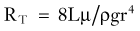CHAPTER 192 Cerebrospinal Fluid Devices
History of Cerebrospinal Fluid Shunt Devices
Before 1900, treatment of hydrocephalus was often uninformed and usually ineffective. Head bandaging, intraventricular injection of a strong iodine solution, exposure of the head to bright sunlight, and irradiation of the choroid plexus were among the more extreme procedures advocated.1 Direct ventricular puncture and repeated lumbar puncture were rarely sufficient to control hydrocephalus, and efforts were directed toward internal diversion of CSF. In the 1890s, Miculicz developed a gold, flanged hollow tube that diverted CSF from the ventricle to the subgaleal space, but this valveless device was only rarely effective.2 Other valveless CSF diversion techniques involving the use of glass, rubber, or silver tubes and linen threads in the subdural or subgaleal space were all equally unsuccessful and carried high mortality. Attention then turned to the choroid plexus, with Walter Dandy promoting the technique of extirpation of the choroid plexus in an attempt to reduce production of CSF.3 Before 1950 this was probably the most common procedure undertaken for infantile hydrocephalus,4 but success remained limited,5–7 and it was largely abandoned by the 1970s after reports of high failure rates.8,9 Interestingly, there has been a recent resurgence of interest in ablation of the choroid plexus, the efficacy of which may have been underestimated.10–12 In 1914, Heile described the first diversion of CSF from the lumbar subarachnoid space to the peritoneum with the use of a valveless rubber tube, but this too was unsuccessful.13 In 1939, Torkilsden described a shunt from the lateral ventricles to the cisterna magna for obstructive hydrocephalus that was modestly successful.14 In 1949, Matson described a shunt from the lumbar subarachnoid space to the ureter, which became popular in the 1950s.15 The procedure necessitated nephrectomy and was often complicated by hypochloremic alkalosis, which was frequently fatal, and with the introduction of valved shunt systems, the technique was abandoned.
The advent of the “modern” era of CSF shunt devices was heralded by the publication of Nulsen and Spitz’s ventriculojugular shunt with a ball and spring differential pressure valve.16 The first shunt to use silicone was the Spitz-Holter valve, a slit valve designed by engineer John Holter for his son who had hydrocephalus.17 At around the same time, Robert Pudenz also designed both a distal-slit and a sleeve valve, both differential pressure silicone valves for use in ventriculoatrial shunts.18 Silicone has since become the material of choice for implanted shunts. Although the initial preferred site for shunt placement was the vascular system, the risks, particularly infection and associated shunt nephritis or pulmonary hypertension,19,20 and identification of the peritoneal cavity as a suitable site for CSF absorption21 led to the peritoneum becoming the preferred site for distal catheter placement. Despite several new shunt and catheter designs over the past 50 years, many of the problems associated with shunts, such as blockage, overdrainage, and infection, still persist. The search for the “ideal” shunt system or an alternative, more efficacious treatment continues.
Cerebrospinal Fluid Shunt Hydrodynamics
Flow and Resistance
where r is the radius of the tube, L is the length of the tube, µ is the viscosity of the fluid (CSF), and r the diameter of the tube.
Laboratory studies have demonstrated that a 90-cm-long distal catheter provides an additional resistance to flow that is roughly equivalent to that provided by a differential pressure valve.22,23
The increase in CSF viscosity (e.g., proteinaceous CSF) seen in patients with optic pathway gliomas does not have a great impact in that even the most proteinaceous CSF reduces CSF flow by only around 7%.24,25 More importantly, CSF viscosity decreases with increasing temperature, with flow rates at body temperature some 30% higher than at room temperature, which has important implications for in vitro testing of new shunt designs, particularly those in which CSF flow occurs through a very small orifice, such as in the flow-controlled Orbis Sigma II valve.
Shunt catheter resistance rises as a fourth power of the radius, and this has been exploited in designing valveless shunt systems such as the “Mexican shunt,” which has an internal diameter of 0.51 mm as opposed to a standard catheter diameter of 1.0 to 1.6 mm.26 The relationship of shunt catheter resistance to radius has also been exploited as a means of reducing excessive drainage through lumbar shunt catheters.
Although RT has linear pressure-flow characteristics, flow through the narrow orifices of valves, despite being laminar at low flow rates, may become turbulent at higher rates of flow. Therefore, the resistance of the shunt valve (RV) is not constant in the range of physiologic flow rates, and a nonlinear pressure-versus-flow relationship is seen. Debris and air bubbles in the shunt valve or catheter will significantly increase turbulence and restrict the diameter of the lumen, both of which will significantly increase resistance to flow; although this does not necessarily occlude the shunt, it may have a major impact on shunt performance.23
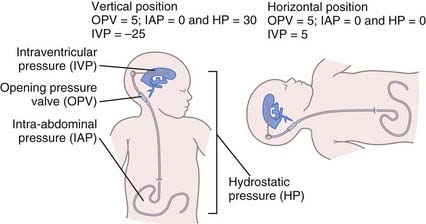
The pressure gradient driving CSF flow in a ventriculoperitoneal shunt system is determined by the formula ΔP = IVP + ρhg − OPV − IAP, where IVP is intraventricular pressure, ρhg (h being the difference in vertical height between the head and distal drainage site) is hydrostatic pressure, OPV is the opening pressure of the valve, and IAP is intra-abdominal pressure. Thus, in the upright position, the predominant influence on the pressure gradient (and therefore CSF flow) is hydrostatic pressure, not OPV (Fig. 192-1).
Siphoning
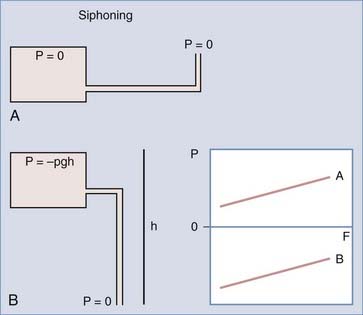
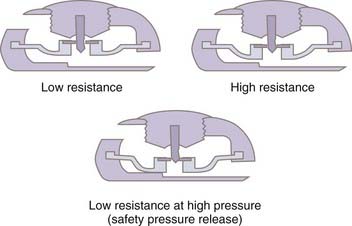
With differential pressure valves (see later), once the patient moves to the upright position and the valve opens, the hydrostatic forces acting on the shunt system will predominate and result in excessively high flow rates despite negative intracranial pressure (ICP). In a valveless system, ICP would continue to fall until IVP equals negative ρhg to balance the siphon effect (Fig. 192-2). Such a drop in ICP does not occur in a normal brain because there is no posture-related change in the CSF–sagittal sinus pressure gradient.23 The exception occurs in patients who have undergone a wide decompressive craniectomy, in which the head is essentially exposed to atmospheric pressure. In these patients in the upright position, CSF will continue to siphon until the ventricles are emptied and the craniectomy is maximally sunken, which may result in marked shift and deformation of the underlying brain tissue and have significant neurological sequelae, the “sinking skin-flap syndrome.”27
The excessively negative pressures generated by siphoning are surprisingly well tolerated by the majority of patients, but around 10% of patients will experience low-pressure symptoms.28,29 Other serious sequelae include ventricular collapse with tearing of bridging veins and subdural hematoma formation, premature suture closure, acquired aqueductal stenosis, Parinaud’s syndrome, and slit ventricle syndrome.28,30–33
Proximal and Distal Shunt Catheters
All shunt catheters (both proximal and distal) are made of artificial silicone rubber or polyurethane. The catheters are stiff enough to resist kinking but compliant enough to minimize the risk of brain injury as the ventricles reduce in size and the catheter comes in contact with the ependyma. Catheters are available in a range of internal and external diameters, the smaller internal diameters being used in neonates or valveless shunt systems to add further resistance to CSF flow. Most modern catheter designs are impregnated with tantalum or barium to facilitate radiologic identification. The latter is associated with an increased rate of distal shunt catheter deterioration and host reaction leading to calcification and loss of elasticity and strength of the catheter tubing.34,35 The catheters then become tethered, typically in the neck, and are prone to fracture, particularly in growing children.36 To mitigate this complication, some manufacturers now produce catheters with a barium strip or coating of pure silicone to reduce host reaction to the tubing.
Packaged catheters carry a static charge and, when opened, can attract airborne dust particles carrying microorganisms; accordingly, non–antibiotic-impregnated catheters should be soaked in sterile saline solution immediately on opening to reduce the risk of contamination. To reduce the risk for shunt infection, manufacturers have introduced specialized catheters, some of which are impregnated with antibiotics, such as the Bactiseal catheter system, which is impregnated with clindamycin and rifampicin (Codman, Johnson & Johnson, Inc., Raynham, MA) and releases the antibiotics in the weeks after implantation to potentially reduce the risk for shunt infection by preventing biofilm-forming organisms from colonizing the catheter. Other manufacturers have developed catheters that are impregnated with silver nanoparticles (Silverline, Speigelberg, Hamburg), which have antibacterial properties,37 or coated with antibiotics to reduce the risk for shunt infection. It should be noted, however, that to date, no prospective multicenter randomized controlled trials have been completed that demonstrate an overall reduction in infection rates with any of these catheters. Some retrospective studies have shown promising results in high-risk populations,38–40 but other series have not demonstrated a benefit,41 and a relative increase in more severe gram-negative infections may offset any benefit from an overall reduction in infection rates. Other measures such as the intraventricular administration of antibiotics at the time of shunt implantation may be of similar efficacy.42
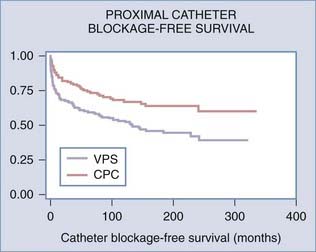
The proximal shunt catheter is usually placed in the lateral ventricle. Occasionally, catheters are placed within the subarachnoid space, arachnoid cysts, syrinx cavities, and subdural hygromas. The most common cause of shunt malfunction is blockage of the proximal catheter, which is usually secondary to ingrowth of choroid plexus. Attempts to identify a preferred site for catheter placement remote from the choroid plexus have been unsuccessful.43 Even endoscopically assisted placement of proximal catheters does not reduce blockage rates,44 although post hoc analysis of the data suggested that if catheters could be accurately positioned away from the choroid plexus, shunt failure could be reduced. A variety of proximal catheter designs with baskets, flanges, or recessed holes, as well as the “J”-shaped Hakim catheter with holes on the inside curve of the “J,” have been produced in an effort to reduce mechanical obstruction by the choroid plexus, but none have been successful in reducing ventricular catheter blockage rates. Endoscopic coagulation of the choroid plexus itself on the side of the shunt may be the most effective means of reducing proximal catheter obstruction (Fig. 192-3).45
The size and number of drainage holes in the tip of the catheter vary, but most holes are redundant. Laboratory studies have shown that even if only a single hole remains patent, there is no significant increase in the total resistance of the shunt system.46 Ventricular catheters come with a stylet to facilitate passage of the catheter through the brain parenchyma. Hollow stylets to allow visualization of CSF when the ventricle is cannulated are now available. A number of devices can be used to facilitate proximal catheter placement, including the Ghajar guide, a tripod designed to ensure a perpendicular trajectory to the ventricle from a coronal approach,47 as well as ultrasound probes and intraluminal ventriculoscopes.44,48 All these devices can assist in either accessing the ventricle or confirming position within the ventricular system (or both). Recently, frameless, image-guided neuronavigation has been used to facilitate catheter placement,49 and the advent of electromagnetic navigation technology has enabled the use of such neuronavigation in infants.50 When contoured or cylinder valves are used, external right-angle guides are used to reduce the risk of shunt migration, but in very small infants these guides may be unduly prominent and increase the risk for scalp breakdown. A variety of rigid connectors (either polyethylene or titanium) are available, either straight, right angled, or “Y,” “X,” or “T” shaped to facilitate the assembly of complex shunt systems. Bur hole reservoirs that redirect the flow of CSF 90 degrees can also be used in conjunction with contoured valves, particularly if the valve has no integral reservoir of its own.
Distal shunt catheters may have a distal slit or a single open-ended lumen. The latter is associated with a significantly lower rate of distal catheter occlusion,51 and we advocate removal of any distal slits before intraperitoneal placement. When the distal catheter is placed in the vascular system, a distal slit valve is required. Recently, distal catheters have been placed with laparoscopic assistance. This may be useful when cosmesis is a major consideration or when coexistent intra-abdominal pathology such as adhesions or obesity may compromise optimal placement, and it allows confirmation of the implanted functioning shunt system.52–57
Shunt Valves
Differential Pressure Valves
Numerous differential pressure one-way valves have been developed and they consist of four broad categories: slit valves, miter valves, diaphragm valves, and ball-in-cone valves.58 These devices all attempt to achieve the same goal of keeping IVP from climbing too high or falling too low. Differential pressure valves are defined by their opening or closing pressure. As IVP rises, the differential pressure across the valve increases. When this exceeds the valve’s opening pressure, the valve opens to allow egress of CSF at a rate determined by the resistance of the entire shunt system; once the pressure falls below the closing pressure, however, flow of CSF ceases. Although it has not been demonstrated in vivo, it is possible that the valve opens and closes with each cardiac cycle, and valves may act by damping the ICP pulse wave, which has been implicated in ventricular enlargement in hydrocephalus.59–61 The valve’s opening pressure is not necessarily the same as its closing pressure because of the phenomenon of hysteresis. Hysteresis occurs because of a slight change in the mechanical properties of the valves, depending on whether they are opening or closing, and occurs most frequently with silicone slit and miter valves. It can have a significant impact on valve performance and potentially lead to overdrainage, even in the absence of siphoning (e.g., during nocturnal vasogenic pressure wave activity or exercise).62 Pumping valve reservoirs can further affect performance, particularly with silicone diaphragm valves.63
Flow-Regulated Valves
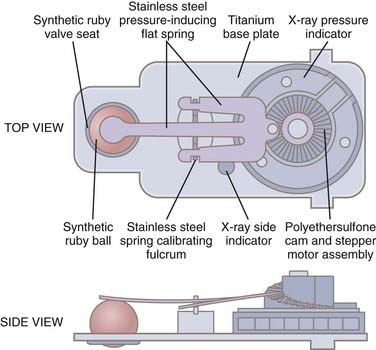
Flow-regulating devices such as the Orbis Sigma II valve (NMT Medical, Inc., Boston) are designed to increase hydrodynamic resistance as the pressure gradient increases in an attempt to keep the flow rate constant.63 It is in fact the differential pressure that controls the resistance, and perhaps these valves should be called pressure-controlled, variable-resistance, constant-flow valves. The valve is composed of a contoured synthetic ruby flow control pin that fits inside a movable synthetic ruby ring (Fig. 192-4). These devices produce pressure-flow curves with a sigmoid shape; at low pressures the valve behaves as a differential pressure valve until flow rates reach about 20 mL/hr. As the pressure increases, the ruby ring is deflected downward, and because the ruby pin is tapered, the flow aperture decreases, which increases resistance and reduces flow. This will tend to maintain flow at a constant level over a range of physiologic pressures (8 to 35 cm H2O). If the pressure increases further, beyond a predetermined threshold, typically around 35 cm H2O, the ruby ring is deflected further downward beyond the pin, thereby increasing the aperture for CSF flow and lowering resistance to allow “venting” of CSF for mitigating any pathologic rise in CSF pressure. At this point the valve behaves as a differential pressure valve and gives rise to a sigmoid curve. Flow-regulating valves are less likely to be associated with siphoning and overdrainage and have been shown to improve symptomatic low ICP in shunted individuals.64 However, flow-regulated valves typically have very small orifices, which makes the valve itself a probable site of obstruction.65,66 In addition, the high resistance has a propensity to cause fluid collections under the scalp in young children unless they are nursed upright with a compressive dressing. A randomized controlled trial failed to demonstrate a reduction in revision rates with flow-controlled valves when compared with other conventional differential pressure valve types.65
Antisiphon Devices
One strategy developed to avoid the complication of overdrainage is the siphon-resistive or antisiphon device (ASD). Several devices are available that can be broadly grouped into three different categories: those using a subcutaneous membrane, those using a gravitational mechanism, and those regulating CSF flow. The most widely used is the PS Medical Delta Chamber (Medtronic PS Medical, Goleta, CA), which also comes integrated with a differential pressure valve: the Delta valve. This device is typically placed under the scalp and has a small diaphragm that reduces the flow of CSF when the pressure inside the shunt falls below atmospheric pressure. Drake and coworkers demonstrated that raised tissue compartment pressure from scar tissue overlying an ASD can lead to functional obstruction.67 Bench testing suggests that the Delta valve probably works predominantly in the horizontal position because in the upright position, the physiologic value of the ICP is sufficiently negative to close the ASD.68 The SiphonGuard ASD (Codman and Shurtleff, Inc., Raynham, MA) is designed to reduce siphoning by having two alternative pathways for drainage of CSF. During normal flow, both the primary and secondary pathways are open. In high-flow states, the primary pathway closes and flow is diverted to a high-resistance secondary pathway. The device can be positioned anywhere along the shunt system. The main criticisms of this device derive from bench test data suggesting that in patients with small or slit ventricles, CSF may not be available to produce flow at a high enough rate to activate the high-resistance pathway. Furthermore, children or adults who spend significant time in a semirecumbent position (the elderly) may fail to generate sufficient “siphoning” to trigger the high-resistance pathway but may have flow rates sufficient to cause overdrainage, and there is also a theoretical risk of the valve “locking” in the high-resistance state.69 These risks remain theoretical, and further clinical studies are required to evaluate this device. Gravitational ASDs are discussed in the next section.
Programmable Valves
Programmable valves are more appropriately called externally adjustable differential pressure valves. They act in the same fashion as nonadjustable differential pressure valves, except that the surgeon has the option of altering the opening pressure with an external device, thereby obviating the need for surgical shunt revision. This increases convenience and marginally decreases risk, but it is not clear whether this benefit outweighs the increased cost of using these valves in all patients. Programmable valves include the Codman Medos Hakim Programmable Valve (an adjustable “ball-in-cone” valve with 18 settings from 30 to 200 mm H2O [Fig. 192-5]), the PS Medical Strata Valve (an adjustable, 5-setting Delta valve; also available as a 5-setting differential pressure valve without an ASD, the Strata-NSC), and the Sophy Programmable Pressure Valve (adjustable spring-ball valve). Most valves are adjusted with an external magnet, and some are disposed to inadvertent reprogramming in the presence of strong magnetic fields.68,70 Patients with these valves require reprogramming after magnetic resonance imaging (MRI) and should also be advised to avoid close proximity to strong magnetic fields (some stereo headphones, magnetic toys, televisions, electricity substations, etc.).71–74 Several authors have reported clinical success with these devices,75–77 but to date no adequately powered randomized controlled trials designed to compare efficacy have been undertaken. It has been suggested that this type of shunt is well suited for difficult-to-manage cases of overdrainage (e.g., slit ventricles, subdural collections) or underdrainage (e.g., persistent symptoms of hydrocephalus).78–80 It has also been suggested that externally adjustable shunts are particularly useful in gradually decreasing the size of arachnoid cysts and in reducing the incidence of symptomatic subdural hygromas in patients with normal-pressure hydrocephalus.79,81,82 It is important to note that programmable valves that do not incorporate an ASD are also susceptible to siphoning.
Ventricular Access Devices
Ventricular access devices such as the Ommaya reservoir allow percutaneous access to ventricular CSF. They were initially developed to permit intrathecal administration of chemotherapy.83 These devices can be used for the in situ treatment of CSF shunt infections.84 Because they do not tend to become blocked with choroid plexus, they are now increasingly being placed in patients with CSF shunts to allow emergency ventricular access in the event of shunt blockage.85,86 They have also been advocated after endoscopic third ventriculostomy87–90 when there is a risk for late blockage, which may be fatal, but this indication remains contentious.91 They are useful in facilitating ventricular taps in infants with posthemorrhagic ventricular dilation who are too small for a shunt. In addition, ventricular access devices may be used for ventricular infusion tests and monitoring of ICP for the evaluation of patients with suspected shunt failure but no increase in ventricular size and for CSF drainage studies to evaluate possible shunt responsiveness in patients with normal-pressure hydrocephalus.92,93
Valve Design Trials
It is impossible to sort through the merits of the various shunt valve designs by attempting to evaluate uncontrolled case series. These series are frequently reported by proponents of the devices, who sometimes have financial interests or other incentives. A multicenter randomized trial of CSF shunt valve design for pediatric hydrocephalus failed to demonstrate any difference among valves in the incidence of shunt failure resulting from shunt obstruction, overdrainage, loculations of the cerebral ventricles, or infection over a minimum 1-year period of follow-up.65,94,95 In this trial, 344 hydrocephalic children undergoing their first CSF shunt insertion were randomized to one of three valves: a standard differential pressure valve, a Delta valve, or an Orbis Sigma valve. A randomized trial comparing a conventional valve and a Codman Hakim programmable valve with respect to shunt failure and valve explantation also did not demonstrate any significant difference between the valves.96 It is clear that further prospective randomized controlled trials are required to evaluate the new generation of programmable and gravitational valves. Studies evaluating shunt selection for specific patient subgroups such as those with normal-pressure hydrocephalus are also required. Data from prospective national shunt registries such as the U.K. Shunt Registry, which now has more than 50,000 procedures logged, may also provide valuable insight into long-term valve outcomes.97
In Vivo Shunt Assessment
Neuroimaging plays a major role in the assessment of suspected shunt malfunction. A plain radiographic shunt series can identify shunt fracture or migration and allow identification of the shunt valve type.63 Computed tomography and MRI are particularly useful in the assessment of ventricular size, particularly when a “well baseline” scan is available for comparison. Radioisotope studies, the “shuntogram,” can provide qualitative information on shunt patency. Pumping the shunt reservoir alone does not have sufficient predictive power to detect shunt malfunction.98 Tapping the shunt with a butterfly cannula can confirm proximal catheter patency, and assessment for “siphoning” with the patient inclined 45 degrees can evaluate distal catheter function, but care should be taken to ensure that the assessor understands the relationship between the valve and integral reservoir for any individual shunt system when tapping a shunt. A more elaborate quantitative invasive test is the ventricular infusion study, which can be performed via either a separate ventricular access device or, for shunts in which the reservoir lies proximal to the valve, the shunt reservoir itself. Czosnyka and colleagues have established threshold pressures for different valve types, and this information is extremely useful when evaluating equivocal shunt failure or overdrainage.92 Accessing the shunt carries a risk for infection, so strict asepsis is essential.
Future Developments
Further advances in technology, such as nanocoatings for shunt components, should increase the biocompatibility of shunt systems, thereby prolonging life span.99 Simple measures such as not using barium-impregnated catheters and removing distal slit valves should also prolong shunt survival. Techniques such as percutaneous ultrasonic cavitation may enable recanalization of blocked ventricular catheters.100 Efforts should be directed to establishing the efficacy and cost-effectiveness of existing technology in large randomized multinational controlled trials. Key areas of focus should include comparisons of novel valve types, including gravitational valves and new-generation programmable valves. Novel proximal catheters designed to reduce rates of obstruction with choroid plexus should be compared with other techniques such as choroid plexus coagulation. The development of telemetric ICP monitors will facilitate the diagnosis of shunt failure. Functioning prototype devices have been tested successfully in animal models.101 Unfortunately, the high cost of developing these products and bringing them to market may hamper translation from the laboratory to the bedside. Shunt systems with integrated thermistor sensors that can monitor flow are likely to be marketed in the near future and may be useful for diagnosing shunt blockage. Intelligent shunt systems with servo-controlled feedback mechanisms to regulate CSF flow remain elusive more than 35 years after they were first proposed,102 and the goal of a single shunt that provides physiologic control of ICP and lasts a lifetime is some decades away.
Ames RH. Ventriculoperitoneal shunts in the management of hydrocephalus. J Neurosurg. 1967;27:525.
Aquilina K, Edwards RJ, Pople IK. Routine placement of a ventricular reservoir at endoscopic third ventriculostomy. Neurosurgery. 2003;53:91.
Czosnyka ZH, Czosnyka M, Pickard JD. Shunt testing in-vivo: a method based on the data from the UK shunt evaluation laboratory. Acta Neurochir Suppl. 2002;81:27.
Czosnyka Z, Czosnyka M, Richards HK, et al. Laboratory testing of hydrocephalus shunts—conclusion of the U.K. Shunt Evaluation Programme. Acta Neurochir (Wien). 2002;144:525.
Davidoff LM. Treatment of hydrocephalus: historical review and description of a new method. Arch Surg. 1929;18:1737.
Dickerman RD, McConathy WJ, Morgan J, et al. Failure rate of frontal versus parietal approaches for proximal catheter placement in ventriculoperitoneal shunts: revisited. J Clin Neurosci. 2005;12:781.
Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294.
Drake JM, Sainte Rose C. The Shunt Book. New York: Blackwell Science; 1995. 1-228
Hanlo PW, Cinalli G, Vandertop WP, et al. Treatment of hydrocephalus determined by the European Orbis Sigma Valve II survey: a multicenter prospective 5-year shunt survival study in children and adults in whom a flow-regulating shunt was used. J Neurosurg. 2003;99:52.
Kan P, Kestle J. Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst. 2007;23:773.
Kestle JR, Drake JM, Cochrane DD, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg. 2003;98:284.
Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum. 1952;2:399.
Sainte-Rose C, Piatt JH, Renier D, et al. Mechanical complications in shunts. Pediatr Neurosurg. 1991;17:2.
1 Davidoff LM. Treatment of hydrocephalus: historical review and description of a new method. Arch Surg. 1929;18:1737.
2 Henle A. Beitrag zur Pathologie und Therapie des hydrocephalus. Mitt Grenzgeb Med Chir. 1896;1:264.
3 Dandy WE. Extirpation of the choroid plexus of the lateral ventricle in communicating hydrocephalus. Ann Surg. 1918;68:569.
4 Davis L. Neurological Surgery. Philedelphia: Lea & Febiger; 1936.
5 Putnam TJ. Results of the treatment of hydrocephalus by endoscopic coagulation of the choroid plexus. Arch Pediatr. 1935;52:676.
6 Scarff JE. Endoscopic treatment of hydrocephalus. Arch Neurol Psychiatry. 1936;35:853.
7 Sachs E. Hydrocephalus: an analysis of 98 cases. J Mt Sinai Hosp. 1942;9:767.
8 Milhorat TH. Failure of choroid plexectomy as treatment for hydrocephalus. Surg Gynecol Obstet. 1974;139:505.
9 Milhorat TH, Hammock MK, Chien T, et al. Normal rate of cerebrospinal fluid formation five years after bilateral choroid plexectomy. Case report. J Neurosurg. 1976;44:735.
10 Pople IK, Ettles D. The role of endoscopic choroid plexus coagulation in the management of hydrocephalus. Neurosurgery. 1995;36:698.
11 Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg. 2005;103:475.
12 Warf BC, Campbell JW. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr. 2008;2:310.
13 Heile B. Zur chirurgischen Behandlung des Hydrocephalus internus durch Ableitung der Cerebrospinalflussigkeitnach der bauchole und nach der Pleurakuppe. Arch Klin Chir. 1914;105:501.
14 Torkilsden A. A new palliative operation in cases of inoperable occlusion of the sylvian aqueduct. Acta Chir Scand. 1939;82:117.
15 Matson DD. A new operation for treatment of communicating hydrocephalus: report of a case secondary to generalised meningitis. J Neurosurg. 1949;6:238.
16 Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum. 1952;2:399.
17 Boockvar JA, Loudon W, Sutton LN. Development of the Spitz-Holter valve in Philadelphia. J Neurosurg. 2001;95:145.
18 Pudenz RH, Russell FE, Hurd AH, et al. Ventriculo-auriculostomy; a technique for shunting cerebrospinal fluid into the right auricle; preliminary report. J Neurosurg. 1957;14:171.
19 Arze RS, Rashid H, Morley R, et al. Shunt nephritis: report of two cases and review of the literature. Clin Nephrol. 1983;19:48.
20 Friedman S, Zita-Gozum C, Chatten J. Pulmonary vascular changes complicating ventriculovascular shunting for hydrocephalus. J Pediatr. 1964;64:305.
21 Ames RH. Ventriculoperitoneal shunts in the management of hydrocephalus. J Neurosurg. 1967;27:525.
22 Aschoff A, Bensch C, Kremer P, et al. The solved and unsolved problems of hydrocephalus valves: a critical comment. In: Lorenz R, Klinger M, Brack M, editors. Advances in Neurosurgery, Vol 21. New York: Springer; 1993:103-114.
23 Czosnyka M, Czosnyka Z, Whitehouse H, et al. Hydrodynamic properties of hydrocephalus shunts: United Kingdom Shunt Evaluation Laboratory. J Neurol Neurosurg Psychiatry. 1997;62:43.
24 Brydon HL, Hayward R, Harkness W, et al. Physical properties of cerebrospinal fluid of relevance to shunt function. 2: The effect of protein upon CSF surface tension and contact angle. Br J Neurosurg. 1995;9:645.
25 Brydon HL, Hayward R, Harkness W, et al. Does the cerebrospinal fluid protein concentration increase the risk of shunt complications? Br J Neurosurg. 1996;10:267.
26 Sotelo J, Izurieta M, Arriada N. Treatment of hydrocephalus in adults by placement of an open ventricular shunt. J Neurosurg. 2001;94:873.
27 Han PY, Kim JH, Kang HI, et al. “Syndrome of the sinking skin-flap” secondary to the ventriculoperitoneal shunt after craniectomy. J Korean Neurosurg Soc. 2008;43:51.
28 Pudenz RH, Foltz EL. Hydrocephalus: overdrainage by ventricular shunts. A review and recommendations. Surg Neurol. 1991;35:200.
29 Edwards RJ, Witchell C, Pople IK. Chronic headaches in adults with spina bifida and associated hydrocephalus. Eur J Pediatr Surg. 2003;13(suppl 1):S13.
30 Rekate HL. The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg. 2004;40:259.
31 Rekate HL. Shunt-related headaches: the slit ventricle syndromes. Childs Nerv Syst. 2008;24:423.
32 Weinzweig J, Bartlett SP, Chen JC, et al. Cranial vault expansion in the management of postshunt craniosynostosis and slit ventricle syndrome. Plast Reconstr Surg. 2008;122:1171.
33 Maroulis H, Halmagyi GM, Heard R, et al. Sylvian aqueduct syndrome with slit ventricles in shunted hydrocephalus due to adult aqueduct stenosis. J Neurosurg. 2008;109:939.
34 Elisevich K, Mattar AG, Cheeseman F. Biodegradation of distal shunt catheters. Pediatr Neurosurg. 1994;21:71.
35 Boch AL, Hermelin E, Sainte-Rose C, et al. Mechanical dysfunction of ventriculoperitoneal shunts caused by calcification of the silicone rubber catheter. J Neurosurg. 1998;88:975.
36 Langmoen IA, Lundar T, Vatne K, et al. Occurrence and management of fractured peripheral catheters in CSF shunts. Childs Nerv Syst. 1992;8:222.
37 Izci Y, Secer H, Akay C, et al. Initial experience with silver-impregnated polyurethane ventricular catheter for shunting of cerebrospinal fluid in patients with infected hydrocephalus. Neurol Res. 2009;31:234.
38 Eymann R, Steudel WI, Kiefer M. Infection rate with application of an antibiotic-impregnated catheter for shunt implantation in children—a retrospective analysis. Klin Padiatr. 2009;221:69.
39 Sciubba DM, Noggle JC, Carson BS, et al. Antibiotic-impregnated shunt catheters for the treatment of infantile hydrocephalus. Pediatr Neurosurg. 2008;44:91.
40 Sciubba DM, Stuart RM, McGirt MJ, et al. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg. 2005;103:131.
41 Kan P, Kestle J. Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst. 2007;23:773.
42 Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg. 2006;105:242.
43 Dickerman RD, McConathy WJ, Morgan J, et al. Failure rate of frontal versus parietal approaches for proximal catheter placement in ventriculoperitoneal shunts: revisited. J Clin Neurosci. 2005;12:781.
44 Kestle JR, Drake JM, Cochrane DD, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg. 2003;98:284.
45 Edwards RJ, Pople IK. Endoscopic choroid plexus coagulation reduces proximal shunt catheter revision rates in non-tumoral hydrocephalus. J Neurosurg. 2003;98:682.
46 Ginsberg HJ, Sum A, Drake JM, et al. Ventriculoperitoneal shunt flow dependency on the number of patent holes in a ventricular catheter. Pediatr Neurosurg. 2000;33:7.
47 Ghajar JB. A guide for ventricular catheter placement. Technical note. J Neurosurg. 1985;63:985.
48 Whitehead WE, Jea A, Vachhrajani S, et al. Accurate placement of cerebrospinal fluid shunt ventricular catheters with real-time ultrasound guidance in older children without patent fontanelles. J Neurosurg. 2007;107:406.
49 Tirakotai W, Riegel T, Sure U, et al. Clinical application of neuro-navigation in a series of single burr-hole procedures. Zentralbl Neurochir. 2004;65:57.
50 Clark S, Sangra M, Hayhurst C, et al. The use of noninvasive electromagnetic neuronavigation for slit ventricle syndrome and complex hydrocephalus in a pediatric population. J Neurosurg Pediatr. 2008;2:430.
51 Sainte-Rose C, Piatt JH, Renier D, et al. Mechanical complications in shunts. Pediatr Neurosurg. 1991;17:2.
52 Nfonsam V, Chand B, Rosenblatt S, et al. Laparoscopic management of distal ventriculoperitoneal shunt complications. Surg Endosc. 2008;22:1866.
53 Jea A, Al-Otibi M, Bonnard A, et al. Laparoscopy-assisted ventriculoperitoneal shunt surgery in children: a series of 11 cases. J Neurosurg. 2007;106:421.
54 Bhasin RR, Chen MK, Pincus DW. Salvaging the “lost peritoneum” after ventriculoatrial shunt failures. Childs Nerv Syst. 2007;23:483.
55 Goitein D, Papasavas P, Gagne D, et al. Single trocar laparoscopically assisted placement of central nervous system–peritoneal shunts. J Laparoendosc Adv Surg Tech A. 2006;16:1.
56 Brunori A, Massari A, Macarone-Palmieri R, et al. Minimally invasive treatment of giant CSF pseudocyst complicating ventriculoperitoneal shunt. Minim Invasive Neurosurg. 1998;41:38.
57 Khosrovi H, Kaufman HH, Hrabovsky E, et al. Laparoscopic-assisted distal ventriculoperitoneal shunt placement. Surg Neurol. 1998;49:127.
58 Drake JM, Kestle JR, Tuli S. Cerebrospinal fluid shunt technology. Clin Neurosurg. 2000;47:336.
59 Eide PK. Demonstration of uneven distribution of intracranial pulsatility in hydrocephalus patients. J Neurosurg. 2008;109:912.
60 Eide PK, Sorteberg W. Changes in intracranial pulse pressure amplitudes after shunt implantation and adjustment of shunt valve opening pressure in normal pressure hydrocephalus. Acta Neurochir (Wien). 2008;150:1141.
61 Czosnyka ZH, Cieslicki K, Czosnyka M, et al. Hydrocephalus shunts and waves of intracranial pressure. Med Biol Eng Comput. 2005;43:71.
62 Schuhmann MU, Schneekloth CG, Klinge P, et al. Dynamic shunt testing applying short lasting pressure waves—inertia of shunt systems. Acta Neurochir Suppl. 2002;81:19.
63 Drake JM, Sainte Rose C. The Shunt Book. New York: Blackwell Science; 1995. 1-228
64 Hanlo PW, Cinalli G, Vandertop WP, et al. Treatment of hydrocephalus determined by the European Orbis Sigma Valve II survey: a multicenter prospective 5-year shunt survival study in children and adults in whom a flow-regulating shunt was used. J Neurosurg. 2003;99:52.
65 Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294.
66 Aschoff A, Kremer P, Benesch C, et al. Overdrainage and shunt technology. A critical comparison of programmable, hydrostatic and variable-resistance valves and flow-reducing devices. Childs Nerv Syst. 1995;11:193.
67 Drake JM, da Silva MC, Rutka JT. Functional obstruction of an antisiphon device by raised tissue capsule pressure. Neurosurgery. 1993;32:137.
68 Czosnyka Z, Czosnyka M, Richards HK, et al. Laboratory testing of hydrocephalus shunts—conclusion of the U.K. Shunt Evaluation Programme. Acta Neurochir (Wien). 2002;144:525.
69 Czosnyka Z, Czosnyka M, Pickard JD. Hydrodynamic performance of a new siphon preventing device: the SiphonGuard. J Neurol Neurosurg Psychiatry. 1999;66:408.
70 Lavinio A, Harding S, Van Der Boogaard F, et al. Magnetic field interactions in adjustable hydrocephalus shunts. J Neurosurg Pediatr. 2008;2:222.
71 Utsuki S, Shimizu S, Oka H, et al. Alteration of the pressure setting of a Codman-Hakim programmable valve by a television. Neurol Med Chir (Tokyo). 2006;46:405.
72 Turner SG, Hall WA. Programmable shunt–related suicide attempt. Acta Neurochir (Wien). 2006;148:1307.
73 Anderson RC, Walker ML, Viner JM, et al. Adjustment and malfunction of a programmable valve after exposure to toy magnets. Case report. J Neurosurg. 2004;101:222.
74 Miwa K, Kondo H, Sakai N. Pressure changes observed in Codman-Medos programmable valves following magnetic exposure and filliping. Childs Nerv Syst. 2001;17:150.
75 McGirt MJ, Buck DW2nd, Sciubba D, et al. Adjustable vs set-pressure valves decrease the risk of proximal shunt obstruction in the treatment of pediatric hydrocephalus. Childs Nerv Syst. 2007;23:289.
76 Zemack G, Romner B. Seven years of clinical experience with the programmable Codman Hakim valve: a retrospective study of 583 patients. J Neurosurg. 2000;92:941.
77 Reinprecht A, Czech T, Dietrich W. Clinical experience with a new pressure-adjustable shunt valve. Acta Neurochir (Wien). 1995;134:119.
78 Olson S. The problematic slit ventricle syndrome. A review of the literature and proposed algorithm for treatment. Pediatr Neurosurg. 2004;40:264.
79 Kay AD, Fisher AJ, O’Kane C, et al. A clinical audit of the Hakim programmable valve in patients with complex hydrocephalus. Br J Neurosurg. 2000;14:535.
80 Kamikawa S, Kuwamura K, Fujita A, et al. [The management of slit-like ventricle with the Medos programmable Hakim valve and the ventriculofiberscope.]. No Shinkei Geka. 1998;26:349.
81 Bergsneider M, Black PM, Klinge P, et al. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S29.
82 Germano A, Caruso G, Caffo M, et al. The treatment of large supratentorial arachnoid cysts in infants with cyst-peritoneal shunting and Hakim programmable valve. Childs Nerv Syst. 2003;19:166.
83 Ratcheson RA, Ommaya AK. Experience with the subcutaneous cerebrospinal-fluid reservoir. Preliminary report of 60 cases. N Engl J Med. 1968;279:1025.
84 Brown EM, Edwards RJ, Pople IK. Conservative management of patients with cerebrospinal fluid shunt infections. Neurosurgery. 2006;58:657.
85 Leggate JR, Baxter P, Minns RA, et al. Role of a separate subcutaneous cerebro-spinal fluid reservoir in the management of hydrocephalus. Br J Neurosurg. 1988;2:327.
86 Pople IK, Edwards RJ, Aquilina K. Endoscopic methods of hydrocephalus treatment. Neurosurg Clin N Am. 2001;12:719.
87 Aquilina K, Edwards RJ, Pople IK. Routine placement of a ventricular reservoir at endoscopic third ventriculostomy. Neurosurgery. 2003;53:91.
88 Lipina R, Palecek T, Reguli S, et al. Death in consequence of late failure of endoscopic third ventriculostomy. Childs Nerv Syst. 2007;23:815.
89 Mobbs RJ, Vonau M, Davies MA. Death after late failure of endoscopic third ventriculostomy: a potential solution. Neurosurgery. 2003;53:384.
90 Edwards RJ, Pople IK. Death and third ventriculostomy. J Neurosurg. 2003;98:649.
91 Roux FE. Death and third ventriculostomy. J Neurosurg. 2003;98:650.
92 Czosnyka ZH, Czosnyka M, Pickard JD. Shunt testing in-vivo: a method based on the data from the UK Shunt Evaluation Laboratory. Acta Neurochir Suppl. 2002;81:27.
93 Aquilina K, Pople I, Edwards RJ. External CSF drainage test is superior to CSF outflow resistance (Ro) measurement in predicting shunt responsiveness in idiopathic NPH patients undergoing both investigations. Presented at the Fourth International Hydrocephalus Workshop, Rhodes, Greece, 2007.
94 Drake JM, Kestle J. Rationale and methodology of the multicenter pediatric cerebrospinal fluid shunt design trial. Pediatric Hydrocephalus Treatment Evaluation Group. Childs Nerv Syst. 1996;12:434.
95 Drake JM, Kestle J. Determining the best cerebrospinal fluid shunt valve design: the pediatric valve design trial. Neurosurgery. 1996;38:604.
96 Pollack IF, Albright AL, Adelson PD. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery. 1999;45:1399.
97 O’Kane MC, Richards H, Winfield P, et al. The United Kingdom Shunt Registry. Eur J Pediatr Surg. 1997;7(Suppl 1):56.
98 Piatt JHJr. Pumping the shunt revisited. A longitudinal study. Pediatr Neurosurg. 1996;25:73.
99 Kumbar SG, Bhattacharyya S, Sethuraman S, et al. A preliminary report on a novel electrospray technique for nanoparticle based biomedical implants coating: precision electrospraying. J Biomed Mater Res B Appl Biomater. 2007;81:91.
100 Ginsberg HJ, Drake JM, Peterson TM, et al. Recanalization of obstructed cerebrospinal fluid ventricular catheters using ultrasonic cavitation. Neurosurgery. 2006;59:ONS403.
101 Frischholz M, Sarmento L, Wenzel M, et al. Telemetric implantable pressure sensor for short- and long-term monitoring of intracranial pressure. Conf Proc IEEE Eng Med Biol Soc. 2007:514.
102 Hakim S. Hydraulic and mechanical mis-matching of valve shunts used in the treatment of hydrocephalus: the need for a servo-valve shunt. Dev Med Child Neurol. 1973;15:646.