Chapter 98
Cerebrovascular Disease
Diagnostic Evaluation
A. Ross Naylor, George Markose
The aim of this chapter is to provide the reader with a pragmatic guide for planning safe and reliable evaluation of patients with cerebrovascular disease. The ultimate choice cannot be standardized because it will inevitably reflect (1) access to state-of-the-art or older imaging technologies, (2) experience, (3) opinion regarding what constitutes the “gold standard,” (4) (rapid) availability, (5) cost, (6) robustness in sensitivity analyses, (7) which stenosis measurement method (North American vs. European, see Chapter 16) is being used, (8) whether discrimination between 50% to 69% and 70% to 99% internal carotid artery (ICA) stenoses is desired, and (9) matching to current requirements for performing carotid endarterectomy (CEA) or carotid artery stenting (CAS), for which planning and imaging evaluation are completely different. Several important themes will emerge, but none is more important than the need for rapid investigation and treatment in symptomatic patients. The Carotid Endarterectomy Trialists’ Collaboration (CETC) combined data from the European Carotid Surgery Trial (ECST), the North American Symptomatic, Carotid Endarterectomy Trial (NASCET), and the Veterans Administration Trial (having remeasured all 6000 prerandomization angiograms using the NASCET method) and then performed subgroup analyses1 on the effect of delay to surgery (Table 98-1). As is clearly apparent, any delay reduces the long-term benefit conferred by CEA. Accordingly, any imaging modality that introduces delay (waiting list pressures, image processing time, reporting, etc.) will reduce overall clinical effectiveness, even if it might also prove to be the most accurate on sensitivity analyses. This paradox must be considered in the planning of the most appropriate investigative strategy.
Table 98-1
Effect of Delay in Surgery on Benefit Conferred by Carotid Endarterectomy*
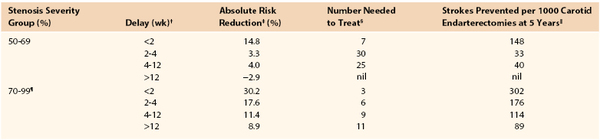
* Data recalculated from the Carotid Endarterectomy Trialists’ Collaboration.
† Delay indicates time from random assignment for treatment to surgery. The mean delay from the onset of symptoms to random assignment was 7 days.
‡ Absolute risk reduction in 5-year risk for ipsilateral stroke conferred by carotid endarterectomy over best medical therapy.
§ Number needed to treat to prevent one ipsilateral stroke at 5 years.
‖ Number of ipsilateral strokes prevented at 5 years by performing 1000 carotid endarterectomies.
¶ Excludes patients with near-occlusion.
Duplex Ultrasonography
Indications
There are a variety of clinical scenarios in which duplex ultrasonography (DUS) can provide useful information for the practitioner (see Chapter 16).
Carotid Artery Disease
In 2006, the UK Health Technology Assessment (HTA) Programme published a meta-analysis of studies evaluating the accuracy of noninvasive imaging (but not including multidetector computed tomographic angiography [MDCTA]) that concluded that although contrast-enhanced MRA (CEMRA) appeared the most accurate imaging modality (Table 98-2), its “clinical effectiveness” was limited by (in)accessibility, (un)availability, and delays.2 As a consequence, the researchers, who “constituted a panel of experts in stroke, imaging, vascular surgery, statistics and health economic modelling,” concluded that DUS should remain the first-line imaging modality for identifying patients with 70% to 99% ICA stenoses because of (1) low cost, (2) the much higher number of strokes likely to be prevented in the long term (because patients are scheduled more rapidly for surgery), (3) robustness in sensitivity analyses (see Table 98-2), and (4) the ability of DUS to match the needs of current surgery (i.e., patients were selected for surgery through attendance at “single visit clinics” incorporating DUS with a low probability of the surgeon encountering unexpected findings at operation that might otherwise compromise patient safety).3 The panel did, however, highlight concerns about DUS accuracy for diagnosing 50% to 69% stenoses. Because the benefit conferred by CEA in patients with 50% to 69% stenoses falls significantly with any delay to surgery (see Table 98-1), the panel recommended that if 4 weeks or more have elapsed after the index event, it would be advisable to perform corroborative imaging (CEMRA or MDCTA) in order to confirm stenosis severity. If, however, DUS has been performed within 4 weeks of the index event, the panel judged it reasonable to proceed to CEA on the basis of DUS findings alone because the number of strokes prevented through rapidly performed surgery exceeds the potential risk to patients with less than 50% stenoses undergoing inappropriate surgery.
Table 98-2
Results of a Meta-Analysis of the Accuracy of Noninvasive Imaging for All Stenosis Severity Groups and Imaging Modalities
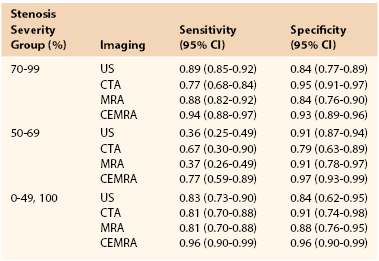
CEMRA, Contrast-enhanced magnetic resonance angiography; CI, confidence interval; CTA, computed tomographic angiography; MRA, magnetic resonance angiography; US, ultrasound.
From Wardlaw JM, et al: Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technol Assess 10:iii, 2006. Available at: http://www.hta.ac.uk/fullmono/mon1030.pdf.
Vertebrobasilar Disease
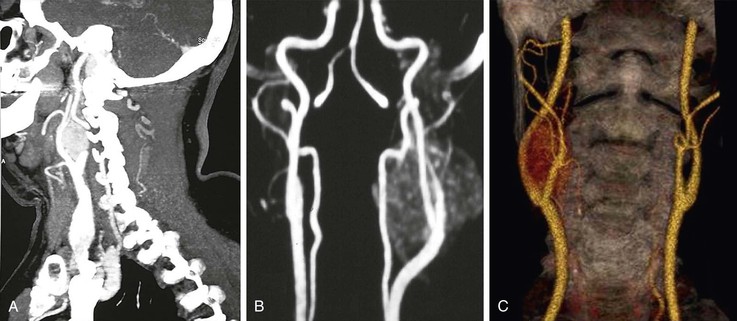
Extracranial DUS offers limited information about flow dynamics in the vertebral arteries (VAs), but nothing about the basilar system (which requires color transcranial DUS). It is not always possible to image the vertebral artery origins, and only those sections running between the transverse processes can be imaged more distally. Accordingly, if a patient presents with suspected vertebrobasilar symptoms, DUS assessment can exclude coexistent carotid disease, but diagnostic imaging with MRA/MDCTA is necessary. DUS is, however, useful for demonstrating complete or partial vertebral artery flow reversal, which suggests the possibility of proximal subclavian or innominate artery disease.
Role in Screening
Although the Society for Vascular Surgery advocates carotid screening in patients older than 55 years with cardiovascular risk factors, the 2007 U.S. Preventive Services Task Force recommends against it.4 A reluctance to screen also exists in Canada, the UK, and Scandinavia. For those committed to screening, DUS is the first-line modality. Portable DUS machines are available, but there is little data about their accuracy. Accordingly, users of portable machines are advised to undertake a corroborative DUS study in an accredited vascular laboratory (or use MDCTA/CEMRA) if a greater than 50% stenosis is suspected. DUS remains the preferred screening modality prior to coronary artery bypass grafting (CABG) surgery. Screening everyone is not cost-effective (5% yield for 80%-99% stenosis5) but the yield is increased in selected patient cohorts (left mainstem disease, carotid bruit, previous stroke/transient ischemic attack [TIA]). Any patient who is being considered for CABG using the internal mammary artery (IMA) and who has weakness or absence of the radial pulses should undergo assessment of the subclavian, innominate, and vertebral arteries. Where inflow disease or vertebral artery flow reversal is suspected, further imaging (MDCTA/CEMRA) is essential, and the cardiac surgeon should be warned that unless the flow reversal is corrected, subsequent use of the ipsilateral internal mammary artery (as a conduit) could cause postoperative coronary steal syndrome (Fig. 98-1).
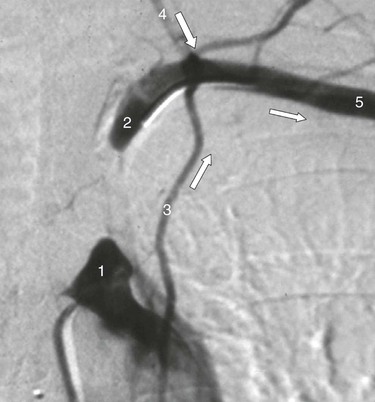
Figure 98-1 Intra-arterial digital subtraction arteriography in a patient with coronary steal syndrome who had previously undergone coronary bypass with use of the left internal mammary artery as a conduit. There is occlusion of the proximal left subclavian artery (1), which refills distally (2, 5) via reversed flow in the left vertebral (4) and internal mammary artery (3). It was treated successfully by angioplasty. Arrows indicate direction of flow.
Role in Trauma
DUS provides a useful screening and surveillance role in patients with zone II carotid artery injuries (between cricoid cartilage and angle of mandible), especially intimal irregularities or small false aneurysms that are being treated conservatively. DUS is, however, less reliable in zone I injuries (clavicle to cricoid cartilage) or zone III injuries (angle of mandible to skull base). MDCTA is now the investigation of choice because vascular imaging can be combined with an evaluation of concurrent bony and soft tissue injuries (see later).
Evaluation of Carotid Body or Glomus Vagale Tumors
Because of their vascularity, DUS is ideally suited for diagnosing carotid body tumor (CBT) and glomus vagale tumor (GVT). Given the rapid availability of DUS, it is unwise to perform biopsy of a “lymph node” in the jugulodigastric region without undertaking DUS. Once such a tumor is suspected, MDCTA or MRA should be performed to exclude bilateral lesions (5% incidence) and to image the upper and lateral extents of the lesion. Large, highly placed lesions require a totally different operative strategy, which must be anticipated preoperatively (temporomandibular subluxation cannot be performed once the operation is under way; see Chapter 104). DUS findings can also alert the surgeon to the possibility that a GVT might be present (Fig. 98-2), thus enabling the patient to be counseled about vagus nerve division (hoarseness, swallowing difficulties). Carotid body tumors cause splaying of the carotid bifurcation. GVTs do not splay the bifurcation, but they do cause displacement of the distal ICA (see Fig. 98-2). In addition, GVTs tend to have vascular serpiginous feeding vessels tracking down the proximal vagus nerve. These usually lie separately from the bifurcation, internal jugular vein, and common carotid artery (CCA).
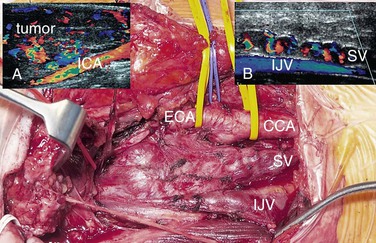
Figure 98-2 Glomus vagale tumor (GVT, main picture). This does not splay the bifurcation but displaces the distal internal carotid artery (ICA) (duplex image, A). Occasionally, preoperative ultrasound features can suggest that this is a GVT and not a carotid body tumor. Note the serpiginous feeding vessels extending down the vagus nerve (SV, main picture and duplex ultrasound scan, B). These are separate from the common carotid artery (CCA) and internal jugular vein (IJV).
Role in the Planning of Carotid Endarterectomy and Carotid Artery Stenting
In many centers, up to 95% of CEAs are undertaken on the basis of DUS findings, and there is no evidence that reliance on DUS compromises safety or operability.3 More importantly, patients can be identified and scheduled for surgery faster than with any other imaging modality, thereby optimizing the long-term benefit conferred by surgery (see Table 98-1). There are, however, important caveats. First, the vascular laboratory should be accredited and should have clear and validated criteria for measuring carotid stenosis. Second, European vascular units must be absolutely clear about which measurement method is being used (NASCET/ECST; see Chapter 16); evidence suggests that there may still be confusion on this matter.6 Third, the physician must be aware of DUS findings that suggest tandem inflow/outflow problems or subocclusion (see later). For either situation, corroborative imaging is mandated (MDCTA, CEMRA). In practice, one of the most frequent pathologic lesions to be missed with DUS is the coiled distal ICA (see Chapter 104). In most situations the coil does not cause undue problems for the surgeon, but it can be a cause of shunt failure (and intraoperative hemodynamic stroke) if the distal shunt tip becomes occluded against the distal loop and the problem is not recognized. This can be avoided by using intraoperative transcranial Doppler ultrasound (TCD) to assess shunt flow. DUS can also be useful in identifying unusual causes of cerebral vascular symptoms, such as the ICA stump syndrome (Fig. 98-3). In this rare condition, an occluded ICA stump (identifiable on DUS) acts as a reservoir for fresh thrombus that can then embolize up the external carotid artery (ECA) and into the brain via retrograde flow through the supraorbital and infraorbital vessels. Finally, some surgeons utilize DUS in the operating room to localize the carotid bifurcation and thus vary the level and orientation of the incision.
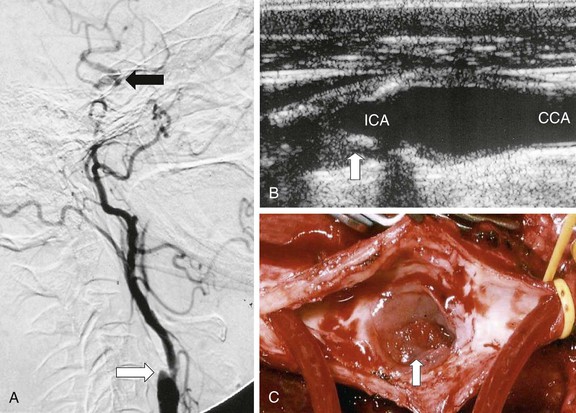
Figure 98-3 Internal carotid artery (ICA) stump syndrome. A, Selective intra-arterial digital subtraction arteriography with delayed imaging confirms the presence of an occluded ICA with a small stump (white arrow). The external carotid artery (ECA) is an important source of collateral flow because there is refilling of the distal ipsilateral carotid syphon (black arrow). B, B-mode ultrasound image of the ICA stump in this patient (arrow). C, Operative image with a shunt inserted between the common carotid artery (CCA) and the ECA. Note the extensive fresh thrombus within the ICA stump (arrow).
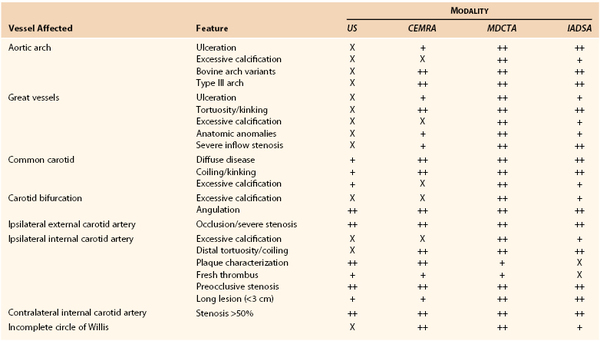
By contrast, CAS cannot be planned on the basis of DUS findings alone. Table 98-3 details anatomic and morphologic features that either are associated with difficulties in performing CAS or might influence the choice of cerebral protection device (CPD) or stent. For example, choice of CPD depends on ECA patency, integrity of the circle of Willis, and whether the distal ICA is straight and nontortuous. Stent selection is determined by varying combinations of vessel tortuosity and plaque friability. DUS can demonstrate (1) the presence of an appropriate ipsilateral stenosis, (2) ECA patency, (3) extent of contralateral disease, (4) tortuosity around the bifurcation, (5) plaque morphology, and (6) the possibility of coexistent inflow and outflow problems. However, DUS cannot provide information regarding (1) type of aortic arch, (2) extent of calcification at the great vessel origins, (3) proximal common carotid artery tortuosity, (4) whether there is an adequate distal landing zone for a filter CPD, or (5) the status of the circle of Willis. If the circle of Willis is not intact (Fig. 98-4), CAS practitioners tend not to deploy the flow reversal type of CPD. This type of information can be provided by only MDCTA or CEMRA (see later). However, it is important to remind CAS practitioners that reliance on additional imaging will introduce delays in planning the management of symptomatic patients. If delays are likely to be excessive (i.e., >2 weeks), patients may be better treated by CEA.
Table 98-3
Anatomic and Morphologic Features That Are Associated with Adverse Outcomes after Carotid Artery Stenting or That Might Influence the Choice of Protection Device or Stent and Relationship with Imaging Modalities
X, Not really suited for imaging; +, basic information provided; ++, highly suitable for imaging; CEMRA, contrast-enhanced magnetic resonance angiography; CI, confidence interval; CTA, computed tomographic angiography; MRA, magnetic resonance angiography; US, ultrasound.
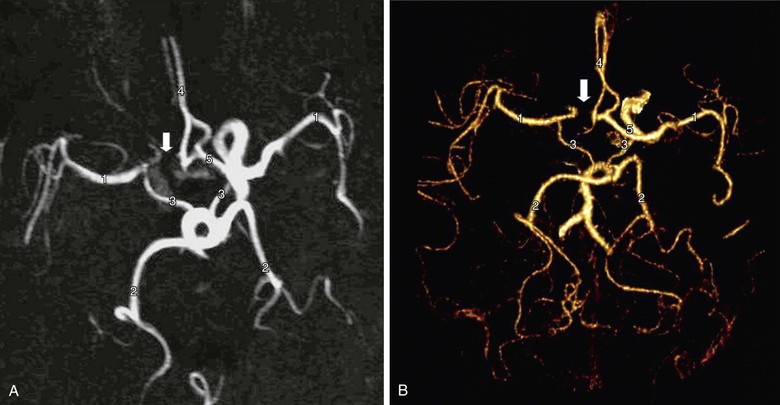
Figure 98-4 Circle of Willis imaged by contrast-enhanced MR angiography (A) and multidetector CT angiography (B). There are normal middle cerebral arteries (1), posterior cerebral arteries (2), posterior communicating arteries (3), and A2 segments of the anterior cerebral arteries (4). The A1 segment of the anterior cerebral artery is intact on one side (5) but absent on the other (arrow). Recognition of this abnormality does not influence the performance of carotid endarterectomy but might influence the choice of protection device during carotid artery stenting (i.e., a filter may be preferred instead of a flow reversal device).
Role in Evaluating Plaque Morphology
DUS enables interpretation of carotid plaque morphology (Table 98-4)7,8. Most practitioners would agree that better methods are needed to identify vulnerable or “at risk “ plaques in asymptomatic patients (as opposed to stenosis severity alone), and high-resolution DUS will likely prove useful to be one of them. In the Asymptomatic Carotid Surgery Trial (ACST), there was no evidence that the Gray-Weale classification (see Table 98-4) predicted outcome in medically treated asymptomatic patients,9 but the Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) study showed that if computerized “image normalization” was undertaken, 94% of strokes destined to occur in a cohort of 1000 asymptomatic patients with 50% to 99% stenosis severity had Geroulakis type 1 to 3 plaques8,10 (see Table 98-4).
The weakness of DUS plaque morphology studies has been a lack of objectivity. This has been partially addressed by gray-scale median (GSM) measurement,11 in which high-resolution B-mode images are combined with a quantitative computer-assisted measurement of plaque echogenicity (Fig. 98-5). The GSM is a numerical measure representing the average echogenicity of the plaque (low values are observed in predominantly echolucent plaques, whereas higher values are observed in more fibrous lesions). Evidence from the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study suggested that a GSM value of 25 or less was associated with a 7.1% stroke rate after CAS, in comparison with 1.5% for a GSM value higher than 25.12 This study was one of the first to suggest that an objective assessment of plaque morphology using DUS could be used to plan management (i.e., when to avoid CAS).
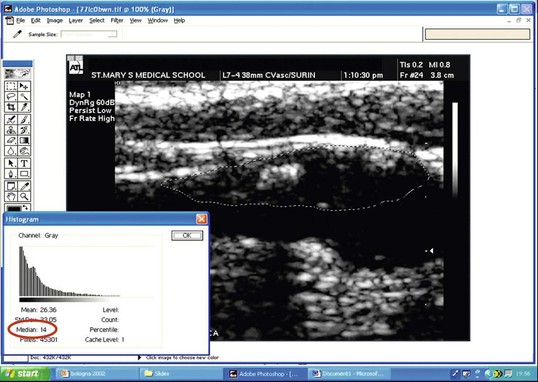
Figure 98-5 Gray-scale median (GSM). A B-mode image of the carotid bifurcation has been image-normalized and a region of interest drawn around the plaque, which shows areas of blackness (low GSM value) and bright areas (higher GSM score). After computerized analysis, an average GSM score (14) is recorded (red circle). (Courtesy Professor Andrew Nicolaides.)
Ultrasonography shows considerable promise in helping identify a cohort of asymptomatic patients with a higher risk for stroke in whom to prioritize CEA or CAS.13 In one study, the presence of three or more plaque ulcers on high-resolution three-dimensional (3D) ultrasound was associated with a significantly higher risk of late ipsilateral stroke (3-year risk 20%) than absence or the presence of up to two micro-ulcers (3-year risk 2%).14 This study also corroborated data from the Asymptomatic Carotid Emboli Study, which showed that plaque morphology predicted late, ipsilateral stroke in asymptomatic patients and that the combination of spontaneous emboli and plaque morphology conferred a greater predictive value than either imaging modality alone.15
The ACSRS group, undertaking a number of outcome analyses in a cohort of 1000 asymptomatic patients with 50% to 99% stenoses who were treated medically, has proposed a relatively simple clinically and DUS-derived scoring system for predicting late stroke on the basis of (1) GSM, (2) stenosis severity, (3) a history of contralateral TIA/stroke, and 4) plaque area.16 This scoring system, shown in Figure 98-6, illustrates how predicted annual rates of ipsilateral stroke might vary from less than 1% to more than 10%.13 This scoring system still requires independent validation but could emerge as a very useful method for predicting individual patient risk in the outpatient clinic.
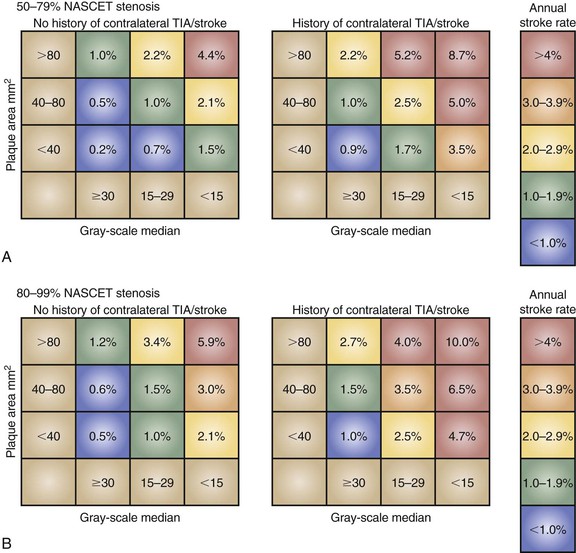
Figure 98-6 The ACSRS (Asymptomatic Carotid Stenosis and Risk of Stroke) scoring system for predicting the risk of stroke in asymptomatic patients with 50% to 99% stenoses as determined by NASCET (North American Symptomatic, Carotid Endarterectomy Trial) classification. The color-coded annual rate of ipsilateral stroke depends on stenosis severity, the gray-scale median, and the plaque area. TIA, transient ischemic attack. (From Naylor AR: Time to rethink management strategies in patients with asymptomatic carotid disease. Nat Rev Cardiol 9:116-124, 2012.)
Perioperative Roles
DUS should be repeated immediately prior to CEA if more than 4 weeks has elapsed since the initial DUS. This policy ensures that the ICA has not become occluded (i.e., CEA is unnecessary). It is also the final opportunity to detect distal disease extension (last chance for consideration of temporomandibular subluxation or alternative exposure techniques) and fulfills an important quality control/internal validation role. Intraoperative DUS can also offer completion assessment following flow restoration, by showing (1) residual filling defects (luminal thrombus), (2) undue turbulence, and (3) evidence of intimal flaps/residual stenoses (see Chapter 100). No randomized trial has determined whether a completion assessment by DUS reduces procedural risk. One overview suggested that it made no difference,17 but this assertion should be interpreted with caution because all events (fatal cardiac, intraoperative/postoperative strokes, all stroke etiologies) were combined in the analyses.
Postoperative Roles
Because of its versatility, DUS is invaluable in guiding the management of postoperative neurologic deficits. These often occur when other imaging modalities are unavailable and DUS can be brought to the patient’s bedside. Air in the deep tissues can interfere with insonation early after surgery, but it is usually possible to exclude thrombosis. Diagnosing the exact cause of the deficit can be difficult, and the role of DUS is enhanced if combined with TCD (see later).
The role of serial DUS surveillance after CEA and CAS remains enduringly controversial. As with screening, such surveillance is rarely undertaken in the UK and Scandinavia but remains common practice in North America, Australasia, and mainland Europe. It might be argued that an early postoperative DUS to assess the technical result of the operation and a follow-up study at 1 year would identify the majority of patients who will experience significant intimal hyperplastic recurrent lesions. However, the contrary opinion is that the identification of these lesions is of little clinical utility because this pathology is usually a benign phenomenon (see Chapters 100 and 101). On a practical note, those who offer DUS surveillance after CAS must be aware that stents change the physical properties of the carotid artery, this requiring different diagnostic criteria.18
DUS is, however, useful in warning about the possibility of late prosthetic patch infection (Fig. 98-7). DUS is the first-line investigation in patients returning with pulsatile neck swellings, discharging sinus tracts, or neck pain, and the recognition of patch “corrugation” may precede onset of clinical symptoms by up to a year19 (i.e., recognition of this phenomenon can alert the surgeon to the possibility of patch infection and antibiotics can be started early). DUS can also identify “leaks” into early false aneurysms (see Fig. 98-7).
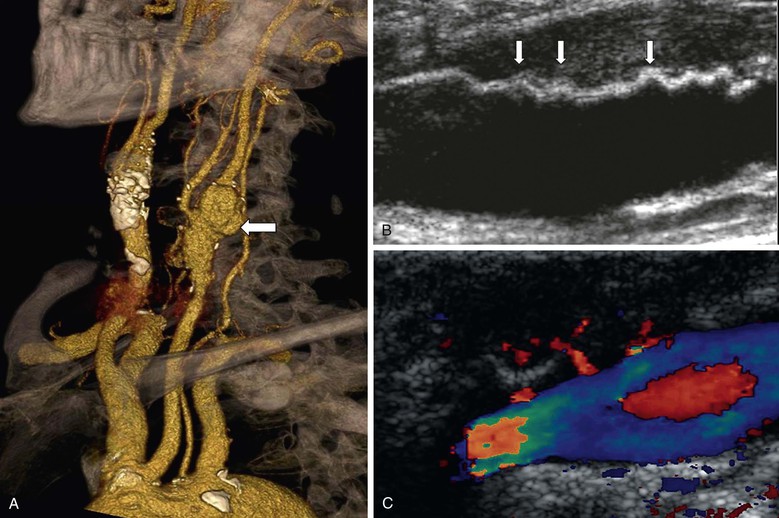
Figure 98-7 Prosthetic patch infection 15 years after carotid endarterectomy. A, Multidetector CT angiography shows a false aneurysm (arrow) at the left carotid bifurcation. B, Corrugation of the polyester patch on B-mode ultrasound imaging (arrows). C, In a different patient, color duplex ultrasound imaging showing jets of blood into a false aneurysm secondary to patch infection.
Contraindications
There are no contraindications to DUS, but the examination should only be undertaken by an operator who has been adequately trained (see Chapter 16).
Accuracy and Limitations in Clinical Practice
The most important determinants for the diagnostic accuracy of DUS are the experience and skill of the operator. Second is awareness of the wide array of stenosis thresholds being used in individual centers (>50%, >60%, >70%) and the spectrum of peak systolic velocity (PSV) and velocity ratios being used to diagnoses stenosis subgroups. This uncertainty has been lessened by publication of North American DUS consensus criteria.20
Third is establishing the ECST or NASCET measurement criteria used. UK experience suggests that many vascular laboratories remain unsure about this matter.6 Interestingly, the North American consensus document provides no advice for grading the large carotid bulb that contains large amounts of atherothrombotic material. This could score 0% with the NASCET measurement method (i.e., no need for CEA) but 70% stenosis with the ECST method (i.e., CEA justified). Future guideline makers must address this anomaly.
Fourth, in some situations, caution should be exercised in interpretation of DUS findings, and corroborative imaging should be considered. These include (1) inability to image high bifurcations, (2) inability to image above the plaque, (3) damped waveforms in the proximal common carotid artery suggesting inflow stenosis, (4) high-resistance flow in the distal ICA suggesting a distal tandem lesion, (5) the presence of a contralateral occlusion or very severe stenosis, because peak systolic velocity values may be increased in the contralateral ICA (due to hyperemic collateralization), leading to the potential for overestimation of degree of ipsilateral stenosis on the basis peak systolic velocity measurement, (6) excessive calcification (which prevents accurate velocity measurement because of acoustic shadowing), and (7) suspicion of subocclusion. The last condition has aroused considerable controversy. Although subocclusion (i.e., a severe ICA stenosis that does not open out into a normal-caliber lumen) was previously considered to be a marker of increased stroke risk, the Carotid Endarterectomy Trialists’ Collaboration observed that it is not a high-risk predictor for stroke and that CEA does not confer long-term benefit for patients with the condition.1
The Need for Corroborative Imaging
Corroborative imaging is necessary for the following conditions (1) uncertainty about DUS findings, (2) when intervention is planned in cases with borderline benefit (e.g., symptomatic patients with 50%-69% stenoses, especially when more than 4 weeks has elapsed since presentation), (3) a new (inexperienced) technologist performs DUS, and (4) a patient being considered for CAS.
Magnetic Resonance Imaging/Angiography
Indications
MRA has been available since 1996, but only after the introduction of contrast enhancement has it been considered a potential rival to angiography for the title of gold standard (see Chapter 23). The main advantage of CEMRA is the avoidance of exposure to radiation. Because of the way images are acquired, bony structures are not present on the resultant image, thus avoiding the need for complicated post-processing. However, adjacent soft tissue structures are not well visualized unless additional MR imaging is performed, and calcium within plaque is not well defined.
Carotid Disease
MRA has assumed an increasingly important role in the evaluation of patients with carotid artery disease. Table 98-2 summarizes the sensitivity analyses from the HTA systematic review for time-of-flight (TOF) MRA and CEMRA. The sensitivity and specificity for diagnosing a 70% to 99% stenosis with TOF MRA is identical to that for DUS and similarly poor for diagnosing 50% to 69% stenoses. Two-dimensional (2D) TOF MRA provides strong vascular signals, even when flow is low (useful for differentiating occlusion from subocclusion), whereas 3D TOF MRA offers better spatial resolution for measuring severity of stenosis and also enables assessment of flow directionality (useful in evaluating steal phenomena). However, in order to minimize the long acquisition times and artifacts (flow-related, motion and boundary), the field of view has to be restricted. Accordingly, although it is possible to image both carotid bifurcations simultaneously, two further data acquisitions are required to image the aortic arch/great vessels and the circle of Willis with TOF MRA. This limits its overall versatility and accessibility and means that, for the most part, TOF MRA offers little in addition to DUS.
CEMRA uses the paramagnetic agent gadolinium and obtains images more rapidly than TOF MRA. CEMRA incurs fewer flow-related artifacts and provides a much greater field of view that enables high-resolution imaging from the aortic arch (Fig. 98-8) up to the circle of Willis while retaining the ability to also evaluate flow directionality. The HTA systematic review and meta-analysis (see Table 98-2) concluded that CEMRA was the best (noninvasive) imaging modality (although MDCTA was not evaluated) and it has completely superseded TOF MRA. However, some centers still do not have rapid access to CEMRA imaging. Accordingly, if it is going to take several weeks for CEMRA to be performed, the symptomatic patient will gain greater clinical benefit by undergoing expedited CEA on the basis of DUS findings alone (see Table 98-1). Another advantage of CEMRA is that it can be combined with MR functional brain imaging, although (unlike MDCTA) doing so requires an additional period of data acquisition. The use of blood-pool contrast agents now facilitates high-resolution image acquisition by allowing equilibrium-phase imaging, thereby enlarging the temporal window.
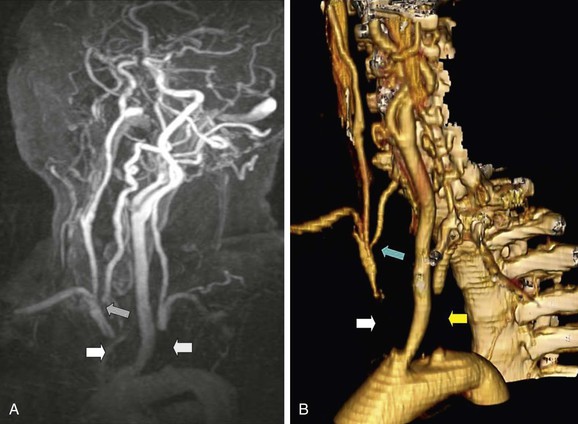
Figure 98-8 Contrast-enhanced magnetic resonance angiography (CEMRA; A) and multidetector computed tomographic angiography (MDCTA; B) of a 60-year-old woman with a vertebrobasilar stroke and recurring unsteadiness of gait. There is complete occlusion of the innominate artery (white arrow) and left subclavian artery (yellow arrow). The right vertebral artery has a stenosis at its origin (blue arrow). CEMRA and MDCTA have shown identical features, but MDCTA is probably easier to interpret because of venous contamination in the CEMRA image.
There is emerging evidence that the presence of infarction on CT/MRI can identify patients at increased risk of suffering an early recurrent stroke after presenting with a TIA or minor stroke (Table 98-5). The ABCD2 score is a clinically based scoring system that predicts the 48-hour and 7-day risk of stroke on the basis of age, blood pressure, clinical presentation, duration of symptoms, and diabetes.21 The maximum score is 7, and the risk of recurrent stroke increases as the score increases (see Table 98-5). Accordingly, the presence of infarction on CT/MRI in a patient with a recent TIA or minor stroke should alert the surgeon about an increased risk of early recurrent stroke, prompting expedited CEA. Interestingly, one study found that where no infarction was present, a high ABCD2 score no longer predicted an increased risk of recurrent stroke.22
Table 98-5
Early Risk of Stroke after Presentation with a Transient Ischemic Attack Stratified for the ABCD2 Score and Presence/Absence of Infarction on Computed Tomography/Magnetic Resonance Imaging
| Stroke Risk at 48 Hours (%) | Stroke Risk at 7 Days (%) | |
| ABCD2 score 0-320 | 1.0 | 1.2 |
| ABCD2 score 4-520 | 4.1 | 5.9 |
| ABCD2 score 6-720 | 8.1 | 11.7 |
| Infarction on CT/MRI + ABCD2 score 0-321 | 2.3 | |
| Infarction on CT/MRI + ABCD2 score 4-521 | 8.9 | |
| Infarction on CT/MRI + ABCD2 score 6-721 | 15.0 | |
| No infarction on CT/MRI + ABCD2 score 0-321 | 0.2 | |
| No infarction on CT/MRI + ABCD2 score 4-521 | 1.4 | |
| No infarction on CT/MRI + ABCD2 score 6-721 | 3.3 |
Role in Vertebrobasilar Disease
One of the advantages of TOF MRA and CEMRA is that they can provide information regarding flow directionality. This information is useful in evaluating steal phenomena (subclavian, coronary). Both modalities, however, are limited by an increased likelihood of artifact at the vertebral artery origins due to vessel tortuosity, respiratory motion, and cardiac pulsation. In practice, MDCTA is probably the preferred (noninvasive) investigation for evaluating patients with suspected vertebrobasilar symptoms.
Role in Screening
MRA has no role as a screening modality. It is simply not cost-effective.
Role in the Evaluation of Trauma
MRI/MRA studies are seldom undertaken in trauma patients. The reason is logistical issues, including scanner location (in relation to the emergency department or operating room), prolonged acquisition times, and poor access to an unstable patient for monitoring. In practice, MDCTA is the preferred modality because of its rapid acquisition times (5 seconds for aortic arch to circle of Willis) and its ability to image adjacent bony and soft tissue structures (including the brain) with a single scan.
Role in the Evaluation of Carotid Body or Glomus Vagale Tumors
MRA and CEMRA provide basic information, which is useful for planning surgical management and for excluding contralateral lesions (Fig. 98-9). However, MRI is limited by the fact that no “bony landmarks’ ” are retained on the image. Accordingly, MDCTA is probably the best imaging modality in this situation, largely because of the high-resolution axial images that detail the full extent of the lesion and its relationship to adjacent bony structures.
Role in the Planning of Carotid Endarterectomy and Carotid Artery Stenting
Surgeons who regularly perform CEA on the basis of DUS alone are unlikely to find any additional role for CEMRA and little or no use for TOF MRA. In the event that DUS findings are nondiagnostic, MDCTA is probably preferable to CEMRA (see later). However, CAS requires much more imaging information during the planning process than CEA (see Table 98-3). Accordingly, the choice among CEMRA, MDCTA, or digital subtraction angiography (DSA) for planning CAS will reflect resources, clinician preferences, and local expertise. CEMRA is preferable to conventional spiral CT, whereas MDCTA is generally accepted to be superior because of its versatility and ability to combine anatomic imaging with the identification of calcification, vessel tortuosity, and vascular anomalies in addition to the ability to perform functional brain imaging during one period of data acquisition.
CEMRA can identify vessel anomalies, but if arteries are occluded, they can sometimes be missed because of the way images are acquired. As is apparent from Table 98-3, the main limitation of CEMRA is its inability to display calcification. Documenting extent of calcification in the arch and origins of the great vessels is not a major problem for CEA, but with increasing age, the arch and great vessels become more tortuous and calcified. These features make CAS more challenging (especially for less experienced practitioners23) and are associated with greater procedural risks. A further problem with CEMRA is the tendency for multiple vessels to be included in the final image (some venous). This can lead to difficulties in overall image interpretation (see Fig. 98-8).
Role in Evaluating Plaque Morphology
There is growing interest in the role of MRI for evaluating subtle changes in carotid plaque morphology, plaque type, and plaque volume that may help identify a subgroup of patients who may be at higher risk of suffering a stroke if left untreated (see Chapter 99). In this respect, MRA has shown considerable research potential, although it still requires relatively long periods of data acquisition (i.e., plaque morphology data cannot be acquired during vessel or brain imaging studies). MRI is probably the best modality for visualization of the fibrous cap24 and lipid core,25 whereas inflammatory molecules within the plaque can be imaged using MR spectroscopy (still very much a research tool). Newer contrast agents that facilitate prolonged imaging now permit higher imaging resolutions to be achieved without compromising the signal-to-noise ratio. They allow greater accuracy in the depiction of ulcers and of subtle plaque surface irregularities.26
Considerable interest has focused on the ability of MRI to diagnose intraplaque hemorrhage (IPH), thought by many to be one of the most important predictors of increased stroke risk. In a multivariate analysis, the High-Resolution Magnetic Resonance Imaging in Atherosclerotic Stenosis of the Carotid Artery (HIRISC) study showed that an MRI diagnosis of IPH was associated with increasing stenosis severity (measured using both the European and North American measurement methods), hemispheric (as opposed to retinal) events, and very recent symptoms.27 In a series of themed projects, Altaf et al28–31 showed that an MRA diagnosis of IPH was associated with (1) increased spontaneous embolization in recently symptomatic patients, (2) increased embolization during the operative dissection portion of CEA in symptomatic patients, (3) an increased prevalence of white matter hyperintense lesions (leukoaraiosis), and (4) a much higher risk of ipsilateral recurrent stroke/TIA in symptomatic patients with 70% to 99% stenoses awaiting CEA and in recently symptomatic patients with 30% to 69% stenoses managed medically.
One of the key imaging goals is to identify the asymptomatic patient a who is at high risk of stroke, and the HIRISC study showed (in a multivariate analysis) that MRI evidence of IPH in asymptomatic patients was associated with increasing stenosis severity (using the European but not the North American measurement method27). A few studies have now evaluated MRI plaque features in asymptomatic patients, although most have been restricted to patients with less severe carotid stenosis (i.e., 50%-79%). Takaya et al32 observed that asymptomatic patients with MRI evidence of a thinned or ruptured fibrous cap, large lipid core, or IPH faced a higher risk of late ipsilateral stroke than those without these features. Similarly, Singh et al33 showed that 6 of 36 asymptomatic patients (17%) with MRI evidence of IPH suffered an ipsilateral stroke during follow-up, compared with 0 of 62 who did not exhibit this feature on MRI.
Perioperative Roles
There is no role for MRI in the perioperative setting.
Postoperative Roles
MRI/MRA does not have a role in the investigation of patients suffering a neurologic deficit after CEA or CAS. The exception might be the rare patient suffering a suspected hyperperfusion syndrome stroke in whom CT suggests there is an evolving ischemic infarct (usually in the posterior circulation). Perfusion MRI usually demonstrates that this area of “infarcted” brain tissue remains normally perfused and that the area of low attenuation on CT represents vasogenic as opposed to cytotoxic edema.34 New hyperintense white matter lesions (attributed to microembolization) have been well documented in the early period after CEA (and particularly after CAS35), but there is no evidence (as yet) that theses lesions predispose to declining cognitive function in the long term. Finally, CEMRA has no role in surveillance after CEA/CAS, partly because it will never be cost-effective, but also because of the metallic stents used in CAS.
Contraindications
Contraindications to MRI include (1) selected metallic implants (cardiac pacemakers, implantable defibrillators, metallic stents, joint replacements), (2) claustrophobia, and (3) obesity. Caution should be exercised in patients with chronic renal impairment in whom nephrogenic systemic fibrosis is more likely to develop following gadolinium exposure.36
Accuracy and Limitations in Clinical Practice
Diagnostic accuracy is related to available technology and expertise. For reasons alluded to earlier, 2D and 3D TOF MRA are limited by a tendency to overestimate stenosis severity, the need for multiple data acquisitions in order to image the inflow and outflow vessels accurately, and the high rate of artifacts (motion, flow-related). These problems are less of a practical issue with CEMRA, although the contraindications (listed previously) still apply. The relative inability of CEMRA to image calcification makes it less attractive for evaluation of patients for CAS, whereas the lower spatial resolution can lead to difficulties in identifying subtle tandem lesions. Anomalous vessels that have become occluded may not be apparent on CEMRA. In practice, however, the main limitations of CEMRA in clinical practice remain availability, lack of rapid access, and the need for dedicated sequences and hardware for plaque imaging.
Positron Emission Tomography–Computed Tomography
Indications
Positron emission tomography–computed tomography (PET-CT) has a limited role in the evaluation of patients with cerebral vascular disease.
Carotid Artery Disease
PET-CT has no role in the routine evaluation of patients being considered for CEA or CAS. However, because of its ability to measure cerebral blood flow (CBF) and oxygen extraction fraction (OEF), PET remains the gold standard for evaluating the hemodynamic effect of extracranial disease,37 particularly autoregulatory failure and impaired cerebral vascular reserve. To date, a measurement of perfusion reserve has never found a role in the investigation of patients with classic thromboembolic carotid disease, but it could evolve into an important role in identifying patients with carotid occlusion, hemodynamic failure, and recurrent carotid territory symptoms who might benefit from extracranial-intracranial bypass. This possibility is currently being evaluated in ongoing research studies. There is also evidence from a systematic review and meta-analysis that the presence of impaired cerebral vascular reserve in patients with asymptomatic carotid stenoses may serve as a predictor of increased late stroke risk if treated medically.38 Although this could become a novel role for PET in the future, it is likely that less expensive, more accessible, and less technologically complicated imaging strategies will assume this function.
Role in Evaluating Plaque Morphology
PET scanners offer (very) good spatial resolution (2 mm) and many are now combined with CT scanners (PET-CT devices) to allow tomographic radionuclide imaging (with anatomic correlation). This raises the possibility that a number of inflammatory processes could be targeted within the carotid plaque (inflammation, macrophages, apoptosis, angiogenesis, protease enzyme function39). The ability to link metabolic activity with plasma biomarkers and plaque imaging could have important implications for the future (patient selection, monitoring of drug therapy). Some studies have utilized novel PET tracers to differentiate plaque types, but (currently), PET-CT cannot compete with DUS or MRI.
Other Roles
PET-CT has no role in the evaluation of vertebrobasilar disease, screening, the evaluation of trauma, perioperative management, or the evaluation of carotid body/glomus tumors.
Accuracy and Limitations in Clinical Practice
PET is limited by inaccessibility, high cost, and lack of relevance to clinical practice. Only time will tell whether an evaluation of hemodynamic reserve can reliably identify patients with chronic carotid occlusions for extracranial-intracranial bypass or high-risk asymptomatic patients for CEA/CAS.
Computed Tomographic Angiography
CTA has proven to be a very valuable modality in the evaluation of patients with cerebral vascular disease (see Chapter 22).
Carotid Artery Disease
CTA is less likely to overestimate stenosis severity, than MRA. In the 2006 HTA Programme meta-analysis of noninvasive imaging modalities (summarized in Table 98-22), only CTA with spiral CT was evaluated. The published data suggested little overall advantage (compared with DUS and TOF MRA) with regard to diagnosing surgically important (and irrelevant) carotid disease. Accordingly, those increasingly rare centers with this older type of CT technology will preferably use CEMRA if DUS provides discordant findings.
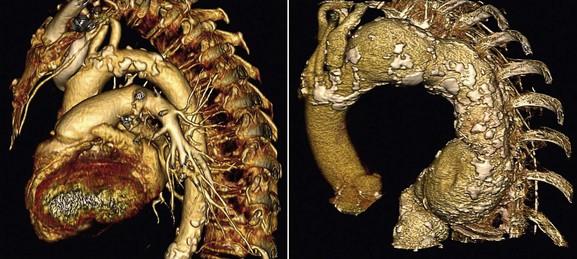
Since the HTA panel reported their deliberations in 2006, 64-, 256-, and 320-section MDCTA have been developed and are widely available. MDCTA has revolutionized the role of CTA because (1) it is extremely fast (5 seconds to perform a scan from aortic arch to circle of Willis; Fig. 98-10), (2) it is cheaper than CEMRA, (3) it offers submillimeter spatial resolution40 (0.3-mm spatial resolution compared with 0.8-mm for CEMRA and 0.2-mm for DSA), (4) sophisticated imaging workstations enable the production of high-quality 2D and 3D reformatted images from the aortic arch to circle of Willis that are easy to interpret (see Fig. 98-8), (5) it offers faster processing times, (6) it can visualize soft tissue, bone, and vessel at the same time, (7) it can rapidly demonstrate vascular anomalies even if vessels are occluded (Fig. 98-11), and (8) it is able to provide data regarding the extent of vessel calcification, especially in the aortic arch (Fig. 98-12). These characteristics make it possible to define extracranial and intracranial vascular anatomy in combination with functional brain imaging during one period of data acquisition. Stenoses (NASCET or ECST) can be measured directly with electronic microcalipers.
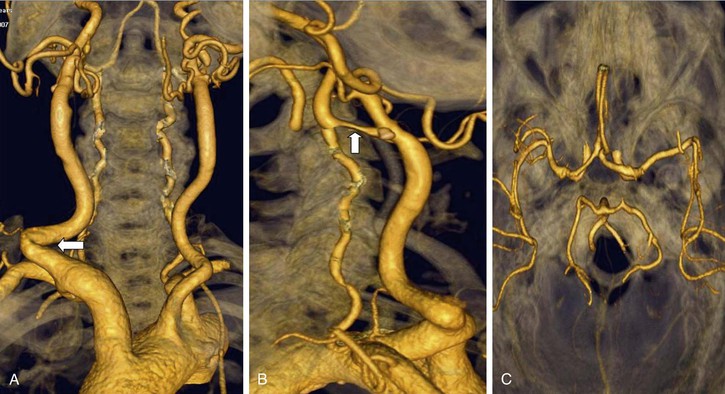
Figure 98-10 Multidetector computed tomographic angiography of the aortic arch and great vessels (A), carotid and vertebral arteries (B), and circle of Willis (C). This scan took 10 seconds to acquire. Note the acutely angled right common carotid artery (CCA) (A, arrow), which would pose a significant challenge to safe passage of a wire and sheath for carotid artery stenting. The left CCA is less angulated and would not pose such a problem. A severe, relatively smooth stenosis is seen in the internal carotid artery (B, arrow).
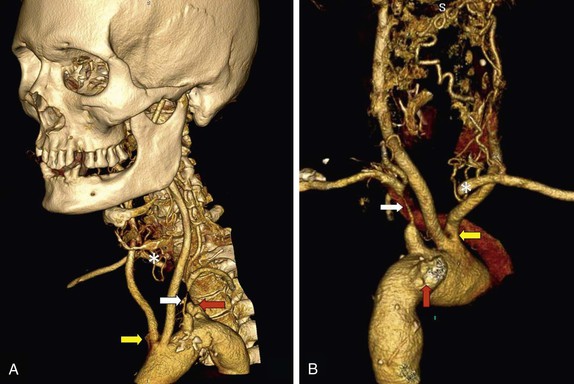
Figure 98-11 Multidetector computed tomographic angiography showing an unexpected anomaly of the great vessels of the aortic arch. A, Arch viewed from its anterior aspect. B, Arch viewed from its posterior aspect. Both common carotid arteries arise from a joint origin (yellow arrow), and there is a long, severe stenosis of the proximal left subclavian artery (white arrow). The right subclavian artery arises from an aberrant position and is occluded just beyond its origin (red arrow) with refilling distally (asterisk). The red arrow marks the diverticulum of Kommerl.
Vertebrobasilar Disease
Imaging the vertebral arteries has always presented a challenge (see Fig. 98-10). The vertebral artery has a tortuous course, a thick bony covering, adjacent veins, and a large variation in normal vessel caliber, and it is not unusual to find that one is hypoplastic. MDCTA provides better images of the extracranial and intracranial vertebrobasilar system than CEMRA, although the latter is preferable if only spiral CT is available. One important advantage of MDCTA is that it retains bony landmarks (see Fig. 98-10) and vessel coverings (which can then be “virtually dissected off” if required). Vessel tortuosity is easily demonstrated, and there is no problem with artifact at the vertebral artery origins. MDCTA cannot, however, provide information regarding flow directionality. If this is required (subclavian, coronary steal phenomena), CEMRA (or DUS) should be undertaken.
Role in Screening
There is no role for CTA as a screening tool. It is not cost-effective, and exposing patients to the radiation involved would be unethical.
Role in the Evaluation of Trauma
MDCTA has an important role in the evaluation of trauma, largely because it can rapidly provide anatomic vascular images from the arch to the circle of Willis, together with bony, soft tissue, and functional brain imaging during a single scan, even in a patient undergoing mechanical ventilation.
Role in the Evaluation of Carotid Body or Glomus Vagale Tumors
Owing to its better spatial resolution and easy interpretation, MDCTA is probably the optimum imaging modality for evaluating tumor circulation (see Fig. 98-9), the extent of the tumor, whether it is bilateral, and the relationship between tumor and adjacent soft tissue structures. This information is usually more than enough to plan a safe surgical resection and can be relied upon to show whether a high or difficult dissection might be encountered (i.e., enabling the surgeon to plan in advance for temporomandibular subluxation as required).
Role in the Planning of Carotid Endarterectomy and Carotid Artery Stenting
Like CEMRA, MDCTA is not needed before CEA in most patients. However, if there is evidence of inflow/outflow disease or excessive calcification on DUS, MDCTA can reliably provide the additional information required to plan management. MDCTA is, however, currently the optimal noninvasive imaging modality for planning CAS (see Table 98-3). The reason is partly the production of easily interpretable 3D images from the aortic arch to the circle of Willis (see Fig. 98-10) but also the ease of demonstrating vessel tortuosity (see Fig. 98-10), abnormalities of the circle of Willis (see Fig. 98-4), patterns of calcification in the aortic arch (see Fig. 98-12), and status of the origins of the great vessels (see Fig. 98-8). Unlike CEMRA, MDCTA is unaffected by occluded anomalous vessels arising from the arch (see Fig. 98-11). Procedural risks after CAS increase with age, probably in relation to progressive calcification/tortuosity of the arch and great vessels, which can lead to difficulties in accessing the carotid arteries with guide wires, sheaths, and so on (see Fig. 98-12). Although many of the adverse features detailed in Table 98-4 may not “deter” the experienced interventionist, they should help the less experienced operator (on his/her learning curve) to identify patients in whom CAS would be unsuitable or suboptimal.
Role in Evaluating Plaque Morphology
At present, the role of CT in evaluating plaque morphology is limited (compared with DUS and MRI). MDCTA has been used to divide carotid plaques into three subgroups on the basis of plaque density measurement (measured in Hounsfield units [HU]). Other visualization or postprocessing tools, such as MIP (maximum intensity projection), MPR (multiplanar reformat), and VR (volume rendition), are less effective (especially MIP) at evaluating plaque components. A “soft” plaque (<50 HU) usually has a soft lipid-rich core and is more likely to be associated with symptoms and perhaps a higher procedural risk after CAS. At the other extreme is the “calcified” plaque (>120 HU), which seems to be associated with a lower risk of symptoms. In between is the “intermediate” plaque (50-119 HU).41,42 These findings have been corroborated in a systematic review on the association between carotid plaque calcification and stroke risk, in which Kwee43 observed that plaques with the lowest volume and proportion of calcification were more likely to be clinically symptomatic, possibly because of the effect on shear stress.
The preceding section on MRI (see Table 98-5) observed that the presence of infarction on CT/MRI is highly predictive of an increased risk of stroke in the early time after onset of TIA or minor stroke symptoms22 irrespective of the ABCD2 score. This finding should alert the surgeon of the need to consider expedited CEA.
With regard to plaque morphology, the available evidence suggests that MRI is better than CT for visualizing thrombus, the fibrous cap, and its rupture (largely because MRI is better than CT at differentiating soft tissues structures), but there is evidence that MDCTA may be better at identifying plaque ulcers.44,45 IPH (provided it is >1 mm in diameter) can be visualized indirectly on MDCTA but is not as reliably predicted as with MRI. MDCTA can readily measure carotid artery wall thickness (CAWT), with a thicker (>1 mm) wall being associated with an increased risk of stroke.46
Perioperative Roles
There is no role for CTA in the perioperative management of the patient with cerebral vascular disease.
Postoperative Roles
MDCTA has a potentially important role in the evaluation of any patient suffering a stroke or other neurologic problem in the postoperative period after CEA and CAS. In the first 12 hours, the likelihood of intracranial hemorrhage (ICH) is remote. Accordingly, the patient should generally be considered for re-exploration, which should not be delayed in order to perform a CT scan (in most circumstances). However, because MDCTA can often be performed very rapidly, it may be useful in selected cases of early postoperative neurologic deficit, especially if completion assessment DUS or DSA findings were normal and intracranial embolism is suspected. After 12 hours has elapsed, there is an increasing likelihood that the stroke is due to intracranial hemorrhage or the hyperperfusion syndrome. In this situation, it is important to avoid an unnecessary (and potentially dangerous) return of the patient to the operating room. An emergency CT scan excludes hemorrhage but may demonstrate features associated with the hyperperfusion syndrome (Fig. 98-13). In the latter condition, middle cerebral artery velocities are generally elevated (frequently in association with hypertension) and it is not unusual for the CT scan to display areas of low attenuation (usually in the posterior fossa) that can sometimes be misinterpreted as representing an evolving infarct.34 Diffusion-weighted MRI studies have shown that the white matter edema seen in the hyperperfusion syndrome on CT is vasogenic (if it was an evolving infarct, it would be cytotoxic), whereas perfusion studies show that there is no loss of blood supply.47 Quite why the hyperperfusion syndrome causes vasogenic edema in the posterior circulation has never been adequately explained.
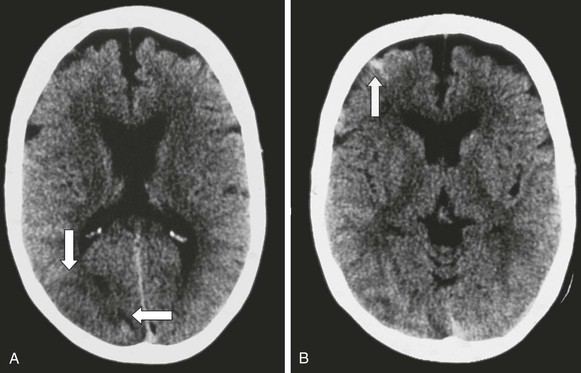
Figure 98-13 CT scan in a patient who underwent right carotid endarterectomy and was seen 5 days later with seizures, hypertension, and left-sided weakness. The CT scan revealed an area of white matter edema in the right posterior circulation (A, arrows) and a focal petechial hemorrhage in the right frontal region (B, arrow). White matter edema in the posterior circulation is not an uncommon finding in this syndrome and is often mistaken for an evolving infarct. (Reproduced with permission from Naylor AR, et al: Seizures after carotid endarterectomy: hyperperfusion, dysautoregulation or hypertensive encephalopathy? Eur J Vasc Endovasc Surg 26:39, 2003.)
If intracranial hemorrhage and hyperperfusion syndrome have been excluded, there remains the possibility that the neurologic deficit was thromboembolic in origin (see later section on TCD). DUS of the carotid bifurcation can be difficult to interpret in the early postoperative period (air in the deeper tissues, edema, overlying hemostatic gauze), and it may not be possible to exclude an intraluminal filling defect. In this situation, MDCTA quickly provides high-quality images of the extracranial circulation, which will not unduly delay return of the patient to surgery if necessary. Finally, MDCTA does not have a routine role in serial surveillance after CEA and CAS; this is better achieved with DUS.
Contraindications
A history of allergy or reaction to iodinated contrast agents may be a contraindication to CTA, although in milder cases, parenteral steroid therapy may allow safe completion of the test. The risk of anaphylaxis (1 : 500-5000 procedures) is higher in patients with a history of asthma and food allergies. Caution must be exercised in patients with impaired renal function, who require intravenous hydration beforehand.
Limitations in Practice
MDCTA has superseded all previous CT technologies and is rapidly becoming the new gold standard. By implication, therefore, older CT technology will not be able to answer many of the clinical questions posed in this section. Notwithstanding this caveat, MDCTA is limited by the need for an intravenous contrast agent (see earlier), there is a potential risk of contrast-related nephropathy (hence the occasional need for hydration); MDCTA cannot provide information on flow directionality (DUS or CEMRA is required); and there is the inevitable exposure to radiation. Newer CT scanners, however, have automatic exposure control systems that continuously modulate the x-ray tube (based on patient size and tissue density), thereby allowing for potential reductions in radiation dose. Finally, there are occasional situations in which MDCTA (and CEMRA) cannot reliably provide accurate and diagnostic imaging information, for example, in selected complex patients, especially those with tandem syphon disease or stenoses within coiled segments of the ICA (Fig. 98-14). In such situations, high-quality selective carotid DSA imaging remains the gold standard (see later).
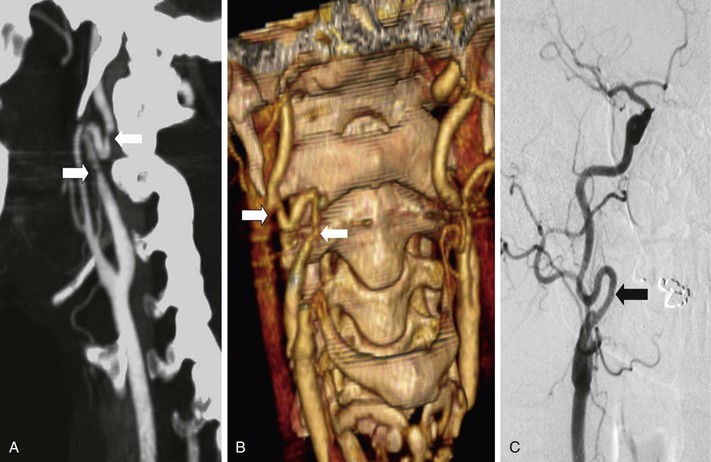
Figure 98-14 Image processing in patients with distal internal carotid artery (ICA) loops can lead to the creation of artifacts that can be interpreted as clinically important stenoses. A, Multidetector computed tomographic angiography (MDCTA) maximum intensity projection; note the normal bifurcation and a distal looped ICA with apparent inflow and outflow stenoses (arrows). B, Three-dimensional MDCTA in the same patient suggests that the stenoses were real (arrows). C, Selective intra-arterial digital subtraction arteriography shows the looped ICA but no stenoses. The “stenoses” identified on A and B were caused during image processing.
Digital Subtraction Angiography
Indications
Conventional catheter angiography was previously the gold standard for imaging the extracranial and intracranial circulation prior to surgery (see Chapter 19). Used in four out of five randomized trials comparing CEA with best medical therapy, its easily interpreted images found favor among surgeons and neurologists. However, the performance of angiography came at a price. In the Asymptomatic Carotid Atherosclerosis Study (ACAS),48 angiography-related stroke accounted for 50% of the procedural risk following CEA. In a comprehensive review of complications after 23,416 carotid angiograms,49 groin hematomas occurred after 4% of procedures, and 2.6% of patients suffered a transient or permanent neurologic deficit. The prevalence of anaphylaxis was 0.03%, and death complicated 0.06% of all carotid angiograms. The risk of suffering a neurologic deficit after catheter angiography was significantly higher in patients with carotid atherosclerosis (hazard ratio 2.5, 95% confidence interval [CI] 1.9-3.3) and in patients with frequent TIAs (hazard ratio 1.7, 95% CI 1.3-2.1). The prevalence of a transient neurologic deficit following angiography in 7614 patients with atherosclerotic carotid disease was 3.2%, that of minor strokes 0.6%, and that of permanent strokes 0.2%. Angiography-related neurologic deficits have significantly declined with time (reflecting better case selection and improved technology and techniques) from 3.8% in 1988 to 0.6% in 2003.49
Carotid Artery Disease
There is no role for routine catheter diagnostic arteriography in modern cerebrovascular practice. However, there are still situations in which intra-arterial digital subtraction angiography (IADSA) can provide valuable diagnostic information (see Fig. 98-14). Improvements in CEMRA and (especially) MDCTA mean that it is rarely necessary to undertake IADSA in order to look at the origins of the great vessels (see Figs. 98-8 and 98-11). Similarly, MDCTA can reliably image patients with heavily calcified plaques in the arch or great vessel origins (see Fig. 98-12). However, CEMRA and MDCTA can provide misleading information in the patient with severe distal ICA disease (Fig. 98-15) and the patient with marked coiling of the ICA in the upper reaches of the neck (see Fig. 98-14). In the former, slow flow through a tiny lumen can be missed. In the latter, unless extreme care is taken with image processing (i.e., ensuring that the axial measurements are in a true perpendicular cross-sectional plane), it is possible that an artifact introduced during image processing may persuade the surgeon or interventionist that significant carotid disease exists.
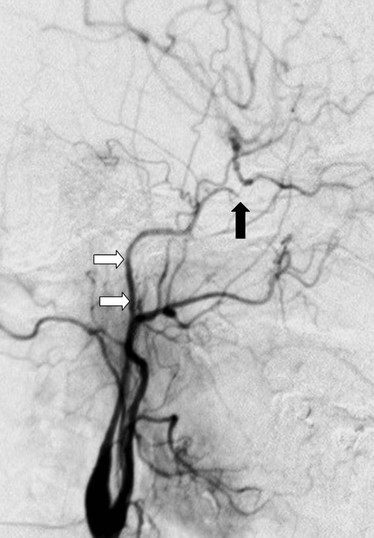
Figure 98-15 Selective intra-arterial digital subtraction arteriography (IADSA) in a 38-year-old woman with stroke (middle cerebral territory infarct on CT scan). Duplex ultrasound imaging showed slow, high-resistance flow in the carotid artery but a normal bifurcation. Contrast-enhanced magnetic resonance angiography suggested a subocclusion but could not image the distal internal carotid artery (ICA). This delayed IADSA image shows a normal-caliber carotid bifurcation with a narrow-caliber ICA in its midsection (white arrows where flow was extremely slow). In the very distal ICA (black arrow), vessel diameter dwindles toward complete occlusion. The images are probably consistent with acute dissection and compression of the true lumen by thrombus in the false lumen.
Vertebrobasilar Disease
There is no role for routine IADSA in the investigation of patients with suspected vertebrobasilar disease. CEMRA and MDCTA are now the investigations of choice, and it is rare that a diagnostic IADSA is necessary.
Role in Screening
There is no role for IADSA as a screening tool in carotid or vertebrobasilar disease.
Role in Trauma
Angiography was previously the first-line imaging modality in patients with a suspected extracranial arterial injury. This role has, once again, been superseded by MDCTA, which can perform a diagnostic study from arch to circle of Willis in 5 seconds. However, IADSA remains an important secondary option in those situations in which MDCTA image quality is compromised or its findings difficult to interpret. Furthermore, angiography retains the unique distinction of allowing one to proceed with endovascular therapeutic interventions (coil/balloon occlusion, insertion of a covered stent).
Role in the Evaluation of Carotid Body or Glomus Vagale Tumors
There is no role for IADSA in the routine evaluation of patients being considered for resection of carotid body tumor or GVT. It provides no information on the overall extent or operability of the lesion. However, preoperative embolization of ECA feeding vessels is considered by some to reduce perioperative bleeding (Fig. 98-16), whereas insertion of a covered stent into the ECA can significantly reduce bleeding in patients with very large tumors.50
Figure 98-16 A, Preoperative intra-arterial digital subtraction arteriography of carotid vessels in a patient with a large carotid body tumor. Most of the tumor circulation is derived from external carotid artery (ECA) branches. B, All major feeding ECA branches have been coiled. The patient was taken directly from the angiography suite to the operating theater for an uneventful tumor resection.
Role in the Planning of CEA/CAS
Most centers now perform the majority of CEAs without preoperative IADSA. Large studies suggest that reliance on such a strategy does not compromise patient safety and the surgeon rarely encounters operative scenarios that cannot be dealt with safely.3 In reality, the safe performance of CEA does not depend on preoperative imaging of inflow or distal vessels (unless DUS suggests an abnormal flow pattern), and there is no evidence that imaging the intracranial circulation alters outcome. The preceding section does, however, summarize those situations in which a diagnostic IADSA would be advisable.
By contrast, some CAS practitioners have been reluctant to dispense with diagnostic IADSA (usually as an arch angiogram) as part of the routine evaluation of their patients. Advocates of this strategy must, however, remember that the need for a diagnostic angiogram (in place of or in addition to CEMRA/MDCTA) will inevitably delay treatment and so potentially reduce the overall effectiveness of any procedure (see Table 98-1) while exposing the patient to two catheterization procedures. The advent of CEMRA and (especially) MDCTA, together with the drive toward expedited treatment, has probably rendered this practice obsolete. In practical terms, this reliance on an additional diagnostic arch angiogram could mean that on very rare occasions, a patient may be evaluated for CAS solely on the basis of CEMRA/MDCTA findings only to have the procedure abandoned following the diagnostic IADSA that immediately precedes the planned CAS procedure. In reality, this is unlikely to happen because of excellent pre-interventional imaging and planning.
Role in Evaluating Plaque Morphology
DSA has no role in evaluating plaque morphology other than demonstrating surface irregularity, and angiography is not particularly good at identifying intraluminal thrombus.51 Although NASCET and ECST showed that plaque irregularity (using angiography) was associated with an increased risk of stroke in medically treated patients, as well as an increased risk of major cardiac events during follow-up,51 there is no evidence to justify IADSA for plaque morphology evaluation.
Perioperative Roles
Intraoperative completion angiography can identify technical errors (intimal flaps, luminal thrombus, residual stenoses) following flow restoration but has been superseded by color DUS (facilitated by dedicated L-shaped probes) or angioscopy. Completion angiography can be cumbersome; it requires the presence of a C-arm and radiology technician in the operating room and (for most patients), represents an unnecessary exposure to radiation.
Angiography does, however, retain a useful diagnostic role in the rare patient who suffers a stroke due to carotid thrombosis or acute distal dissection in the early postoperative period after CEA or CAS (Fig. 98-17). After CEA, the key to ensuring the best outcome is early re-exploration (preferably within 1 hour). At re-exploration, it is not uncommon to find a “pulse” in the ICA (suggesting to the unwary surgeon that the vessel is still patent). In this situation, it is important to avoid manipulation of the bifurcation, which could precipitate major embolization. Despite the apparent pulsation, it is not unusual for the angiogram to demonstrate complete occlusion (see Fig. 98-17). Angiography can also identify the very rare case of acute distal dissection that may follow shunt trauma (see Fig. 98-17). In the latter situation, the diagnostic angiogram can be followed by insertion of a stent/covered stent into the true lumen, whereas embolic occlusion of intracranial vessels can be treated by intra-arterial, low-dose thrombolysis or catheter-directed embolus retrieval.
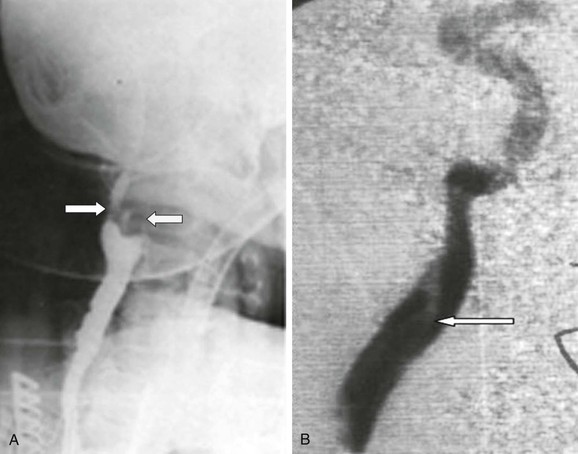
Figure 98-17 A, On-table conventional carotid angiogram in a patient who underwent re-exploration after the onset of a dense neurologic deficit following uneventful carotid endarterectomy. The external carotid artery (left arrow) has a partial filling defect. The internal carotid artery (ICA) had a pulse but was completely occluded (right arrow). This patient was treated before routine postoperative transcranial Doppler echocardiography (TCD) monitoring was used to prevent this complication. B, On-table intra-arterial digital subtraction arteriogram in a patient who recovered from anesthesia with a new neurologic deficit. TCD showed flow rates consistent with carotid occlusion, but there had been no preceding embolization. At re-exploration, dissection of the distal ICA was found to extend to the base of the skull (starting at the arrow).
Thromboembolic stroke in the first few hours after CAS raises the possibility of in-stent thrombosis (Fig. 98-18). For a detailed discussion of CAS and the prevention and management of its complications, see Chapter 101.
Figure 98-18 Selective intra-arterial digital subtraction arteriography in a patient who had undergone uneventful carotid stenting of an extensive symptomatic plaque at the origin of the internal carotid artery. He was readmitted on day 5 with extensive hemiplegia. This image shows extensive in-stent thrombosis
Postoperative Roles
There is no role for IADSA in the serial surveillance of patients after CEA or CAS.
Contraindications
The only contraindication to IADSA is previous allergic reaction to iodinated contrast media.
Accuracy and Limitations in Clinical Practice
IADSA is now largely relegated to a “supporting role” because of exposure to radiation, the inevitable delays to performance of studies, and the risk of procedural neurologic deficits. In addition, formal angiography is associated with a small but important risk of access complications (bleeding, dissection, thromboembolism) and contrast-induced nephropathy.
The Need for Corroborative Imaging
DSA can image only what is outlined by the contrast agent and cannot, therefore, provide any information regarding soft tissues, or direct visualization of processes affecting the vessel wall. Accordingly, the original true lumen of the vessel cannot be assessed. MRI or CT is required if extrinsic compression is suspected from angiography.
Transcranial Doppler Ultrasound
Indications
Although not part of the routine evaluation of most patients with cerebral vascular disease, TCD can provide useful diagnostic information in selected cases. For a discussion of the details of Doppler or duplex ultrasonography for this application, see Chapter 16.
Carotid Artery Disease
TCD has no role in imaging carotid disease, but it can provide a simple evaluation of the hemodynamic effect of a stenosis (compared with PET) by measuring the proportional increase in middle cerebral artery (MCA) velocity in response to intravenous administration of acetazolamide (a cerebral vasodilator). TCD is, however, the only modality capable of diagnosing in vivo embolization. This is because the larger embolus reflects more back-scattered signal than red cells, and despite initial skepticism, there are now well-accepted and validated criteria for diagnosing embolization and for excluding artifact.52 Embolization is more commonly encountered in the MCA ipsilateral to a symptomatic carotid stenosis and is indicative of an unstable plaque. Recognition of this phenomenon should prompt the surgeon to undertake an expedited CEA, and although it seems intuitively obvious that patients with these findings might be suboptimal candidates for CAS, this assumption has never been properly evaluated.
TCD-diagnosed spontaneous embolization may, however, assume an increasingly important role in identifying a cohort of patients with asymptomatic carotid disease who are at high risk for stroke. A systematic review and meta-analysis of six prospective observational studies involving 1144 patients with asymptomatic 70% to 99% stenosis observed that the presence of a single embolic signal during TCD monitoring was associated with a near eightfold increase in stroke risk during follow-up (odds ratio 7.6, 95% CI 2.3-24.7).53 A further systematic review and meta-analysis of the relationship among spontaneous embolization, carotid stenosis severity, and silent cerebral infarction has shown that (1) spontaneous embolization was significantly more common in symptomatic than asymptomatic patients, (2) the prevalence of spontaneous embolization was significantly higher in patients with a 70% to 99% asymptomatic stenosis (as opposed to a stenosis <70%), (3) that the rate of ipsilateral stroke in patients with asymptomatic embolization was significantly higher than in those with no embolization (10% vs. 1%), and (4) patients with evidence of type A silent cerebral infarction (defined as cortical or subcortical, in or adjacent to the anterior or MCA territories or basal ganglia/thalamic infarctions) were nearly five times more likely to suffer a late stroke (but only if they had a 60%-99% stenosis).54
Vertebrobasilar Disease
One useful practical role for TCD in the vertebrobasilar circulation involves patients who report isolated dizziness or vertigo following head movements. In the past, many were labeled as having suffered vertebrobasilar TIAs due to compression of the vertebral arteries within the transverse processes of the upper cervical vertebrae during neck movement. Subsequent research has shown this to be rarely the case. In a series of 46 symptomatic patients undergoing extracranial DUS and intracranial TCD with the head rotated into provocative positions, not one patient exhibited flow reversal or flow reduction,55 suggesting that most historical cases were probably misdiagnosed. In reality, most patients with such symptoms have inner ear pathologies.
Role in the Evaluation of Trauma
TCD has no role in the management of trauma other than being used to monitor the effect of carotid ligation/endovascular balloon occlusion in an unconscious patient if reconstruction proves impossible. During CEA, provided that mean MCA velocity is higher than 15 cm/sec, most patients tolerate carotid ligation (balloon occlusion) without suffering a stroke.56 Whether this velocity threshold also applies to patients with head trauma has never been evaluated.
Intraoperative Monitoring
The main reason that intraoperative monitoring and/or completion quality control assessment have failed to gain wider acceptance is a simple failure to ask the appropriate questions.44 TCD warns of embolization (enabling the surgeon to modify technique), but it cannot prevent a stroke due to embolization of luminal thrombus after restoration of flow. Some other form of quality control completion assessment is required for that. Accordingly, TCD is used to answer only four questions during CEA57: (1) is there spontaneous embolization during operative carotid dissection (i.e., warning the surgeon of an unstable plaque, thereby allowing modification of dissection technique); (2) is the shunt working (3% of shunts malfunction following insertion);58 (3) is MCA velocity higher than 15 cm/sec (if not flow can be increased by pharmacologic elevation of blood pressure); and (4) is this patient one of the very rare ones destined to have ICA thrombosis before the operation is completed (Fig. 98-19). At present, the role of TCD monitoring during CAS has not been clarified, but the potential to answer some of the questions posed previously during CEA could also be relevant for CAS.
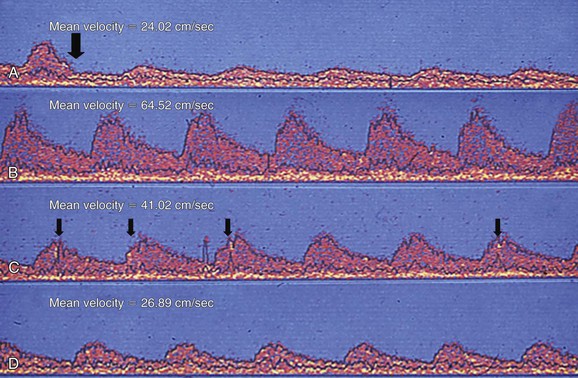
Figure 98-19 Transcranial Doppler ultrasound diagnosis of a patient suffering an on-table carotid thrombosis. A, Last cardiac cycle (arrow) before the carotid clamp is applied. After clamping, middle cerebral artery (MCA) flow velocity falls to 24.02 cm/sec. B, MCA waveform after restoration of flow (i.e., after endarterectomy). MCA flow velocity is now 64.52 cm/sec. C, Increasing embolization is detected within 12 minutes of restoration of flow (arrows), which is associated with a gradual decline in MCA flow velocity. D, Within 23 minutes of restoration of flow, the internal carotid artery is occluded, as demonstrated by the fact that MCA flow velocity (26.89 cm/sec) is now virtually identical to that noted during carotid clamping (A).
Role in Evaluating Postoperative Strokes
TCD has a useful (but unexploited) role in determining the cause of intraoperative and postoperative stroke. Intraoperative stroke follows inadvertent technical error. Hemodynamic failure accounts for only 20% of intraoperative events, with the remainder being thromboembolic.57 In a large, prospective audit involving more than 2000 patients undergoing CEA, intraoperative stroke was virtually abolished with the use of TCD and completion angioscopy.59 Although intimal flaps were occasionally diagnosed on angioscopy, 4% to 5% of patients had retained luminal thrombus (despite venting and irrigation), which were derived from transected vasa vasorum.59
For practitioners without recourse to angioscopy or other quality control methods, the patient wakening with a new neurologic deficit poses a considerable management dilemma. However, if TCD-derived MCA velocity is now the same as was observed during carotid clamping, it is highly likely that the carotid artery has thrombosed (see Fig. 98-19). If MCA velocity is unchanged but embolization is ongoing, it is likely that there is an evolving thrombus within the endarterectomy zone. Both of these scenarios warrant immediate reoperation.
However, the etiology of postoperative stroke is multifactorial. Most strokes occurring more than 24 hours after surgery are due to intracranial hemorrhage or the hyperperfusion syndrome. Unfortunately, no TCD protocol has been developed to reliably anticipate and prevent either of these situations. In the first 24 hours after CEA, postoperative carotid thrombosis remains the most frequent etiology of stroke.60 Although it was previously considered unpredictable, there is now growing evidence that postoperative carotid thrombosis is probably related to patient-mediated factors (e.g., increased platelet sensitivity to adenosine diphosphate61) and may be preventable.
In the past, the Leicester Vascular Unit performed TCD monitoring for 3 hours after CEA.57 The aim was to identify the 5% cohort of patients with high rates of early postoperative embolization (who faced a 50% risk of progression to thrombotic stroke62), in whom to target incremental-dose dextran therapy.57 However, a randomized trial subsequently showed that the addition of a single 75-mg dose of clopidogrel the night before surgery (in addition to routine aspirin therapy) virtually abolished postoperative embolization.63 A subsequent prospective audit observed that perioperative dual-antiplatelet strategy was associated with virtual abolition of significant postoperative embolization with no progression to thromboembolic stroke.64 Consequently, all postoperative TCD monitoring in the Leicester Vascular Unit ceased in 2009.
Accuracy and Limitations in Clinical Practice
The main limitation of TCD is operator experience. This improves with time, and most experienced technologists can identify an accessible cranial window in about 90% of patients. Common pitfalls include a lack of knowledge about intracranial anatomy (about 40% of circles of Willis are incomplete) as well as of practical tips on how to proceed if the initial study suggests an inaccessible window.
Laboratory Investigations
All patients being investigated for carotid disease should undergo a number of relatively simple but important laboratory investigations at the initial consultation.
Baseline Evaluations
The provision of “best medical therapy” involves more than just recommending that the patient stop smoking, have blood pressure checked, and start aspirin and statin therapy. A complete blood count will exclude (1) thrombocytopenia (potential bleeding risk [i.e., be careful about choice and dose of antiplatelet agent], (2) thrombocytosis (prothrombotic tendency), (3) anemia (possible neoplasm), (4) polycythemia (prothrombotic tendency), and (5) sickle cell disease (important in susceptible patient groups). Baseline blood biochemistry analysis should identify chronic renal impairment (important if CAS is being considered) and exclude undiagnosed diabetes. Fasting total cholesterol and triglyceride levels should be checked along with high-density lipoprotein and low-density lipoprotein cholesterol levels and the total cholesterol to high-density lipoprotein cholesterol ratio. Finally, a simple measurement of plasma viscosity or erythrocyte sedimentation rate may warn of the possibility that there may be an underlying vasculitis rather than atherosclerosis.
Secondary Evaluations
Secondary blood evaluations are reserved for selected patients (usually the younger age group) who exhibit clinical or demographic features that warrant further investigation, usually because there is a significant possibility that a nonatheromatous or prothrombotic disorder is responsible. A full thrombophilia screen (including lupus anticoagulant and fasting homocysteine levels), should be undertaken in young patients (<50 years) who present with an ischemic stroke or TIA or who have a personal (or strong family history) of thrombosis. Similarly, a full autoantibody screen should be performed in any patient with an elevated erythrocyte sedimentation rate or plasma viscosity, or a clinical history suggestive of an autoimmune disorder (e.g., telangiectatic facial rash, arthralgias, purpura). It is also important to remember that drug abuse (particularly cocaine and methamphetamines) is an important cause of stroke and TIA, especially in younger patients.
Antiplatelet Resistance
Most clinicians start their carotid patients on a “standard” dose of aspirin (or clopidogrel) irrespective of age, gender, and body weight. Unfortunately, between 5% and 10% of patients are aspirin resistant and more than 20% exhibit a partial response to aspirin.65 Similarly, clopidogrel resistance may affect up to 31% of patients at 5 days and 15% at 30 days,66 with little evidence that raising the dose reduces resistance. The clinical relevance of testing for clopidogrel resistance has not been extensively evaluated in patients with carotid artery disease, but parallels may be drawn from the cardiac literature. In a series of patients presenting with non–ST segment elevation myocardial infarction (NSTEMI) undergoing percutaneous coronary interventions, 40% of patients whose response to clopidogrel put them in the lowest quartile of responders (i.e., resistant) suffered a recurrent cardiovascular event. This compares with only 6.7% of patients in the second quartile and 0% in patients in the third and fourth quartiles (i.e., more/most sensitive to clopidogrel).67
Role of Carotid Biomarkers
In cardiac disease, a troponin assay has become invaluable in diagnosing acute coronary syndromes. Unfortunately, no parallel biomarkers have assumed a validated clinical role in the investigation and management of carotid artery disease. The rationale for serial biomarker measurement in patients with cerebrovascular disease (especially asymptomatic patients) is that it will identify patients at high risk of suffering a stroke owing to development of an unstable carotid plaque.
To date, numerous biomarkers have been tested (in the research environment), but none has developed a sufficiently sensitive or specific role in clinical practice. Two biomarkers, however, show potential promise. Several studies have shown that highly selective C-reactive protein (hs-CRP) is significantly elevated in patients with (histologically proven) unstable carotid plaques.68,69 However, an hs-CRP elevation has not been shown to predict the presence of an unstable plaque in previously asymptomatic patients.68 This is an important limitation in terms of identifying asymptomatic patients who might benefit from prophylactic intervention.
The second carotid biomarker under close scrutiny is lipoprotein-associated phospholipase A2 (Lp-PLA2). It is a macrophage-derived enzyme that metabolizes low-density lipoprotein (LDL) into the proinflammatory metabolites lysophosphatidyl-choline (LPC) and oxidized fatty acids. Research has observed a direct relationship between Lp-PLA2, LPC, and proinflammatory cytokine production within carotid plaques, suggesting that LPC and Lp-PLA2 may have an important role as mediators of atherosclerotic plaque instability.70 A number of studies have evaluated circulating Lp-PLA2 levels with regard to predicting plaque instability and recurrent stroke risk. Sarlon-Bartoli et al68 observed not only that (unlike hs-CRP) elevations of Lp-PLA2 predicted unstable carotid plaques (overall) but also that Lp-PLA2 was significantly elevated in neurologically asymptomatic patients with severe carotid stenoses who were found to have unstable histologic features. In addition, the Northern Manhattan Stroke Study found that patients with the highest quartile levels of Lp-PLA2 were two times more likely to suffer a recurrent stroke during follow-up.69
Selected Key References
Biasi GM, Froio A, Dietrich EB, Galimberti S, Mingazzini P, Nicolaides AN, Griffin M, Raithel D, Reid DB, Valsecchi MG. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) Study. Circulation. 2004;110:756.
Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, Katarick S, Needleman L, Pellerito J, Polak JF, Rholl KS, Wooster DL, Zierler E, Society of Radiologists in Ultrasound. Carotid artery stenosis: gray-scale and Doppler US diagnosis: Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340.
Macdonald S, Lee R, Williams R, Stansby G, Delphi Carotid Stenting Consensus Panel. Towards safer carotid artery stenting: a scoring system for anatomic suitability. Stroke. 2009;40(5):1698.
Nicolaides A, Kakkos SK, Kyriacou E, Griffin M, Thomas DJ, Geroulakos G, Labropoulos N, Doré CJ, Morris TP, Naylor R, Abbott AL, Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52:1486.
Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ, for the Carotid Endarterectomy Trialists’ Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915.
Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, Berry E, Young G, Rothwell P, Roditi G, Gough M, Brennan A, Bamford J, Best J. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technol Assess. 2006;10:iii http://www,hta,ac,uk/fullmono/mon1030,pdf.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Rothwell PM, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915.
2. Wardlaw JM, et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technol Assess. 2006 http://www.hta.ac.uk/fullmono/mon1030.pdf.
3. Loftus IM, et al. Carotid endarterectomy without angiography does not compromise operative outcome. Eur J Vasc Endovasc Surg. 1998;16:489.
4. US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2007;147:854.
5. Naylor AR, et al. Stroke during coronary artery bypass surgery: a critical review of the role of carotid artery disease. Eur J Vasc Endovasc Surg. 2002;23:283.
6. Walker J, et al. Ultrasound based diagnosis of “carotid stenosis >70%”: an audit of UK practice. Eur J Vasc Endovasc Surg. 2006;31:487.
7. Gray-Weale AC, et al. Carotid artery atheroma: comparison of pre-operative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg. 1988;29:676.
8. Geroulakos G, et al. Characterisation of symptomatic and asymptomatic carotid plaques in high-resolution real time ultrasonography. Brit J Surg. 1993;80:1274.
9. Asymptomatic Carotid Surgery Trial Collaborators. The MRC Asymptomatic Carotid Surgery Trial (ACST): carotid endarterectomy prevents disabling and fatal carotid territory strokes. Lancet. 2004;363:1491.
10. Nicolaides AN, et al. Effect of image normalisation on carotid plaque classification and the risk of ipsilateral ischemic events: results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study. Vascular. 2005;13:211.
11. Griffin M, et al. Normalisation of ultrasonic images of atherosclerotic plaques and reproducibility of gray scale median using dedicated software. Int Angiol. 2007;26:372.
12. Biasi GM, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) Study. Circulation. 2004;110:756.
13. Naylor AR. Time to rethink management strategies in patients with asymptomatic carotid disease. Nat Rev Cardiol. 2012;9:116.
14. Madani A, et al. High risk asymptomatic carotid stenosis: ulceration on 3D ultrasound vs. TCD microemboli. Neurology. 2011;77:744.
15. Topakian R, et al. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic individuals. Neurology. 2011;77:751.
16. Nicolaides A, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52:1486.
17. Rockman CB, et al. Intra-operative imaging: does it really improve peri-operative outcomes of carotid endarterectomy? Sem Vasc Surg. 2007;20:236.
18. Spies C, et al. Carotid artery stent type influences duplex ultrasonography derived peak systolic velocity: findings of an in-vitro model. Catheter Cardiovasc Interv. 2007;70:304.
19. Lazaris A, et al. Patch corrugation on duplex ultrasonography may be an early warning of prosthetic patch infection. Eur J Vasc Endovasc Surg. 2005;29:91.
20. Grant EG, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis: Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340.
21. Johnston SC, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283.
22. Giles MF, et al. Early stroke risk and ABCD2 score performance in tissue versus time defined TIA: a multicentre study. Neurology. 2011;77:1222.
23. Macdonald S, et al. Towards safer carotid artery stenting: a scoring system for anatomic suitability. Stroke. 2009;40:1698.
24. Hatsukami TS, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic plaque in vivo with high resolution magnetic resonance imaging. Circulation. 2000;102:959.
25. Cai J, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high resolution, contrast enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437.
26. Anzidei M, et al. Preliminary experience with MRA in evaluating the degree of carotid stenosis and plaque morphology using high-resolution sequences after gadofosveset trisodium (Vasovist) administration: comparison with CTA and DSA. Radiol Med. 2010;115:634.
27. Turc GT, et al. Relationships between recent intraplaque hemorrhage and stroke risk factors in patients with carotid stenosis: the HIRISC Study. Arterioscler Thromb Vasc Biol. 2012;32:492.
28. Altaf N, et al. Carotid intraplaque haemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke. 2007;38:1633.
29. Altaf N, et al. Magnetic resonance imaging detected carotid intraplaque haemorrhage predicts embolisation during carotid endarterectomy. J Vasc Surg. 2007;46:31.
30. Altaf N, et al. Detection of intraplaque haemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008;47:337.
31. Altaf N, et al. Cerebral white matter hyperintense lesions are associated with unstable carotid plaques. Eur J Vasc Endovasc Surg. 2006;31:8.
32. Takaya N, et al. Association between carotid plaque characteristics and subsequent ischaemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke. 2006;37:818.
33. Singh N, et al. Moderate carotid artery stenosis: MR imaging depicted intraplaque haemorrhage predicts risk of cerebrovascular ischaemic events in asymptomatic men. Radiology. 2009;252:502.
34. Naylor AR, et al. Seizures after carotid endarterectomy: hyperperfusion, dysautoregulation or hypertensive encephalopathy? Eur J Vasc Endovasc Surg. 2003;26:39.
35. Bonati LH, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8:908.
36. Kuo PH, et al. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647.
37. Powers WJ, et al. The use of positron emission tomography in cerebrovascular disease. Neuroimag Clin N Am. 2003;13:741.
38. Gupta A, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke. 2012;43:2884–2991.
39. Van der Vaart MG, et al. Application of PET/SPECT imaging in vascular disease. Eur J Vasc Endovasc Surg. 2008;35:507.
40. Lell MM, et al. New techniques in CT angiography. Radiographics. 2006;26:S45.
41. De Weert TT, et al. Characterisation and quantification of atherosclerotic plaque components with multidetector computed tomography and histopahological correlation. Arterioscler Thromb Vasc Biol. 2006;26:2366.
42. Nandalur KR, et al. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is non-calcified plaque on MDCT. AJR. 2005;184:295.
43. Kwee R. Systematic review on the association between calcification in carotid plaques and clinical ischaemic symptoms. J Vasc Surg. 2010;51:1015.
44. Randoux B, et al. Carotid artery stenosis: prospective comparison of CT, 3-dimensional gadolinium enhanced MR, and conventional angiography. Radiology. 2001;220:179.
45. Saba L, et al. CT and US in the study of ulcerated carotid plaque compared to surgical results: advantages of multidetector-row CT angiography. AJNR. 2007;28:1061.
46. Saba L, et al. Carotid artery wall thickness and ischemic symptoms evaluation using multi-detector row CT. Angiography Eur Radiol. 2008;18:1962.
47. Baptista MV, et al. Conflicting images. Lancet. 1998;351:414.
49. Kaufman TJ, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243:812.
50. Tripp HF, et al. New approach to preoperative vascular exclusion for carotid body tumor. J Vasc Surg. 2003;38:389.
51. Naylor AR, et al. Overview of the principal results and secondary analyses from the European and the North American randomised trials of carotid endarterectomy. Eur J Vasc Endovasc Surg. 2003;26:115.
52. Smith JL, et al. Differentiation between emboli and artefacts using dual gated transcranial Doppler ultrasound. Ultrasound Med Biol. 1996;22:1031.
53. Markus HS, et al. Asymptomatic embolisation for prediction of stroke in the asymptomatic carotid emboli study: a prospective observational study. Lancet Neurol. 2010;9:663.
54. Jayasooriya G, et al. Silent cerebral events in asymptomatic carotid stenosis. J Vasc Surg. 2011;54:227.
55. Sultan MJ, et al. Extracranial and transcranial ultrasound assessment in patients with suspected positional “vertebrobasilar ischaemia”. Eur J Vasc Endovasc Surg. 2008;38:10.
56. Halsey JH, et al. Blood flow velocity in the middle cerebral artery and regional cerebral blood flow during carotid endarterectomy. Stroke. 1989;20:53.
57. Naylor AR, et al. Reducing the risk of carotid surgery: a seven year audit of the role of monitoring and quality control assessment. J Vasc Surg. 2000;32:750.
58. Ghali R, et al. Transcranial Doppler intra-operative monitoring during carotid endarterectomy: experience with regional or general anaesthesia, with and without shunting. Ann Vasc Surg. 1997;11:9.
59. Naylor AR, et al. Closing the loop: a 20-year audit of strategies for preventing stroke and death following carotid endarterectomy. Eur J Vasc Endovasc Surg. 2013.
60. Gaunt ME, et al. A comparison of quality control methods applied to carotid endarterectomy. Eur J Vasc Endovasc Surg. 1996;11:4.
61. Hayes PD, et al. Patients’ thromboembolic response after carotid endarterectomy is related to the platelets’ sensitivity to adenosine diphosphate. J Vasc Surg. 2003;38:1226.
62. Horn J, et al. Identification of patients at risk for cerebral ischaemic complications after carotid endarterectomy with TCD monitoring. Eur J Vasc Endovasc Surg. 2005;30:270.
63. Payne DA, et al. Beneficial effects of clopidogrel combined with aspirin in reducing cerebral emboli in patients undergoing carotid endarterectomy. Circulation. 2004;109:1476.
64. Sharpe RY, et al. Dual antiplatelet therapy prior to carotid endarterectomy reduces post-operative embolisation: post-operative transcranial Doppler monitoring is now unnecessary. Eur J Vasc Endovasc Surg. 2010;40:162.
65. Gum PA, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001;88:230.
66. Gurbel PA, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;17:2908.
67. Matetzky S, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171.
68. Sarlon-Bartoli G, et al. Circulating lipoprotein-associated phospholipase A2 in high grade carotid stenosis: a new biomarker for predicting unstable plaque. Eur J Vasc Endovasc Surg. 2012;43:154.
69. Elkind MSV, et al. Lipoprotein associated phospholipase A2 activity and risk of recurrent stroke. Cerebrovasc Dis. 2009;27:42.
70. Goncalves I, et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol. 2012;32:1505.