CHAPTER 188 Cerebrospinal Fluid Physiology
Cerebrospinal Fluid Physiology of the Developing Fetus
Cerebrospinal Fluid Pathways
The mechanism of ventricular formation appears to be well conserved in vertebrates, with ventricular shape being determined by adjacent cellular proliferation. The initial ventricular fluid is not dependent on the presence of blood vessels. The CSF within the ventricles contains hormones, proteoglycans, and ions and its composition varying with time and from site to site within the ventricles, depending on adjacent parenchymal development.1 Ventricular enlargement is present in early development but steadily decreases to its size at term by approximately 30 weeks’ gestation.2,3
The mesenchyme surrounding the brain thins out in a definite, organized pattern to form the pia-arachnoid membrane, the cisterns, and the SAS. The residual mesenchyme forms the trabecular meshwork of the arachnoid. Ultrasonography has largely been supplanted by magnetic resonance imaging (MRI) for study of the ventricular system and SAS in the fetus.2 The width of the SAS in early development is fairly large until 32 to 34 weeks’ gestation, when it declines to its size at term. The volume of the SAS is related to CSF formation, CSF absorption, and fetal CNS development. An enlarged SAS can indicate chromosomal abnormalities, infection, and CNS underdevelopment. Enlargement of the cisterna magna (CM) can be seen with trisomy 18 and 21, Dandy-Walker malformation, cerebellar hypoplasia, and posterior fossa cysts.2 A large CM by itself can be an isolated finding and compatible with normal development. A small CM can also be seen in patients with Chiari malformations.
The SAS and its configuration are virtually complete at birth.4 The SAS develops independently of choroid plexus CSF secretion and does not require the presence of CSF circulation. There is no movement of fluid out of the ventricular system during early development of the SAS.5 The outlets to the fourth ventricle are covered with a membrane, even after the choroid plexus begins to secrete CSF. This membrane does not appear to impair outflow of CSF from the ventricles because drainage occurs via intracellular pores in the membrane.6 The membrane subsequently becomes progressively attenuated, and larger and larger holes develop until it is no longer present.
Resistance to outflow from the ventricles increases as gestation progresses, but it does not change to any degree after birth.7 Resistance to CSF drainage in turn is the end product of differentiation of the cells that make up the pathways. Glycoconjugates appear to influence development of the matrix of the drainage pathways and to determine the degree of resistance.8 Presumedly, impaired function or absence of normal glycoconjugates could lead to increased resistance, which if significant, would result in hydrocephalus.
Choroid Plexus
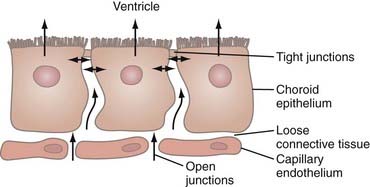
Various genetic factors that result in malformation or abnormal function of the choroid plexus have been described in animal models but not thus far in humans.9 Tight junctions between the epithelial cells of the choroid plexus become operative early in development and limit the free passage of proteins (Fig. 188-1). Synthesis or transport of proteins (or both) into and out of the CSF by the choroid plexus appears to influence neurogenesis.10,11 When developed, the choroid plexus, in addition to secreting CSF, also performs regulatory functions such as transporting various substances out of the CSF, neutralizing substances that could be harmful to the CNS, and helping maintain the homeostasis needed for normal CNS function.
The locations of the lateral, third, and fourth ventricular choroid plexus may also play a unique role in CNS development inasmuch as trophic substances can be added or removed from CSF at specific sites. The CSF in the lateral ventricles is in continuity with the germinal matrix, the main site for cortical cell proliferation and subsequent migration, whereas the CSF in association with the choroid plexus of the fourth ventricle may more likely influence structures in the basal cisterns.12
Development of the Cerebrospinal Fluid–Blood-Brain Barriers
The earliest CSF is most likely an ultrafiltrate of plasma. With development of the CSF-blood-brain barriers, CSF becomes a secretion. The concept that these barriers are less developed in the fetus and infant is based on observations that blood-borne dyes stain the immature brain more extensively, that the concentration of protein in CSF is higher in the newborn, and that metabolites and various solutes enter more readily and reach higher concentrations in the fetal brain than in the adult brain.13
However, many problems exist in trying to determine the permeability of the CSF-blood-brain barriers in an immature brain because there are many different and continual changes occurring during gestation. The very early embryonic brain has no blood vessels, so exchanges with blood in vessels external to the CNS must occur indirectly. The main route of transfer appears to be from blood into CSF and then into the parenchyma, and it seems likely that the choroid plexus and early CSF have important roles in nutritional supply to the developing brain.14
After vascularization begins, the initial low density of blood vessels increases steadily with gestation. The number of blood vessels and cerebral blood flow increase in relation to metabolic needs. The ECS appears to be larger at certain stages, so there is less restriction to free diffusion. In addition, an increase in ventricular volume takes place. This increases the volume distribution, which in turn decreases CSF/plasma concentration ratios for small, passively defusing molecules and may not reflect changes in permeability.15 The volume of the brain is initially small relative to its surface area, and the amount of CSF is proportionally higher. The kinetics of CSF circulation is different in the fetus such that removal of dyes and other markers of permeability, such as inulin, is not the same as in the adult CNS.
Finally, the concentration of various substances in the CNS depends on many different active transport mechanisms, which mature independently. It has been shown that the developing choroid plexus distinguishes between different types of albumin.16 All these factors produce alterations that make it difficult to assess any changes in the passive permeability characteristics of the CSF-blood-brain barriers.17 Despite these complexities, experimental models have been designed and are able to answer some of the questions regarding the development of barriers particular to the CNS.
The CNS barriers are indeed more permeable in the fetus, but this greater permeability does not relate to the tightness of the junctions at either the brain capillary endothelium18 or the choroid plexus epithelium.19,20 Indeed, the intracellular junctions at these locations are well formed very early in fetal development and do not differ significantly from those in adults. The degree of permeability relates instead to the size of the intracellular channels, and it is a decrease in the size and perhaps the number of these “pores” that tightens the barrier.11,21
For the barrier to tighten, however, it is necessary for astrocytes to be present. It has been shown that capillaries of CNS origin grown outside the CNS lose their normal barrier properties whereas non-CNS capillaries grown in the CNS acquire the appropriate characteristics.22,23 Additional evidence to support the contention of the inductive influence of astrocytes is the loss of normal capillary barrier function in the mature brain at the site of tumors.
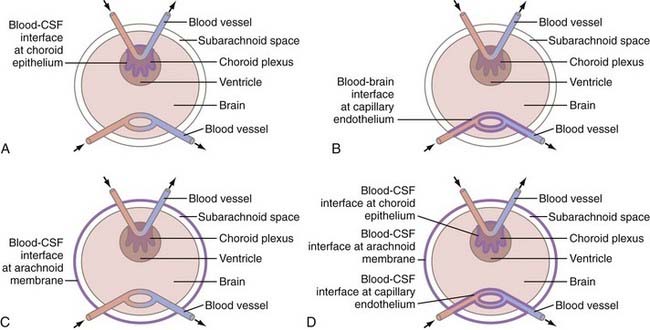
For the brain to have its protected environment, there must be a barrier with the equivalent of tight junctions at the arachnoidal membrane comparable to those present in the capillary endothelium and the choroid plexus epithelium. The timing of completion of the arachnoidal barrier in the fetus is unknown, but it may coincide with the development of tight junctions in the blood vessels and choroid plexus (Fig. 188-2).
The protein content of the CSF of fetuses, premature infants, and infants has been studied extensively.24 Total protein levels reach a peak concentration at 20 weeks’ gestation and then fall steadily. For full-term infants younger than 2 months, it is normal to find a protein level of up to 100 mg/mL. Premature infants have an even higher protein level. The concentration of protein in CSF varies with conceptual age but not with birth weight or with postnatal life span, thus indicating that maturation of the barrier, as reflected by a decline in protein, is not influenced by the timing of birth. The protein level represents a steady state at the time of sampling and is dependent on multiple factors, including the CSF secretion rate, volume of distribution, CSF circulation, and absorption rates of macromolecules. The CSF acts as a sink to clear macromolecules. Consequently, the protein level is not dependent solely on the degree of barrier permeability at the time of sampling. The proteins do not appear to emanate from the brain side of the barrier because they have an electrophoretic pattern similar to that of plasma.11
Formation and Absorption of Cerebrospinal Fluid
Formation of CSF is dependent on a number of transporters and enzymes, the most important of which appear to be carbonic anhydrase, sodium-potassium adenosine triphosphatase (Na+,K+-ATPase), and aquaporin-1. Low levels of carbonic anhydrase and Na+,K+-ATPase are present early in fetal development, but whether these enzymes are functional when they first appear is not known.21,25 Their presence coupled with aquaporin-1 water channels and concomitant enlargement of the ventricles does, however, lend support to at least some degree of CSF formation fairly early in fetal development.14
The relationship of CSF formation to brain maturation has been studied in several animal species but not in humans.26 The data indicate that CSF production increases at a rate greater than can be accounted for by the corresponding increase in choroid plexus weight or brain weight. Thus, the increased production of CSF may reflect maturation of the enzyme systems involved. Oversecretion of CSF does occur in the presence of a choroid plexus papilloma. In utero, hydrocephalus secondary to the presence of a choroid plexus papilloma has been demonstrated as well.27
No studies exist regarding CSF absorption in fetal animals or humans. Arachnoid villi, visible only microscopically, and arachnoid granulations, visible with the unaided eye, have long been thought to be the site of CSF absorption and are not found in the fetus. These structures begin to be present at birth and increase in size and number with age, with villi becoming arachnoid granulations. If they are not present, how is CSF absorbed? Although no fetal studies have been performed, neonatal animal studies confirm CSF absorption via the extracranial lymphatics, with drainage into the venous sinuses through the arachnoidal structures being secondary.28
Hydrocephalus
Normal dilation of the ventricular system is needed for the cells of the germinal matrix to multiply and migrate to form the normal cortical architecture. Various factors are found to be altered with hydrocephalus, but it is difficult to determine cause and effect.29,30 One study has shown that CSF taken from hydrocephalic animals in and of itself can inhibit neurogenesis but does not alter cell migration from the germinal matrix.31 Experimental models of hydrocephalus are discussed in Chapter 189.
Currently, the only genetic defect directly linked to fetal hydrocephalus is the X-linked L1-NCAM mutation; however, the CNS is severely altered as well.32,33 The ventricular dilation seen with extensive structural CNS abnormalities may have other different underlying genetic causations.
Simpson and colleagues attempted to delineate the dynamics of fetal intracranial pressure in utero at the time of therapeutic abortion.34 Although their studies suffered from having only seven patients with diverse CNS malformations, they could not correlate the type of CNS lesions with the intracranial pressure found in fetuses with an excessive amount of CSF. In hydrocephalic fetuses, an attempt has been made to correlate ventricular size with velocity waveforms of pulsed Doppler recordings of cerebral blood flow, but no correlation has been found.35,36
In utero imaging studies now make it possible to detect developmental abnormalities, mass lesions, and evidence of infection and hemorrhage, any of which can result in hydrocephalus. The antenatal diagnosis of hydrocephalus may influence the timing of delivery, the mode of delivery, and the possibility of terminating the pregnancy. In a situation that is fairly analogous to in utero hydrocephalus, a retrospective analysis was undertaken to assess the efficacy of aggressive surgical management of progressive hydrocephalus in preterm neonates with intracranial hemorrhage. The overwhelming factor in determining the outcome of this patient group was the extent of intracranial hemorrhage and parenchymal damage. The degree of hydrocephalus and aggressive treatment of it were not significant.37 There is no evidence that early control of hydrocephalus significantly improves neurological function because functional outcome is determined by the underlying insult to the CNS rather than the hydrocephalus.
Blood-Brain Barrier
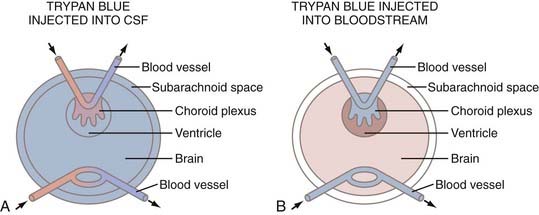
In 1885, Ehrlich demonstrated that many dyes injected into the systemic circulation of laboratory animals stain virtually all the organs in the body except the brain and spinal cord.38 Ehrlich’s disciple Goldman continued these experiments and showed that intravenously administrated trypan blue fails to stain the CNS and CSF,39,40 although the choroid plexuses and meninges were stained. In his second paper, he demonstrated that interventricularly injected trypan blue rapidly stains brain parenchyma. Thus, it was concluded that there is a barrier between blood and the brain and that this barrier could be circumvented by direct injection of dye into CSF (Fig. 188-3).
Electron microscopic cytochemical studies from the late 1960s duplicated Goldman’s first and second experiments.41,42 Horseradish peroxidase (HRP) injected intravenously was found in the lumen of brain microvessels, but no further movement of the label beyond the endothelial membrane was observed. When HRP was injected interventricularly, it readily infused across the ependyma and along the basement membranes of capillaries, but it did not enter blood via the endothelial membrane. These experiments established the anatomic concept of the blood-brain barrier and suggested that the endothelial membrane restricts free exchange of substances between blood and the brain because of the presence of tight junctions in the cerebral capillary endothelium.
During the past several decades, accumulating information has challenged the anatomic concept of an impermeable blood-brain barrier by showing that what is true for some vital dyes and HRP does not hold for many other biologically important molecules43,44 or for cells of the immune system. In addition, several classes of metabolic substrates, regulatory peptides, transport plasma proteins, steroid hormones, ions, and various groups of centrally active pharmacotherapeutics are able to use specialized shuttle services at the blood-brain barrier. In fact, the concept of a blood-brain barrier should be replaced with that of a blood-brain interface because the endothelial layer in reality regulates homeostasis of the neural milieu by numerous highly specific transport, enzymatic, receptor, and cell-mediated mechanisms rather than simply impeding exchange of solutes between blood and the brain.
Formation of Cerebrospinal Fluid
Formation Sites
It is generally agreed that most CSF is formed within the ventricular system. Possible sites of origin include the choroid plexus, the ependyma, and the parenchyma. A method has not been developed to separate the function of the ependyma from the remainder of the parenchyma, so the role of the ependyma in bulk CSF formation is not known, although from a morphologic standpoint its contribution is most likely to be insignificant. However, the choroidal epithelium has histologic features characteristic of epithelia specialized for transcellular transport of solutes and solvents.45,46 The discussion that follows is limited solely to the bulk secretion of CSF.
Results from isolated choroid plexus preparations would indicate that 80% or more of CSF production is derived from this source alone.26,47,48 However, perfusion of a portion of the ventricular system devoid of choroid plexus has demonstrated that 30% to 60% of CSF is produced from nonchoroidal sources,49,50 which may explain the failure of choroid plexectomy in the clinical setting to control progressive hydrocephalus.49 It may be added that this operative procedure removes the choroid plexus only from the lateral ventricles and not from the third and fourth ventricles. The contributions of the remaining intact choroid plexus to the formation of CSF is not clear, and whether it can compensate for the portion of the choroid plexus removed is not known.
The various lines of evidence showing the ECS to be approximately 15% of the brain’s volume has been summarized by Welch.26 The established presence of substantial ECS, the lack of ependymal resistance to free exchange between fluid in the ECS and CSF, and the similar composition of ECS fluid and CSF have a direct bearing on the possibility that the parenchyma may be the main source of nonchoroidal CSF formation.26,41,51,52 In summary, it appears that normally roughly 80% of CSF secretion is derived from the choroid plexus, with the remaining portion probably originating from the parenchyma. The obvious candidate for the parenchymal source is the capillary endothelium because its high content of mitochondria could provide the metabolic energy required for such a function.53
Mechanism of Cerebrospinal Fluid Formation
The first step in the formation of CSF is passage of an ultrafiltrate of plasma through choroidal capillary endothelium without tight junctions by hydrostatic pressure into the surrounding connective tissue stroma beneath the epithelium of the villus. The ultrafiltrate is subsequently transformed into a secretion (namely, CSF) by an active metabolic process within the choroidal epithelium. This mechanism, the formation rate, and alterations in the formation rate are discussed in detail in Chapter 33.
Absorption of Cerebrospinal Fluid
Absorptive Forces
That the rate of CSF absorption is dependent on pressure and is relatively linear over a fairly wide physiologic range has been well established.54–59 Resistance to flow appears to diminish at higher than normal physiologic pressures60,61 and may relate to the opening of channels not available at lower pressures.
Weed proposed an incremental colloid osmotic force, in addition to a hydrostatic force, that would by necessity require the presence of a semipermeable membrane between CSF and the site of absorption.62 Subsequent physiologic studies have shown that a colloid osmotic force does not exist; instead, Weed’s previous observations are explained by particulate matter or an increase in viscosity occluding the absorptive sites, thereby slowing bulk flow.60,63,64 Studies have shown that the presence of pinocytotic vesicles in the arachnoid endothelial cells lining the venous sinuses is influenced by pressure.65,66 However, the process may not be metabolically dependent in that the absorption process is reported to be unaltered by death of the animal.67,68 Thus, the only proven force responsible for bulk CSF absorption is that of a hydrostatic gradient.
Absorption via the Arachnoid Villus
The arachnoid villus would seem to be ideally situated to drain CSF from the SAS into the major dural sinuses inasmuch as it consists of a cell cluster that projects from the SAS into the lacunae laterales adjacent to these venous structures. Electron microscopic studies have shown that the villi are covered by a layer of endothelium with tight junctions that are continuous with the undersurface of the venous sinuses.69,70 These villi, also called “arachnoid granulations” or “pacchionian bodies,” are grossly visible and are functionally similar to those that are not.71 Key and Retzius72 and Weed73 firmly established that these structures drain CSF. Welch and Friedman, using a flux chamber containing a section of monkey superior sagittal sinus with arachnoid villi, found unidirectional flow from the SAS to the venous sinuses when a critical opening pressure was exceeded.64
A point of controversy regarding the structure of the arachnoid villus is the existence of open channels connecting the arachnoid side with the venous side, for the presence or absence of such channels would mean a basic physiologic difference in the manner in which CSF and its constituents drain. The open villus model would be solely pressure responsive and would allow passive escape of macromolecules, whereas a villus covered with a continuous endothelial membrane with tight junctions would add the factors of osmosis and filtration, and macromolecules would require an active transport process to cross the barrier. Earlier anatomic studies were fairly evenly divided between these two possibilities; more recent ones, however, support the open-channel pathway. The discrepancy in findings may relate in part or entirety to the manner in which the villus is prepared for histologic study: a zero pressure gradient between the arachnoid and the venous side of the villus during fixation would allow its collapse, and as a result the open channels would not be apparent.74
Another possible mechanism that could bridge the gap between the open- versus closed-channel theory of CSF drainage was proposed by Tripathi.75 He reported the presence of a dynamic transendothelial vacuolization process that temporarily creates an open channel across the villus endothelium through which CSF and its constituents can flow from the SAS to blood.76 The effect of pressure on this mechanism was not investigated.
Attempts have been made to determine the size of passageways in the arachnoid villus77 and also to see whether they are responsive to pressure, which they were not.78 The size of the passageways in the arachnoid villus is only pertinent if this site is virtually the exclusive location for bulk egress of CSF into the bloodstream. If a significant fraction of CSF and its constituents drains elsewhere, the size of the channels in the arachnoid villus is less relevant.
Absorption into the Lymphatic System
Weed’s work firmly established that the arachnoid villus is a major site for bulk CSF outflow.73 It is rarely mentioned, however, that Weed acknowledged drainage of his injected solutions into the mucosa of the paranasal sinuses, nasal mucosa, cranial nerve root sheaths, and cervical lymph nodes; he thought that these routes were accessory. The idea that a proportion of CSF could and did drain via the lymphatics was gradually relegated to obscurity, and for more than a generation, standard texts and teachings limited CSF drainage solely to the arachnoid villus.
The concept of CSF draining via the lymphatic system has been given additional support by a number of laboratory investigations in which it was indicated that a significant quantity of CSF, and under certain circumstances even the majority, can drain via lymphatic channels.79–98 Substances with different molecular weights infused into the lateral ventricles can be found in the same concentration in the deep cervical lymph, thus indicating that the process of transport is by way of bulk flow.79 Additional studies have shown that elevations in interventricular pressure will increase the volume of CSF directed into the lymphatic pathways.74,88 Conversely, blocking access to the lymphatic pathways reduces CSF lymphatic drainage.28,99,100
Lymphatic drainage declines with age but may relate to reduced CSF formation and thus turnover rate rather than increased resistance to drainage.101 At present, no studies show to what extent lymphatic drainage of CSF exists in humans, but some support of this concept comes from the clinical observation that parents of children with CSF diverting shunts will occasionally report nasal congestion and periorbital or facial swelling when their child’s shunt becomes obstructed. With improved imaging techniques, it may be possible to visualize this pathway.
Absorption via the Brain
A question debated for some time is whether CSF can be absorbed by the brain. Penetration of substances into the periventricular region of hydrocephalic animals has been well documented.102–104 With the advent of computed tomography and MRI, periventricular hypodensity may be seen in the presence of acute hydrocephalus and has been shown to be the result of CSF migrating into the area surrounding the ventricles in the face of increased interventricular pressure.105 CSF in the parenchyma, indicative of migration, however, does not necessarily equate with absorption. Bulk flow of CSF is usually measured by the clearance of various reference macromolecules, such as radioiodinated serum albumin (RISA), which by necessity would have to enter the lumen of the blood vessel and be removed by the systemic circulation. It has been shown that the cerebral capillaries have low permeability to RISA and that most of any given quantity of RISA injected into the brain can be recovered from lymph and CSF with little being lost to blood.80 Additional studies have found that HRP, which has nearly the same molecular weight as albumin, could penetrate into the basal lamina of the capillary endothelium but not beyond.106 In addition to the impermeability of the capillaries to various reference markers, clearance of which is a measure of CSF absorption, Welch has pointed out that because absorption occurs in response to a drop in pressure, higher pressure would be required outside the lumen of the capillary than inside, which would obviously lead to its collapse and preclude absorption.26 The ECS in the brain, which amounts to 15%, readily allows flow of fluid in the parenchyma. This flow of fluid within the parenchyma is present under normal physiologic conditions,107 and its velocity and direction are responsive to changes in hydrostatic108 and osmotic pressure gradients.107,109 Macromolecules injected into the CSF of the ventricles or SAS have been observed to readily penetrate the ECS of the parenchyma and vice versa.41,106,110 Evidence thus supports the contention that the brain, rather than absorbing CSF, is acting as a conduit for fluid to move from the ventricles to the SAS, there being no barrier at the pial surface, just as there is no barrier at the ventricular ependymal surface of the ventricles.92
Absorption via Blood Vessels
As noted in the discussion on the absorption of CSF via the brain, there is no evidence to support CSF being absorbed by the capillary endothelium. However, this does not preclude net changes in water when disequilibrium in the blood-brain osmotic gradient occurs because there is no barrier in this regard. One experimental study found that carbon black injected into the parenchyma could later be traced to the SAS, the walls of cerebral blood vessels, the adventitia of the internal carotid artery outside the cranium, and the cervical lymph nodes.110 Two newer studies are at variance with this observation that macromolecules travel extracranially in the adventitia of the major cerebral blood vessels, for in these studies, RISA injected into the brain or SAS stopped abruptly when the blood vessels exited the SAS80,111; however, a more recent study is inconclusive.112 The parenchymal vessels appear to provide a passageway for macromolecules to reach the SAS, where absorption via the lymphatic system or arachnoid granulations occurs.
Absorption at the Nerve Root Sleeves
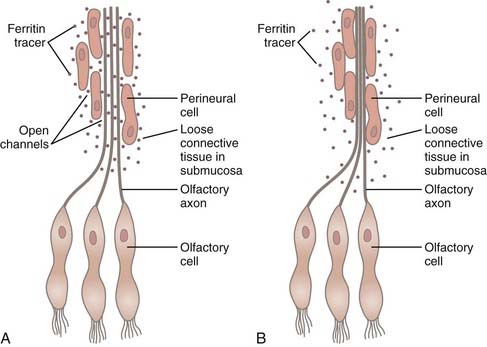
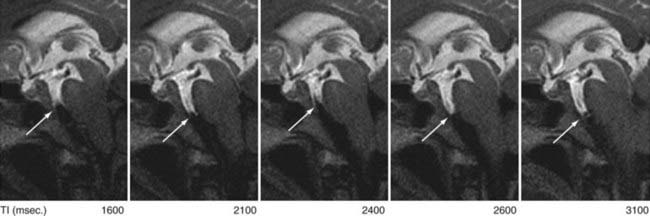
Drainage of CSF into the nasal submucosa was first postulated by Schwalbe,113 and this finding has been confirmed on many occasions since.72,73,114–118 Yoffrey and Drinker noted that the best injections in the nasal submucosa were achieved by placing tracers in the cranial SAS.118 The nasal submucosa has a dense network of lymphatic channels that subsequently drain into the deep cervical nodes.72,73,113,114,116,118 The pathway of CSF into the nasal submucosa is via an extension of the SAS that surrounds each olfactory filament as it passes through the lamina cribrosa, and this pathway can be blocked if the continuity of the space is disrupted.81,119 The pia-arachnoid layer progressively thins and blends into a perineural sheath as the olfactory filaments pass through the cribriform plate. This perineural sheath becomes but a single-cell layer in the submucosa. The perineural space between the filament and the sheath is in continuity with the SAS.114,118 A previous point of uncertainty has been whether open channels connect the perineural spaces (and thus the SAS) with the ECS of the submucosa. The presence or absence of open channels would mean basic physiologic difference in the manner in which CSF and its constituents drain, just as with the arachnoid villus. An electron microscopic study indicated that a tight junction endothelial membrane is not present, thus allowing passive escape of macromolecules via bulk fluid flow on a pressure-responsive basis alone (Fig. 188-4).83 Two additional studies have shown that the SAS surrounding the optic nerve divides into numerous tortuous channels to form a “subarachnoid trabecular meshwork” containing “microcanals” that allow the passage of ferritin to reach the posterior intraorbital connective tissue. Once again, the passageways were open and similar to those found in the olfactory region.84,91 A barrier present at the sclera prevented entrance of tracer into the choroidal interstitium.
A study looking at CSF drainage from the spinal nerve roots indicated that the same physiologic process operative at the cranial nerves occurs in the spinal nerves as well.89,93 Drainage of CSF from the spinal nerve root sleeves is yet to be studied from a morphologic standpoint, but the evidence thus far favors an open-channel passageway similar to that found in the optic and olfactory nerves.
Absorption from the Subarachnoid Space
Experiments have documented that CSF drains from the SAS surrounding the cranial and spinal nerves and enters the lymphatic system, but the question of egress of fluid from the membrane itself remains unsettled. Dandy and Blackfan contended that CSF absorption was a diffuse process from the SAS, with the arachnoid villi accounting for only a small percentage of the fluid drained.120 Weed found that under normal physiologic conditions, the arachnoid membrane acted as a barrier but could readily be breached with cellular damage.71 Bowsher injected radioisotope-labeled protein into the SAS of cats and found uptake at the arachnoid villi, around the blood vessels of the cortex, and along the cranial and spinal nerve root sheaths, but no penetration through the arachnoid membrane,121 thus confirming the work of Weed.
Electron microscopic studies have shown several layers of arachnoid cells between the SAS and the dura mater; the cells of the outer portion of these layers exhibit tight junctions with occlusion of intercellular clefts, thereby serving as an effective barrier to large molecules (i.e., they function as a blood-CSF interface or barrier at this location).122 Butler has noted that contrary to findings at normal pressure, the arachnoidal barrier layer is disrupted at higher pressures and HRP can penetrate through the arachnoid membrane to reach the ECS of the dura mater and dural lymphatic channels.123 Normally, it does not seem that much, if any, CSF drains through the arachnoid membrane, but at unphysiologically high pressures, disruption of the barrier may allow significant bulk flow.
Circulation of Cerebrospinal Fluid
CSF functions as a lymphatic system for the CNS. With rapid turnover of CSF, a concentration gradient or “sink” is produced for the clearance of metabolic waste products, including macromolecules.124 The pressure gradient created between the newly secreted CSF and that at sites of absorption produces the major force for bulk movement of CSF. Other factors that influence the circulation of CSF are the ciliary action of the ependyma and choroid plexus, pulsations induced by the arterial tree, and respiration. The newly formed CSF has a protein content of approximately 10 mg/dL; that from the lower spinal SAS is higher than 40 mg/dL. The difference reflects the rate of CSF turnover: the longer that CSF remains within the CNS, the more protein is added from the brain, spinal cord, and leakage from the blood-brain-CSF interface. That portion of the CSF produced in the parenchyma travels via the ECS to reach the SAS or joins the CSF made by the choroid plexus within the ventricular system. The lower pressure at sites of absorption draws CSF from the brain and spinal cord.
Cardiac-gated phase-contrast MRI, until recently, was the only technique available to observe CSF flow noninvasively.125,126 This phase-contrast technique provides “to-and-fro” CSF flow velocity and direction during a period of a single cardiac cycle; however, this method is limited because of high variability of the data, poor visualization of turbulent flow,127,128 and an inability to measure bulk flow.126
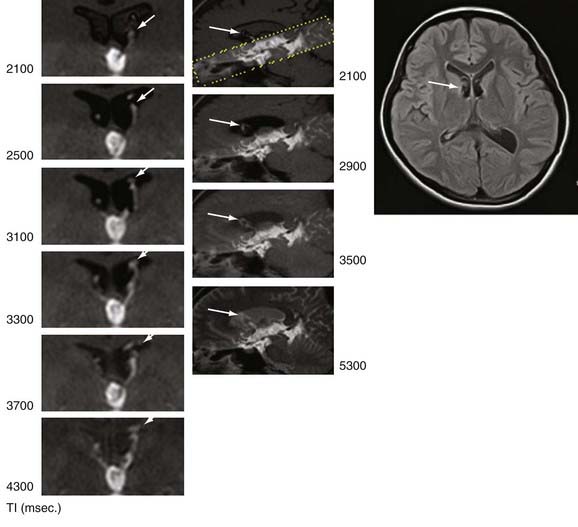
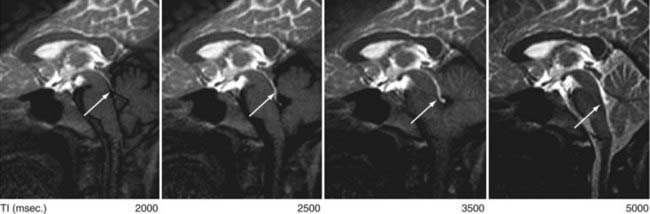
Now available is a nonenhanced MRI technique, time–spatial labeling inversion pulse (SLIP), that can label or tag CSF in a region of interest. The tagged CSF is clearly visualized at inversion times of 1500 to 4500 msec after pulse labeling in both the intracranial and intraspinal compartments. Noninvasive visualization of CSF movement, including bulk and turbulent flow in normal and altered physiologic conditions, is possible with this time-SLIP technique (Figs. 188-5 to 188-7).129 Impressive turbulent flow exists in the third and fourth ventricles and would aid in distributing various substances in the CSF, as well as in helping clear macromolecules from the parenchyma.130,131 The turbulent flow is markedly reduced with ventricular obstruction and readily reestablished with CSF diversion.
The time-SLIP technique shows significant movement of CSF within the SAS during respiration and increases in the mixing effect intraventricularly. One can observe “to-and-fro” flow of CSF, but no bulk flow from the sylvian SAS to the SAS over the hemispheric convexities leading to the superior sagittal sinus, the location of the arachnoid villi/granulations.129 This lack of CSF flow over the dorsal surface of the hemispheres, in both normal and hydrocephalic individuals, adds additional support for the importance of nonarachnoidal granulation absorption of CSF.
Time-SLIP MRI can also be used to observe CSF movement in the spinal SAS. Rapid pulsatile CSF flow in the prepontine SAS is continuous with that in the ventral spinal SAS and progressively diminishes to almost nothing at the terminus of the thecal sac in the sacral region, as had been predicted.132 Imaging an individual turning from the supine to the prone position shows a position-related shift of the spinal cord with a concomitant change in CSF pulsatility from the anterior to the posterior SAS.
Bradbury MW. The Concept of the Blood-Brain Barrier. Chichester, England: Wiley, 1979;465.
Bradbury MW, Bradbury RJ, Westrop MWRJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519-534.
Cutler RW, Page L, Galicich J, et al. Formation and absorption of cerebrospinal fluid in man. Brain. 1968;91:707-720.
Davson H, Hollingsworth G, Segal MB. The mechanism of drainage of the cerebrospinal fluid. Brain. 1970;93:665-678.
Davson H, Welch K, Segal M. Physiology and Pathophysiology of the Cerebrospinal Fluid. Edinburgh: Churchill Livingstone; 1987.
Erlich SS, McComb JG, Hyman S, et al. Ultrastructural morphology of the olfactory pathway for cerebrospinal fluid drainage in the rabbit. J Neurosurg. 1986;64:466-473.
Heisey SR, Held D, Pappenheimer JR. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol. 1962;203:775-781.
Johnston M, Zakharov A, Papaiconomou C, et al. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2.
Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6.
McComb JG, Hyman S. Lymphatic drainage of cerebrospinal fluid in the primate. In: Johansson BB, Owman CH, Widner H, editors. Pathophysiology of the Blood-Brain Barrier. Amsterdam: Elsevier; 1990:421-438.
McLone DG. The subarachnoid space: a review. Childs Brain. 1980;6:113-130.
Mollanji R, Papaiconomou C, Boulton M, et al. Comparison of cerebrospinal fluid transport in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1215-1223.
Ohata K, Marmarou A. Clearance of brain edema and macromolecules through the cortical extracellular space. J Neurosurg. 1992;77:387-396.
Oldendorf WH, Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967;17:196-205.
Reulen HJ, Tsuyumu M, Tack A, et al. Clearance of edema fluid into cerebrospinal fluid. A mechanism for resolution of vasogenic brain edema. J Neurosurg. 1978;48:754-764.
Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42-F49.
Saunders NR. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25(suppl):523-550.
Welch K. The principles of physiology of the cerebrospinal fluid in relation to hydrocephalus including normal pressure hydrocephalus. Adv Neurol. 1975;13:247-332.
Williams MA, McAllister JP, Walker ML, et al. Priorities for hydrocephalus research: report from a National Institutes of Health–sponsored workshop. J Neurosurg. 2007;107(5 suppl):345-357.
Yamada S, Miyazaki M, Kanazawa H, et al. Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology. 2008;249:644-652.
1 Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132:2057-2067.
2 Watanabe Y, Abe S, Takagi K, et al. Evolution of subarachnoid space in normal fetuses using magnetic resonance imaging. Prenat Diagn. 2005;25:1217-1222.
3 Girard NJ, Raybaud CA. Ventriculomegaly and pericerebral CSF collection in the fetus: early stage of benign external hydrocephalus? Childs Nerv Syst. 2001;17:239-245.
4 McLone DG. The subarachnoid space: a review. Childs Brain. 1980;6:113-130.
5 Jones H. The movement of fluids out of the cerebral ventricles in fetal and neonatal rats. Z Kinderchir. 1982;37:130-133.
6 Jones H. Intercellular pores between the ependymal cells lining the roof of the fourth cerebral ventricle in mammalian fetuses. Z Kinderchir. 1980;31:309-313.
7 Deane R, Jones H. Cerebrospinal fluid outflow resistance in the developing rat. Z Kinderchir. 1983;38(suppl II):64.
8 McLone DG, Herman J, Higbee RG, et al. Glycoconjugates and the development of the cerebrospinal fluid outflow pathway in the mouse. In: Marlin AE, editor. Concepts in Pediatric Neurosurgery. Basal: Karger; 1988:97-100.
9 Swetloff A, Ferretti P. Changes in E2F5 intracellular localization in mouse and human choroid plexus epithelium with development. Int J Dev Biol. 2005;49:859-865.
10 Swetloff A, Greenwood S, Wade AM, et al. Growth of choroid plexus epithelium vesicles in vitro depends on secretory activity. J Cell Physiol. 2006;208:549-555.
11 Saunders NR. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25(suppl):523-550.
12 Miyan JA, Nabiyouni M, Zendah M. Development of the brain: a vital role for cerebrospinal fluid. Can J Physiol Pharmacol. 2003;81:317-328.
13 Rapport S. Blood-Brain Barrier in Physiology and Medicine. New York: Raven Press, 1976;316.
14 Johansson PA, Dziegielewska KM, Liddelow SA, et al. The blood-CSF barrier explained: when development is not immaturity. Bioessays. 2008;30:237-248.
15 Johansson PA, Dziegielewska KM, Ek CJ, et al. Blood-CSF barrier function in the rat embryo. Eur J Neurosci. 2006;24:65-76.
16 Knott GW, Dziegielewska KM, Habgood MD, et al. Albumin transfer across the choroid plexus of South American opossum (Monodelphus domestica). J Physiol. 1997;499:179-194.
17 Bradbury MW. The Concept of the Blood-Brain Barrier. Chichester, England: Wiley, 1979;465.
18 Mollgard K, Luritzen B, Saunders NR. Complex tight junctions of epithelial and of endothelial cells in early fetal brain. J Neurocytol. 1975;4:453-468.
19 Mollgard K, Lauritzen B, Saunders NR. Double replica technique applied to choroid plexus from early foetal sheep: completeness and complexity of tight junctions. J Neurocytol. 1979;8:139-149.
20 Tauc M, Vignon X, Bouchaud C. Evidence for the effectiveness of the blood-CSF barrier in the fetal rat choroid plexus. A freeze-fracture and peroxidase diffusion study. Tissue Cell. 1984;16:65-74.
21 Johansson PA, Dziegielewska KM, Saunders N. Low levels of Na, K-ATPase and carbonic anhydrase II during choroid plexus development suggest limited involvement in early CSF secretion. Neurosci Lett. 2008;442:77-80.
22 Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail-chick transplantation chimeras. Dev Biol. 1981;84:183-192.
23 Svengaard NA, Kjorklund A, Hardebo JE, et al. Axonal degeneration associated with a defective blood-brain barrier in cerebral implants. Nature. 1975;255:837-842.
24 Statz A, Felgenhauer K. Development of the blood-CSF barrier. Dev Med Child Neurol. 1983;25:152-161.
25 Catala M. Carbonic anhydrase activity during development of the choroid plexus in the human fetus. Childs Nerv Syst. 1997;13:364-368.
26 Welch K. The principles of physiology of the cerebrospinal fluid in relation to hydrocephalus including normal pressure hydrocephalus. Adv Neurol. 1975;13:247-332.
27 Pilu G, De Palma L, Romero R, et al. The fetal subarachnoid cisterns: an ultrasound study with report of a case of congenital communicating hydrocephalus. J Ultrasound Med. 1986;5:365-372.
28 Papaiconomou C, Bozanovic-Sosic R, Zakharov A, et al. Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol Regul Integr Comp Physiol. 2002;283:R869-876.
29 Heep A, Bartmann P, Stoffel-Wagner B, et al. Cerebrospinal fluid obstruction and malabsorption in human neonatal hydrocephaly. Childs Nerv Syst. 2006;22:1249-1255.
30 Mashayekhi F, Salehi Z. Expression of nerve growth factor in cerebrospinal fluid of congenital hydrocephalic and normal children. Eur J Neurol. 2005;12:632-637.
31 Mashayekhi F, Draper CE, Bannister CM, et al. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain. 2002;125:1859-1874.
32 Rolf B, Kutsche M, Bartsch U. Severe hydrocephalus in L1-deficient mice. Brain Res. 2001;891:247-252.
33 Williams MA, McAllister JP, Walker ML, et al. Priorities for hydrocephalus research: report from a National Institutes of Health–sponsored workshop. J Neurosurg. 2007;107(5 suppl):345-357.
34 Simpson GF, Edwards MS, Callen P, et al. Pressure, biochemical, and culture characteristics of CSF associated with the in utero drainage of various fetal CNS defects. Am J Med Genet. 1988;29:343-351.
35 Kirkinen P, Muller R, Baumann H, et al. Cerebral blood flow velocity waveforms in hydrocephalic fetuses. J Clin Ultrasound. 1988;16:493-498.
36 van den Wijngaard JA, Reuss A, Wladimiroff JW. The blood flow velocity waveform in the fetal internal carotid artery in the presence of hydrocephaly. Early Hum Dev. 1988;18:95-99.
37 Levy ML, Masri LS, McComb JG. Outcome for preterm infants with germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery. 1997;41:1111-1117.
38 Ehrlich P. Das Sauerstoff-Bedurfins des Organismus: Eine farbenanalytische Studie. Berlin: Hirschwald; 1885.
39 Goldman E. Die aussere ind innere Sekretion des Gesunden und kranken Organismus im Lichte der “vitalen Farbung.”. Beitr Klin Chirurg. 1909;64:192.
40 Goldman E. Vitalfarbung am Zentralnervensystem. Abh Preuss Akad Wiss Phys Math Kl. 1913;1:1.
41 Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648-677.
42 Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207-217.
43 Pardridge WM. Peptide Drug Delivery to the Brain. New York: Raven Press; 1991.
44 Segal MB, Zlokovic BV. The Blood-Brain Barrier, Amino Acids and Peptides. Boston: Kluwer; 1990.
45 Davson H, Welch K, Segal M. Physiology and Pathophysiology of the Cerebrospinal Fluid. Edinburgh: Churchill Livingstone; 1987.
46 Dohrmann GJ. The choroid plexus in experimental hydrocephalus. A light and electron microscopic study in normal, hydrocephalic, and shunted hydrocephalic dogs. J Neurosurg. 1971;34:56-69.
47 Miner LC, Reed DJ. Composition of fluid obtained from choroid plexus tissue isolated in a chamber in situ. J Physiol. 1972;227:127-139.
48 Welch K. Secretion of cerebrospinal fluid by choroid plexus of the rabbit. Am J Physiol. 1963;205:617-624.
49 Milhorat TH. Hydrocephalus and the Cerebrospinal Fluid. Baltimore: Williams & Williams; 1972.
50 Pollay M, Curl F. Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am J Physiol. 1967;213:1031-1038.
51 Brightman MW. The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice. Prog Brain Res. 1968;29:19-40.
52 Rall DP. Transport through the ependymal linings. Prog Brain Res. 1968;29:159-172.
53 Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409-417.
54 Bering EAJr, Sato O. Hydrocephalus: changes in formation and absorption of cerebrospinal fluid within the cerebral ventricles. J Neurosurg. 1963;20:1050-1063.
55 Cutler RW, Page L, Galicich J, et al. Formation and absorption of cerebrospinal fluid in man. Brain. 1968;91:707-720.
56 Heisey SR, Held D, Pappenheimer JR. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol. 1962;203:775-781.
57 Katzman R, Hussey F. A simple constant-infusion manometric test for measurement of CSF absorption. I. Rationale and method. Neurology. 1970;20:534-544.
58 Mortensen OA, Weed LH. Absorption of isotonic fluids from the subarachnoid space. Am J Physiol. 1934;108:458-468.
59 Rubin RC, Henderson ES, Ommaya AK, et al. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg. 1966;25:430-436.
60 Davson H, Hollingsworth G, Segal MB. The mechanism of drainage of the cerebrospinal fluid. Brain. 1970;93:665-678.
61 Mann JD, Butler AB, Johnson RN, et al. Clearance of macromolecular and particulate substances from the cerebrospinal fluid system of the rat. J Neurosurg. 1979;50:343-348.
62 Weed LH. Forces concerned in the absorption of the cerebrospinal fluid. Am J Physiol. 1935;114:40-45.
63 Prockop LD, Schanker LS, Brodie BB. Passage of lipid-insoluble substances from cerebrospinal fluid to blood. J Pharmacol Exp Ther. 1962;135:266-270.
64 Welch K, Friedman V. The cerebrospinal fluid valves. Brain. 1960;83:454-469.
65 Alksne JF, White LEJr. Electron-microscope study of the effect of increased intracranial pressure on the arachnoid villus. J Neurosurg. 1965;22:481-488.
66 Gomez DG, Potts G, Deonarine V, et al. Effects of pressure gradient changes on the morphology of arachnoid villi and granulations of the monkey. Lab Invest. 1973;28:648-657.
67 Potts DG, Deonarine V, Welton W. Perfusion studies of the cerebrospinal fluid absorptive pathways in the dog. Radiology. 1972;104:321-325.
68 Wolfson LI, Katzman R. Infusion manometric test in experimental subarachnoid hemorrhage in cats. Neurology. 1972;22:856-862.
69 Alksne JF, Lovings ET. The role of the arachnoid villus in the removal of red blood cells from the subarachnoid space. An electron microscope study in the dog. J Neurosurg. 1972;36:192-200.
70 Shabo AL, Maxwell DS. The morphology of the arachnoid villi: a light and electron microscopic study in the monkey. J Neurosurg. 1968;29:451-463.
71 Weed LH. The absorption of cerebrospinal fluid into the venous system. Am J Anat. 1923;31:188-221.
72 Key E, Retzius M. Studien in der Antomie des Nervensystems und des Bindegewebes. 1875.
73 Weed LH. Studies on cerebrospinal fluid. No III. The pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J Med Res. 1914;31:51-91.
74 Levine JE, Povlishock JT, Becker DP. The morphological correlates of primate cerebrospinal fluid absorption. Brain Res. 1982;241:31-41.
75 Tripathi RC. Ultrastructure of the arachnoid mater in relation to outflow of cerebrospinal fluid. A new concept. Lancet. 1973;2:8-11.
76 Tripathi BJ, Tripathi RC. Vacuolar transcellular channels as a drainage pathway for cerebrospinal fluid. J Physiol. 1974;239:195-206.
77 Welch K, Pollay M. Perfusion of particles through arachnoid villi of the monkey. Am J Physiol. 1961;201:651-654.
78 James AEJr, McComb J, Christian JG, et al. The effect of cerebrospinal fluid pressure on the size of drainage pathways. Neurology. 1976;26:659-663.
79 Bradbury MW, Cole DF. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol. 1980;299:353-365.
80 Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol. 1981;240:F329-F336.
81 Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519-534.
82 Bradbury MWB, Westrop R. Lymphatics and the drainage of cerebrospinal fluid. In: Shapiro K, Marmarou A, Portnoy H, editors. Hydrocephalus. New York: Raven Press; 1984:69-82.
83 Erlich SS, McComb JG, Hyman S, et al. Ultrastructural morphology of the olfactory pathway for cerebrospinal fluid drainage in the rabbit. J Neurosurg. 1986;64:466-473.
84 Erlich SS, McComb JG, Hyman S, et al. Ultrastructure of the orbital pathway for cerebrospinal fluid drainage in rabbits. J Neurosurg. 1989;70:926-931.
85 Gomez DG, Fenstermacher JD, Manzo RP, et al. Cerebrospinal fluid absorption in the rabbit: olfactory pathways. Acta Otolaryngol. 1985;100:429-436.
86 Gomez DG, Manzo RP, Fenstermacher JD, et al. Cerebrospinal fluid absorption in the rabbit. Optic pathways. Graefes Arch Clin Exp Ophthalmol. 1988;226:1-7.
87 Love JA, Leslie RA. The effects of raised ICP on lymph flow in the cervical lymphatic trunks in cats. J Neurosurg. 1984;60:577-581.
88 McComb JG, Davson H, Hyman S, et al. Cerebrospinal fluid drainage as influenced by ventricular pressure in the rabbit. J Neurosurg. 1982;56:790-797.
89 McComb JG, Hyman S, Weiss MH. Contribution of the spinal compartment to cerebrospinal fluid drainage. Presented at the American Association of Neurological Surgeons, Pediatric Section, Salt Lake City 1984.
90 McComb JG, Hyman S. Lymphatic drainage of cerebrospinal fluid in the primate. In: Johansson BB, Owman CH, Widner H, editors. Pathophysiology of the Blood-Brain Barrier. Amsterdam: Elsevier; 1990:421-438.
91 Shen JY, Kelly DE, Hyman S, et al. Intraorbital cerebrospinal fluid outflow and the posterior uveal compartment of the hamster eye. Cell Tissue Res. 1985;240:77-87.
92 Yamada S, DePasquale M, Patlak CS, et al. Albumin outflow into deep cervical lymph from different regions of rabbit brain. Am J Physiol. 1991;261:H1197-H1204.
93 Bozanovic-Sosic R, Mollanji R, Johnston MG. Spinal and cranial contributions to total cerebrospinal fluid transport. Am J Physiol Regul Integr Comp Physiol. 2001;281:R909-916.
94 Johnston M, Zakharov A, Papaiconomou C, et al. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2.
95 Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6.
96 Mollanji R, Papaiconomou C, Boulton M, et al. Comparison of cerebrospinal fluid transport in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1215-1223.
97 Walter BA, Valera VA, Takahashi S, et al. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol Appl Neurobiol. 2006;32:388-396.
98 Zakharov A, Papaiconomou C, Johnston M. Lymphatic vessels gain access to cerebrospinal fluid through unique association with olfactory nerves. Lymphat Res Biol. 2004;2:139-146.
99 Mollanji R, Bozanovic-Sosic R, Zakharov A, et al. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1593-1599.
100 Nagra G, Li J, McAllister JP2nd, et al. Impaired lymphatic cerebrospinal fluid absorption in a rat model of kaolin-induced communicating hydrocephalus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1752-1759.
101 Nagra G, Johnston MG. Impact of ageing on lymphatic cerebrospinal fluid absorption in the rat. Neuropathol Appl Neurobiol. 2007;33:684-691.
102 James AEJr, Strecker EP, Sperber E, et al. An alternative pathway of cerebrospinal fluid absorption in communicating hydrocephalus. Transependymal movement. Radiology. 1974;111:143-146.
103 Tamaki N, Yamashita H, Kimura M, et al. Changes in the components and content of biological water in the brain of experimental hydrocephalic rabbits. J Neurosurg. 1990;73:274-278.
104 Wislocki GB, Putnam TJ. Absorption from the ventricles in experimentally produced internal hydrocephalus. Am J Anat. 1921;29:313-320.
105 Hiratsuka H, Tabata H, Tsuruoka S, et al. Evaluation of periventricular hypodensity in experimental hydrocephalus by metrizamide CT ventriculography. J Neurosurg. 1982;56:235-240.
106 Zervas NT, Liszczak TM, Mayberg MR, et al. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg. 1982;56:475-481.
107 Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42-F49.
108 Reulen HJ, Tsuyumu M, Tack A, et al. Clearance of edema fluid into cerebrospinal fluid. A mechanism for resolution of vasogenic brain edema. J Neurosurg. 1978;48:754-764.
109 Ohata K, Marmarou A. Clearance of brain edema and macromolecules through the cortical extracellular space. J Neurosurg. 1992;77:387-396.
110 Casley-Smith JR, Foldi-Borsok E, Foldi M. The prelymphatic pathways of the brain as revealed by cervical lymphatic obstruction and the passage of particles. Br J Exp Pathol. 1976;57:179-188.
111 McComb JG, Song SH, Hyman S, et al. The adventitia of the major cranial blood vessels does not provide a pathway for lymphatic drainage of cerebrospinal fluid. Presented at the America Association of Neurological Surgeons, San Francisco 1992.
112 Johnston M, Armstrong D, Koh L. Possible role of the cavernous sinus veins in cerebrospinal fluid absorption. Cerebrospinal Fluid Res. 2007;4:3.
113 Schwalbe G. Der Arachnoiddalraum ein Lymphraum und sein Zusammenhang mit den Perichoriodalraum. Zentralbl Med Wiss. 1869;7:465.
114 Brierley JB, Field EJ. The connexions of the spinal sub-arachnoid space with the lymphatic system. J Anat. 1948;82:153-166.
115 DiChiro G, Stein SC, Harrington T. Spontaneous cerebrospinal fluid rhinorrhea in normal dogs. Radioscopic studies of an alternative pathway of CSF drainage. J Neuropathol Exp Neurol. 1972;31:447-453.
116 Faber FW. The nasal mucosa and the subarachnoid space. Am J Anat. 1937;62:121-148.
117 Schurr PH, McLaurin RL, Ingraham FD. Experimental studies on the circulation of the cerebrospinal fluid and methods of producing communicating hydrocephalus in the dog. J Neurosurg. 1953;10:515-525.
118 Yoffrey JM, Drinker CK. Some observations on the lymphatics of the nasal mucous membrane in the cat and monkey. J Anat. 1939;74:45.
119 Galkin W. Uber die Bedentung der “Nasenbahn” fur Arbfluss aus subarachnoidal Raum. Z Gesamte Exp Med. 1930;72:65.
120 Dandy WE, Blackfan K. Internal hydrocephalus. An experimental, clinical and pathological study. Am J Dis Child. 1914;8:406-482.
121 Bowsher D. Pathways of absorption of protein from the cerebrospinal fluid: an autoradiographic study in the cat. Anat Rec. 1957;128:23-39.
122 Nabeshima S, Reese TS, Landis DM, et al. Junctions in the meninges and marginal glia. J Comp Neurol. 1975;164:127-169.
123 Butler A. Correlated physiologic and structural studies of CSF absorption. In: Shapiro K, Marmarou A, Portnoy H, editors. Hydrocephalus. New York: Raven Press; 1984:41-58.
124 Oldendorf WH, Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967;17:196-205.
125 Alperin N, Vikingstad EM, Gomez-Anson B, et al. Hemodynamically independent analysis of cerebrospinal fluid and brain motion observed with dynamic phase contrast MRI. Magn Reson Med. 1996;35:741-754.
126 Wagshul ME, Chen JJ, Egnor MR, et al. Amplitude and phase of cerebrospinal fluid pulsations: experimental studies and review of the literature. J Neurosurg. 2006;104:810-819.
127 Malko JA, Hoffman JCJr, McClees EC, et al. A phantom study of intracranial CSF signal loss due to pulsatile motion. AJNR Am J Neuroradiol. 1988;9:83-89.
128 Sherman JL, Barkovich AJ, Citrin CM. The MR appearance of syringomyelia: new observations. AJR Am J Roentgenol. 1987;148:381-391.
129 Yamada S, Miyazaki M, Kanazawa H, et al. Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology. 2008;249:644-652.
130 Agnati LF, Genedani S, Lenzi PL, et al. Energy gradients for the homeostatic control of brain ECF composition and for VT signal migration: introduction of the tide hypothesis. J Neural Transm. 2005;112:45-63.
131 Scammell T, Gerashchenko D, Urade Y, et al. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci U S A. 1998;95:7754-7759.
132 Greitz D, Hannerz J. A proposed model of cerebrospinal fluid circulation: observations with radionuclide cisternography. AJNR Am J Neuroradiol. 1996;17:431-438.