Cerebrospinal fluid and lumbar puncture
Anatomy and physiology
The brain and spinal cord are surrounded by three layers of meninges: thick dura mater, trabeculated arachnoid mater and thin pia mater. The trabeculae of the arachnoid traverse the subarachnoid space, which contains cerebrospinal fluid (CSF). The CSF is essentially an ultrafiltrate of plasma with some differences: for example, 80% of proteins are transudated and 20% are synthesized locally. In order to reach CNS tissue, substances must cross one of two relative barriers: the blood–brain barrier (BBB) and blood–CSF barrier (BCB).
The CSF is clear in colour and its total volume is 140 ml. The rate of CSF production is 500 ml per day: 70% from the choroid plexuses within the lateral, third and fourth ventricles and 30% from the capillaries and metabolic water. CSF circulates from the lateral ventricles to the third ventricle, through the aqueduct of Sylvius to the fourth ventricle. From here it enters the subarachnoid cistern around the medulla. It is then reabsorbed in the arachnoid villi because of the pressure difference between CSF and venous blood in the superior sagittal sinus and venous circulation. CSF is freely interchangeable with CNS extracellular fluid and is under a pressure of 100–200 mmH2O in the supine individual. The normal CSF contains no red cells and very few leucocytes. Plasma proteins are present in inverse proportion to their molecular size, consistent with an ultrafiltration process. Ions are present in very similar concentrations to plasma and the glucose concentration is about 60% of that in plasma. The problems related to intracranial pressure are discussed on pages 48–49.
Abnormalities of CSF constituents
Routine tests
Microscopy
Microscopy is performed on a fresh, spun CSF specimen. Excess leucocytes may be present in infection, malignancy and inflammatory conditions, for example sarcoidosis. A high neutrophil count (often >800/ml) is usually due to acute bacterial meningitis. Excess mononuclear cells are seen in chronic meningitis (e.g. tuberculous or fungal), viral meningoencephalitis, partially treated bacterial meningitis, chronic inflammation and malignant meningeal infiltration. Special stains may be used to identify microorganisms: Gram stain, Ziehl–Neelsen for acid-fast bacilli and fungal stains. Abnormal cells may be seen in malignant meningeal infiltration, but the sensitivity of cytology in proven malignant meningitis is only about 50% and repeated lumbar puncture may be required. Red cells are seen in subarachnoid haemorrhage but may represent a traumatic CSF tap. Xanthochromia confirms subarachnoid haemorrhage.
Glucose
Glucose levels in the CSF are compared with those in blood measured at the same time. CSF glucose is normally 60% of blood glucose levels. It is profoundly reduced (often <1 mmol/l) in acute bacterial meningitis and tuberculous meningitis. Milder reductions are seen in viral infections, malignant meningitis and inflammatory conditions.
Total protein
Total protein is elevated in many situations, including most causing CSF cellularity. A rise without an increased cell count is seen in inflammatory neuropathies, especially Guillain–Barré syndrome (‘albuminocytologic dissociation’) and in blockage to CSF flow of any cause. Elevated protein is a non-specific manifestation of diabetes mellitus.
Specialized tests
Oligoclonal bands
Oligoclonal bands are visible as an increased concentration of restricted bands of IgG after isoelectric focusing and immunofixation of IgG. A serum sample is taken at the time of the lumbar puncture as oligoclonal bands may be present in CSF alone or CSF and blood. If present in both, they may represent systemic infection, autoimmune disease, sarcoidosis or neoplasia. Local CNS synthesis implies local CNS disease, especially multiple sclerosis but sometimes other CNS inflammation, infection or neoplasia. The sensitivity in clinically definite multiple sclerosis is above 95%.
Polymerase chain reaction (PCR)
DNA amplification is available for increasing numbers of infections, including herpes simplex encephalitis and Mycobacterium tuberculosis meningitis. The sensitivity and specificity of PCR is improving and it may become the diagnostic test of choice for these otherwise difficult to diagnose infections.
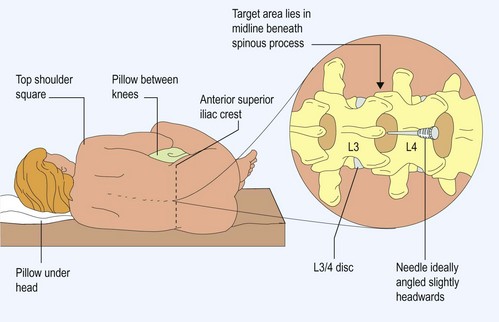
How to do a lumbar puncture
The potential indications for and contraindications of lumbar puncture (LP) are summarized in Box 1 and Table 1. Following appropriate guidelines, LP under these circumstances is a safe and invaluable procedure. If an LP is performed when contraindicated, in the presence of obstructive hydrocephalus, permanent disability or death may result from herniation of the intracranial contents.
Box 1 The contraindications of lumbar puncture
* In some situations, LP may be deemed safe after neuroimaging has excluded obstruction to CSF flow
Table 1 Examples of abnormal cerebrospinal fluid (CSF) results
| Indication | CSF picture | Significance |
|---|---|---|
| Acute headache | Increased red cells, xanthochromia and raised protein | Recent subarachnoid haemorrhage Spectrophotometry for xanthochromia |
| Acute meningitis | Neutrophil leucocytosis, elevated protein, very low CSF glucose | Acute bacterial infection, early tuberculous meningitis |
| Chronic meningitis | Mononuclear leucocytosis, elevated protein, low sugar | Tuberculosis, fungal or neoplastic; meningitis |
| Acute or chronic meningoencephalitis | Mononuclear leucocytosis, elevated protein, normal sugar | Viral meningitis or meningoencephalitis, neoplastic meningitis, inflammatory conditions, e.g. sarcoidosis |
| Demyelinating neuropathy: acute or chronic | Elevated protein, normal cell count | Supports inflammatory cause, e.g. Guillain–Barré syndrome |
| Inflammatory CNS disease | Isolated oligoclonal bands in CSF, not blood | Localized CNS inflammatory response; supports multiple sclerosis, sometimes tumours or infection |
| Inflammatory CNS and systemic disease | Oligoclonal bands in blood and CSF | CNS inflammation as part of systemic disease, e.g. viral infection or sarcoidosis |
Technique
Problems with lumbar puncture
Failed tap
Failure to obtain CSF is usually due to technical difficulties. This is more common with degenerative spine disease (narrow disc spaces), obesity (difficulty in identifying landmarks), kyphoscoliosis, ankylosing spondylitis (bamboo spine calcified throughout) and rarely infiltrative intraspinal lesion causing a ‘dry tap’.
Bloody tap
The differential diagnosis lies between a subarachnoid haemorrhage and a traumatic tap. After subarachnoid haemorrhage, the CSF is uniformly blood stained in all bottles and haemoglobin breakdown products (xanthochromia) appear 12 h after onset. Spectroscopy may help define xanthochromia.
With a traumatic tap, a cannulation of venous plexus, the cell count falls in successive bottles as the proportion of CSF increases and there is no xanthochromia.
Complications
The most common complication of LP is headache. This is due to continued leakage of CSF after the procedure causing a decrease in CSF pressure. When present, it is made worse by standing or sitting, which reduce intracranial pressure. Remaining supine after the procedure does not prevent headache. The headache usually resolves spontaneously. In refractory cases, 10 ml of the patient’s own venous blood can be introduced into the lumbar epidural space to seal the hole: ‘autologous blood patch’. The risk of headache is reduced by using smaller needles (22G rather than 18 or 20G) and by using a bullet- or pencil-pointed needle rather than a traditional bevelled needle. Thirty per cent of patients develop a headache if a bevelled 20G needle is used, compared with 7% when a 22G bullet or pencil point is used. Serious complications are very rare. Persistent dural leak is treated as above, but very rarely may result in intracranial subdural haematoma. Introduction of infection, causing meningitis, can occur.