempts at spur removal.9,10 A large soft disc can be approached via the axilla or the shoulder of the nerve depending on where the fragment predominates. A small group of patients have a conjoint nerve, so care must be taken when “sweeping” the nerve in the axilla to avoid damaging a conjoint nerve. The incision into a disc fragment should be very shallow (just enough to release the fragment). The vertebral artery is deep to the nerve and can be damaged. Hemostasis can be readily established (particularly in the sitting position) with judicious microbipolar use or a small piece of hemostatic agent.
Benzel E.C. Biomechanics of spine stabilization. Rolling Meadows, IL: American Association of Neurological Surgeons; 2001.
Fehlings M.G., Arvin B. Surgical management of cervical degenerative disease: the evidence related to indications, impact, and outcome. J Neurosurg Spine. 2009;11:97-100.
Henderson F.C., Geddes J.F., Vaccaro A.R., et al. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005;56:1101-1113.
Kaptain G.J., Simmons N.E., Replogle R.E., et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93:199-204.
Matz P.G., Anderson P.A., Holly L.T., et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:104-111.
Maurer P.K., Ellenbogen R.G., Ecklund J., et al. Cervical spondylotic myelopathy: treatment with posterior decompression and Luque rectangle bone fusion. Neurosurgery. 1991;28:680-683.
Mummaneni P.V., Kaiser M.G., Matz P.G., et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130-141.
1. Matz P.G., Anderson P.A., Holly L.T., et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:104-111.
2. Fehlings M.G., Arvin B. Surgical management of cervical degenerative disease: the evidence related to indications, impact, and outcome. J Neurosurg Spine. 2009;11:97-100.
3. Adams C.B., Logue V. Studies in cervical spondylotic myelopathy. II. The movement and contour of the spine in relation to the neural complications of cervical spondylosis. Brain. 1971;94:568-586.
4. Henderson F.C., Geddes J.F., Vaccaro A.R., et al. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005;56:1101-1113.
5. Maurer P.K., Ellenbogen R.G., Ecklund J., et al. Cervical spondylotic myelopathy: treatment with posterior decompression and Luque rectangle bone fusion. Neurosurgery. 1991;28:680-683.
6. Mummaneni P.V., Kaiser M.G., Matz P.G., et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130-141.
7. Ryken T.C., Heary R.F., Matz P.G., et al. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:142-149.
8. Kaptain G.J., Simmons N.E., Replogle R.E., et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93:199-204.
9. Benzel E.C. Biomechanics of spine stabilization. Rolling Meadows, IL: American Association of Neurological Surgeons; 2001.
10. Henderson C.M., Hennessy R.G., Shuey H.M.Jr., et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
11. Black S., Ockert D.B., Oliver W.C.Jr., et al. Outcome following posterior fossa craniectomy in patients in the sitting or horizontal positions. Anesthesiology. 1988;69:49-56.
12. Kelleher M.O., Tan G., Sarjeant R., et al. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: a prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8:215-221.
13. Resnick D.K., Anderson P.A., Kaiser M.G., et al. Electrophysiological monitoring during surgery for cervical degenerative myelopathy and radiculopathy. J Neurosurg Spine. 2009;11:245-252.