CHAPTER 357 Cerebral Venous and Sinus Thrombosis
History and Clinical Significance
Cerebral venous thrombosis (CVT) is a pathologic condition encompassing thrombosis of the cortical and deep cerebral veins and the dural sinuses. It was first described by Ribes in the early 19th century in a 45-year-old man harboring a systemic malignancy who had thrombosis of the superior sagittal sinus demonstrated at autopsy.1 CVT is thought to be a rare clinical problem, but the exact incidence is unknown. Because of the rarity of the disease and its nonspecific clinical findings, the diagnosis of CVT is difficult to make and frequently delayed.
Pathogenesis
When thrombosis occurs in the cerebral veins and dural sinuses, the resulting venous hypertension causes hypoxia of the brain with subsequent neuronal ischemia, similar to the symptomatology of dural arteriovenous fistulas.2 The spectrum ranges from varying degrees of cerebral edema to massive hemorrhage and often bilateral cerebral infarcts. The potential causes of and risk factors associated with CVT are numerous. The underlying mechanisms are related to alterations in the physical properties of the dural sinuses and veins, the chemical properties of blood, or the hemodynamic properties of blood flow.
Vascular injury from trauma causes local endothelial damage and altered hemodynamics. Trauma, neoplasms, and other mass lesions can cause vascular compression and altered hemodynamics as well. The association between dural arteriovenous fistulas and CVT has been recognized3 and is thought to be related to the aberrant rheology induced by the fistulous connection.
Protein C is a vitamin K–dependent, thrombin-activating regulatory protein. Activated protein C has been reported to have potent anticoagulation properties.4 Acquired protein C resistance can also cause a hypercoagulable state,5 and it has been reported as a cause of CVT.6–8 Furthermore, factor V gene mutation (factor V Leiden)7,9 and prothrombin (factor II) gene mutation10 have been determined to be risk factors for CVT. A hypercoagulable state may be present in patients with lupus anticoagulants,11 or circulating autoantibodies directed primarily against phospholipids, and in patients with anticardiolipin antibodies,12 both of which may represent contributing factors in the pathogenesis of CVT.
A number of coagulation system abnormalities that are rarely associated with CVT have been reported in various conditions, including Behçet’s disease,13–15 inflammatory bowel diseases (e.g., ulcerative colitis),16–18 Wegener’s granulomatosis,19 and Cogan’s syndrome,20 among others.
Infection is thought to cause CVT by altering the coagulation cascade and inducing a hypercoagulable state in patients with active infection. Infection used to be a major cause of CVT but, with the advent of modern antibiotic therapy, has become less common. Ameri and Bousser reported that 8.2% of CVT cases are due to infection.21 Cavernous and transverse sinus thrombosis is most frequently associated with infections such as sinusitis, otitis, and mastoiditis. Staphylococcus aureus is the most frequently reported pathogen. In chronic forms, gram-negative rods and fungi such as Aspergillus species are most commonly isolated.22,23
CVT is more common in young women than in other groups,13,22,24–27 and CVT developing during the puerperium is still common in developing countries.13,28 Oral contraceptives may also play a role in the pathogenesis of CVT in young women.7,13,28 Ameri and Bousser reported that 8% of their cases of CVT were thought to be caused by the use of oral contraceptives.21 Martinelli and colleagues reported an association between oral contraceptive use and the development of CVT.7 The risk is much higher in women using oral contraceptives who also have the prothrombin gene mutation. CVT was reported to develop in a postmenopausal woman taking estrogen-progesterone therapy.29
Despite a long list of possible causes of CVT, the inciting factors remain undetermined in some cases. Some investigators estimate that as many as 40% of cases are idiopathic.30,31
Incidence
Although the exact incidence of CVT is unknown, it is agreed that it is a rare disease.21 Most of the estimates are derived from autopsy studies, but autopsy cases may introduce bias because the most severe cases with the worst outcome are overrepresented. Ehler and Courville found 16 cases of superior sagittal sinus thrombosis in a series of 12,500 autopsies.32 Over a 20-year period, Barnett and Hyland found only 39 cases of noninfective CVT that were proved by autopsy.33 Later autopsy studies have reported the incidence to be as low as 0.03%34 and as high as 9% in 182 adult cases.35 Scotti and coworkers observed CVT in approximately 4% of cerebral angiograms performed in 240 children.36 In our experience, clinically significant CVT occurs in patients 2 to 51 years of age.
CVT affects all age groups and both sexes, but with a strong preponderance in women between 20 and 40 years of age.37 This may reflect the fact that women in that age group are more likely to use oral contraceptives38 and undergo changes associated with the puerperium.
Clinical Findings
The clinical manifestations of CVT are highly variable.21,26,27,39–41 Headache is the most common and often the earliest symptom21 and is seen in nearly 80% of patients.13 Nausea, vomiting, and visual changes are other symptoms experienced by patients. Increased intracranial pressure is thought to be the underlying cause of these symptoms. Papilledema is seen in about half of those afflicted, and confusion, agitation, and other changes in mental status occur in about 25% of patients.21 As the disease progresses, focal neurological deficits are present in 50% to 75%, often caused by venous hypertension and cerebral infarction or hemorrhage.13 Aphasia, hemianopia, or hemisensory deficits may also occur. Seizure is another common symptom, with simple or generalized seizures complicating nearly 33% of cases.42
The clinical features also vary according to the site and extent of thrombosis. When thrombosis is limited to the superior sagittal sinus or transverse sinus, the most frequent pattern of findings is isolated intracranial hypertension. If the thrombosis extends to the cortical veins, focal deficits and seizures may occur. Bilateral deficits are typical as late signs of superior sagittal sinus thrombosis. Transverse sinus CVT may be associated with otalgia, otorrhea, cervical tenderness, and lymphadenopathy from an underlying infection such as mastoiditis or otitis media. In cavernous sinus CVT, symptoms often include eyelid edema, chemosis, retro-orbital pain, and exophthalmos. Paralysis of cranial nerves III, IV, V1, V2, and VI may occur because of their anatomic location within the cavernous sinus. When the thrombosis involves the deep venous system, the patient can have akinetic mutism, coma, or decerebration. Mild cases causing memory disturbances or minor confusion may occur. Cortical vein thrombosis without sinus involvement can be manifested as stroke syndromes.43 Cerebellar vein thrombosis is extremely rare and often lethal.24
Diagnostic Evaluation
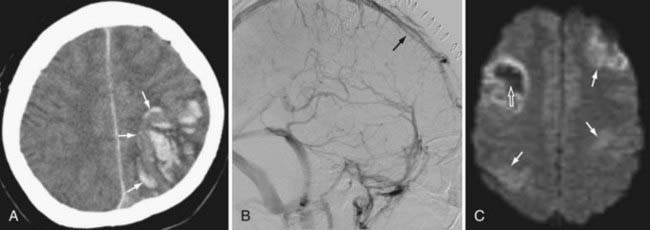
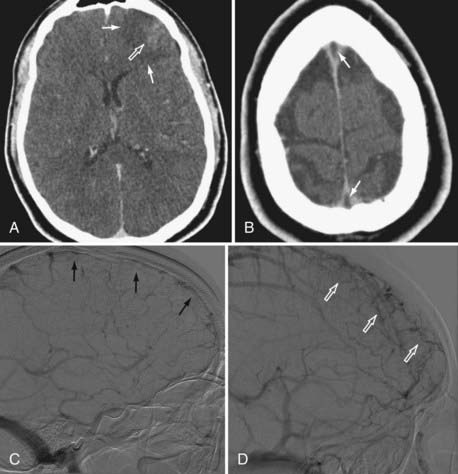
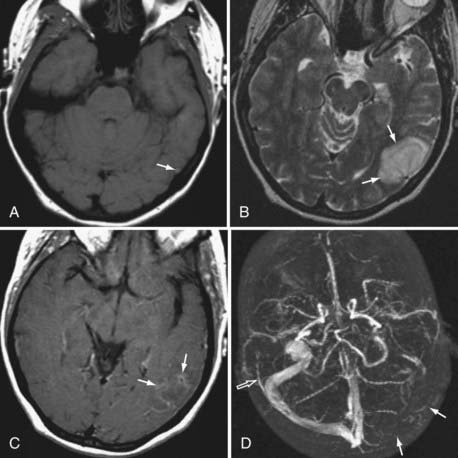
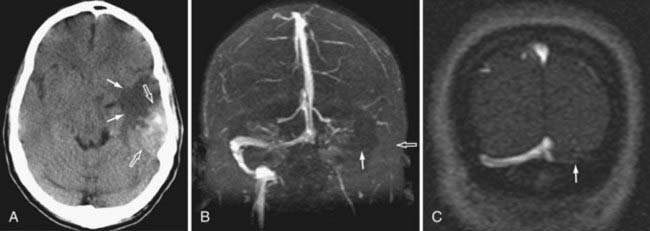
Because the clinical features are highly variable and the symptoms are usually nonspecific, the key to making the diagnosis of CVT is to have a high level of suspicion. Historically, CVT was first documented radiographically after direct injection of the superior sagittal sinus.21,24 Diagnostic modalities currently used for the confirmation of CVT include computed tomography (CT), magnetic resonance imaging (MRI), and cerebral angiography (Figs. 357-1 to 357-4).
CT is usually the initial diagnostic test performed on patients suspected of having CVT or those exhibiting acute deterioration in mental status.21,24 The dense vein and cord sign may be seen in approximately 20% of patients on standard non–contrast-enhanced head CT.30,44,45 The cord sign and the dense triangle sign correspond to spontaneously hyperdense cortical veins or sinuses, respectively. They can be seen during the first 1 to 2 weeks after thrombosis and may be falsely positive in neonates, dehydrated individuals, and those with elevated hemoglobin. Sometimes a dense triangle, also known as the delta sign, may be seen as the thrombus occupies the superior sagittal sinus. On contrast-enhanced CT, the empty delta sign consists of peripheral dural leaf enhancement along with a central nonopacified thrombus; it occurs in 10% to 30% of patients with superior sagittal sinus thrombosis.46–49 Unfortunately, the false-negative rate for the CT diagnosis of CVT is high, estimated at between 4% and 25%. CT can also show hemorrhagic infarctions as a result of poor drainage secondary to CVT. Such infarctions are usually bilateral (see Figs. 357-1 to 357-4).
MRI has recently become the modality of choice for detecting CVT.41,50–54 With magnetic resonance angiography (MRA) and magnetic resonance venography (MRV), the arterial and venous phases can be examined concomitantly or separately. MRA/MRV is now the best method for detecting CVT. MRI offers certain advantages: the thrombus can be directly visualized, and cortical lesions such as edema and hemorrhagic infarction can be detected. These MRI changes are best seen with thrombosis of the superior sagittal sinus, transverse sinus, straight sinus, and vein of Galen; they are far less obvious with thrombosis of the cavernous sinus and cortical vein, which sometimes remain difficult to diagnose. Although MRA/MRV is the most sensitive modality for detecting CVT, it may give inaccurate results in some circumstances. False-positive identification may occur when the sinuses are congenitally absent or hypoplastic. False-negative identification may occur when the signal of methemoglobin mimics that of flowing blood, when loss of signal occurs secondary to magnetic susceptibility artifact, or when the patient cannot cooperate and the study is technically poor (see Figs. 357-1 to 357-4).
Although traditionally considered to be the standard in diagnosing CVT,55 cerebral angiography is now indicated only in cases in which the MRI diagnosis is uncertain or when intervention is desired. The typical finding is nonvisualization of all or part of a sinus during the venous phase of an angiogram. In cortical vein thrombosis, there is a sudden nonvisualization of the occluded vein, which may be surrounded by dilated collateral “corkscrew vessels.“21
The value of conventional transcranial Doppler ultrasonography was examined by Canhao and colleagues.56 It was suggested that using transcranial ultrasound to detect the increase in velocity in the deep middle cerebral vein and basal vein of Rosenthal aided the diagnosis of superior sagittal sinus thrombosis. Others have stated that ultrasonography is of little benefit.57 Given the prevalence and ease of MRI in this era, ultrasonography is of limited value.
Treatment
Therapy for patients with CVT should be directed at treating the underlying causative process, symptoms secondary to elevated intracranial pressure, and seizures or focal deficits caused by cerebral edema and infarction. If an underlying cause is found, it should be treated. If an infectious process is identified, broad-spectrum antibiotics or drainage of purulent collections should be part of the treatment.21,24 Patients with CVT should initially be stabilized with appropriate airway and circulatory management. Subsequent supportive measures should be instituted. Increased intracranial pressure can be treated with osmotic agents (mannitol, hypertonic saline), temporary short-term hyperventilation, drainage of cerebrospinal fluid by ventriculostomy or lumbar puncture, barbiturate coma, or surgical craniectomy.21,24,58 Cerebral perfusion pressure should be kept at an adequate level to prevent secondary ischemic insults. Seizure is a frequent symptom and should be treated with anticonvulsants.
Antithrombotics
The natural course of CVT is variable, and the guidelines directing treatment are controversial. The use of heparin for the treatment of CVT was initially reported by Stansfield in 1942.59 Since then, several other authors have reported beneficial effects of heparin in patients with CVT,13,14,60 but there has been only one prospective, randomized, controlled study using heparin. Einhaupl and colleagues published the results of their series of 20 patients in 1991.61 The efficacy of systemic heparin was compared with that of placebo in a double-blinded, randomized study. The group of patients receiving heparin had a better outcome at 3 days, 8 days, and 3 months. Although the power of this study was reduced because the investigators did not meet all the criteria for a double-blinded, randomized study, the advantage of heparin in treating CVT was strongly suggested.
Another randomized, placebo-controlled trial looking at systemic anticoagulation was performed by de Bruijn and Stam.62 Sixty patients were randomized to receive either subcutaneous heparin or placebo for 3 weeks, followed by 3 months of oral anticoagulation. No patients were lost to follow-up. Although their study failed to show statistical significance, there was again a trend toward better clinical outcome in patients treated early with systemic anticoagulation.
Despite the risk for intracerebral hemorrhage, the benefit of using systemic heparin was examined in a large retrospective study by Ameri and Bousser.21 Their 82 patients were treated with heparin for CVT, and no mortality or worsening of clinical status was found. This has also been corroborated by other studies.62
Levine and associates found that patients treated with anticoagulation and antibiotics for cavernous sinus thrombosis in the early stage (<7 days after initial onset) had significantly less morbidity than did patients treated with antibiotics alone.63 However, if anticoagulant treatment began at a later stage of the disease, no beneficial effect was found.
The current trend is to use heparin.21,27,64 The goal of heparin anticoagulation is to maintain the activated partial thromboplastin time at 2 to 2.5 times normal. Once the patient’s condition has stabilized, warfarin therapy is initiated. There are few scientific data or guidelines on the dosage and duration of heparin and warfarin therapy in the literature on CVT. If no underlying cause of the CVT is found, the therapeutic international normalized ratio is kept between 2 and 3 for 6 months; lifelong therapy is warranted in patients with hypercoagulable disease states.
Systemic Thrombolytics
Thrombolytic agents such as streptokinase, urokinase, and tissue plasminogen activator (t-PA) have been used systemically in small experimental series.65 Unfortunately, only anecdotal reports are available on clinical management with thrombolytic agents.66–70
The major risk associated with the systemic administration of thrombolytics is gastrointestinal or intracranial hemorrhage. Contraindications to thrombolytic therapy include recent childbirth, history of a bleeding diathesis, recent major surgery, recent major trauma, active gastrointestinal bleeding, or inflammatory bowel disease. It has been suggested that local infusion of thrombolytic agents via interventional neuroradiologic techniques can minimize the side effects seen with systemic thrombolytic therapy. There are no strong data to support the use of systemic thrombolytics in patients with this disease, although an isolated case report detailed the successful use of intravenous recombinant tissue plasminogen activator (rt-PA) and subsequent heparin infusion in treating acute middle cerebral artery occlusion in a patient with angiographically diagnosed CVT. Follow-up imaging demonstrated significant sinus recanalization, and the patient was left with just minor neurological deficits.71
Interventional Neuroradiology
Recent advances in endovascular techniques have not only enabled the local delivery of thrombolytic agents to selective venous channels where the thrombus is located but have also allowed direct mechanical manipulation and removal of the clot. Local infusion of thrombolytics offers advantages, including minimization of systemic effects and local clot lysis with high concentrations of thrombolytic agents (Tables 357-1 and 357-2). Routes of access include the transfemoral and transjugular routes, as well as direct puncture of the dural sinuses.
TABLE 357-1 Endovascular Urokinase Treatment of Cerebral Venous Thrombosis Reported in the Literature
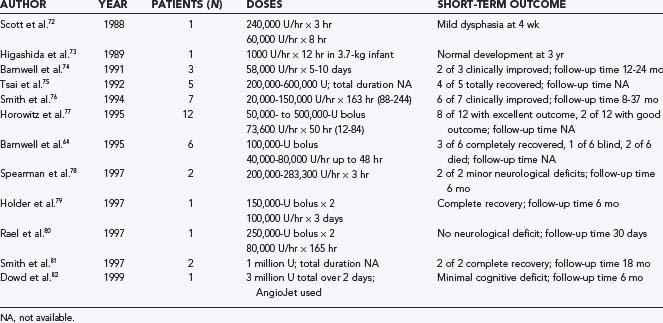
TABLE 357-2 Endovascular Treatment of Cerebral Venous Thrombosis with Tissue Plasminogen Activator Reported in the Literature

Historically, Scott and colleagues reported the first use of local fibrinolytic therapy.72 A young patient with CVT and a rapidly deteriorating clinical course was treated by midline craniotomy and direct catheterization of the superior sagittal sinus. Urokinase was then infused at 4000 U/min for 3 hours and then 1000 U/min for 8 hours. The patient progressed from being unresponsive initially to having just mild dysphasia at 4 weeks. Higashida and associates proposed a more aggressive modality of therapy for CVT patients with clinical deterioration.73 These investigators successfully performed direct puncture of the superior sagittal sinus in a neonate and infused urokinase, which resulted in clot lysis over a 12-hour period. This was the first reported attempt at locally infused thrombolytic therapy for CVT without a craniotomy.
Barnwell and coworkers reported direct transjugular endovascular thrombolytic therapy in three patients.74 All three patients had dural arteriovenous fistulas, and all three were treated by direct transjugular infusion of urokinase. The period of continuous infusion for thrombolysis ranged from 5 to 10 days. In two patients, the clinical signs and symptoms improved, with angiographic evidence of clot lysis and dural sinus recanalization. Angiography indicated that one patient had partial resolution of a clot in the torcular Herophili and transverse sinus, but that patient showed no clinical improvement. These preliminary results were encouraging and suggested that transjugular local infusion of thrombolytic agents can be an effective and safe treatment of symptomatic, thrombosed dural sinuses.
Tsai and colleagues reported five cases of successful direct transfemoral thrombolytic treatment.75 Smith and associates reported on seven patients with symptomatic dural sinus thrombosis who had failed a trial of medical treatment and were treated by direct infusion of urokinase into the thrombosed sinus.76 The patients received urokinase dosages ranging from 20,000 to 150,000 U/hr, with a mean infusion time of 163 hours (range, 88 to 244 hours). Patency of the affected dural sinus was achieved along with antegrade flow in all patients. Six patients either improved neurologically over their prethrombolysis state or were healthy after thrombolysis. The only complications were an infected femoral access site and transient hematuria.
Horowitz and coworkers added 12 more cases to the literature.77 They reported that despite the presence of preinfusion infarcts in 5 patients, 4 of which were hemorrhagic, they incurred no major therapeutic morbidity. Functional sinus patency was achieved in 11 of 12 patients, with the only true failure occurring in an individual with symptoms of at least 2 months’ duration. Good to excellent clinical outcomes were achieved in 10 of the 12 patients.
Barnwell and colleagues reported an additional six cases of local thrombolytic therapy.64 Three patients did well and made complete recoveries. Two of these patients had extensive thrombosis and severe clinical problems but made full functional recoveries. One patient, with extensive dural sinus thrombosis that had been observed but not treated for 3 weeks, showed minimal improvement on the post-therapy angiogram and became blind from optic neuropathy, probably secondary to optic nerve damage from increased intracranial pressure. Two patients with extensive dural sinus thrombosis, bilateral parietal lobe infarcts, and coma did not improve and died, both from intractable intracranial hypertension and stroke.
Spearman and associates reported two patients with CVT who were deteriorating rapidly.78 Endovascular thrombolysis was performed, and both patients survived with minimal deficits. Holder’s and Rael’s groups each contributed one additional case to the literature.79,80 Smith and colleagues reported two additional cases.81
Currently, urokinase is not available in the United States; t-PA remains the only thrombolytic agent readily available. rt-PA offers several theoretical advantages over urokinase: a shorter half-life (5 versus 15 minutes) and better clot lysis in in vitro clot assays.87 Kim and Suh presented one of the earliest series of patients receiving local rt-PA for CVT.83 After bringing the delivery catheter proximal to the site of thrombosis, a bolus dose of 10 mg of rt-PA was given over a 10-minute period, followed by 50 mg over a 3-hour period. A continuous infusion was then started at 5 mg/hr until complete thrombolysis or 100 mg had been reached (mean total dose, 135 mg). Concomitant heparin infusion was performed with bridging to warfarin. None of their 9 patients suffered intracranial bleeding: 1 had oozing at the groin site, and a small retroperitoneal hematoma developed in another. They achieved angiographic recanalization of the dural sinuses and resolution of symptoms in all patients. Frey and colleagues presented a series of 12 patients in whom they infused rt-PA with concomitant systemic heparinization.85 The presence of an intracranial hemorrhage did not preclude a patient from receiving local rt-PA. They infused 1-mg boluses every 1 to 2 cm along the length of the thrombosis, followed by 1 to 2 mg/hr. Although they achieved a good outcome in 10 of their 12 patients, 2 experienced worsening of their preexisting intracranial hemorrhage. The average dose to achieve complete restoration of flow (6 patients) was 49 mg (over a 31-hour period). Yamini and coauthors reported a patient in whom they used more aggressive dosing of rt-PA, 25 mg infused in 2-mg boluses every centimeter along the thrombosed segment.86 After this did not induce lysis, the catheter was readvanced and rt-PA infused at 1 mg/min (60 mg/hr) for 19 hours. This produced complete sinus recanalization and a great clinical outcome. Renowden and colleagues reported a similar patient in whom they initially infused 30 mg of rt-PA progressively along the thrombosed segment followed by another pass of 35 mg when the first infusion did not produce adequate angiographic results.84 They, too, did not have any hemorrhagic complications.
Soleau and associates published a series of CVT patients who underwent a wide spectrum of treatments from medical observation to endovascular thrombectomy.87 Ten of their patients were treated by chemical thrombolysis in the form of urokinase or t-PA. Both patients treated with t-PA experienced significant hemorrhagic complications, although it is not clear from the article what dosing of rt-PA was used.
Most recently, Tsai and colleagues reported a series of 25 patients undergoing the gamut of therapy for CVT, including systemic anticoagulation only, systemic anticoagulation with endovascular thrombolytic therapy, and endovascular thrombolytic therapy only.88 The dose of t-PA that they used was just 3 to 10 mg. An inflated soft balloon was placed distally to contain the t-PA at the site of thrombosis to increase efficacy. They had no adverse outcomes in the 13 patients who received rt-PA. Nine of the 10 patients receiving systemic anticoagulation had symptom-free recoveries; the 10th died of a severe intracranial hemorrhage. Seven patients underwent balloon thrombectomy after failing anticoagulation: 3 with symptom-free recovery, 2 with minor weakness, 1 with a gait disturbance, and 1 in whom a dural fistula developed. Eight patients went straight to endovascular thrombolytic therapy; 5 of them experienced symptom-free recovery and 3 had some mild weakness. They concluded that timely treatment with endovascular thrombolytic therapy is indicated in patients with evidence of cerebral venous congestion.
Mechanical endovascular thrombectomy can be performed by direct puncture of the jugular vein and use of a wire to access the thrombus. Soleau and associates presented a small series of eight patients in whom a Fogarty catheter was used to pull the thrombus down into the jugular access point in the presence of systemic heparinization.87 Seven of their patients experienced clinical improvement and one died.
Dowd and associates presented a novel application of a transvascular rheolytic thrombectomy system for the treatment of symptomatic CVT.82 An AngioJet catheter (Possis Medical, Minneapolis, MN) was directed over a micro-guidewire to the thrombus. The AngioJet catheter was activated, and partial sinus thrombolysis was achieved via the hydrodynamic thrombolytic action of the catheter as it was slowly withdrawn to the jugular bulb. Concomitant infusion of urokinase was performed as well, and the patient had only a minimal neurological deficit at 6 months’ follow-up. Chow and coworkers and Agner and coauthors reported similar techniques in which a rheolytic catheter was navigated to the thrombus and thrombectomy performed.89,90 They also combined accompanying thrombolytic therapy with good results. The thrombi are usually approached via a transfemoral approach. Should tortuous anatomy or lack of support preclude a transfemoral approach, puncture of the jugular can be performed. Generally, the stiffness of these rheolytic catheters confines their use to the dural sinuses.
Local infusion of thrombolytics can be achieved by transfemoral venous catheterization and cannulation of the cerebral venous system. This is usually done after a diagnostic cerebral angiogram demonstrates venous thrombosis. A small catheter is navigated to the venous channel where the clot resides, and a thrombolytic agent is infused at a predetermined rate. An exact regimen for local thrombolytic therapy has not been definitively determined. Current trends at major centers with neurointerventional capability advocate the administration of urokinase initially by bolus (100,000 IU or less if the patient has a hemorrhagic infarct present on CT), followed by a constant infusion of 40,000 to 80,000 IU/hr for up to 72 hours. Response to thrombolytic therapy is assessed by serial venograms obtained approximately every 6 hours and ultimately by arteriography when therapy is stopped. Although three groin hematomas and one retroperitoneal hematoma were reported by Horowitz and coauthors in their series of 12 patients,77 no intracranial hemorrhage as a direct result of endovascular urokinase treatment has been reported in the previously cited series.
Despite the lack of conclusive data regarding the efficacy of local thrombolytic agents in the treatment of CVT, it appears that endovascular local thrombolysis and mechanical thrombectomy are generally safe and effective in opening venous occlusions and that patients treated successfully have a better clinical outcome than can be achieved with the use of other available agents. Currently, only rt-PA is available in the United States; urokinase has been withdrawn from the market. There are no defined guidelines for the use of this drug in the treatment of CVT, and it should be used with caution. Although some groups have had success with rt-PA,83,85,86 it may have a higher hemorrhagic complication rate than seen with the use of urokinase.87
Surgery
Surgical treatment may be indicated in patients with CVT complicated by malignant intracranial hypertension, acute visual loss, or intracranial hemorrhage. Ventriculostomy can serve as a method for diversion of cerebrospinal fluid and monitoring of intracranial pressure. In cases in which medical therapy is inadequate and endovascular thrombectomy is not technically possible, there have been reports in the literature of direct puncture of the dural sinuses after craniotomy and performance of thrombectomy at that time. Chahlavi and colleagues reported two cases in which the AngioJet was used via direct transcranial puncture with great success.91
Outcome
Like the clinical manifestations of CVT, the outcome of patients with CVT is highly variable. Earlier series reported mortality rates ranging from 30% to 80%.46 More recent series have reported mortality rates of less than 20%.13,92,93 Extreme cases exist in which patients either die acutely or recover rapidly. However, the mortality associated with CVT (an entity that was once thought to be almost invariably lethal) has been drastically decreased by thrombolytic therapy and antithrombotic treatment. Factors that suggest a bad prognosis are the presence of coma, extremes of age (high mortality in infants and the elderly),49 site of thrombosis in the deep venous system or cerebellar system,94 severely increased intracranial pressure, and underlying sepsis or malignancy.13
Preter and colleagues conducted a retrospective follow-up of 77 patients with CVT evaluated from 1975 through 1990.93 The age of the patients ranged from 18 to 77 years (mean, 38.5 years). Sixty-six patients (85.7%) had no neurological sequelae during follow-up. Eleven patients (14.3%) remained neurologically impaired. Two who were initially seen with isolated intracranial hypertension had blindness secondary to optic atrophy. The other 9 had focal signs at the time of CVT and were left with various cognitive or focal deficits. Four of the 28 patients (14.3%) who had seizures during the acute state had recurrent seizures. A dural arteriovenous fistula developed in 1 of the 51 patients with transverse sinus thrombosis. Nine of the 77 patients (11.7%) suffered a second CVT, all but one in the first year. Noncerebral thrombotic events occurred in 11 patients (14.3%). No recurrence of CVT was noted during later pregnancies, but 1 patient had postpartum deep venous thrombosis. The authors concluded that CVT has an essentially good long-term prognosis. The incidence of long-standing epilepsy was low, which suggests that long-term anticonvulsant treatment is not necessary in most cases. A second CVT or another thrombotic episode occurred in 20% of patients, thus stressing the need for long-term anticoagulation in a minority of patients.
Ferro and coauthors recently published the largest prospective series of CVT patients in the literature; they collected data on clinical findings and outcomes for a total of 624 patients from 1998 to 2001.92 Mortality and prognosis appeared to be better than previously thought: just 8% of patients died as a direct result of the CVT or an underlying condition. All patients included in the study received systemic anticoagulation after diagnosis, typically with heparin. A minority of patients (2.1%) received local thrombolytic therapy. Follow-up information was available for 98.7% of the patients, who had mean follow-up time of 18.6 months. At this time point, 79% of patients had a modified Rankin scale of 1 or less, which is essentially a complete recovery. They identified various factors associated with a worse outcome: male sex, age older than 37 years, coma, mental status disorder, intracranial hemorrhage on admission, thrombosis involving the deep venous system, central nervous system infection, and malignancy. Seizures (10%) and new thrombotic events (4%) were the most frequent complications identified in this study.
Agner C, Deshaies EM, Bernardini GL, et al. Coronary AngioJet catheterization for the management of dural venous sinus thrombosis. Technical note. J Neurosurg. 2005;103:368.
Alexander LF, Yamamoto Y, Ayoubi S, et al. Efficacy of tissue plasminogen activator in the lysis of thrombosis of the cerebral venous sinus. Neurosurgery. 1990;26:559.
Barnwell SL, Higashida RT, Halbach VV, et al. Direct endovascular thrombolytic therapy for dural sinus thrombosis. Neurosurgery. 1991;28:135.
Barnwell SL, Nesbit GM, Clark WM. Local thrombolytic therapy for cerebrovascular disease: current Oregon Health Sciences University experience (July 1991 through April 1995). J Vasc Interv Radiol. 1995;6:78S.
Chow K, Gobin YP, Saver J, et al. Endovascular treatment of dural sinus thrombosis with rheolytic thrombectomy and intra-arterial thrombolysis. Stroke. 2000;31:1420.
de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30:484.
Duvelleroy Hommet C, De Toffol B, Corcia P, et al. Cerebral venous thrombosis and estrogen-progesterone therapy. Eur Neurol. 1998;39:245.
Ferro JM, Canhao P, Stam J, et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664.
Fisher WI. Intracranial Venous Occlusive Diseases. Baltimore: Williams & Wilkins; 1996. 1907-1919
Frey JL, Muro GJ, McDougall CG, et al. Cerebral venous thrombosis: combined intrathrombus rtPA and intravenous heparin. Stroke. 1999;30:489.
Higashida RT, Helmer E, Halbach VV, et al. Direct thrombolytic therapy for superior sagittal sinus thrombosis. AJNR Am J Neuroradiol. 1989;10:S4.
Kim SY, Suh JH. Direct endovascular thrombolytic therapy for dural sinus thrombosis: infusion of alteplase. AJNR Am J Neuroradiol. 1997;18:639.
Levine SR, Twyman RE, Gilman S. The role of anticoagulation in cavernous sinus thrombosis. Neurology. 1988;38:517.
Rael JR, Orrison WWJr, Baldwin N, et al. Direct thrombolysis of superior sagittal sinus thrombosis with coexisting intracranial hemorrhage. AJNR Am J Neuroradiol. 1997;18:1238.
Rippe DJ, Boyko OB, Spritzer CE, et al. Demonstration of dural sinus occlusion by the use of MR angiography. AJNR Am J Neuroradiol. 1990;11:199.
Smith AG, Cornblath WT, Deveikis JP. Local thrombolytic therapy in deep cerebral venous thrombosis. Neurology. 1997;48:1613.
Smith TP, Higashida RT, Barnwell SL, et al. Treatment of dural sinus thrombosis by urokinase infusion. AJNR Am J Neuroradiol. 1994;15:801.
Soleau SW, Schmidt R, Stevens S, et al. Extensive experience with dural sinus thrombosis. Neurosurgery. 2003;52:534.
Spearman MP, Jungreis CA, Wehner JJ, et al. Endovascular thrombolysis in deep cerebral venous thrombosis. AJNR Am J Neuroradiol. 1997;18:502.
Tsai FY, Higashida RT, Matovich V, et al. Acute thrombosis of the intracranial dural sinus: direct thrombolytic treatment. AJNR Am J Neuroradiol. 1992;13:1137.
Tsai FY, Kostanian V, Rivera M, et al. Cerebral venous congestion as indication for thrombolytic treatment. Cardiovasc Intervent Radiol. 2007;30:675.
Tsai FY, Wang AM, Matovich VB, et al. MR staging of acute dural sinus thrombosis: correlation with venous pressure measurements and implications for treatment and prognosis. AJNR Am J Neuroradiol. 1995;16:1021.
Yuh WT, Simonson TM, Wang AM, et al. Venous sinus occlusive disease: MR findings. AJNR Am J Neuroradiol. 1994;15:309.
1 Ribes M. Des rescherches faites sur la phlébite. Revue Médicale Française et Etrangère et Journal de Clinique de lHôtel-Dieu et de la Charité de Paris. 3, 1825.
2 Sanders MD, Hoyt WF. Hypoxic ocular sequelae of carotid-cavernous fistulae. Study of the causes of visual failure before and after neurosurgical treatment in a series of 25 cases. Br J Ophthalmol. 1969;53:82.
3 Kuether TA, ONeill O, Nesbit GM, et al. Endovascular treatment of traumatic dural sinus thrombosis: case report. Neurosurgery. 1998;42:1163.
4 Seegers WH, Marlar RA, Walz DA. Anticoagulant effects of autoprothrombin II-A and prothrombin fragment 1. Thromb Res. 1978;13:233.
5 Pugliese D, Nicoletti G, Andreula C, et al. Combined protein C deficiency and protein C activated resistance as a cause of caval, peripheral, and cerebral venous thrombosis—a case report. Angiology. 1998;49:399.
6 Dulli DA, Luzzio CC, Williams EC, et al. Cerebral venous thrombosis and activated protein C resistance. Stroke. 1996;27:1731.
7 Martinelli I, Landi G, Merati G, et al. Factor V gene mutation is a risk factor for cerebral venous thrombosis. Thromb Haemost. 1996;75:393.
8 Vuillier F, Moulin T, Tatu L, et al. Isolated cortical vein thrombosis and activated protein C resistance. Stroke. 1996;27:1440.
9 Deschiens MA, Conard J, Horellou MH, et al. Coagulation studies, factor V Leiden, and anticardiolipin antibodies in 40 cases of cerebral venous thrombosis. Stroke. 1996;27:1724.
10 Biousse V, Conard J, Brouzes C, et al. Frequency of the 20210 G→A mutation in the 3′-untranslated region of the prothrombin gene in 35 cases of cerebral venous thrombosis. Stroke. 1998;29:1398.
11 Levine SR, Kieran S, Puzio K, et al. Cerebral venous thrombosis with lupus anticoagulants. Report of two cases. Stroke. 1987;18:801.
12 Carhuapoma JR, Mitsias P, Levine SR. Cerebral venous thrombosis and anticardiolipin antibodies. Stroke. 1997;28:2363.
13 Bousser MG, Chiras J, Bories J, et al. Cerebral venous thrombosis—a review of 38 cases. Stroke. 1985;16:199.
14 Karabudak R, Caner H, Oztekin N, et al. Thrombosis of intracranial venous sinuses: aetiology, clinical findings and prognosis of 56 patients. J Neurosurg Sci. 1990;34:117.
15 Wechsler B, Vidailhet M, Piette JC, et al. Cerebral venous thrombosis in Behçet’s disease: clinical study and long-term follow-up of 25 cases. Neurology. 1992;42:614.
16 Bridger S, Evans N, Parker A, et al. Multiple cerebral venous thromboses in a child with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1997;25:533.
17 Derdeyn CP, Powers WJ. Isolated cortical venous thrombosis and ulcerative colitis. AJNR Am J Neuroradiol. 1998;19:488.
18 Johns DR. Cerebrovascular complications of inflammatory bowel disease. Am J Gastroenterol. 1991;86:367.
19 Hammans SR, Ginsberg L. Superior sagittal sinus thrombosis in Wegener’s granulomatosis. J Neurol Neurosurg Psychiatry. 1989;52:287.
20 Gilbert WS, Talbot FJ. Cogan’s syndrome. Signs of periarteritis nodosa and cerebral venous sinus thrombosis. Arch Ophthalmol. 1969;82:633.
21 Ameri A, Bousser MG. Cerebral venous thrombosis. Neurol Clin. 1992;10:87.
22 DiNubile MJ. Septic thrombosis of the cavernous sinuses. Arch Neurol. 1988;45:567.
23 Sekhar LN, Dujovny M, Rao GR. Carotid-cavernous sinus thrombosis caused by Aspergillus fumigatus. Case report. J Neurosurg. 1980;52:120.
24 Bousser MG. Cerebral Venous Thrombosis. New York: Academic Press; 1997. 385-389
25 Fisher WI. Intracranial Venous Occlusive Diseases. Baltimore: Williams & Wilkins; 1996. 1907-1919
26 Toms S, Chyatte D. Cerebral Sinus and Venous Thrombosis. New York: Academic Press; 1997. 528-532
27 Wakhloo A, Johnson B, Kraus G, et al. Neurovascular Surgery. New York: McGraw-Hill; 1995. 1337-1363
28 Estanol B, Rodriguez A, Conte G, et al. Intracranial venous thrombosis in young women. Stroke. 1979;10:680.
29 Duvelleroy Hommet C, De Toffol B, Corcia P, et al. Cerebral venous thrombosis and estrogen-progesterone therapy. Eur Neurol. 1998;39:245.
30 Chiras J, Bousser MG, Meder JF, et al. CT in cerebral thrombophlebitis. Neuroradiology. 1985;27:145.
31 Diaz JM, Schiffman JS, Urban ES, et al. Superior sagittal sinus thrombosis and pulmonary embolism: a syndrome rediscovered. Acta Neurol Scand. 1992;86:390.
32 Ehler H, Courville C. Thrombosis of internal cerebral veins in infancy and childhood: review of literature and report of five cases. J Pediatr. 1936;8:600.
33 Barnett HJ, Hyland HH. Noninfective intracranial venous thrombosis. Brain. 1953;76:36.
34 Erez N, Babuna C, Uner A. Low incidence of thromboembolic disease. An evaluation of obstetric and gynecologic patients in Istanbul. Obstet Gynecol. 1966;27:833.
35 Towbin A. The syndrome of latent cerebral venous thrombosis: its frequency and relation to age and congestive heart failure. Stroke. 1973;4:419.
36 Scotti LN, Goldman RL, Hardman DR, et al. Venous thrombosis in infants and children. Radiology. 1974;112:393.
37 Cantu C, Barinagarrementeria F. Cerebral venous thrombosis associated with pregnancy and puerperium. Review of 67 cases. Stroke. 1993;24:1880.
38 Buchanan DS, Brazinsky JH. Dural sinus and cerebral venous thrombosis. Incidence in young women receiving oral contraceptives. Arch Neurol. 1970;22:440.
39 Crawford SC, Digre KB, Palmer CA, et al. Thrombosis of the deep venous drainage of the brain in adults. Analysis of seven cases with review of the literature. Arch Neurol. 1995;52:1101.
40 Daif A, Awada A, al-Rajeh S, et al. Cerebral venous thrombosis in adults. A study of 40 cases from Saudi Arabia. Stroke. 1995;26:1193.
41 Perkin GD. Cerebral venous thrombosis: developments in imaging and treatment. J Neurol Neurosurg Psychiatry. 1995;59:1.
42 Krayenbuhl HA. Cerebral venous and sinus thrombosis. Clin Neurosurg. 1966;14:1.
43 Jacobs K, Moulin T, Bogousslavsky J, et al. The stroke syndrome of cortical vein thrombosis. Neurology. 1996;47:376.
44 Ford K, Sarwar M. Computed tomography of dural sinus thrombosis. AJNR Am J Neuroradiol. 1981;2:539.
45 Rao KC, Levine H, Itani A, et al. CT findings in multicentric glioblastoma: diagnostic-pathologic correlation. J Comput Assist Tomogr. 1980;4:187.
46 Buonanno FS, Moody DM, Ball MR. CT scan findings in cerebral sinovenous occlusion. Neurology. 1979;29:1433.
47 Buonanno FS, Moody DM, Ball MR, et al. Computed cranial tomographic findings in cerebral sinovenous occlusion. J Comput Assist Tomogr. 1978;2:281.
48 Shinohara Y, Yoshitoshi M, Yoshii F. Appearance and disappearance of empty delta sign in superior sagittal sinus thrombosis. Stroke. 1986;17:1282.
49 Virapongse C, Cazenave C, Quisling R, et al. The empty delta sign: frequency and significance in 76 cases of dural sinus thrombosis. Radiology. 1987;162:779.
50 Padayachee TS, Bingham JB, Graves MJ, et al. Dural sinus thrombosis. Diagnosis and follow-up by magnetic resonance angiography and imaging. Neuroradiology. 1991;33:165.
51 Rippe DJ, Boyko OB, Spritzer CE, et al. Demonstration of dural sinus occlusion by the use of MR angiography. AJNR Am J Neuroradiol. 1990;11:199.
52 Tsai FY, Wang AM, Matovich VB, et al. MR staging of acute dural sinus thrombosis: correlation with venous pressure measurements and implications for treatment and prognosis. AJNR Am J Neuroradiol. 1995;16:1021.
53 Tsuruda JS, Shimakawa A, Pelc NJ, et al. Dural sinus occlusion: evaluation with phase-sensitive gradient-echo MR imaging. AJNR Am J Neuroradiol. 1991;12:481.
54 Yuh WT, Simonson TM, Wang AM, et al. Venous sinus occlusive disease: MR findings. AJNR Am J Neuroradiol. 1994;15:309.
55 Halbach VV, Higashida RT, Hieshima GB, et al. Venography and venous pressure monitoring in dural sinus meningiomas. AJNR Am J Neuroradiol. 1989;10:1209.
56 Canhao P, Batista P, Ferro JM. Venous transcranial Doppler in acute dural sinus thrombosis. J Neurol. 1998;245:276.
57 Ries S, Steinke W, Neff KW, et al. Echocontrast-enhanced transcranial color-coded sonography for the diagnosis of transverse sinus venous thrombosis. Stroke. 1997;28:696.
58 Hanley DF, Feldman E, Borel CO, et al. Treatment of sagittal sinus thrombosis associated with cerebral hemorrhage and intracranial hypertension. Stroke. 1988;19:903.
59 Stansfield F. Puerperal cerebral thrombophlebitis treated by heparin. BMJ. 1942;4:436.
60 Dorndorf D, Wessel K, Kessler C, et al. Thrombosis of the right vein of Labbe: radiological and clinical findings. Neuroradiology. 1993;35:202.
61 Einhaupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597.
62 de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30:484.
63 Levine SR, Twyman RE, Gilman S. The role of anticoagulation in cavernous sinus thrombosis. Neurology. 1988;38:517.
64 Barnwell SL, Nesbit GM, Clark WM. Local thrombolytic therapy for cerebrovascular disease: current Oregon Health Sciences University experience (July 1991 through April 1995). J Vasc Interv Radiol. 1995;6:78S.
65 Alexander LF, Yamamoto Y, Ayoubi S, et al. Efficacy of tissue plasminogen activator in the lysis of thrombosis of the cerebral venous sinus. Neurosurgery. 1990;26:559.
66 Castaigne P, Laplane D, Bousser MG. Superior sagittal sinus thrombosis. Arch Neurol. 1977;34:788.
67 Di Rocco C, Iannelli A, Leone G, et al. Heparin-urokinase treatment in aseptic dural sinus thrombosis. Arch Neurol. 1981;38:431.
68 Fletcher AP, Alkjaersig N, Lewis M, et al. A pilot study of urokinase therapy in cerebral infarction. Stroke. 1976;7:135.
69 Meyer JS, Gilroy J, Barnhart MI, et al. Therapeutic thrombolysis in cerebral thromboembolism. Double-blind evaluation of intravenous plasmin therapy in carotid and middle cerebral arterial occlusion. Neurology. 1963;13:927.
70 Vines FS, Davis DO. Clinical-radiological correlation in cerebral venous occlusive disease. Radiology. 1971;98:9.
71 Misra V, Elliott DG, Gonzalez-Toledo E, et al. Demonstration of significant resolution of cerebral sino-venous thrombosis associated with intravenous recombinant tissue plasminogen activator. J Neuroimaging. 2007;17:348.
72 Scott JA, Pascuzzi RM, Hall PV, et al. Treatment of dural sinus thrombosis with local urokinase infusion. Case report. J Neurosurg. 1988;68:284.
73 Higashida RT, Helmer E, Halbach VV, et al. Direct thrombolytic therapy for superior sagittal sinus thrombosis. AJNR Am J Neuroradiol. 1989;10:S4.
74 Barnwell SL, Higashida RT, Halbach VV, et al. Direct endovascular thrombolytic therapy for dural sinus thrombosis. Neurosurgery. 1991;28:135.
75 Tsai FY, Higashida RT, Matovich V, et al. Acute thrombosis of the intracranial dural sinus: direct thrombolytic treatment. AJNR Am J Neuroradiol. 1992;13:1137.
76 Smith TP, Higashida RT, Barnwell SL, et al. Treatment of dural sinus thrombosis by urokinase infusion. AJNR Am J Neuroradiol. 1994;15:801.
77 Horowitz M, Purdy P, Unwin H, et al. Treatment of dural sinus thrombosis using selective catheterization and urokinase. Ann Neurol. 1995;38:58.
78 Spearman MP, Jungreis CA, Wehner JJ, et al. Endovascular thrombolysis in deep cerebral venous thrombosis. AJNR Am J Neuroradiol. 1997;18:502.
79 Holder CA, Bell DA, Lundell AL, et al. Isolated straight sinus and deep cerebral venous thrombosis: successful treatment with local infusion of urokinase. Case report. J Neurosurg. 1997;86:704.
80 Rael JR, Orrison WWJr, Baldwin N, et al. Direct thrombolysis of superior sagittal sinus thrombosis with coexisting intracranial hemorrhage. AJNR Am J Neuroradiol. 1997;18:1238.
81 Smith AG, Cornblath WT, Deveikis JP. Local thrombolytic therapy in deep cerebral venous thrombosis. Neurology. 1997;48:1613.
82 Dowd CF, Malek AM, Phatouros CC, et al. Application of a rheolytic thrombectomy device in the treatment of dural sinus thrombosis: a new technique. AJNR Am J Neuroradiol. 1999;20:568.
83 Kim SY, Suh JH. Direct endovascular thrombolytic therapy for dural sinus thrombosis: infusion of alteplase. AJNR Am J Neuroradiol. 1997;18:639.
84 Renowden SA, Oxbury J, Molyneux AJ. Case report: venous sinus thrombosis: the use of thrombolysis. Clin Radiol. 1997;52:396.
85 Frey JL, Muro GJ, McDougall CG, et al. Cerebral venous thrombosis: combined intrathrombus rtPA and intravenous heparin. Stroke. 1999;30:489.
86 Yamini B, Loch Macdonald R, Rosenblum J. Treatment of deep cerebral venous thrombosis by local infusion of tissue plasminogen activator. Surg Neurol. 2001;55:340.
87 Soleau SW, Schmidt R, Stevens S, et al. Extensive experience with dural sinus thrombosis. Neurosurgery. 2003;52:534.
88 Tsai FY, Kostanian V, Rivera M, et al. Cerebral venous congestion as indication for thrombolytic treatment. Cardiovasc Intervent Radiol. 2007;30:675.
89 Agner C, Deshaies EM, Bernardini GL, et al. Coronary AngioJet catheterization for the management of dural venous sinus thrombosis. Technical note. J Neurosurg. 2005;103:368.
90 Chow K, Gobin YP, Saver J, et al. Endovascular treatment of dural sinus thrombosis with rheolytic thrombectomy and intra-arterial thrombolysis. Stroke. 2000;31:1420.
91 Chahlavi A, Steinmetz MP, Masaryk TJ, et al. A transcranial approach for direct mechanical thrombectomy of dural sinus thrombosis. Report of two cases. J Neurosurg. 2004;101:347.
92 Ferro JM, Canhao P, Stam J, et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664.
93 Preter M, Tzourio C, Ameri A, et al. Long-term prognosis in cerebral venous thrombosis. Follow-up of 77 patients. Stroke. 1996;27:243.
94 Eick JJ, Miller KD, Bell KA, et al. Computed tomography of deep cerebral venous thrombosis in children. Radiology. 1981;140:399.