Chapter 232 Cervical Disc Herniation with Radiculopathy
Anterior Cervical Discectomy with Interbody Fusion
Anterior cervical discectomy and fusion (ACDF) is a common and accepted treatment for the patient with cervical disc herniation with radiculopathy. Successful results have been reported since the original descriptions of the operative techniques of Smith and Robinson1 and Bailey and Badgley2 and the refined techniques and instruments developed by Cloward.3,4 Although single-level ACDF has a generally high fusion rate by several techniques, argument continues as to the added benefit of plating for treatment of single-level disease. Proponents of plating cite reduction in interbody graft migration, diminished rates of pseudarthrosis, improved immediate postoperative segmental rigidity, reduction in kyphotic angulation, and the lack of need for cervical orthosis.
Case for Fusion without Plating
Satisfactory Clinical Outcomes without Plating
Samartzis et al.5 demonstrated an increased fusion rate in noninstrumented patients (100%) using autologous iliac crest compared to patients undergoing ventral plating (90.3%), though the finding was noted not to be statistically significant. Cervical plating may theoretically produce detrimental stress shielding of the segment that is intended to undergo arthrodesis. Distractive forces imparted by a rigid plate-screw construct may reduce desirable compressive stress at the graft–end-plate interface and could contribute to delayed fusion and pseudarthrosis.6–8
Epstein9 reviewed her series of 178 patients undergoing ACDF with autograft iliac crest bone grafting. Dynamic radiographs and computed tomography showed fusion or pseudarthrosis without motion in 99% of patients undergoing single-level surgery.
Use of autologous iliac crest, once considered the gold standard for interbody fusion material, is known to carry significant graft site complications. In light of this, many surgeons have turned to the use of allogeneic bone sources and have employed cervical instrumentation as a means to augment fusion rates out of concern for increased risk of pseudarthrosis. However, multiple reports indicate favorable radiographic and clinical outcomes with allogeneic grafting material independent of the presence of fixation. Martin et al.10 reviewed 317 cases of ACDF surgery using freeze-dried fibular allograft without ventral plating. They reported a 90% fusion rate in single-level surgeries in a population of which more than 25% were habitual smokers.
Jagannathan et al.11 reviewed 170 patients undergoing single-level ACDF using a modified Cloward technique with freeze-dried allograft. Rigid cervical orthosis was not utilized, though patients were offered soft cervical collars for comfort. They reported a 94% fusion rate and 96% of patients demonstrating favorable neurologic outcomes.
Matz et al.12 recently performed an excellent evidence-based review of the current literature with regard to various techniques for the treatment of anterior cervical nerve root compression. They identified 17 prior studies that compared ACDF with and without fixation and concluded that the addition of an anterior cervical plate did not improve long-term clinical outcomes but may reduce pseudarthrosis rates and graft-related problems.
Increased Cost Associated with Fixation
In today’s ever cost-conscious health-care climate, the added expense of cervical instrumentation cannot be completely ignored. Angevine et al.13 performed a retrospective cost analysis with regard to the addition of an anterior cervical plate following ACDF with allograft. Outcome in this cost-effectiveness analysis was measured in quality-adjusted life-years (QuaLYs). The improvement in quality of life following ACDF surgery with allograft alone came at a cost of $496 per QuaLY. The additional gains following ACDF with plating increase the cost to $32,560 per QuaLY.
Branch has previously reported on the increased cost of ACDF with plating versus ACDF alone.14 Sources of increased cost include increased operating time required for placement of fixation, surgical fees, and charges for hardware costs. The hospital charges for single-level ACDF with plating were approximately $3123 higher than charges for similar cases without internal fixation.
A recent European study examined the cost effectiveness of various surgical treatments of single-level cervical radiculopathy.15 Patients were allotted to one or four groups: cage only, autograft and plating, cage with allograft particles and plating, and disc arthroplasty. All groups had similar improvements in neck and arm pain scores, and all patients who underwent fusion procedures demonstrated radiographic evidence of fusion at 1 year. The average total cost was least in the cage-only group (≤1930), which was the most cost-effective treatment option for single-level ACDF.
Alternatives to Ventral Cervical Plating
Anchored Interbody Spacers
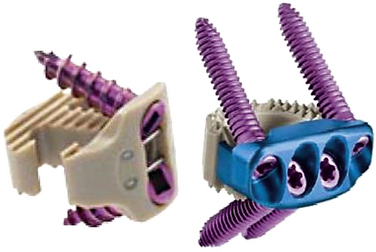
Several recent devices have come to market that share the properties of being a low-profile interbody spacer that allows for fixation to the adjacent vertebral bodies without a formal anterior cervical plate (Fig. 232-1). The polyetheretherketone (PEEK) Prevail cervical interbody device (Medtronic Sofamor Danek, Memphis, TN) is an interbody device with rostral and caudal midline flanges that allow for a screw interface with the ventral corner of the adjacent vertebral body. The device utilizes nitinol wires as a locking mechanism to prevent screw backout.
The Zero-P device (Synthes, West Chester, PA) has similar properties but does not require preparation of the anterior surface of the vertebral body. This “anchored spacer” consists of a polyetheretherketone interbody cage with a ventral titanium housing that allows for anchoring screws to be placed diagonally into the adjacent vertebral bodies. Scholz et al.16 recently examined the biomechanical properties of this stand-alone cervical interbody device. This device demonstrated decreased range of motion compared to interbody cage alone (no fixation) and no significant difference when compared to the addition of a locking or dynamic anterior cervical plate.
Cervical Total Disc Arthroplasty
In the absence of significant coexisting cervical disc disease at other levels, total disc arthroplasty is another option for treatment of cervical disc herniation with radiculopathy. Mummaneni et al.17 recently presented the 3- to 5-year results of a prospective randomized trial of ACDFP versus arthroplasty with the Prestige device (Medtronic Sofamor Danek). Of 541 patients included in the study, 276 underwent discectomy and disc arthroplasty. Neck disability index and neck pain scores were significantly better in the arthroplasty group at 3 years but were similar to the ACDFP group at 5 years. The arthroplasty devices maintained a mean of 7.1 degrees of flexion-extension motion, and the arthroplasty group had fewer secondary surgeries than did the ACDFP group.
Angevine P.D., Zivin J.G., McCormick P.C. Cost-effectiveness of single-level anterior cervical discectomy and fusion for cervical spondylosis. Spine (Phila Pa 1976). 2005;340:1989-1997.
Branch C.L.Jr. Anterior cervical fusion: the case for fusion without plating. Clin Neurosurg. 1999;45:22-24.
Jagannathan J., Shaffrey C.I., Oskouian R.J., et al. Radiographic and clinical outcomes following single-level anterior cervical discectomy and allograft fusion without plate placement or cervical collar. J Neurosurg Spine. 2008;8:420-428.
Matz P.G., Ryken T.C., Groff M.W., et al. Techniques for anterior cervical decompression for radiculopathy. J Neurosurg Spine. 2009;11:183-197.
Mummaneni PV, Haid RW Jr., Traynelis VC, et al. 3-year and 5-year follow up from the prospective randomized US FDA IDE trial of the Prestige cervical disc arthroplasty. Presented at the 25th Annual Meeting of the AANS/CNS Section on Disorders of the Spine and Peripheral Nerves. March 11–14, 2009. Phoenix, AZ.
Schloz M., Reyes P.M., Schleicher P., et al. A new stand-alone cervical anterior interbody fusion device. Biomechanical comparison with established anterior cervical fixation devices. Spine (Phila Pa 1976). 2009;34:156-160.
1. Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
2. Bailey R.Q., Badgley C.E. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg [Am]. 1960;42:565-594.
3. Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
4. Cloward R.B. The anterior surgical approach to the cervical spine: the Cloward Procedure: past, present, and future. The presidential guest lecture, Cervical Spine Research Society. Spine. 1988;13:823-827.
5. Samartzis D., Shen F.H., Lyon C., et al. Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J. 2004;4:636-643.
6. Wang J.C., McDonough P.W., Endow K.K., et al. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467-471.
7. Shapiro S. Banked fibula and the locking anterior cervical plate in anterior cervical fusion following cervical discectomy. J Neurosurg. 1996;84:161-165.
8. Capen D, Rah A, Nagelberg S, et al. Clinical significance of instrumented versus noninstrumented anterior cervical discectomy and fusion. Paper presented at the Annual Meeting of the North American Spine Society; October 25, 1997; New York.
9. Epstein N.E. Anterior cervical discectomy and fusion without plate instrumentation in 178 patients. J Spinal Disord. 2000;13:1-8.
10. Martin G.J., Haid R.Q., MacMillan M., et al. Anterior cervical discectomy with freeze-dried allograft. Overview of 317 cases and literature review. Spine (Phila Pa 1976). 1999;24:852-859.
11. Jagannathan J., Shaffrey C.I., Oskouian R.J., et al. Radiographic and clinical outcomes following single-level anterior cervical discectomy and allograft fusion without plate placement or cervical collar. J Neurosurg Spine. 2008;8:420-428.
12. Matz P.G., Ryken T.C., Groff M.W., et al. Techniques for anterior cervical decompression for radiculopathy. J Neurosurg Spine. 2009;11:183-197.
13. Angevine P.D., Zivin J.G., McCormick P.C. Cost-effectiveness of single-level anterior cervical discectomy and fusion for cervical spondylosis. Spine (Phila Pa 1976). 2005;340:1989-1997.
14. Branch C.L.Jr. Anterior cervical fusion: the case for fusion without plating. Clin Neurosurg. 1999;45:22-24.
15. Bhadra A.K., Raman A.S., Casey A.T.H., et al. Single-level cervical radiculopathy: clinical outcome and cost-effectiveness of four techniques of anterior cervical discectomy and fusion and disc arthroplasty. Eur Spine J. 2009;18:232-237.
16. Schloz M., Reyes P.M., Schleicher P., et al. A new stand-alone cervical anterior interbody fusion device. Biomechanical comparison with established anterior cervical fixation devices. Spine (Phila Pa 1976). 2009;34:156-160.
17. Mummaneni PV, Haid RW Jr, Traynelis VC, et al. 3-year and 5-year follow up from the prospective randomized US FDA IDE trial of the Prestige cervical disc arthroplasty. Presented at the 25th Annual Meeting of the AANS/CNS Section on Disorders of the Spine and Peripheral Nerves. March 11–14, 2009. Phoenix, AZ.
Anterior Cervical Discectomy with Interbody Fusion with Plating
Anterior cervical discectomy with interbody fusion (ACDF) is a very common surgical procedure that can be used to treat multiple cervical pathologies. ACDF surgery is commonly used to treat radiculopathy and myelopathy due to degenerative cervical disease. ACDF was initially done without instrumentation and demonstrated very good results in treating radiculopathy and myelopathy.1 However, pseudarthrosis and graft extrusion are known complications of ACDF.2 To address these complications, plating was added to ACDF, as first described by Bohler and Gaudermak in 1964.3
Description of Procedure
The following is a description of our technique for ACDF.
The patient is placed supine on the operating room table. Once the anesthesiologist’s endotracheal tube and lines and are appropriately secured, the patient’s head should be placed in a neutral position or in slight extension. This can be done with a small shoulder roll or by dropping the head of the table. The head can be placed on a donut to secure it in position or in traction if desired. The shoulders should be pulled down toward the feet either with tape or with wrist straps if necessary to visualize the lower cervical levels. To avoid injury to the brachial plexus, care should be taken not to pull too tightly. Electrophysiologic monitoring can be used to minimize the risk for iatrogenic injury due to positioning, decompression, and instrumenting as desired by the primary surgeon.4
External landmarks can be used to estimate cervical spine levels, which should then be confirmed with fluoroscopy. The hyoid bone is close to the C2-3 interspace. The top of the thyroid cartilage is close to C3-4, while the inferior border can be estimated as C4-5. The cricoid ring is roughly at the C5-6 level. One finger breadth above the clavicle is estimated as C7-T1.5
The neck is then prepped and draped in the usual sterile fashion. A transverse incision over the level of interest should be adequate for most surgical exposures, though if a particularly long ventral construct is being performed, then a longitudinal incision along the medial border of the sternocleidomastoid muscle provides greater exposure.6
The transverse incision should be just off midline and extend laterally to the sternocleidomastoid. The incision is normally performed on the left side to reduce the risk of injury to the recurrent laryngeal nerve, though it is unclear whether this actually reduces the risk.7 The skin is opened with a knife, exposing the platysma. The platysma is then divided and undermined. After the sternocleidomastoid (SCM) has been identified and the carotid has been palpated, a plane medial to SCM and carotid sheath and lateral to the trachea and esophagus should be identified. With the use of blunt dissection, this avascular plane is followed down to prevertebral fascia. The omohyoid muscle may obstruct this approach at the C5 level and can be divided without significant consequences (Fig. 232-2).
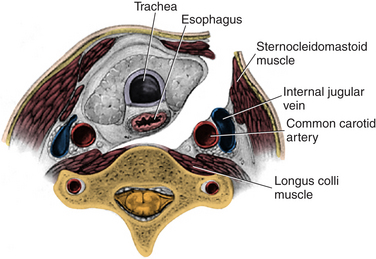
FIGURE 232-2 The corridor for anterior cervical discectomy and fusion approaches.
(Used with permission from Barrow Neurological Institute.)
By using cautery, the longus colli muscles are then detached medially to laterally, and the retractor system can be placed. Placement of the retraction blades on the longus colli muscles is important to reduce risk of injury to the underlying carotid and esophagus. One theory about injury to the recurrent laryngeal nerve is that it is a compressive injury of the endolaryngeal segment of the nerve between the retractor and the endotracheal tube rather than a direct dissection injury. Apfelbaum et al. reported that if the endotracheal tube cuff was deflated after the retractor blades were placed and was then reinflated, their rate of injury went from 6.4% to 1.7%.8
The graft is then inserted into the disc space. Autograft or allograft can be used. Autograft has a slightly higher fusion rate, quoted as being between 83% and 97%, but there is the risk for donor site complications, and the allograft fusion rate of 82% to 94% is nearly as good.9 The graft is slightly oversized and the vertebral bodies are slightly distracted so that the graft is under compression, which helps with fusion. Distracting the vertebral bodies can be done with distractor pins, gentle cervical traction, or disc spacers. Care is taken not to oversize the graft so that the vertebral bodies are not overdistracted. Once the graft is in place, the distraction force is removed, and a radiograph is taken to evaluate alignment and graft position.
The plate should be placed in the midline—straight up and down. Unicortical screws are often used. The screws should be toed in, or directed medially in the horizontal direction, and toed out, or directed away from the graft, in the vertical direction to help reduce pull-out. The screws should not violate the end plate, as this will predispose the patient to adjacent-segment disease (Fig. 232-3).
Complications
ACDF surgery has been shown to have a great deal of success in treating cervical radiculopathy, but there are some well-known complications. One study quoted the following complication rate: recurrent laryngeal nerve palsy, 3.1%; postoperative dysphagia, 9.5%; postoperative hematoma, 5.6%; dural perforation, 0.5%; esophageal perforation, 0.3%; wound infection, 0.1%; and hardware failure, 0.1%.10 With knowledge of these complications, care can be taken to avoid them.
Plate versus Uninstrumented
The act of placing a plate in ACDF adds to operative time, cost, and blood loss. Many studies show that patients get an excellent fusion in single-level ACDF without instrumentation. Fuse rates for single-level surgery have been quoted as being greater than 90% with or without plating.11 However, plating is recommended in patients who are having an operation on multiple levels, as the pseudarthrosis rate is significantly higher in noninstrumented patients. A study by Wang et al. shows a pseudarthrosis rate of 25% in two-level ACDF patients without instrumentation and no pseudarthrosis in patients with plating.12 Also, some patients are unable to wear hard collars; by placing the plate over the graft and screwing it into the vertebral body, the orthosis is moved interiorly so that collars or halos are often not required.
Dynamic versus Fixed
During the last 20 years, there have been many developments in the design of the plating systems. The different plating systems have different retractors and distractors but, most important, different screw plate interfaces. The major choice in selection of the plate system is whether a dynamic or fixed system is chosen. In a fixed system, the screw-plate interface is constrained. This provides for a more stable construct, but it also stress-shields the graft and diminishes load sharing between plate and bone graft. Grafts are best incorporated into constructs when they are subjected to compressive forces. This can be achieved by oversizing the graft and distracting the vertebral bodies, but a fixed anterior cervical plate will still shield the graft from the normal daily compressive forces that help bones to remodel (Fig. 232-4).
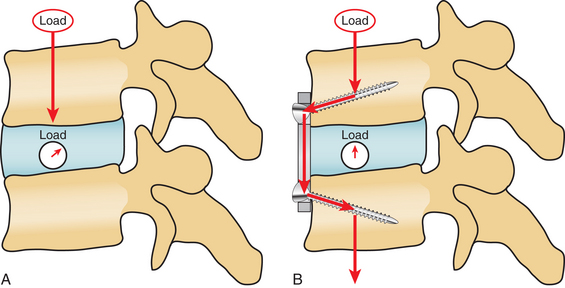
FIGURE 232-4 Biomechanical differences of force vectors in the uninstrumented (A) and instrumented (B).
(From Rhee JM, Riew KD: Dynamic anterior cervical plates. J Am Acad Orthop Surg 15:640–646, 2007.)
Dynamic plating systems allow movement at the screw-plate interface. This may decrease the rigidity of the construct, but it allows compressive forces to run through the graft. In theory, this should help to promote fusion. However, several studies comparing the fusion rate between dynamic and fixed plate systems show similar fusion rates. One study demonstrated slightly better outcomes from a pain perspective with the dynamic plates,13 but in general, the few studies that have been completed showed minimal difference between fixed and dynamic plates.14
Bartolomei J., Sonntag V. Anterior approach including cervical corpectomy. In Youmans J., editor: Neurological surgery, ed 5, Philadelphia: WB Saunders, 2004.
Fountas K.N. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976). 2007;32:2310-2317.
Kilburg C., Sullivan H.G., Mathiason M.A. Effect of approach side during anterior cervical discectomy and fusion on the incidence of recurrent laryngeal nerve injury. J Neurosurg Spine. 2006;4:273-277.
Kim S.H., Sebastian C.T., Nathan D.J., et al. Operative techniques: anterior cervical decompression, fusion, and instrumentation. In: Kim D.H., Ludwig S.C., Vaccaro A.R., editors. Atlas of spine trauma: adult and pediatric. Philadelphia: WB Saunders, 2008.
Rhee J.M., Riew K.D. Dynamic anterior cervical plates. J Am Acad Orthop Surg. 2007;15:640-646.
Smith G.W., Robinson R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
1. Smith G.W., Robinson R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
2. Connolly P.J., Esses S.I., Kostuik J.P. Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
3. Bohler J., Gaudernak T. Anterior plate stabilization for fracture-dislocations of the lower cervical spine. J Trauma. 1980;20:203-205.
4. Slimp J.C. Electrophysiologic intraoperative monitoring for spine procedures. Phys Med Rehabil Clin North Am.. 2004;15:85-105.
5. Bartolomei J., Sonntag V. Anterior approach including cervical corpectomy. In Youmans J., editor: Neurological surgery, ed 5, Philadelphia: WB Saunders, 2004.
6. Kim S.H., Sebastian C.T., Nathan D.J., et al. Operative techniques: anterior cervical decompression, fusion, and instrumentation. In: Kim D.H., Ludwig S.C., Vaccaro A.R., editors. Atlas of spine trauma: adult and pediatric. Philadelphia: WB Saunders, 2008.
7. Kilburg C., Sullivan H.G., Mathiason M.A. Effect of approach side during anterior cervical discectomy and fusion on the incidence of recurrent laryngeal nerve injury. J Neurosurg Spine. 2006;4:273-277.
8. Apfelbaum R.I., Kriskovich M.D., Haller J.R. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976). 2000;25:2906-2912.
9. Samartzis D. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451-459.
10. Fountas K.N. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976). 2007;32:2310-2317.
11. Samartzis D., Shen F.H., Lyon C., et al. Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J. 2004;4:636-643.
12. Wang J.C., McDonough P., Endow K., et al. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2000;25:41-45.
13. Saphier P.S., Arginteanu M.S., Moore F.M., et al. Stress-shielding compared with load-sharing anterior cervical plate fixation: a clinical and radiographic prospective analysis of 50 patients. J Neurosurg Spine. 2007;6:391-397.
14. Rhee J.M., Riew K.D. Dynamic anterior cervical plates. J Am Acad Orthop Surg. 2007;15:640-646.
Anterior Cervical Discectomy with Artificial Disc Spacer
Over the past few decades, ventral cervical spine decompression and fusion surgery using the Smith-Robinson technique1 has become commonplace. Anterior cervical discectomy allows direct decompression of the spinal canal and neural foramen in the treatment of myelopathy and cervical radiculopathy.1,2 The question arises, “What should be placed in the disc space after total discectomy?”
Currently, most surgeons prefer to perform anterior cervical discectomy with fusion (ACDF) using an interbody graft supplemented with an anterior ventral plate to restore disc height, provide immediate structural stability, and maintain cervical lordosis. ACDF produces highly predictable, favorable, and reproducible results.3–5 However, limitation of the cervical range of motion and the potential of accelerating adjacent-segment disease (ASD) are drawbacks with ACDF. Biomechanical6 and clinical7–10 data both demonstrate limited range of motion and probable progression of ASD in the cervical spine after arthrodesis. Hilibrand et al.8 demonstrated an ASD rate of 2.9%/year following ACDF. Other studies by Katsuura et al.9 and Goffin et al.7 reported cumulative ASD rates as high as 30% to 50% over several years.
The advantage of disc arthroplasty is the ability to preserve motion while maintaining stability.11 Theoretically, the maintenance of motion after discectomy results in lower stresses on the adjacent disc space. Two-year data available from Food and Drug Administration (FDA) trials shows that artificial disc spacers maintain normal segmental motion at the index surgical level and have a lower reoperation rate than ACDF.12–14 Further studies are pending regarding the long-term benefits of disc arthroplasty as it relates to ASD.
Indications and Contraindications for Cervical Arthroplasty
The first cervical arthroplasty devices were approved in the United States by the FDA in 2007 to treat single-level disc herniations or single-level cervical spondylosis in adult patients.13,14 This approval was based on Investigational Device Exemption (IDE) trials conducted by the FDA and on European data. The European experience has included use of cervical arthroplasty in single-level as well as multilevel disease. There are currently ongoing trials for multilevel cervical arthroplasty in the United States.15
Relative contraindications for cervical arthroplasty include patients with cervical kyphosis or spondylosis with incompetent facets. Flexion-extension cervical radiographs should be performed before consideration of disc arthroplasty. Patients with more than 3.5 mm of subluxation should not undergo placement of the device. Arthroplasty should not be considered in cervical trauma with ligamentous or facet injury. Osteoporosis or metabolic conditions that cause weakening of the bone are also important relative contraindications, as these processes can lead to subsidence of the device.12–14
Preoperative Planning
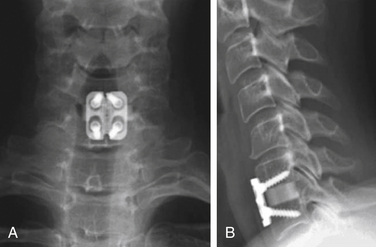
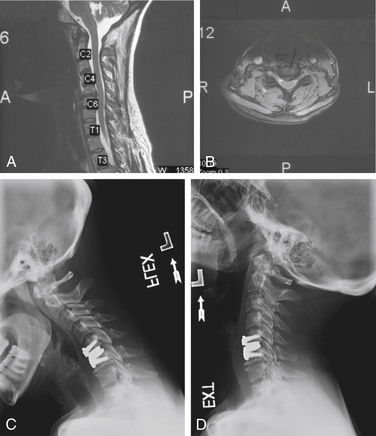
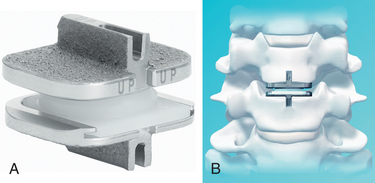
Before considering arthroplasty, the surgeon should review all appropriate imaging including anteroposterior (AP), lateral, flexion-extension radiographs, MRI, and/or CT of the cervical spine (Figs. 232-5A and B). There are several arthroplasty devices to choose from in the United States (three are currently FDA approved for single-level placement).12–14 The Prestige ST (Medtronic, Memphis, TN) incorporates a stainless steel ball-and-trough design. Vertebral body screws are used to fix the device’s position in the interspace. The device is considered semiconstrained with regard to motion (Fig. 232-6). The ProDisc-C (Synthes Spine, Paoli, PA) device has two cobalt chrome alloy end plates and an ultra-high-molecular-weight polyethylene inlay in a ball-and-socket configuration. Instead of vertebral screws, it uses a central keel for primary fixation. This device is also semiconstrained (Fig. 232-7). The third device approved by the FDA is the Bryan Cervical Disc (Medtronic). This artificial disc spacer consists of two titanium alloy shells articulating with a polyurethane core. It is held in the disc space with only a tight fit. Bone ingrowth eventually bonds the metallic device faces to the vertebrae. The device is considered unconstrained16 (Fig. 232-8).

FIGURE 232-6 The Prestige ST artificial disc spacer.
(Courtesy of Medtronic Sofamor Danek, Memphis, TN)

FIGURE 232-8 The Bryan Cervical Disc artificial disc spacer.
(Courtesy of Medtronic, Memphis, TN; the Bryan Cervical Disc System incorporates technology developed by Gary K. Michelson, MD.)
Patient Counseling
The risks of implant failure, new or residual radiculopathy, migration, subsidence, and reoperation should also be discussed. Many devices contain stainless steel, which can cause significant artifact on MRI scans. Patients should be aware of the possible need to undergo a more invasive CT myelogram instead of MRI for workup of new neck problems. Second-generation artificial discs that contain components that are MRI-compatible are currently undergoing FDA IDE clinical trials
Intraoperative Technique
Sizing and Placement of the Prestige ST Artificial Disc
Following the discectomy and removal of the posterior longitudinal ligament and bilateral uncovertebral joints, the distraction pins are removed, and an implant trial is placed snugly in the disc space to confirm the appropriate arthroplasty size. It is important to ensure that the front lipping osteophytes are burred flat to the ventral surface of the vertebral body prior to placing the trials. Avoid oversizing the trial because an oversized artificial disc may limit the normal range of motion. True AP and lateral fluoroscopic radiographs are obtained to ensure that the trial fits and is centered in the disc space. Ideally, the trial (and subsequently the arthroplasty device) should occupy over 80% of the vertebral end plate. The Prestige ST disc is inserted, care being taken to ensure that it remains centered in the midline. Four holes are drilled through the attached drill guide, and the vertebral body screws are placed through the screw apertures in the device. The inserter is removed, and the vertebral screws are tightened. Locking screws are placed to prevent backout of the vertebral body screws. Again AP and lateral fluoroscopy is used to confirm position and size of the artificial disc. The platysma and skin are then closed in a layered fashion, and a small soft tissue drain may be left in place subfascially overnight. After wound closure and before the patient is taken from the operating room, the neck is manually flexed and extended on the operating room table while the implant is visualized by using lateral fluoroscopy to ensure proper motion of the device.13
Postoperative Treatment
Patients are started on nonsteroidal anti-inflammatory drugs on postoperative day one, and this medication is continued for 2 weeks to lower the rate of heterotopic ossification. Most patients are discharged the day after surgery without the use of a cervical immobilization collar. Lateral, AP, and flexion-extension radiographs are performed at routine follow-up appointments to assess motion. (see Figs. 232-5C and D).
Complications
Implant migration is a rare potential problem. Migration of a few millimeters is often tolerated. However, conversion to fusion should be considered for significant migration or if the patient develops new symptoms from implant migration.12–14
Summary
Ventral cervical discectomy and fusion and posterior foraminotomy are both proven and effective methods to treat a cervical disc herniation. Posterior foraminotomy has excellent results for lateral disc herniations that cause radicular pain, especially when done in a minimally invasive fashion.17–20 ACDF is an effective treatment for central and paracentral disc herniations, radial osteophytes, and uncovertebral joint spurs.3–5
Advancements in the technique and implants used in ACDF have increased the fusion rates, reduced postoperative immobilization, and improved clinical results.8,21 Although ACDF benefits the target level, it may accelerate degeneration of the adjacent levels.7–10 ASD following ACDF may result in another surgical procedure. The reported incidence of symptomatic ASD after ACDF ranges from 2.2% to 25%.3,8,10,22 Hilibrand et al.8 reported that surgery is necessary in 2.9% of patients per year owing to symptomatic ASD after ACDF.
Cervical disc arthroplasty has the potential to maintain anatomic disc space height, normal segmental lordosis, and physiologic motion patterns following surgery. These characteristics may help to reduce or delay the onset of degenerative disc disease at adjacent levels.23–26 Attempts at placement of artificial disc spacers date back to the 1990s.25 European and Australian clinical trials have demonstrated improved clinical outcomes with disc arthroplasty compared to ACDF using validated clinical measurements.24,27–31
The recent FDA IDE trials (evaluating the Prestige ST, ProDisc-C, and Bryan Cervical Disc) show comparative effectiveness of arthroplasty in comparison to ACDF.12–14 The Prestige ST trial showed an improved neurologic success rate, shorter return-to-work time (16 day difference), and greater improvement in the neck disability index for patients undergoing arthroplasty compared to those who had a fusion. The complication rates between the groups were comparable. All patients in the arthroplasty group maintained device motion at 2 years. There was a statistically significant lower rate of secondary or adjacent-level surgeries in the arthroplasty group during the trial.13
The ProDisc-C trial also showed a statistically significant difference in the number of secondary surgeries required in the ACDF group (8.5%) compared to the investigational group (1.8%) at 2 years. Patients maintained motion of the device on radiographic follow-up. There was statistically significant improvement in neurologic outcomes compared to preoperative values. However, there was no statistical difference between the arthroplasty and fusion groups in neurologic success at the 2-year time point.14
The Bryan cervical disc has also completed 2 years of follow-up in an FDA IDE trial. In a recent publication, investigators found that the investigational group had a statistically greater improvement in primary outcome variables compared to the control group (ACDF). Also there was a lower rate of implant-associated adverse events in the investigational group. Patients who received the artificial disc spacer returned to work nearly 2 weeks earlier than the patients who underwent fusion.12
Five-year and 10-year data will be needed to validate the excellent 2-year results seen in these trials. The ultimate goal is to stabilize the involved segment, preserve motion, and mimic the kinematics of the healthy disc without changing the shear stress at the unoperated level. Early results indicate that cervical arthroplasty might provide the benefits of neural decompression without the drawback of placing adjacent motion segments at risk for accelerated degeneration.12–14 The encouraging results from recent FDA IDE trials will likely continue to drive the development and use of arthroplasty devices in the future.
Goffin J., Geusens E., Vantomme N. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79-85.
Heller J.G., Sasso R.C., Papadopoulos S.M., et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976). 2009;34:101-107.
Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
Mummaneni P.V., Burkus J.K., Haid R.W., et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198-209.
Murrey D., Janssen M., Delamarter R., et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275-286.
1. Smith G.W., Robinson R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
2. Hoff J.T., Wilson C.B. Microsurgical approach to the anterior cervical spine and spinal cord. Clin Neurosurg. 1979;26:513-528.
3. Bohlman H.H., Emery S.E., Goodfellow D.B., Jones P.K. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298-1307.
4. Gore D.R., Sepic S.B. Anterior cervical fusion for degenerated or protruded discs. A review of one hundred forty-six patients. Spine (Phila Pa 1976). 1984;9:667-671.
5. Robinson R.A., Walker A.E., Ferlic D.C., et al. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg [Am]. 1962;44:1569-1587.
6. Eck J.C., Humphreys S.C., Lim T.H., et al. Biomechanical study on the effect of cervical spine fusion on adjacent level intradiscal pressure and segmental motion. Spine (Phila Pa 1976). 2002;27:2431-2434.
7. Goffin J., Geusens E., Vantomme N. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79-85.
8. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
9. Katsuura A., Hukuda S., Saruhashi Y. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J. 2001;10:320-324.
10. Robertson J.T., Papadopoulos S.M., Traynelis V.C. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417-423.
11. Albert T.J., Eichenbaum M.D. Goals of cervical disc replacement. Spine J. 2004;4:S292-S293.
12. Heller J.G., Sasso R.C., Papadopoulos S.M., et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976). 2009;34:101-107.
13. Mummaneni P.V., Burkus J.K., Haid R.W., et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198-209.
14. Murrey D., Janssen M., Delamarter R., et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275-286.
15. Pimenta L., McAfee P., Cappuccino A., et al. Superiority of multilevel cervical arthroplasty outcomes versus single-level outcomes: 229 consecutive PCM prostheses. Spine (Phila Pa 1976). 2007;20:1337-1344.
16. Baaj A.A., Uribe J.S., Vale F.L., et al. History of cervical disc arthroplasty. Neurosurg Focus. 2009;3:E10.
17. Adamson T.E. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95(Suppl 1):51-57.
18. Fager C.A. Posterolateral approach to ruptured median and para-median cervical disk. Surg Neurol. 1983;20:443-452.
19. Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(Suppl 5):S37-S45.
20. Southwick W.O., Robinson R.N. Surgical approaches to the vertebral bodies in the cervical and lumbar regions. J Bone Joint Surg [Am]. 1959;39:631-643.
21. Bose B. Anterior cervical instrumentation enhances fusion rates in multilevel reconstruction in smokers. J Spinal Disord. 2001;14:3-9.
22. Baba H., Furusawa N., Imura S., et al. Late radiographic findings after anterior surgical fusion for spondylotic myeloradiculopathy. Spine (Phila Pa 1976). 1993;18:2167-2173.
23. Cummins B.H., Robertson J.T., Gill S.S. Surgical experience with an implanted artificial cervical joint. J Neurosurg. 1998;88:943-948.
24. Goffin J., Van Calenbergh F., van Loon J., et al. Intermediate follow-up after treatment of degenerative disc disease by the Bryan Cervical Disc Prosthesis: single-level and bi-level. Spine (Phila Pa 1976). 2003;28:2673-2678.
25. Mummaneni P.V., Haid R.W. The future in the care of the cervical spine: interbody fusion and arthroplasty. J Neurosurg Spine. 2004;1:155-159.
26. Wigfield C.C., Gill S., Nelson R., et al. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg. 2002;96(Suppl 1):17-21.
27. Bertagnoli R., Yue J.J., Pfeiffer F., et al. Early results after ProDisc-C cervical disc replacement. J Neurosurg Spine. 2005;2:403-410.
28. Lafuente J., Casey A.T., Petzold A., Brew S. The Bryan cervical disc prosthesis as an alternative to arthrodesis in the treatment of cervical spondylosis: 46 consecutive cases. J Bone Joint Surg [Br]. 2005;87:508-512.
29. Porchet F., Metcalf N.H. Clinical outcomes with the Prestige II cervical disc: preliminary results from a prospective randomized clinical trial. Neurosurg Focus. 2004;17(3):E6.
30. Robertson J., Metcalf N. Long-term outcome after implantation of the Prestige I disc in an end-stage indication: 4-year results form a pilot study. Neurosurg Focus. 2004;17(3):E10.
31. Wigfield C.C., Gill S., Nelson R., et al. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine (Phila Pa 1976). 2002;27:2446-2452.
Anterior Cervical Discectomy without Fusion
Since its introduction, ACD has been described in conjunction with placement of an interbody graft for fusion.1–3 Originally, this graft was harvested from the patient’s iliac crest; hence, this additional surgical step contributed its own set of risks and complications. Years later, surgeons began advocating discectomy without fusion for the treatment of this disease process. The results were fairly good, and complications associated with graft harvest and placement were avoided.4,5 With the introduction of various interbody graft materials and the improvement in the rate of fusion associated with these materials, many neurosurgeons now routinely perform fusion procedures. However, conclusive data are lacking to support the superiority of interbody fusion compared to a simple discectomy for the treatment of radiculopathy related to a cervical disc herniation. We therefore review the existing data in the hope of clarifying this controversial issue.
ACD with or without fusion employs the approach described by Cloward, Robinson et al., and Smith and Robinson.1–3 The discectomy is performed under magnification, with resection of the posterior longitudinal ligament and extruded disc fragments. Foraminotomies are performed to fully decompress the nerve roots. Finally, in the fusion procedure, an interbody graft is inserted, and its position is confirmed under fluoroscopy. A cervical plate spanning the interspace may then be placed.
In 1983, Rosenørn et al. published a prospective randomized study evaluating ACD with and without fusion.6 Sixty-three patients with symptomatic cervical disc herniations were randomized either to discectomy alone (DE, 32 patients) or to discectomy and fusion using freeze-dried bone grafts (DEF, 31 patients). Clinical outcome was graded as “excellent” if patients returned to work without symptoms, “good” if they returned to work with minor symptoms, “fair” if they returned to work with the same symptoms or had to change occupations, and “poor” if they did not return to work and their status was unchanged or worse. At both 3-month and 12-month follow-up examinations, the clinical outcomes for DE were significantly better than those associated with DEF. However, if the “excellent” and “good” results were grouped together, the difference in outcomes between DE and DEF was insignificant at 12 months. There were no significant differences in length of hospital stay between the two groups, and there was no difference in the complication rate.
Another prospective randomized study presented in 1998 by Savolainen et al. compared three different surgical techniques for single-level cervical root compression: (1) discectomy alone, (2) discectomy and fusion with autologous bone graft, and (3) discectomy and fusion with bone graft plus plating.7 After 4 years of follow-up, there were no significant differences between the three groups in the achievement of good clinical outcomes (i.e., no symptoms): 76% for discectomy, 82% for discectomy + graft, and 73% for discectomy + graft/plate. There were no poor outcomes in the discectomy group compared to 4% poor outcomes in both of the other groups (results not significant). All patients who underwent grafting, plating, or both grafting and plating achieved a solid fusion, with 90% of all simple discectomy patients achieving solid fusion as well. Finally, there were no significant differences in the development of kyphosis among the three groups (63% for discectomy, 41% discectomy + graft, 44% discectomy + graft/plate). The authors therefore argued that interbody fusion seemed unnecessary for single-level cervical nerve root compression.
In 1999, Dowd and Wirth reported their experience after enrolling 84 patients in a prospective randomized trial comparing ACD with ACD and fusion (ACDF).8 Patients who underwent placement of iliac crest bone graft were most likely to develop fusion (97% vs. 70%). At final follow-up (average 4.5 years), however, there were no differences between the two groups in terms of patient satisfaction or return to preoperative activity.
Proponents of fusion propose that placement of an interbody graft after discectomy prevents collapse of the disc space, segmental kyphosis, and the resultant neck pain and avoids the consequent narrowing of the neural foramina and nerve root impingement. To some degree, Murphy et al. supported this proposal with radiologic studies.9 They evaluated the foraminal area before and after ACD alone or after discectomy with interbody graft placement and fusion. As expected, the foraminal area decreased significantly after discectomy alone (P < .001) and increased after placement of an interbody graft. However, when the authors compared absolute changes in disc height between the two groups, the difference was not significant. Most important, they found no significant correlation between foraminal area and clinical outcome.
Oktenoglu et al. compared disc height and foraminal area at index and adjacent levels and evaluated symptom relief of patients undergoing ACD or ACDF with plating.10 Patients were examined preoperatively and in the immediate and 1-year postoperative periods. There were no significant differences between the two groups in terms of disc height and foraminal areas at adjacent levels. At 1 year, both groups showed decreases in disc height at the index level compared to preoperative values, but the ACDF with plating group maintained greater disc height (P = .007). Both groups showed a decrease in foraminal area, with no significant difference between ACD and ACDF with plating (P = .603). Importantly, pain scores for arm pain decreased similarly and significantly for all patients after surgery, regardless of type of surgery.
White and Fitzgerald tested the hypothesis that the degree of disc space settling and kyphosis after discectomy directly correlates with preoperative disc height, greater degrees of angulation and kyphosis being noted after resection of normal discs and with only minimal changes after resection of collapsed, degenerated discs.11 Furthermore, they projected that with greater degrees of settling, angulation, and foraminal closure, the clinical outcomes after discectomy alone in patients with normal disc height would be worse than outcomes in patients with restored disc height after interbody graft placement. The authors evaluated preoperative and postoperative kyphosis in both groups (discectomy alone and discectomy plus fusion). Discectomy alone resulted in a greater degree of kyphosis if the preoperative disc space was more than 4 mm in height and resulted in only minimal change in sagittal alignment if the disc space was narrower than 4 mm. As clinical correlates to these findings, they noted an increase in adverse outcomes when small disc spaces were grafted and when larger disc spaces were left ungrafted. Of their 15 complications, 12 (80%) fell into one of these two categories. Therefore, they concluded that the decision regarding whether to graft should be based on preoperative evaluation of the height of the disc space on plain lateral radiographs, and they recommended placing a graft when disc height is greater than or equal to 4 mm.
Xie and Hurlbert also evaluated segmental kyphosis after ACD compared with after ACDF or ACDF with plating.12 In their prospective randomized study of 42 consecutive patients with cervical radiculopathy, segmental kyphosis was noted in 75% of ACD patients after surgery compared with 17% before surgery. In contrast, there was no change in sagittal balance in the ACDF or ACDF with plating groups. Nonetheless, after 2 years of follow-up, there were no differences with respect to arm pain, neck pain, or interscapular pain among the three groups of patients (P > .05).
With the advent of synthetic interbody graft materials, the obvious negative factors associated with graft harvest (e.g., pain, potential graft site complications) could be avoided. Therefore, despite the lack of class I evidence to confirm a clinical benefit, the technique of fusion after discectomy continued to gain widespread acceptance. In 2008, Hauerberg et al. published their results evaluating ACD versus fusion with a titanium cage.13 They randomized 86 patients with cervical radiculopathy to undergo discectomy alone (46 patients) or to undergo ACDF with a Ray titanium cage (40 patients). Neither group was placed in an external orthosis postoperatively. After 2 years of follow-up, 86% of the patients who underwent titanium cage fusion reported a “good” clinical outcome compared to 77% of the discectomy alone group. Consistent with previous studies, this difference was not statistically significant. The authors also found no differences in self-reported satisfaction, arm pain, or neck pain between the two groups 3 months or 24 months after surgery. The rate of fusion was similar between the two groups: 81% in the discectomy alone group versus 83% in the titanium cage group.
In 2005, White and Fitzgerald provided a different angle from which to view this controversy.11 Perhaps the real challenge is to develop an algorithm that predicts who may or may not benefit from a fusion procedure. Several factors could be included in such an algorithm, such as preoperative sagittal alignment, disc space height, single-level or multilevel disease, bone condition, and presence of concomitant neck pain14 (Fig. 232-9).
In Sonntag’s experience, patients with single-level or two-level disease, strictly radicular symptoms, and no neck pain have done well after discectomy alone15 (Figs. 232-10 to 232-12). However, in patients with multilevel disease, loss of cervical curvature, or any evidence of instability, Sonntag performs discectomy followed by fusion. The presence of axial neck pain is also a strong indication for fusion, which provides excellent relief of symptoms. This trend was noted by Oktenoglu et al., who found that neck pain scores improved significantly only in patients who underwent interbody fusion (P = .008).10
Other indications for fusion include acute disc herniation with concomitant cervical vertebral body fracture, extensive vertebral body resection (large dorsal osteophytes), previous laminectomy, or when future laminectomy is anticipated.15,16 As has been noted in several studies, postoperative transient interscapular and axial neck pain after ACD are common; however, without treatment, both usually resolve within a few weeks of surgery.
Finally, the economic costs of adding interbody fusion and instrumentation must be considered. In the absence of clear indications for fusion, such as instability, neck pain, or existing kyphotic angulation, simple discectomy can help to avoid the extra costs associated with a fusion procedure. These costs include the interbody graft, possible instrumentation, extra operative time, the orthosis, and intraoperative and follow-up radiographs to evaluate the position of the graft and fusion. Nonetheless, ACD with interbody fusion and plating has become almost universally accepted as the standard treatment for radiculopathy related to cervical disc herniation. It may take many more years of patient follow-up and evaluation to determine whether the costs associated with placing a graft and possible instrumentation are justified in regard to long-term outcomes and patient satisfaction.
Dan N.G. Anterior cervical graftless fusion for soft disc protrusion. A review of 509 disc excisions in 476 patients. J Clin Neurosci. 1998;5:172-177.
Jagannathan J., Shaffrey C.I., Oskouian R.J., et al. Radiographic and clinical outcomes following single-level anterior cervical discectomy and allograft fusion without plate placement or cervical collar. J Neurosurg Spine. 2008;8:420-428.
Konduru S., Findlay G. Anterior cervical discectomy: to graft or not to graft? Br J Neurosurg. 2009;23:99-103.
Matz P.G., Holly L.T., Groff M.W., et al. Indications for anterior cervical decompression for the treatment of cervical degenerative radiculopathy. J Neurosurg Spine. 2009;11:174-182.
Matz P.G., Ryken T.C., Groff M.W., et al. Techniques for anterior cervical decompression for radiculopathy. J Neurosurg Spine. 2009;11:183-197.
1. Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
2. Robinson R.A., Walker A.E., Ferlic D.C., Wiecking D.K. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg [Am]. 1962;44:1569-1587.
3. Smith G.W., Robinson R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
4. Murphy M.G., Gado M. Anterior cervical discectomy without interbody bone graft. J Neurosurg. 1972;37:71-74.
5. Hirsch C., Wickbom I., Lidstroema A., et al. Cervical-disc resection. A follow-up of myelographic and surgical procedure. J Bone Joint Surg [Am]. 1964;46:1811-1821.
6. Rosenørn J., Hansen E.B., Rosenørn M.A. Anterior cervical discectomy with and without fusion. A prospective study. J Neurosurg. 1983;59:252-255.
7. Savolainen S., Rinne J., Hernesniemi J. A prospective randomized study of anterior single-level cervical disc operations with long-term follow-up: surgical fusion is unnecessary. Neurosurgery. 1998;43:51-55.
8. Dowd G.C., Wirth F.P. Anterior cervical discectomy: is fusion necessary? J Neurosurg. 1999;90:8-12.
9. Murphy M.A., Trimble M.B., Piedmonte M.R., Kalfas I.H. Changes in the cervical foraminal area after anterior discectomy with and without a graft. Neurosurgery. 1994;34:93-96.
10. Oktenoglu T., Cosar M., Ozer A.F., et al. Anterior cervical microdiscectomy with or without fusion. J Spinal Disord Tech. 2007;20:361-368.
11. White B.D., Fitzgerald J.J. To graft or not to graft: rationalizing choice in anterior cervical discectomy. Br J Neurosurg. 2005;19:148-154.
12. Xie J.C., Hurlbert R.J. Discectomy versus discectomy with fusion versus discectomy with fusion and instrumentation: a prospective randomized study. Neurosurgery. 2007;61:107-116.
13. Hauerberg J., Kosteljanetz M., Boge-Rasmussen T., et al. Anterior cervical discectomy with or without fusion with ray titanium cage: a prospective randomized clinical study. Spine (Phila Pa 1976). 2008;33:458-464.
14. Theodore N., Sonntag V.K. Decision making in degenerative cervical spine surgery. Clin Neurosurg. 2001;48:260-276.
15. Sonntag V.K., Klara P. Controversy in spine care. Is fusion necessary after anterior cervical discectomy? Spine (Phila Pa 1976). 1996;21:1111-1113.
16. Hadley M.N., Sonntag V.K. Cervical disc herniations. The anterior approach to symptomatic interspace pathology. Neurosurg Clin N Am. 1993;4:45-52.
Laminoforaminotomy
The motives for selection of a ventral approach are as follows:
• Direct access to a ventral fragment
• Less postoperative initial mechanical neck pain owing to reduced muscle dissection
• Historical concerns regarding postoperative kyphosis or instability in patients with any facet resection via a dorsal approach1
Dorsal laminoforaminotomy offers a number of significant advantages in the treatment of cervical radiculopathy; these are discussed next.1,2
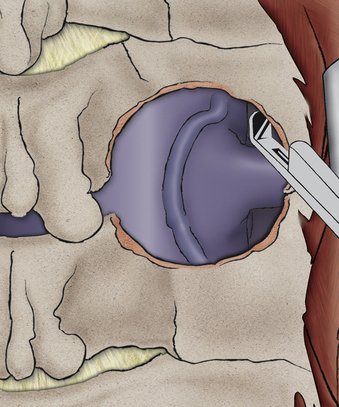
Although the exposure is dorsal to the nerve root, the expanse of nerve root decompression that can be achieved is extensive (Fig. 232-13). Even resection of less than 30% to 50% of the medial facet provides wide decompression of the nerve.2 While I generally clear the fragment from ventral to the nerve root, there are abundant data to confirm that nerve root decompression alone is sufficient to treat the problem.2 This is important in that in patients with spurs or fixed fragments, it is not necessary to be unduly aggressive with exploration and dissection ventral to the nerve root.
Dorsal laminoforaminotomy is the “original” motion preservation technique. Although there have long been concerns that dorsal procedures, regardless of technique, impart some degree of spinal instability, there is substantial evidence to refute this claim. In vitro studies suggest that resection of less than 50% of the facet does not lead to clinically significant instability or progression of kyphosis.3 Significantly, a recent series of 162 cases treated with laminoforaminotomy failed to show a significant increase in kyphosis, even when 50% or more of a unilateral facet was resected.1 Clarke et al. reviewed 303 patients with single-level dorsal foraminotomy, noting same-segment disease in only 3.2% of patients at 5 years and in 5% at 10 years.4 Although the ventral surgery proponents often portray the disc fusion option as less intrusive on spinal dynamics, in fact, the hypermotility and adjacent-segment disease after ventral cervical disc and fusion may reach a rate of 2.9% per year, with recurrent surgical procedures conducted in 7.0% of patients within 10 years of ventral cervical disc fusion procedures.5 Jagannathan et al. and Henderson et al. both had recurrent surgical procedure requirements of 5.0% or less at 10 years using a standard dorsal laminoforaminotomy approach.1,2
Technique
The procedure can be safely accomplished in either the prone or sitting position.1,2 While more extensive monitoring is required in a sitting craniotomy procedure, the data suggest that in reasonably healthy patients, no Foley catheter, arterial line, or central venous catheter is needed in the sitting cervical laminoforaminotomy procedure.2 I do not employ neurologic monitoring in cases of cervical radiculopathy.
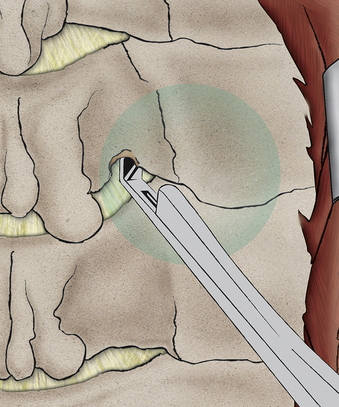
In the initial exposure, a unilateral subperiosteal dissection is achieved through a moderate midline incision (Fig. 232-14). Minimal access techniques can be used to achieve similar exposure.6 It is important to limit the dissection to just lateral to the laminar facet groove. This prevents unnecessary disruption of the facet capsule.
The nerve will exit within 5 to 7 mm of the articular groove of the facet; accordingly, the target zone for the laminoforaminotomy will be the laminar facet groove (for medial-lateral planning) and the facet articular groove (for superior-inferior planning). It is preferable to begin the laminotomy somewhat medial to the laminar facet groove, as this will allow exposure of the lateral thecal sac (see Fig. 232-13). Once the thecal sac has been identified with a removal of the medial facet (usually less than 30% of the facet), the origin of the nerve root will become evident. (see Fig. 232-13). It is now necessary only to extend the foraminotomy directly dorsal to the nerve root to achieve full and effective decompression. Beginning the dissection medially allows a carefully targeted and minimized facet resection by removing only the portion of facet that is directly dorsal to the nerve.
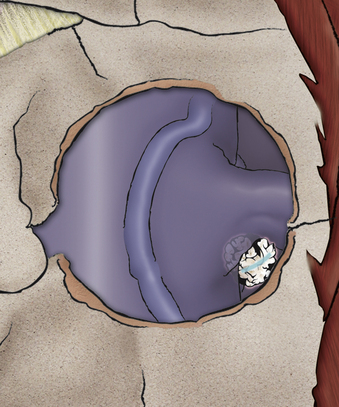
If a soft disc fragment is lodged ventral to the nerve, the nerve can be mobilized in a superior direction under magnified vision. Occasionally, the fragment will be around the shoulder of the nerve root. A shallow incision in the fragment in the direction of the nerve can be made to release the fragment. A small but important 4% to 6% subgroup may have a conjoint nerve root origin, with the motor root in the ventral-inferior position. The conjoint root, when stretched over a disc fragment, can be subtle to visualize. Being certain of the full movement of the edge of the nerve root dura, magnification, and attention to this anatomic variant will reduce the risk of nerve injury. (Fig. 232-15).
Summary
The literature supports exclusive dorsal laminoforaminotomy to achieve decompression without the need to remove spurs.2 Over the last 10 years, I have removed soft fragments but do not drill off ventral hard spurs. This option reduces the risk of nerve injury, and simple foraminotomy appears quite effective in this setting.
Clarke M.J., Ecker R.D., Krauss W.E., et al. Same-segment and adjacent-segment disease following posterior cervical foraminotomy. J Neurosurg Spine. 2007;6:5-9.
Fehlings M.G., Gray R.J. Posterior cervical foraminotomy for the treatment of cervical radiculopathy. J Neurosurg Spine. 2009;10:343-344.
Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(Suppl 5):S37-S45.
Henderson C.M., Hennessy R.G., Shuey H.M.Jr., et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
Jagannathan J., Sherman J.H., Szabo T., et al. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years’ clinical and radiographic follow-up. J Neurosurg Spine. 2009;10:347-356.
1. Jagannathan J., Sherman J.H., Szabo T., et al. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years’ clinical and radiographic follow-up. J Neurosurg Spine. 2009;10:347-356.
2. Henderson C.M., Hennessy R.G., Shuey H.M.Jr., et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
3. Fehlings M.G., Gray R.J. Posterior cervical foraminotomy for the treatment of cervical radiculopathy. J Neurosurg Spine. 2009;10:343-344.
4. Clarke M.J., Ecker R.D., Krauss W.E., et al. Same-segment and adjacent-segment disease following posterior cervical foraminotomy. J Neurosurg Spine. 2007;6:5-9.
5. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
6. Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(Suppl 5):S37-S45.