Chapter 42 Cervical Interbody Strut Techniques
Since the 1980s, extensive ventral decompression via corpectomy for cervical spondylotic myelopathy and spinal deformity has become routine.1 Although neurologic outcomes remain similar between multilevel anterior discectomy and corpectomy,2–5 certain clinical scenarios favor corpectomy (e.g., ossification of the posterior longitudinal ligament, trauma, osteomyelitis, neoplasms). Moreover, fusion rates after anterior decompression procedures across more than two disc levels may be higher for corpectomy than discectomy, particularly in uninstrumented cases.4,6,7 Therefore, spine surgeons must be comfortable with anterior decompression by corpectomy and also with the subsequent intervertebral strut grafting, the focus of this chapter.
Technologic advances now permit a wide variety of materials to be used as interbody devices; these newer products are covered elsewhere in this textbook (see Chapters 41 and 43). Furthermore, in the majority of clinical situations today, anterior corpectomy strut grafting is supplemented with anterior spinal plate instrumentation to reduce graft migration and enhance fusion rates.4,6–8 However, certain scenarios, for either clinical or logistical reasons, may dictate uninstrumented strut grafting. The techniques of interlocking bone grafting discussed in this chapter are most germane to the latter category of corpectomy cases. Even in instrumented strut grafts, however, some of the principles delineated here remain important for successful integration of the bone graft.
Fundamentals of Grafting
Three fundamental concepts need to be recognized for successful strut grafting. First is a clear understanding of the surgical objectives of the procedure in general. The primary goal for cervical spondylotic myelopathy typically is adequate and durable decompression of the neural elements. Although this generally would seem obvious, concerns over reconstruction can alter the operative plan and possibly subvert the primary goals of the surgery (Fig. 42-1). Ideally, the reconstruction must be fit to the decompression, and not vice versa.
The second essential component of strut grafting is an understanding of the factors affecting spinal stability (Box 42-1).9 An uninstrumented, unstable spine requires prolonged external bracing (e.g., halo brace or Minerva jacket). This is relatively independent of the surgical fusion technique. The stable spine reconstructed with a short-segment strut graft may be managed with a rigid cervical orthosis.
Bone Graft
Both the origin of the graft material and its proper handling are important considerations in bone graft selection. Autogenous iliac crest tends to fuse rapidly, which is a distinct advantage. Its incorporation, however, can be compromised by suboptimal harvesting techniques (see Chapter 123), osteoporosis, and injudicious tailoring. Technical constraints typically limit its use to replacing two or three vertebral segments. In fashioning iliac crest to the bony defect, it is ideal to preserve at least two contiguous cortical surfaces from one end of the graft to the other to optimize axial loading strength. Surgeons must also keep in mind the real complications associated with iliac crest harvest, which fortunately only rarely result in long-term problems.
With a fibular implant, however, there are different characteristics to consider: (1) it is a strong, circumferential cortical strut with a higher modulus of elasticity than mixed cortical–cancellous implants, and as such must be used with caution in the osteoporotic spine; (2) it can be tailored to any needed length; and (3) it provides a central channel for the packing of autograft cancellous bone to enhance fusion. The disadvantage of fibula is the mismatch of the density with that of the vertebral body. As a general rule, the receiving vertebra will fail before the fibula graft does. This generally results in “pistoning,” in which the fibula penetrates through the vertebral body and can even enter the next motion segment. Some subsidence may be unavoidable, especially in osteoporosis, but is usually of no significant clinical consequence (Fig. 42-2). Subsidence may theoretically be limited by using minimal distraction during graft placement and by using an orthotic brace postoperatively to limit flexion. Too much graft loading and excessive neck flexion early in recovery predispose to graft pistoning. Minimal disruption of the vertebral body graft bed site is also important in maintaining the final height of the fusion. A fibula grafted to a partial corpectomy will almost invariably result in substantial subsidence and loss of height. If necessary, an additional vertebral level may need to be resected to preserve the resistance to subsidence at the graft site.
Allograft fibula is slower to incorporate than autologous iliac crest.8 Autograft fibula is less commonly used owing to the increased operative times and blood loss and significant complications associated with its harvest.10 One method to enhance fusion but attenuate graft harvest morbidity is to use allograft fibula packed with autograft cancellous bone, taken from the iliac crest or from the resected corpectomy bone itself.11 Autogenous cancellous bone may be accessed via the superficial surface of the iliac crest through a 3-cm skin incision. The medial and outer surfaces of the iliac crest are not disturbed, as would be needed for the harvest of tricortical grafts. This ideally reduces blood loss and postoperative pain. A 1-cm cortical defect is created in the iliac crest with a high-speed bur, and cancellous bone is taken with a large curette. This, in turn, is packed into the central canal of the allograft fibula with a 3-mm diameter rod. No bone need be placed around the outside of the fibula strut after insertion.
Despite the differences between iliac crest structural autograft and fibular allograft, a significant difference in pseudarthrosis rates has not been consistently demonstrated.2,5,12,13
Strut Graft
Preparation of Vertebral Defect for Strut Grafting
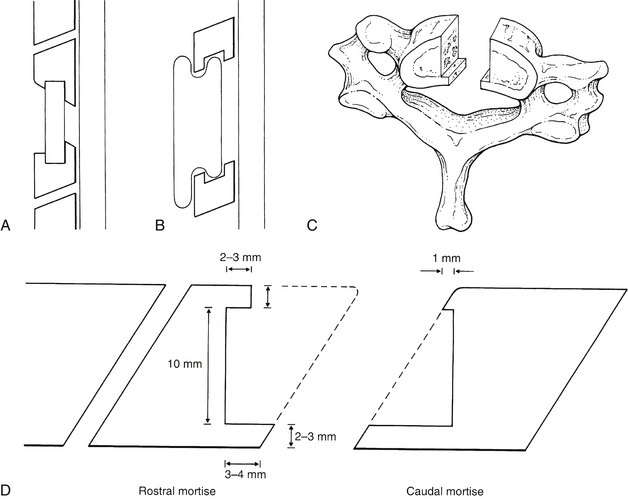
The paramount concern in preparing the vertebral end plates for arthrodesis is the prevention of graft displacement. Although plates and screws prevent graft displacement and improve graft incorporation, even instrumented grafts in rare cases can retropulse toward the spinal cord. The bed for the graft must be prepared in such a manner that the avenue toward the spinal canal is shorter or narrower than the graft itself. If graft migration were to occur, the direction should be away from the spinal cord. When anterior plating is used, deep slots or mortises in the vertebral body are limited by the need for adequate remaining vertebral body volume for screw purchase. When hardware insertion is not anticipated, spinal canal protection may be attained by one of four strategies (Figs. 42-3A–C): (1) the keystone mortise and tenon, (2) the dovetail technique, (3) the lateral step method, or (4) the anterior peg method of Niu et al.11 The primary focus here is on the keystone method.
Keystone Technique
The keystone graft method places the graft close to the middle column of the vertebral body. It is secured by means of mortises or slots in the opposing vertebral end plates (see Fig. 42-3A).
Proper preparation of the mortises in the keystone technique requires consideration of the angling of the cervical disc space (Fig. 42-3D). This disc space angling is the consequence of the ventral vertebral surface being slightly more caudal than the dorsal vertebral surface. The caudal mortise can be fashioned into the face of the vertebral end plate without removal of the anterior cortical corner of the vertebra. Thus, the sloping of this end plate away from the spinal canal provides the opportunity for creating the ideal mortise. The dorsal mortise lip is longer than its ventral counterpart. This ensures that any potential displacement of the graft occurs across the shallower ventral mortise lip. Because the caudal vertebral mortise can be readily fashioned with preservation of the cortical vertebral margins, this is the strongest mortise construct (see Fig. 42-3D).
Creation of the rostral mortise is more complex. Again, the critical consideration is the disc space angle. At the caudal end plate of the rostral mortise, the angle is such that to ensure a shorter ventral mortise lip, a portion of the anterior vertebral body must be resected. To avoid undue anterior resection while ensuring adequacy of the posterior mortise lip, appreciable resection of the dorsal vertebral margin in the decompression is precluded. Should any dorsal vertebral body decompression be pursued, the remaining vertebral body may be inadequate for proper mortising (see Fig. 42-3D).
Dovetail Technique
The dovetail grafting method refers to fashioning a segment of the graft that is placed ventral to the anterior vertebral surfaces and is longer than the length of the decompression defect. Dovetail refers to the bipartite shape of both ends of the graft, one slightly longer than the other. This is not unlike the tail of a dove (see Fig. 42-3B). The shorter of the two “tail feathers” at both ends is placed into matching slots drilled into the opposing end plates of the cephalic and caudal vertebrae. The rostral slot is of a depth such that the respective dovetail can be inserted to a depth that allows the clearance of the distal “tail” into its respective slot with moderate cervical traction. The graft, thus in place, is then shifted distally for a final locking-in position. The advantage of this construct is that it can be prepared in such a way that it is unequivocally too large to be displaced into the spinal canal. The disadvantages are that it can place excessive vertical loads on the ventral vertebral body cortex and may not allow significant impaction of the cancellous components of the graft and vertebra. In theory the graft is located within the anterior column. Therefore, vertebral failure may not be via impaction but via anterior displacement. Obviously, this construct does not lend itself to plating. Finally, the dovetail graft may lead to some increase in postoperative dysphagia given its position anterior to the anterior vertebral body walls.
Lateral Bone Step Technique
As described by Awasthi and Voorhies,1 lateral bone steps can be fashioned on either side of the anterior spinal canal, after completion of decompression by widening of the trough superficially (see Fig. 42-3C). The graft is then tailored so that it is wider than the width of the decompression and is placed superficial to the lateral steps. Potential disadvantages of this technique include possible inadequate canal decompression to maintain the posterior vertebral body wall steps and possible trapping of epidural bleeding behind the steps and graft with subsequent epidural hematoma (a fortunately rare complication14).
Preparation of the Strut Graft
The keystone graft (see Fig. 42-3A) is tailored for intimate lateral surface contact with the sides of the decompression trough. This fit should not require more than firm pressure for positioning. Forcefully hammering a slightly wide graft past a tight lateral contact point risks subsequent displacement by a lateral levering mechanism, which may occur with minimal neck movement. Width tailoring is usually accomplished with a high-speed bur or oscillating saw. A rongeur may cause cortical microfractures, which may lead to subsequent postoperative midshaft graft fracture. The width is repeatedly checked by placing both ends of the graft into the vertebral trough until a fit that allows no lateral play is achieved. By a similar tailoring sequence, the rostral tip of the graft is fashioned to fit its mortise exactly. Because the ventral mortise lip is foreshortened deliberately, the strut can be angled into the mortise, and the fit can be assessed before the final determination of the strut length. After the exact graft width and rostral fit have been determined, the length is ascertained by marking the caudal aspect of the graft with the graft fully positioned rostrally while manual cervical traction is applied. Traction will usually provide at least 1 mm of trough distraction. This, in turn, results in the graft marked 1 mm longer than the defect. The caudal mortise graft fit is then tailored similar to the rostral end. The graft can then be put into place, rostral end first, using firm pressure or very light tapping with a small mallet. It is important to advance the caudal portion of the graft into the trough until it contacts the posterior mortise and lies deep to the anterior mortise lip. Caution should be used to avoid overdistracting the spine, resulting in “too tight” a fit, because this may predispose it to increased axial loading and fracture of the caudal vertebrae.
Similarly, the dovetail graft (see Fig. 42-3B) is fastidiously tailored. However, as already noted, the strategy of locking the graft by caudal engagement requires a greater vertebral slot or mortise depth. Because tailoring of the anterior mortise lips, as in the keystone method, is not necessary with the dovetail technique, the anterior vertebral cortical edges should ensure the utmost vertebral resistance to fracture. A cortical surface of the iliac crest graft should be placed toward the depth of the decompression to ensure a strong graft construct. The positioning of the cortical margin of the bone graft within the confines of the vertebral body (i.e., dorsal to the ventral vertebral body margins) helps minimize the chance of ventral bone graft migration. Because the fibula has a tendency to cut or penetrate into its receiving vertebral bodies, it should be used sparingly for this purpose.
Complications of Strut Grafting
Strut grafts across the cervicothoracic junction are subjected to unique forces due to the long lever arm of the thoracic cage.15 The potential for fracture (either of the vertebra, strut, or both) with the use of a multisegment intervertebral graft may be significant and merits consideration of anterior plating with or without supplemental posterior instrumentation.
In general, correction of kyphotic deformity is ideal in anterior reconstructions, in particular to prevent the spinal cord from draping over the ventral apex of a kyphotic spine.16 Nevertheless, forceful correction of such a deformity often loads anterior strut grafts substantially, subsequently risking graft complications. In these situations, segmental posterior instrumentation should be strongly considered.
Complications of anterior cervical approaches in general are discussed elsewhere in this volume (see Chapters 35 and 40). Fortunately, hematoma and infection of the anterior neck are uncommon, but there are no specific management schemes unique to strut grafting for preventing these complications. The use of a suction drain for 24 hours may lessen their incidence. Persistent severe neck pain early in the course of recovery should raise concern for infection. Infection, especially after the first postoperative week, should prompt suspicion of esophageal leakage. Infection alone may not require graft or hardware removal, but typically incision and drainage are necessary.
Graft Displacement
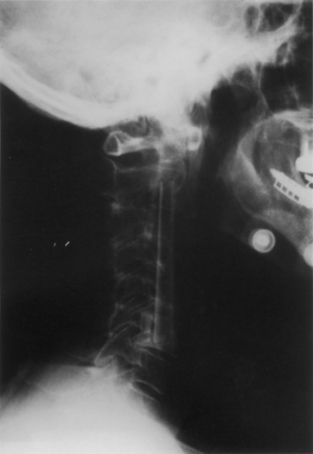
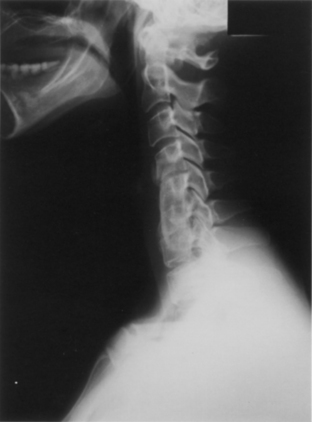
Displacements and displaced graft fractures almost always occur early after surgery and are usually best handled by repeat surgery. The incidence of graft migration increases with the number of levels involved and the proximity to the cervicothoracic junction.15 Displacement alone often reflects a technical error and is often accompanied by an associated vertebral fracture (Fig. 42-4). Vertebral fractures are usually caudal and, unless minor, will require extending the fusion across the next motion segment. This does not require further decompression but does require a new strut and the creation of a bed across the fractured vertebra. In such a situation, many surgeons will opt for anterior plating, prolonged bracing, or circumferential fixation after graft revision.
Graft Pistoning
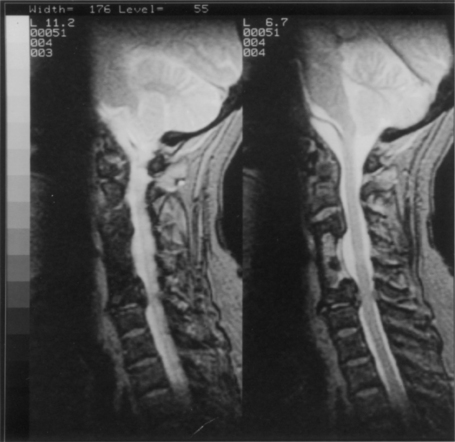
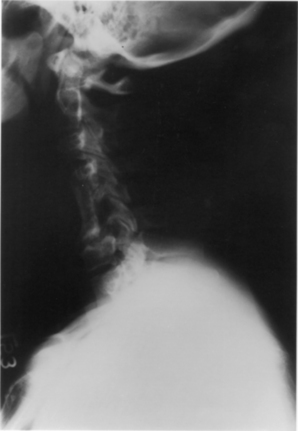
As mentioned above, subsidence, or pistoning, is frequently seen to at least a minor extent, particularly when fibula is used (see Fig. 42-2).17 It is important to avoid circumferential sharpening of the fibula strut ends. This may in part be averted by not overdistracting at the time of graft insertion. The degree of penetration may appear alarming on a radiograph. An average of 6 to 7 mm of settling of the fibula strut graft is typical after two- or three-level corpectomy; this has been reported to have no impact on postoperative pain, neurologic outcomes, or fusion rates.16,17 When the graft penetrates into the adjacent disc space (frequently the caudal disc space), the options are to observe clinically ot to revise the graft. Anecdotally, both approaches may result in good outcomes.
Graft Angulation
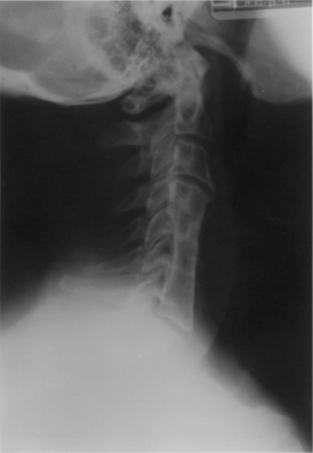
Graft angulation, usually at the rostral mortise, occurs infrequently. The incidence of this complication is not necessarily proportional to strut graft length (Fig. 42-5). Clearly, plating should minimize the incidence of this problem. The revision of an angulated graft is not typically necessary. Extensive bracing with a halo vest or Minerva jacket may be the most appropriate first line of treatment.
Pseudarthrosis
Late complications of strut grafts include pseudarthrosis (Fig. 42-6) and midshaft graft fracture18,19 (Fig. 42-7). If these are associated with compressive osteophyte formation or persistent neck pain, a posterior instrumented arthrodesis is a viable revision option. Ventral revision of a pseudarthrosis is also feasible. However, a simultaneous posterior fusion and stabilization procedure may be prudent if stability has been significantly threatened. Anterior revision of a midgraft fracture requires a new strut graft and plating. Late graft fractures may heal with the passage of time alone (see Fig. 42-7).
The clinical significance of pseudarthrosis after strut grafting is as uncertain as it is after an anterior cervical dissection and fusion. The incidence of this complication in uninstrumented cases has been reported as less than 5% (typically single-level cases with autograft) to as high as 30% (higher rates associated with multilevel corpectomies and possibly with allograft).2,5,12,13 Instrumentation clearly improves fusion rates4,6 and tends to eliminate differences between autograft and allograft.8,20 Late recurrent myelopathy may occur in these patients due to a pseudarthrosis with hypertrophic changes or new adjacent-segment disease.2
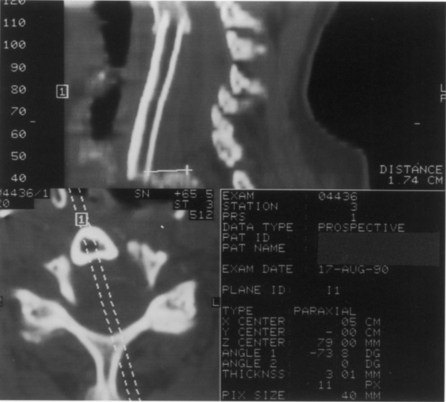
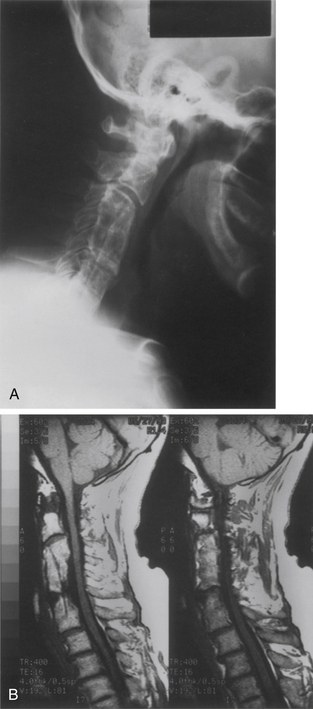
Treatment options after radiographic pseudarthrosis are largely based on clinical symptomatology. If pseudarthrosis is associated with intolerable neck pain, the patient may be offered a posterior arthrodesis. When the etiology of persistent neck pain is unclear, a posterior exploration of segmental motion may be undertaken and instrumented arthrodesis employed if abnormal mobility is found. This latter strategy is plausible during the period of anterior graft immaturity in the first year postoperatively. Radiographic findings after this time on CT and flexion/extension x-rays are such that a determination of nonunion is somewhat more straightforward (Fig. 42-8). Patients with fibrous unions and late graft fractures can potentially be relieved of persistent neck pain by successful posterior fusion.
Emery S.E., Bohlman H.H., Bolesta M.J., Jones P.K. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two- to 17-year follow-up. J Bone Joint Surg [Am]. 1998;80:941-951.
Hankinson T.C., O’Toole J.E., Kaiser M.G. Multilevel ACDF vs. corpectomy for cervical kyphosis. In: Mummaneni P.V., Lenke L.G., Haid R.W., editors. Spinal deformity: a guide to surgical planning and management. St. Louis: Quality Medical, 2008.
Hughes S.S., Pringle T., Phillips F., Emery S. Settling of fibula strut grafts following multilevel anterior cervical corpectomy: a radiographic evaluation. Spine (Phila Pa 1976). 2006;31:1911-1915.
Ikenaga M., Shikata J., Tanaka C. Anterior corpectomy and fusion with fibular strut grafts for multilevel cervical myelopathy. J Neurosurg Spine. 2005;3:79-85.
Nirala A.P., Husain M., Vatsal D.K. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. Br J Neurosurg. 2004;18:227-232.
Saunders R.L. Anterior reconstructive procedures in cervical spondylotic myelopathy. Clin Neurosurg. 1991;37:682-721.
Wang J.C., Hart R.A., Emery S.E., Bohlman H.H. Graft migration or displacement after multilevel cervical corpectomy and strut grafting. Spine (Phila Pa 1976). 2006;28:1016-1021.
1. Saunders R.L. Anterior reconstructive procedures in cervical spondylotic myelopathy. Clin Neurosurg. 1991;37:682-721.
2. Emery S.E., Bohlman H.H., Bolesta M.J., Jones P.K. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to 17-year follow-up. J Bone Joint Surg [Am]. 1998;80:941-951.
3. Hankinson T.C., O’Toole J.E., Kaiser M.G. Multilevel ACDF vs. corpectomy for cervical kyphosis. In: Mummaneni P.V., Lenke L.G., Haid R.W., editors. Spinal deformity: a guide to surgical planning and management. St. Louis: Quality Medical; 2008:257-290.
4. Mummaneni P.V., Kaiser M.G., Matz P.G., et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130-141.
5. Nirala A.P., Husain M., Vatsal D.K. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. Br J Neurosurg. 2004;18:227-332.
6. Fraser J.F., Hartl R. Anterior approaches to fusion of the cervical spine: a meta-analysis of fusion rates. J Neurosurg Spine. 2007;6:298-303.
7. Galler R.M., Dogan S., Fifield M.S., et al. Biomechanical comparison of instrumented and uninstrumented multilevel cervical discectomy versus corpectomy. Spine (Phila Pa 1976). 2007;32:1220-1226.
8. Eleraky M.A., Llanos C., Sonntag V.K. Cervical corpectomy: report of 185 cases and review of the literature. J Neurosurg. 1999;90:35-41.
9. White A.A., Panjabi M.M. Clinical biomechanics of the spine, ed 2, Philadelphia: Lippincott-Raven, 1990.
10. Nassr A., Khan M.H., Ali M.H., et al. Donor-site complications of autogenous nonvascularized fibula strut graft harvest for anterior cervical corpectomy and fusion surgery: experience with 163 consecutive cases. Spine J. 2009;9:893-899.
11. Niu C.C., Hai Y., Fredrickson B.E., Yuan H.A. Anterior cervical corpectomy and strut graft fusion using a different method. Spine J. 2002;2:179-187.
12. Fernyhough J.C., White J.I., LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine (Phila Pa 1976). 1991;16:S561-S564.
13. Ikenaga M., Shikata J., Tanaka C. Anterior corpectomy and fusion with fibular strut grafts for multilevel cervical myelopathy. J Neurosurg Spine. 2005;3:79-85.
14. Lee J.Y., Schwartz D.M., Anderson D.G., Hilibrand A.S. Epidural hematoma causing dense paralysis after anterior cervical corpectomy. A report of two cases. J Bone Joint Surg [Am]. 2006;88:198-201.
15. Wang J.C., Hart R.A., Emery S.E., Bohlman H.H. Graft migration or displacement after multilevel cervical corpectomy and strut grafting. Spine (Phila Pa 1976). 2006;28:1016-1021.
16. Herman J.M., Sonntag V.K. Cervical corpectomy and plate fixation for postlaminectomy kyphosis. J Neurosurg. 1994;80:963-970.
17. Hughes S.S., Pringle T., Phillips F., Emery S. Settling of fibula strut grafts following multilevel anterior cervical corpectomy: a radiographic evaluation. Spine (Phila Pa 1976). 2006;31:1911-1915.
18. Hanks S.E., Kang J.D. Late stress fracture of a well-incorporated autologous fibula strut graft in the cervical spine: a case report. J Spinal Disord Tech. 2004;17:526-530.
19. Jones J., Yoo J., Hart R. Delayed fracture of fibular strut allograft following multilevel anterior cervical spine corpectomy and fusion. Spine (Phila Pa 1976). 2006;31:E595-E599.
20. Epstein N.E. Reoperation rates for acute graft extrusion and pseudarthrosis after one-level anterior corpectomy and fusion with and without plate instrumentation: etiology and corrective management. Surg Neurol. 2001;56:73-80.