Chapter 8 Cervical and Lumbar Sympathetic Blocks
 Sympathetic blocks provide a valuable diagnostic, prognostic, and therapeutic value to sympathetically maintained pain syndromes.
Sympathetic blocks provide a valuable diagnostic, prognostic, and therapeutic value to sympathetically maintained pain syndromes. Fluoroscopy is a reliable method for identifying bony surfaces, which facilitates identifying C6 and C7 transverse processes; however, this is only a surrogate marker for the cervical sympathetic trunk. It is defined by the fascial plane of the prevertebral fascia, which cannot be visualized with fluoroscopy.
Fluoroscopy is a reliable method for identifying bony surfaces, which facilitates identifying C6 and C7 transverse processes; however, this is only a surrogate marker for the cervical sympathetic trunk. It is defined by the fascial plane of the prevertebral fascia, which cannot be visualized with fluoroscopy. Ultrasound-guided stellate ganglion block may improve the precision of the procedure by identifying the fascial plane anterolateral to the longus coli muscle.
Ultrasound-guided stellate ganglion block may improve the precision of the procedure by identifying the fascial plane anterolateral to the longus coli muscle. When performing lumbar sympathetic block, contrast should be injected with real-time fluoroscopy to minimize the risk of vascular uptake. The sensitivity of the aspiration test and static radiography are very low.
When performing lumbar sympathetic block, contrast should be injected with real-time fluoroscopy to minimize the risk of vascular uptake. The sensitivity of the aspiration test and static radiography are very low. Ultrasound-guided stellate ganglion block may improve the safety of the procedure by direct visualization of vascular structures (inferior thyroidal, cervical, vertebral, and carotid arteries) and soft tissue (thyroid, esophagus, nerve roots).
Ultrasound-guided stellate ganglion block may improve the safety of the procedure by direct visualization of vascular structures (inferior thyroidal, cervical, vertebral, and carotid arteries) and soft tissue (thyroid, esophagus, nerve roots). When performing cervical sympathetic block at C6, the ganglion primarily blocked is the middle cervical ganglion; the cervicothoracic ganglion is blocked if the injectate spreads down to the T1 level.
When performing cervical sympathetic block at C6, the ganglion primarily blocked is the middle cervical ganglion; the cervicothoracic ganglion is blocked if the injectate spreads down to the T1 level. There is no safer level for performing stellate ganglion block (C6 level vs. C7 level). However; there may be a safer tool (ultrasound vs. fluoroscopy).
There is no safer level for performing stellate ganglion block (C6 level vs. C7 level). However; there may be a safer tool (ultrasound vs. fluoroscopy). Stellate ganglion block may be associated with serious complications even in experienced hands. Mastering anatomy is of paramount importance.
Stellate ganglion block may be associated with serious complications even in experienced hands. Mastering anatomy is of paramount importance.Cervical Sympathetic Block
Anatomy
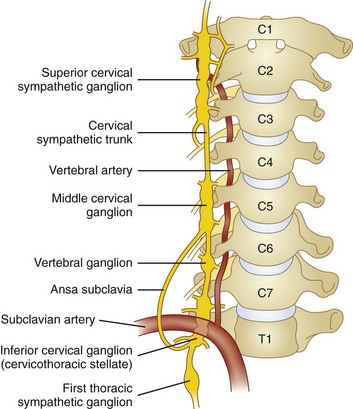
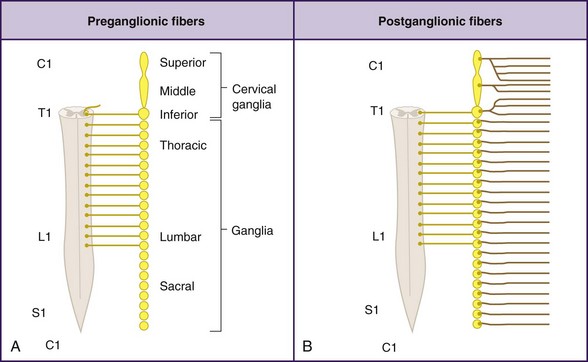
The cervical sympathetic chain is composed of superior, middle, and inferior cervical ganglia. However, in approximately 80% of the population, the inferior cervical ganglion is fused with the first thoracic ganglion, forming the cervicothoracic ganglion, also known as the stellate ganglion (Fig. 8-1).
The superior cervical ganglion sends somatic branches via the gray rami communicantes to the cervical plexus (C1-C4), innervating the structures of the neck. The middle and inferior (stellate) ganglia contribute somatic postganglionics to the brachial plexus (C5-T1), innervating the upper extremities.1–3 The superior cervical ganglion sends its vascular branches along the internal and external carotid arteries to reach structures in the cranium, orbit, face, nasal and oral cavities, and pharynx. Blockade of efferents to this ganglion is what results in ptosis, miosis, anhydrosis, and enophthalmos, the classic Horner syndrome. The middle ganglion sends vascular branches along the inferior thyroid artery to the larynx, trachea, and upper esophagus. The inferior (stellate) ganglion sends branches to travel along the subclavian and vertebral arteries. All three cervical ganglia are known to provide visceral branches that contribute to the cardiac plexus. The superior ganglion contributes to the superficial cardiac plexus, and the middle and inferior ganglia contribute to the deep cardiac plexus.
Most preganglionic sympathetic efferents innervating the head, neck, and upper extremity either pass through or synapse at the stellate ganglion. This provides us with an ideal target for blockade of sympathetic efferents to the head, neck, and upper limbs. Occasionally, additional sympathetic innervation to the upper extremity exits the sympathetic chain via gray rami communicantes at T2 and T3 and goes on to the distal upper extremity without ever passing through the stellate ganglion.4,5 The prevalence of this anomaly is unknown but should be kept in mind when interpreting the results of the block because it could result in failed sympathetic denervation despite adequate blockade of the stellate ganglion.
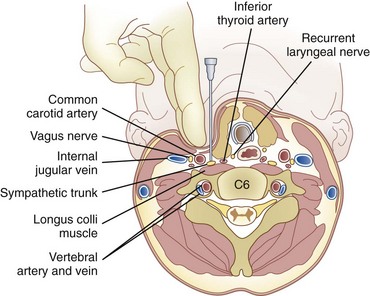
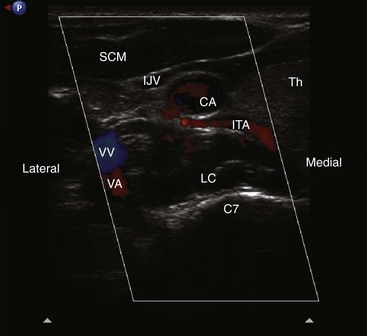
The stellate ganglion is located medial to the scalene muscles; lateral to the longus colli muscle, esophagus, and trachea along with the recurrent laryngeal nerve (RLN); anterior to the transverse processes and prevertebral fascia; superior to the subclavian artery and the posterior aspect of the pleura; and posterior to the vertebral vessels at the C7 level.6 This explains why there may be increased risk of pneumothorax and vertebral artery injury with blockade at the C7 level.
The stellate ganglion measures approximately 2.5 cm long, 1 cm wide, and 0.5 cm thick (anteroposterior [AP] diameter). It is usually located posteriorly in the chest in front of the neck of the first rib and may extend to the seventh cervical (C7) vertebral body.6–8 If the inferior cervical ganglion and first thoracic ganglion are not fused, the inferior cervical ganglion lies in front of the C7 tubercle, and the first thoracic ganglion rests over the neck of the first rib.6–8 Accordingly, by using the blind technique at C6, the ganglion primarily blocked is the middle cervical ganglion, and the cervicothoracic ganglion is blocked if the injectate spreads down to the T1 level.
Indications
Other less commonly encountered indications include hyperhidrosis, Ménière disease, accidental intraarterial injection of intravenous medications, and angina pectoris.9 More recently, stellate ganglion block has been used for the treatment of patients with hot flashes and posttraumatic stress disorders.10,11
Technique
C6 Anterior Approach
The stellate ganglion block is most commonly performed with an anterior approach at C6 transverse process (Chassaignac tubercle).12–14 The anatomical landmarks allow this block to be performed either with or without fluoroscopic guidance.
The patient is placed in the supine position with support under the shoulders and the head resting flat on the table. This position provides slight extension of the neck and facilitates palpation of the necessary anatomical landmarks. The C6 transverse process can be easily located by first palpating the cricoid cartilage. Chassaignac tubercle is located between the cricoid cartilage and the medial border of the sternocleidomastoid. With the index and middle fingers of the nondominant hand, pressure is applied to compress the subcutaneous tissues and identify the C6 transverse process. The pulsations of the carotid artery should be palpated, and an attempt should be made to retract the carotid artery laterally to keep it out of the path of the needle (Fig. 8-2). The cranial and caudal borders of the transverse process are identified with the index and middle fingers, and the block needle is inserted directly between the two fingers to ensure contact with bone.
After negative gentle aspiration, an initial test dose of 0.5 to 1.0 mL of local anesthetic should be injected. Intravascular injection of less than 1 mL of local anesthetic has been reported to cause loss of consciousness and seizure activity.15 If no signs or symptoms of intravascular injection have been observed, a solution of 5 to 10 mL may be injected with re-aspiration after every 3 to 5 mL to help ensure persistent extravascular needle placement.
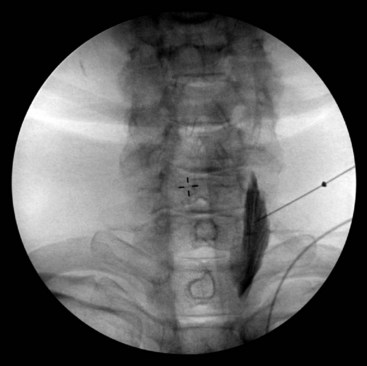
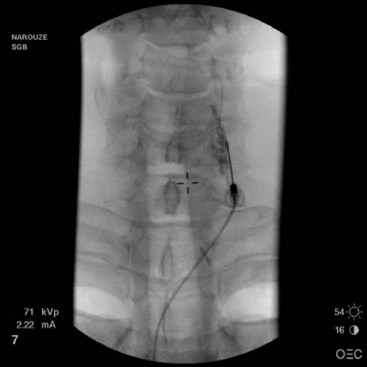
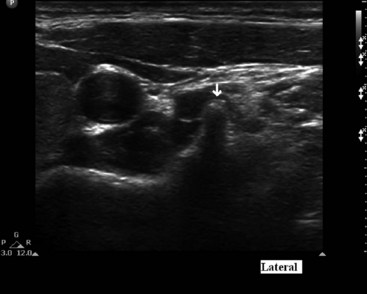
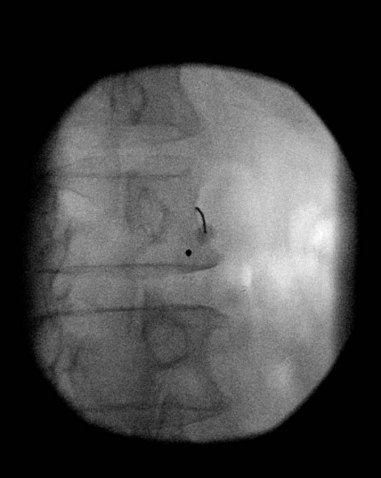
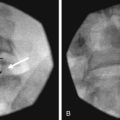
If using fluoroscopy for the procedure, injection of 1 to 2 mL of nonionic contrast should be injected before injection of local anesthetic. It should be visualized traveling inferiorly with minimal resistance. If the needle tip has been placed medially along the transverse process, contrast spread my have a striated appearance, indicating tip placement within the longus colli muscle (Fig. 8-3). If this is the case, the needle should be withdrawn slightly and contrast reinjected to demonstrate the characteristic “honeycomb” appearance indicating that the needle tip is in the appropriate fascial plane anterior to the muscle (Fig. 8-4). Real-time fluoroscopy should be used for careful assessment of any sign of intravascular injection.
C7 Anterior Approach
The stellate ganglion block can also be performed at C7.16 The approach is nearly identical to the C6 approach; however, the anatomical landmarks are more difficult to identify because the C7 vertebra has only a vestigial tubercle that is not readily palpable. Hence, the procedure is usually performed with fluoroscopy. When performing the block without fluoroscopic guidance, it is easier to first palpate the C6 tubercle and then move one fingerbreadth inferior as an estimate of the C7 tubercle. At this level, the risk of pneumothorax and vertebral artery injury is higher.17
An oblique fluoroscopic approach18 targeting the junction between the uncinate process and the vertebral body at the C7 level was described in an effort to decrease those risks.
The C7 approach does offer a few advantages, however. Because the needle is closer in proximity to the stellate ganglion, which resides directly anterior to the C7 transverse process, a smaller volume of solution can be injected to produce a more reliable and consistent blockade.19 This may be of particular value when the patient has failed previous block at the C6 level.
Limitations and Evolution of Current Techniques
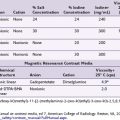
Because the stellate ganglion is located in close proximity to various critical structures, its blockade may be associated with a number of complications, some of which are life threatening. Accordingly, techniques for blockade have evolved and varied from the use of the standard blind technique to the use of radionuclide tracers,20 computed tomography,21 and magnetic resonance imaging.6,22 However, these techniques are not practical in daily clinical practice because they are time consuming, cost-ineffective, and involve radiation exposure. Fluoroscopy has been suggested as a safer and more effective way to perform stellate ganglion block than the traditional blind approach.18,23
Vascular structures (inferior thyroidal, cervical, vertebral, and carotid arteries) and soft tissue (thyroid, esophagus, nerve roots) are also not seen with fluoroscopy—contrary to with ultrasonography—and are therefore at risk of injury with fluoroscopy-guided techniques.24
Ultrasound-guided stellate ganglion block may improve the safety of the procedure by direct visualization of the related anatomical structures, and accordingly the risk of vascular and soft tissue injury may be minimized. Also, ultrasound guidance allows direct monitoring of the spread of the injectate, so complications such as RLN palsy, intrathecal, epidural, or intravascular spread may be minimized as well. The absence of the spread of local anesthetic during the real-time injection raises the suspicion of intravascular injection.25
Ultrasound-Guided Cervical Sympathetic Block
Kapral et al26 first described ultrasound imaging for stellate ganglion block. In their case series, 12 patients received the classical “blind” stellate ganglion block followed by ultrasound-guided block the next day. The blind technique resulted in “asymptomatic” hematoma formation in three of 12 patients, with no hematoma occurring with the ultrasound technique. The spread of the local anesthetic was observed under real-time sonography, and the proximity of the local anesthetic to the RLN and nerve root correlated well with complications such as hoarseness and paresthesia. In this study, 5 mL of local anesthetic was administered, and all patients in the ultrasound-guided group developed sympathetic block compared with 10 out of 12 in the blind group.
Shibata et al27 noted more caudad injectate distribution and better sympathetic blockade with less incidence of hoarseness with ultrasound-guided subfascial injection compared with suprafascial injection.25 Contrary to the fluoroscopy-guided method, the end point of the needle is not the contact with bone but the prevertebral fascia.24,27
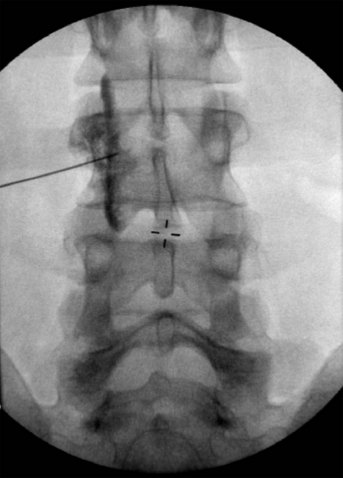
With injection anterior to the prevertebral fascia, the solution tends to spread around the carotid sheath (Fig. 8-5).28 In this case, the risk of hoarseness is higher, probably secondary to proximity of the vagus nerve in the carotid sheath and the RLN medial to the carotid and lateral to the trachea.27,28
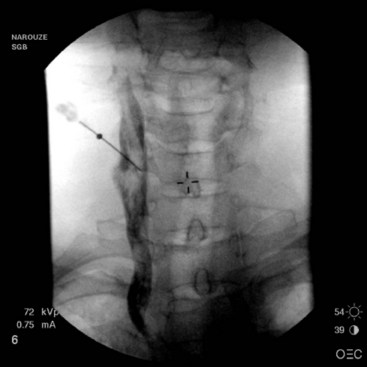
Fig. 8-5 Anteroposterior view showing the spread of the contrast agent along the carotid sheath.
(Reprinted with permission from the Ohio Pain and Headache Institute.)
The vertebral artery runs anteriorly at the C7 level before it enters the foramen of C6 transverse process in about 90% of cases. However, it enters at C5 or higher in the remaining cases.29 This makes it vulnerable to injury during lower cervical sympathetic block, not only at the C7 level but at C6 as well, a possibility that can be avoided by ultrasound imaging.24
The inferior thyroid vessels may be a major source of retropharyngeal hematoma because of their vulnerable and variable anatomy.30 The inferior thyroid artery originates from the thyrocervical trunk of the subclavian artery and ascends anteriorly to the vertebral artery and the longus colli muscle and then curves medially behind the carotid sheath to enter posteriorly the inferior part of the thyroid lobe. It is vulnerable to injury because it lies anterior to the vertebral artery at the C7 level or more commonly when it crosses (at the C6-C7 level) behind the carotid artery from lateral to medial to end in the thyroid gland. This is the most critical portion of the vessel to be injured during performing the procedure with the blind technique or even with fluoroscopic guidance. Because the artery has a variable unpredicted anatomy and has a very tortuous serpentine course, it can be easily injured in the path of the needle. This possibility can be prevented with an ultrasound-guided technique (Fig. 8-6).30
Also, ultrasound imaging can identify the esophagus, especially on the left (Fig. 8-7).24 The esophagus usually appears as an outpouching behind the trachea and can be better identified by the change in shape and shadowing during swallowing and the presence of a peripheral arc-shaped echogenic line or a boundary hypoechoic zone, which is suggestive of the striated structure of the digestive tract.31
Placing the needle by ultrasonography closer to the target also minimizes the amount of local anesthetic used and hence improves the patient’s safety; Wulf et al32 reported toxic plasma levels in 30% of patients undergoing stellate ganglion block using 10 mL of bupivacaine 0.5%.
Technique for Ultrasound-Guided Injection
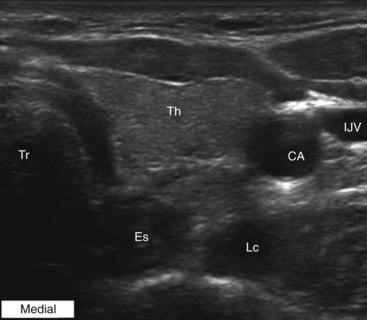
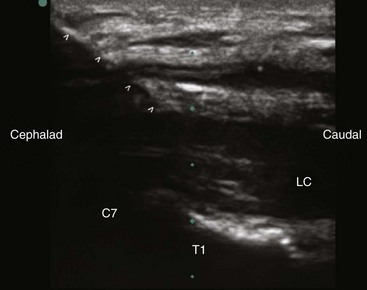
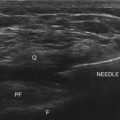
The patient is placed in the supine position with the neck extended in the neutral position. A high-frequency linear transducer (5-12 MHz) is usually used. The transducer is placed horizontally at the root of the neck to obtain a transverse sonogram. Scanning from midline laterally, one can easily identify the trachea, esophagus, thyroid gland, carotid sheath, and longus colli muscle (Fig. 8-7). Then the transducer is moved slowly cephalad until the characteristic sharp anterior tubercle of the transverse process of C6 comes into the image (Fig. 8-8). At this point, the C6 and C7 levels are identified, and a careful scanning is performed to identify all of the relevant vessels in the vicinity of the cervical sympathetic chain. The vertebral artery, inferior thyroid artery, and thyrocervical trunk branches should be looked after and identified.30 A color Doppler scan helps in revealing any variable vascular anatomy. A safe path for the needle should be planned to avoid any puncture to those vital structures. Turning the head to the opposite side usually shifts the carotid sheath more medially, thus creating more room to place the needle lateral to the carotid sheath. The authors usually use an out-of-plane approach, and the ideal placement of the needle tip should be anterolateral to the longus colli muscle and deep to the prevertebral fascia (to avoid spread along the carotid sheath) but superficial to the fascia investing the longus colli muscle (to avoid injecting into the muscle substance). The authors usually use less than 5 mL of local anesthetic, and the injection should be carried out with real-time sonography to monitor the spread of the injectate as above.
Others advocate the use of an in-plane approach with the patient in the lateral position in an effort to minimize the risk of vascular injury.33
T2 Anterior Approach
Vallejo et al34 described the fluoroscopy-guided T2 anterior approach for upper extremity sympathetic blockade. The T2 approach should provide a complete sympathetic block to the upper extremity while using a small volume of local anesthetic because it will allow for blockade of the Kuntz’s fibers (see Anatomy section above), thus enhancing the diagnostic accuracy and the therapeutic benefit as compared with traditional stellate ganglion block. Also, the T2 anterior approach may have a lower risk of pneumothorax than the classic posterior paravertebral block approach at T2.
Narouze35,36 recently described an ultrasound-guided T2 anterior approach. Ultrasonography may improve the safety of the procedure by direct visualization of the related anatomical structures, and accordingly, the risk of vertebral artery or pleura injury may be minimized.
For the T2 anterior approach, the authors prefer to use a high-resolution compact curvilinear array probe (C5-C8) because its small size (however wider footprint) allows room for placing the needle in plane whether in the short- or the long-axis view. The transducer is first applied transversely at the root of the neck to obtain a short-axis view. The C7 can be identified with its characteristic transverse process (lacking the anterior tubercle) as well as its relation to the vertebral artery (Fig. 8-9). By moving the probe caudally, T1 will appear in the image, and then afterward with a caudal tilt, one can identify the T2 level.
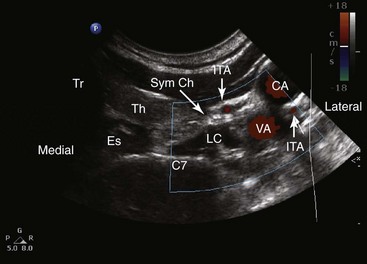
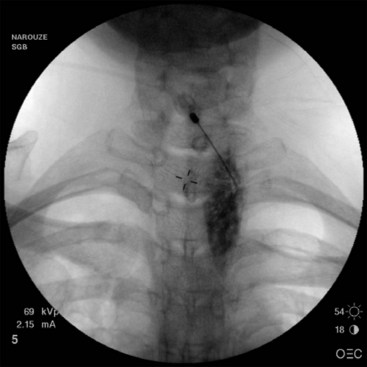
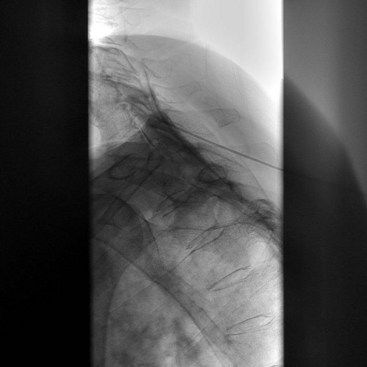
First, a 22- to 25-gauge blunt needle is inserted out of plane in the short-axis view and advanced with real-time sonography so the needle tip will lie just lateral to the longus colli muscle. Caution must be exercised to avoid the vertebral artery as it lies anterior to the sympathetic chain at this level. Then a longitudinal axis view is obtained to monitor the tip of the needle as it is advanced caudally along the lateral border of the muscle (Fig. 8-10). The final needle position may be checked with real-time fluoroscopy after contrast injection to show the cephalocaudal spread without vascular escape (Fig. 8-11).
Complications
Because the anatomy of the stellate ganglion is in close proximity to various critical structures, a number of complications may be potentially associated with its blockade, some of which are life threatening.37 Before performing this block, all patients should have a reliable intravenous catheter placed, and full resuscitative equipment should be readily available. When performed properly by an experienced practitioner, the stellate ganglion block has a low incidence of complications.38 The complications of stellate ganglion block result either from insertion and manipulation of the needle or as a direct result of the injected solution. These complications may include:
RLN block is another side effect that occurs frequently secondary to spread of local anesthetic to the RLN. Hardy and Wells39 reported an incidence of 10% with 10 mL of local anesthetic solution and up to 80% with 20 mL of solution. When unilateral, it results in hoarseness and may cause subjective difficulty breathing. This is rarely of consequence unless the patient has a preexisting contralateral RLN injury, as is common after thyroid surgery. In this circumstance, blockade could produce critical airway obstruction and loss of laryngeal reflexes requiring intubation. The ultrasound-guided stellate ganglion block approach was associated with much less incidence of RLN block (see earlier discussion).26,27
The most serious complications of stellate ganglion block include pneumothorax, subarachnoid injection, intravascular injection, and retropharyngeal hematoma. The proximity of the stellate ganglion to the inferior thyroid, cervical, vertebral, or carotid arteries provides potential for either intravascular injection or vascular trauma with resulting bleeding and hematoma.30,40 Intravascular injection of even small volumes of local anesthetic may result in loss of consciousness, apnea, and seizure. Retropharyngeal hematoma varies in severity from mild and asymptomatic to severe and causing tracheal compression requiring emergency tracheotomy.37,41 The frequency of catastrophic retropharyngeal hematoma after stellate ganglion block has been reported to be one in 100,000 cases with resulting airway compromise and obstruction.37 However, Kapral et al26 reported a much higher incidence of asymptomatic hematoma with the blind technique.
Lumbar Sympathetic Block
Anatomy
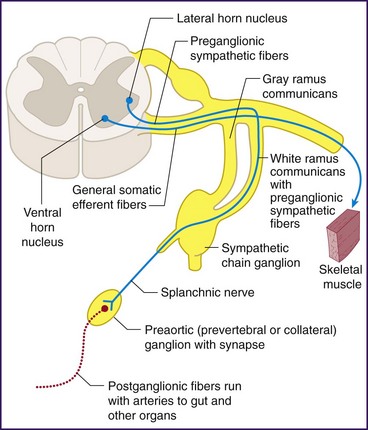
The lumbar sympathetic chain consists of both pre- and postganglionic efferent fibers (Fig. 8-12). The preganglionic sympathetic nerves have their cell bodies located within the intermediolateral cell column of the thoracolumbar spinal cord. After exiting the spinal cord through the ventral root and traveling briefly with the spinal nerve as it exits the spinal canal, the axons continue through the white rami communicantes to join the sympathetic chain on either side of the vertebral column (Fig. 8-13). Within the sympathetic chain, the efferent fibers take different paths. Some travel up or down the sympathetic chain and synapse with postganglionic neurons within the paravertebral sympathetic ganglia. The postganglionic neurons then exit through the gray rami communicantes and follow somatic nerves or vessels to affect vascular smooth muscle, sudomotor cells, and peripheral nociceptors. Other preganglionic efferents pass on to prevertebral ganglia (aortic plexus and the superior and inferior hypogastric plexuses) before synapsing with postganglionic neurons (Fig. 8-14).
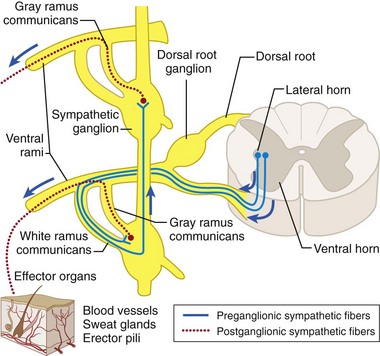
Fig. 8-13 Sympathetic pathway to sweat glands, erector pili muscles, and blood vessels.
(From Bogart BI, Ort VH: Elsevier’s integrated anatomy and embryology. Philadelphia, 2007, Mosby.)
The paravertebral ganglia exist along the entire vertebral column. There are considered to be five paired lumbar ganglia that lie along the anterolateral border of either side of the five lumbar vertebrae. Cadaver dissections, however, have shown significant variability in both the number and location of the ganglia.42 Typically, there are only four ganglia because the L1 and L2 ganglia are commonly fused. The vast majority of sympathetic efferent neurons responsible for vascular tone in the lower extremities pass through the paravertebral ganglia at L2 and L3. Therefore, these ganglia are the targets for lumbar sympathetic blockade. The ganglia at these levels have been most frequently found at the lower third of the L2 vertebra, at the L2-L3 interspace, and at the upper third of the L3 vertebra.43 Also of note, the lumbar arteries at these levels are known to exit the aorta and travel posteriorly across the middle of the vertebral bodies before branching into radicular or segmental medullary arteries. Hence, the ideal site for blockade of the lumbar sympathetic chain is at the lower third of the L2 vertebral body or the upper third of the L3 vertebral body, both targeting the ganglia and avoiding the segmental lumbar arteries and their branches. Also, lumbar sympathetic block at the L2 level showed the lowest incidence of psoas muscle injection of contrast in comparison with lumbar sympathetic block at L3 and L4.44
Indications
Other less commonly encountered indications include hyperhidrosis, phlegmasia alba dolens, acrocyanosis, discogenic pain, and accidental intraarterial injection of intravenous medications.45–47
Technique
There are essentially two frequently used techniques for performing lumbar sympathetic blockade. The most commonly used technique is the paramedian or “classic” approach that was initially described by Mandle48 in 1926. A more lateral approach was later developed by Reid and colleagues49 and published in 1970.
The initial description of the lumbar sympathetic block involved the placement of two separate needles at L2 and L3. Single-needle techniques were described in 1975 by Brown and Kunjappan50 and in 1985 by Hatangdi and Boas.51 The single-needle technique is currently the more popular technique when performing blocks with local anesthetic because it saves significant time, produces less postprocedural discomfort, and has similar results.50,51 One may consider a multiple-needle technique when performing a neurolytic block to minimize the volume of alcohol or phenol injected and decrease the risk of damage to the surrounding tissues.
Lateral Approach
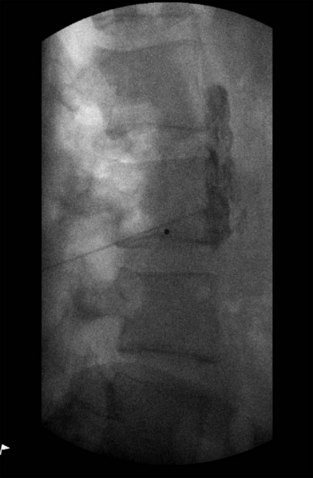
The patient is placed in the prone position, and the C-arm is rotated in an oblique direction until the transverse process of L3 is entirely medial to the lateral border of the vertebral body. The advantage of this approach is that it allows advancement of the needle with less risk of hitting the transverse process or the exiting segmental nerve.5 The skin is marked at what appears on the oblique fluoroscopic image to be the lateral aspect of the L3 vertebral body. Using a 22-gauge, 3.5-inch spinal needle, a skin wheal is raised, and local anesthetic is infiltrated in an oblique path directed toward the L3 vertebral body. A 22-gauge needle is then inserted and advanced under fluoroscopic guidance directly toward the anterolateral border of the vertebral body, taking care not to make bony contact, thereby avoiding patient discomfort (Fig. 8-15). It is also reasonable to use lateral fluoroscopic imaging and advance the needle until the tip sits exactly at the most anterior border of the vertebral body (Fig. 8-16).
Injection
With appropriate needle position confirmed, 1 to 2 mL of contrast is injected and should be visualized tracking in a cephalocaudad direction along the anterolateral surface of L3 and spreading at least one vertebral level above and below the injection site (Fig. 8-17). If most of the contrast is noted extending in a caudal and lateral direction tracking along the psoas muscle, the needle should be advanced slightly and contrast reinjected because local anesthetic injected predominantly along the psoas would result in a suboptimal block.44
After contrast spread is deemed appropriate, a test dose of 5 mL of a short-acting local anesthetic (2% 3-chloroprocaine or 2% lidocaine) may be injected first to facilitate a rapid onset of the sympathetic block and production of subsequent changes in skin temperature. When skin temperature has begun to increase in the affected lower extremity, a volume of 15 to 20 mL of 0.375% bupivacaine is injected. The patient should be observed in the postblock room for confirmation of sustained increase in lower extremity color and temperature. The skin temperature usually reaches a stable level in approximately 30 minutes.52
Temperature Monitoring
The most commonly used objective clinical indicator of sympathetic block is skin temperature measurement over the great toe. Skin temperature changes in the lower extremity have been shown to correlate well with increase in cutaneous blood flow after sympathetic block and may predict the relief of sympathetically maintained pain.52 Measurements are most commonly made using thermocouple probes applied bilaterally to the great toes of each lower extremity. A more sensitive measurement of temperature may be made using infrared thermography.53
The degree of skin temperature changes that signifies adequate sympathetic blockade is not well defined. A mean increase of 3° C has been found to occur after lumbar sympathetic block.51 In one study, a temperature increase of 2° C higher than the contralateral extremity signified complete sympathetic blockade in most but not all patients.54 Other investigators have preferred to use a final temperature of 34° C or higher in determining efficacy.55
Complications
With careful attention to detail on live fluoroscopic imaging, the incidence of complications related to lumbar sympathetic blockade is minimal.56,57 The complications result either from insertion and manipulation of the needle or as a direct result of the injected solution. The known complications are as follows:
Given the close proximity to segmental vessels and their branches, careful attention should be paid to real-time fluoroscopy during injection to minimize the risk of vascular uptake. There is also a small chance of penetrating a dural sleeve with resulting subarachnoid injection. Because of the length and diameter of the sympathectomy needles being used for the block, one should not use aspiration of blood or cerebrospinal fluid as a reliable indicator of vascular or subarachnoid needle placement. The best predictor is real-time fluoroscopic imaging. In one report, the sensitivity of the aspiration test and static radiography were 40.7% and 70.4%, respectively.44
Consequences of sympathectomy are more significant when performing neurolytic blockade. When injected into the body of the psoas, muscular necrosis may result. Intrapsoas injection or lateral spread of neurolytic solution may result in genitofemoral neuralgia or injury to the lumbar plexus.57,58 The symptoms usually resolve within 6 to 12 weeks. A transdiscal technique was reported in an effort to decrease the risk of genitofemoral neuralgia.59
1 Williams PL. Gray’s anatomy, ed 38. New York: Churchill Livingstone; 1995.
2 Fitzgerald MJT. Neuroanatomy: basic and clinical, ed 3. London: WB Saunders; 1996.
3 Tubbs RS, Loukas M, Remy AC, et al. The vertebral nerve revisited. Clin Anat. 2007;20:644-647.
4 Bonica JJ. Sympathetic nerve blocks for pain diagnosis and therapy. New York: Breon Laboratories; 1984.
5 Linn CC, Wu HH. Kuntz’s fiber: the scapegoat of surgical failure in sympathetic surgery. Ann Chir Gynael. 2001;90:170-171.
6 Hogan Q, Erickson SJ. Magnetic resonance imaging of the stellate ganglion: normal appearance. AJR Am J Roentgenol. 1992;158:655-659.
7 Raj PP. Stellate ganglion block. In: Waldman, Wenner. Interventional pain management. Philadelphia: Saunders, 1996.
8 Ellis H, Feldman S. Anatomy for anesthetists, ed 3. Oxford: Blackwell Scientific Publications; 1979. pp 256-262
9 Moore R, Groves D, Hammond C, et al. Temporary sympathectomy in the treatment of chronic refractory angina. J Pain Symptom Manage. 2005;30(2):183-191.
10 Lipov EG, Lipov S, Joshi JR, et al. Stellate ganglion block may relieve hot flashes by interrupting the sympathetic nervous system. Med Hypotheses. 2007;69(4):758-763.
11 Mulvaney SW, McLean B, de Leeuw J. The use of stellate ganglion block in the treatment of panic/anxiety symptoms with combat-related post-traumatic stress disorder; preliminary results of long-term follow-up: a case series. Pain Pract. 2010;10(4):359-365.
12 Bryce-Smith R. Stellate ganglion block. Anaesthesia. 1952;7:154-156.
13 Davies RM. Stellate ganglion block, a new approach. Anaesthesia. 1952;7:151-153.
14 Carron H, Litwiller R. Stellate ganglion block. Anesth Analg. 1975;54:567-570.
15 Raj PP. Practical management of pain, ed 3. St Louis: Mosby; 2000.
16 Moore DC, Bridenbaugh LDJr. The anterior approach to the stellate ganglion. JAMA. 1956;160:158-162.
17 Matsumoto S. Thermographic assessments of the sympathetic blockade by stellate ganglion block (1): comparison between C7-SGB and C6-SGB in 40 patients. Masui. 1991;40(4):562-569.
18 Abdi S, Zhou Y, Patel N, et al. A new and easy technique to block the stellate ganglion. Pain Physician. 2004;7:327-331.
19 Malmqvist ELA, Bengtsson M, Sorensen J. Efficacy of stellate ganglion block: a clinical study with bupivacaine. Reg Anesth. 1992;17:340-347.
20 Baumann JM, Middaugh RE, Cawthon MA, et al. Radionuclide-anesthetic flow study: a new technique for the study of regional anesthesia. J Nucl Med. 1986;27:1487-1489.
21 Hogan QH, Erickson SJ, Abram SE. Computerized tomography (CT) guided stellate ganglion blockade. Anesthesiology. 1992;77:596-599.
22 Slapppendel F, Thijssen H, Crul BJ, Merx JL. The stellate ganglion in magnetic resonance imaging, a quantification of anatomic variability. Anesthesiology. 1995;83:424-426.
23 Elias M. Cervical sympathetic and stellate ganglion blocks. Pain Physician. 2000;3:294-304.
24 Narouze S, Vydyanathan A, Patel N. Ultrasound-guided stellate ganglion block successfully prevented esophageal puncture. Pain Physician. 2007;10:747-752.
25 Peng P, Narouze S. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy and procedures. part I: non-axial structures. Reg Anesth Pain Med. 2009;34:458-474.
26 Kapral S, Krafft P, Gosch M, et al. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. Reg Anesth. 1995;20:323-328.
27 Shibata Y, Fujiwara Y, Komatsu T. A new approach of ultrasound-guided stellate ganglion block. Anesth Analg. 2007;105:550-551.
28 Christie JM, Martinez CR. Computerized axial tomography to define the distribution of solution after stellate ganglion nerve block. J Clin Anesth. 1995;7:306-311.
29 Matula C, Trattnig S, Tschabitscher M, et al. The course of the prevertebral segment of the vertebral artery: anatomy and clinical significance. Surg Neurol. 1997;48:125-131.
30 Narouze S. Beware of the “serpentine” inferior thyroid artery while performing stellate ganglion block. Anesth Analg. 2009;109(1):289-290.
31 Kwak JY, Kim E. Sonographic findings of Zenker diverticula. J Ultrasound Med. 2006;25:639-642.
32 Wulf H, Maier C, Schele H, Wabbel W. Plasma concentration of bupivacaine after stellate ganglion blockade. Anesth Analg. 1991;72:546-548.
33 Gofeld M, Bhatia A, Abbas S, et al. Development and validation of a new technique for ultrasound-guided stellate ganglion block. Reg Anesth Pain Med. 2009;34(5):475-479.
34 Vallejo R, Plancarte R, Benyamin RM, Santiago-Palma J. Anterior cervical approach for stellate ganglion and T2 to T3 sympathetic blocks: a novel technique. Pain Pract. 2005;5(3):244-248.
35 Narouze S. Ultrasound guided percutaneous cervical and upper thoracic sympathetic chain neuroelectrode implant for the treatment of complex regional pain syndrome [abstract]. Pain Med. 2010;11:298.
36 Narouze S, El-Sharkawy H. Ultrasound- guided T2 Sympathetic Block with the Anterior Approach [abstract]. Pain Med. 2009;10:225.
37 Higa KJ, Hirata K, Hirota K, et al. Retropharyngeal hematoma after stellate ganglion block. Anesthesiology. 2006;105:1238-1245.
38 Marples IL, Atkin RE. Stellate ganglion block. Pain Rev. 2001;8:3-11.
39 Hardy PAJ, Wells JCD. Extent of sympathetic blockade after stellate ganglion block with bupivacaine. Pain. 1989;36:193-196.
40 Huntoon MA. The vertebral artery is unlikely to be the sole source of vascular complications occurring during stellate ganglion block. Pain Pract. 2010;10(1):25-30.
41 Okuda Y, Urabe K, Kitajima T. Retropharyngeal or cervicomediastinal hematomas following stellate ganglion block. Eur J Anesthesiol. 2003;20:757-759.
42 Rocco AG, Palombi D, Raeke D. Anatomy of the lumbar sympathetic chain. Reg Anesth. 1995;20(1):3-19.
43 Umeda S, Toshiyuki A, Hatano Y. Cadaver anatomic analysis of the best site for chemical lumbar sympathectomy. Anesth Analg. 1987;66:643-646.
44 Hong JH, Kim AR, Lee MY, et al. A prospective evaluation of psoas muscle and intravascular injection in lumbar sympathetic ganglion block. Anesth Analg. 2010;111(3):802-807.
45 Mekhail N, Malak O. Lumbar sympathetic blockade. Tech Reg Anesth Pain Manage. 2001;5(3):99-101.
46 Boas RA. Sympathetic nerve blocks: in search of a role. Reg Anesth Pain Med. 1998;23(3):292-305.
47 Kosharskyy B, Rozen D. Lumbar discogenic pain. Disk degeneration and minimally invasive interventional therapies [German]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2007;42(4):262-267.
48 Mandle F. Die Paravertebrale Injektion. Vienna: J Springer; 1926.
49 Reid W, Watt JK, Gray RG. Phenol injection of the sympathetic chain. Br J Surg. 1970;57:45-50.
50 Brown EM, Kunjappan V. Single-needle lateral approach for lumbar sympathetic block. Anesth Analg. 1975;4:725.
51 Hatangdi VS, Boas RA. Lumbar sympathectomy: a single needle technique. Br J Anaesth. 1985;57:285.
52 Tran KM, Frank SM, Raja SN. Lumbar sympathetic block for sympathetically maintained pain: changes in cutaneous temperatures and pain perception. Anesth Analg. 2000;90:1396-1401.
53 Sherman RA, Barja RH, Bruno GM. Thermographic correlates of chronic pain: Analysis of 125 patients incorporating evaluations by a blind panel. Arch Phys Med Rehab. 1987;68:273-279.
54 Stevens RA, Stotz A, Kao TC, et al. The relative increase in skin temperature after stellate ganglion block is predictive of a complete sympathectomy of the hand. Reg Anesth Pain Med. 1998;23:266-270.
55 Malmqvist ELA, Bengtsson M, Sorensen J. Efficacy of stellate ganglion block: a clinical study with bupivacaine. Reg Anesth. 1992;17:340-347.
56 Walsh JA, Glynn CJ, Cousins MJ, Basedow RW. Blood flow, sympathetic activity and pain relief following lumbar sympathetic blockade or surgical sympathectomy. Anaesth Intensive Care. 1984;13:18.
57 Cousins MJ, Reeve TS, Glynn CJ, et al. Neurolytic lumbar sympathetic blockade. Duration of denervation and relief of rest pain. Anaesth Intensive Care. 1979;7:121.
58 Raskin NH, Levinson SA, Hoffman PM, et al. Post-sympathectomy neuralgia amelioration with diphenylhydantoin and carbamazepine. Am J Surg. 1974;128:75-78.
59 Ohno K, Oshita S. Transdiscal lumbar sympathetic block: a new technique for a chemical sympathectomy. Anesth Analg. 1997;85:1312-1316.