18 Cerebral Revascularization for Moyamoya Disease
Moyamoya disease
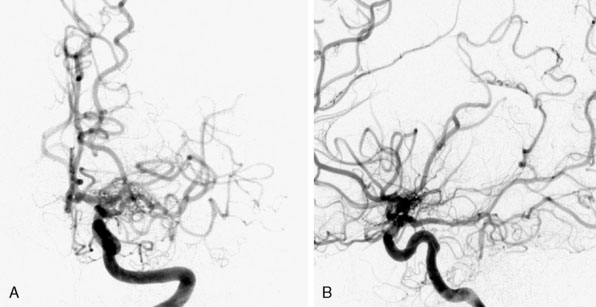
Moyamoya disease (MMD) is a rare cerebrovascular disorder characterized by idiopathic and progressive stenosis or occlusion of the supraclinoid internal carotid arteries bilaterally with frequent involvement of the bilateral anterior and middle cerebral arteries1 (Figure 18–1). The term Moyamoya meaning “a puff of smoke” in Japanese was first coined in 1969 by Suzuki and Takaku to describe the resulting characteristic abnormal vascular networks that form secondarily to the stenosis/occlusion,2 but the disease was first described in the Japanese literature in 1957 by Takeuchi and Shimizu.3 Although genetic links are implicated in MMD,4 the etiology remains unknown. Moyamoya syndrome (MMS) has similar clinical and angiographic characteristics to MMD but is associated with other conditions such as Downs syndrome, neurofibromatosis type-1 (NF-1), prior irradiation, and sickle cell disease.5 Patients with unilateral findings have MMS, although approximately 40% of these patients develop angiographic progression on the contralateral side.6 In this chapter, we will give an overview of MMD and provide a comprehensive surgical view of cerebral revascularization for MMD, emphasizing the technical nuances and complications of the procedure.
Epidemiology
Although MMD was initially described in patients of Asian descent, it recently has been increasingly reported in patients of all demographic groups, although the exact incidence is not completely known.7 Japanese studies report an annual incidence between 0.35 to 0.94/100,000 persons per year and annual prevalence between 3.16 to 10.5 per 100,000 persons.8–10 There is a bimodal distribution of age of onset with the first peak at 5 years of age and a second peak between 45 and 49 years of age. There are twice as many female patients as compared to male patients.8,9 The reported incidence and prevalence of MMD in the United States is significantly lower when compared to Japanese studies. A study of Moyamoya patients from Washington and California identified 298 individuals with MMD for an incidence of 0.086/100,000 persons per year. In a subgroup of Asian Americans, however, the incidence of 0.28/100,000 was closer to those of the Japanese studies.11 A study looking at the epidemiology of MMD in Hawaii, where the cohort was primarily of Asian descent, also revealed a higher incidence when compared to the general population in the United States.12
Presentation
Patients with MMD generally present either with hemorrhage or with ischemia. The rate of hemorrhage is approximately seven times more common in adult patients (14.6%) when compared to pediatric patients (2.1%).5,13 However, this was not true for the 432 patients treated at Stanford from 1991 to 2009, where ischemic symptoms were far more common than hemorrhage.13 Some have suggested that the hemorrhage rate is higher in Asians.14–17 Occasionally patients may also present with seizure, headaches, or behavioral changes. Some studies suggest a relation between headaches and hypoperfusion leading to cortical depression and associated migraine symptoms.18 Others have suggested that dilation of dural and meningeal vessels irritate the pain fibers on the dura, resulting in a migraine-like headache that is refractive to medical therapies.19 This headache initially persisted in 63% of patients even after cerebral revascularization, although it would often improve over time.
Pathophysiology
On histopathologic examination, the internal carotid arteries demonstrate eccentric fibrocellular thickening of the intimal layer, proliferation of smooth muscle cells, and tortuous and often duplicated internal elastic lamina with no inflammatory or atheromatous involvement, resulting in artery stenosis/occlusion.4 The resulting hypoxia induces collateralization through formation of dilated and tortuous perforating arteries. These “Moyamoya” vessels have thinned media with fibrin deposition in the vessel walls, fragmented elastic lamina, and microaneurysm formation that may be secondary to increased expression of numerous growth factors, including hypoxia-inducible factor 1 (HIF-1), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor-beta (TGF-beta), hepatocyte growth factor (HGF), and matrix metalloproteinases (MMPs).4
Genetic factors play a role as up to 15% of MMD patients are familial indicating an autosomal dominant inheritance pattern with incomplete penetrance. A number of genome-wide studies from these familial carriers of MMD suggest links to chromosomal regions including 3p24.2-p26,20 6q25,21 8q23,22 12p12,22 and 17q25.23 Recently, carriers of a mutation in the vascular smooth muscle cell (SMC)–specific isoform of alpha-actin (ACTA2) were found to present with a variety of vascular diseases including premature onset of coronary artery disease, non-Marfan’s thoracic artery aneurysms and dissections, non-MMD early onset stroke, and MMD.24
Natural history and prognosis
There are limited studies on the natural history of MMD, but each of the studies has demonstrated inevitable disease progression if left untreated.6,25,26 In one study, the 5-year cumulative risk of any recurrent ipsilateral stroke in patients treated without surgery with impaired hemodynamic reserve, as measured by increased oxygen extraction fraction on positron emission tomography (PET), was approximately 65%. In patients with bilateral disease, the stroke risk over 5 years was 82%.14 Another North American study found a recurrent stroke rate of 18% in the first year and a 5% per year risk in subsequent years.17 A nationwide survey in Japan of asymptomatic Moyamoya patients found a 3.2% per year stroke rate in medically treated patients.27 In contrast, the estimated rate of perioperative or subsequent stroke or death after surgical revascularization was between 5.5%13 and 17%14 over 5 years, demonstrating the need for surgical treatment of these patients.
Diagnoses and treatment
The diagnoses of MMD or MMS should be considered in any patient, especially in a child or young adult who presents with neurologic deficits or symptoms secondary to cerebral ischemia or hemorrhage. Radiologic evaluation can include CT, MRI, cerebral angiography, and cerebral perfusion studies, including CT perfusion, xenon-enhanced CT, or PET, MR perfusion, and SPECT or CT imaging without and with acetazolomide challenge. Cerebral angiography, CT angiography, and MR angiography imaging can reveal intracerebral vascular occlusion along with the resulting Moyamoya vessels. CT and MRI can reveal hemorrhage or ischemic changes. Cerebral perfusion studies help to characterize blood flow and elucidate the hemodynamic state preoperatively and postoperatively.28
Although there are no randomized studies comparing medical treatment versus surgical revascularization for patients with MMD, we believe that the stroke rate is significantly reduced in surgically treated patients. As discussed in the previous section, the natural history of these patients is to progress and the risk of recurrent stroke is extremely high with medical management only. The main medical treatment option for these patients is antiplatelet therapy. All patients are on aspirin pre- and postoperatively. Full anticoagulation drugs such as warfarin are rarely used.5
Patients with MMD have occlusion of the internal carotid arteries with sparing of the external carotid system. Surgical treatment uses the external carotid system to supply blood to the ischemic cerebrum by either creating a “direct” or an “indirect” revascularization bypass. A direct bypass involves dissection of a branch of the external carotid artery, specifically the superficial temporal artery (STA), which is then directly anastomosed to a distal (usually M4) cortical branch of the middle cerebral artery (MCA). An indirect bypass is performed by placement of vascularized tissue supplied by the external carotid (such as dura, the temporalis muscle, or the STA itself) onto the cortical surface of the brain leading to an ingrowth of new blood vessels to the cortex beneath it. There are no randomized studies comparing the efficacy and safety of indirect versus direct bypass, and a review of the literature failed to reveal a difference in surgical morbidity or stroke rate (perioperative or long term) between methods.29 Many institutions will perform direct revascularization for adults and indirect revascularization for children because of the smaller and more friable caliber of the vessels in children.30,31 However, there are many groups that perform indirect bypass for adult patients as well.14,17,32 Our philosophy is to perform direct bypass on all patients unless the STA or MCA is too small or friable. Direct revascularization procedures have the advantage of providing immediate increase in blood flow to the ischemic cortex. In bilateral MMD, we first revascularize the symptomatic hemisphere. If there are no lateralizing signs or symptoms, we prefer to revascularize the non-dominant hemisphere because of increased incidence of transient neurologic symptoms after surgery on the dominant hemisphere. The contralateral side is generally revascularized 1 week after the first side unless there were significant complications after the first procedure.
Direct revascularization
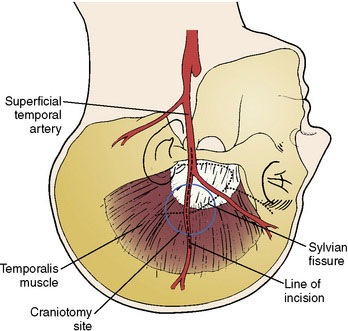
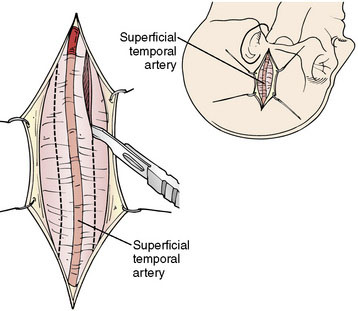
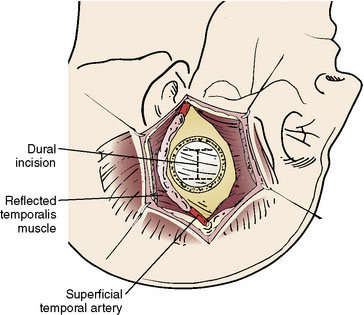
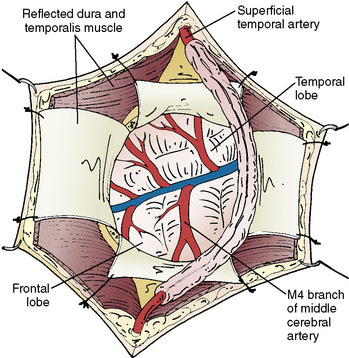
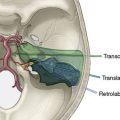
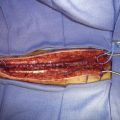
The size of the frontal and parietal branch of the STA is determined preoperatively by cerebral angiography. The larger diameter branch is chosen as the donor vessel. The patient is positioned supine and placed in a three-point pin fixation. The head is elevated above the heart and turned to the side, bringing the frontotemporal region uppermost in the field. Stimulating and recording electrodes are placed for monitoring of bilateral somatosensory evoked potentials (SSEP) and bilateral EEG. Cooling to approximately 33°C is achieved by either a cooling blanket and bladder irrigation, or by placing an intravenous catheter (InnerCool Therapies Philips, Andover, MA) into the inferior vena cava as described previously.33 In general, the InnerCool catheter is placed in the larger patients who are more difficult to cool and rewarm via surface techniques. The course of the superficial temporal artery is mapped out with a Doppler probe. When the parietal branch of the STA is chosen as the donor, the skin incision is planned over the artery along its course (Figure 18–2). If the frontal branch is the donor, the inferior portion of the incision follows the STA but as the artery tracks anterior to the hairline, the incision stays posterior behind the hairline for cosmetic purposes. After shaving the hair over the planned incision, the area is prepped and draped in standard sterile fashion. The operating microscope is positioned and used for careful harvesting of the superficial temporal artery. A length of approximately 7 cm of STA with a generous cuff of soft tissue to protect it is needed for the bypass (Figure 18–3). Then the underlying temporalis fascia muscle is split and dissected from the frontotemporal bone. Burr holes are placed, the craniotomy bone flap is removed over the frontotemporal region, and the dura is opened in a cruciate manner (Figure 18–4).
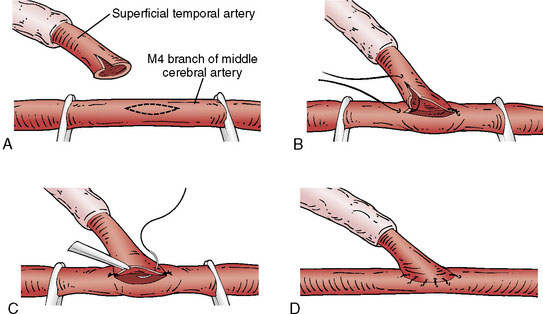
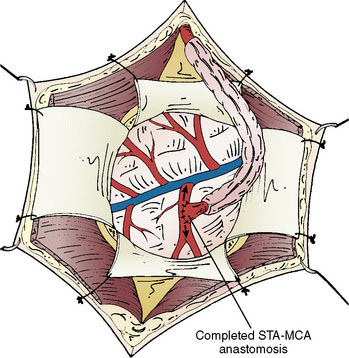
Identification of a sufficiently large M4 branch of the MCA (≥0.6 mm) emerging from the sylvian fissure is paramount for this procedure. The arachnoid overlying this cortical branch of the MCA is then microscopically opened. A 7-mm segment of M4 artery without branches is preferably chosen as the recipient, but any tiny branches arising from this segment can be coagulated and divided if necessary (Figure 18–5). A jeweler-type bipolar is used. High-visibility background is placed under the M4 segment. Papaverine is intermittently instilled over the vessels to prevent spasm. Then the STA is temporarily occluded proximally and sectioned distally. The distal stump of the superficial temporal artery in the scalp is coagulated and the superficial temporal artery is truncated to the proper length for anastomosis and fish-mouthed. Temporary release of the proximal clip is performed to ensure excellent flow. The artery is temporarily occluded again proximally and flushed with heparinized saline. Intravenous thiopental is given to induce burst suppression in the EEG. The mean arterial pressure is raised and then the M4 MCA branch is temporarily occluded with Sugita aneurysm clips. An arteriotomy is made in the M4 branch, removing an elliptical portion of the superior wall, and irrigated with heparinized saline. An end-to-side anastomosis between the STA branch and the MCA branch is performed with 10-0 interrupted suture (Figure 18–6). The Sugita clips are then removed, restoring flow. Flow in all vessels is confirmed with the intraoperative Doppler. An intraoperative indocyanine green (ICG) angiogram is performed by injecting 2 ml of ICG dye intravenously and visualizing the graft with the near-infrared camera on the microscope. If the anastomosis is done correctly, the ICG demonstrates wide patency of the graft with no stenoses and good filling of the pial vasculature. It is our practice to then lay the STA with its soft tissue cuff on the cortical surface to induce an indirect bypass as well as a direct one (Figure 18–7). The dura is closed with 4-0 suture followed by synthetic dura, leaving an opening for the graft to enter without compromise. The bone is replaced using a plating system, also leaving an opening for the graft to enter unimpeded. The temporalis muscle and scalp are closed in several layers in the usual fashion. During the entire procedure care is taken to keep the mean arterial pressure high-normal for that patient and about 10 points higher during the occlusion of the cortical MCA branch for adequate perfusion. Patients are maintained normocarbic during the entire operation to prevent vasoconstriction and ischemia from hyperventilation.
Results and complications
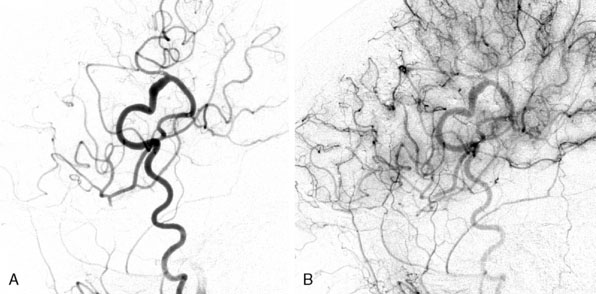
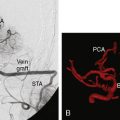
Patients are generally discharged after 3 to 4 hospital nights (the first night is spent in the intensive care unit). They are then evaluated clinically 1 week after the second bypass procedure (or after the first procedure in unilateral disease), and subsequently at 6 months, 3 years, and finally at 10 years. We also perform MRI, SPECT, or Xenon CT, and cerebral angiography at 6 months, 3 years, and 10 years. In addition to internal carotid and vertebral injections, external injections are included to evaluate bypass patency and extent of revascularization (Figure 18–8). In our series of 450 bypasses in 265 patients, 3% (eight patients) or 1.7% of procedures suffered postoperative ischemic stroke (four ipsilateral to the surgical hemisphere and four in the contralateral hemisphere). Fifty percent of these patients made a complete recovery by 6 months. A total of 2.6% (seven patients) suffered a postoperative hemorrhage (1.8% of procedures), all in the ipsilateral hemisphere with bleeding into prior ischemic regions. Of these seven patients, four made a full recovery by 6 months, but two died from hemorrhage within 30 days of the procedure. In nine patients (3.3% or 2.0% of procedures) transient neurologic symptoms without MRI changes occurred with full recovery by postoperative days 3 to 14. Statistical analysis for surgical risk identified several factors that trended toward increased risk; however, none were statistically significant. Patients who presented with transient ischemic attack (TIA) or stroke trended toward an increased risk of postoperative ischemic symptoms. Similarly, patients who presented with hemorrhage trended toward an increased risk of postoperative hemorrhage. Patients with MMD appeared to have a higher risk of postoperative stroke and death. Sex, ethnicity, unilateral versus bilateral, age, and type of revascularization procedure did not affect outcome.13
In long-term follow-up, 71.2% of our patients demonstrated improvement in quality of life. There was a significant reduction in TIAs and >80% of patients who presented with headaches were headache-free at their last follow-up. The overall incidence of ischemic stroke after surgery was 3.8% (eight early and two late events) and the overall incidence of hemorrhagic stroke was 3.4% (seven early and two late events). The overall mortality rate was 2.3% (three early and three late deaths). The 5-year overall cumulative risk of postoperative stroke or death was 5.5%.13 In another study, the 5-year risk of stroke or death was decreased from 65% in medically treated patients to 17% in surgically treated patients.14 One study did fail to show a benefit of surgical treatment, but the authors reported an unusually high initial surgical morbidity and mortality.17
1 Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the Circle of Willis (“Moyamoya” disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997;99(Suppl 2):S238-S240.
2 Suzuki J., Takaku A. Cerebrovascular “Moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288-299.
3 Takeuchi K., Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve. 1957;9:37-43.
4 Achrol A.S., Guzman R., Lee M., et al. Pathophysiology and genetic factors in moyamoya disease. Neurosurg Focus. 2009;26:E4.
5 Scott R.M., Smith E.R. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-1237.
6 Kelly M.E., Bell-Stephens T.E., Marks M.P., et al. Progression of unilateral moyamoya disease: a clinical series. Cerebrovasc Dis. 2006;22:109-115.
7 Hoffman H.J. Moyamoya disease and syndrome. Clin Neurol Neurosurg. 1997;99(Suppl 2):S39-S44.
8 Baba T., Houkin K., Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79:900-904.
9 Wakai K., Tamakoshi A., Ikezaki K., et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997;99(Suppl 2):S1-S5.
10 Kuriyama S., Kusaka Y., Fujimura M., et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008;39:42-47.
11 Uchino K., Johnston S.C., Becker K.J., et al. Moyamoya disease in Washington State and California. Neurology. 2005;65:956-958.
12 Graham J.F., Matoba A. A survey of moyamoya disease in Hawaii. Clin Neurol Neurosurg. 1997;99(Suppl 2):S31-S35.
13 Guzman R., Lee M., Achrol A., et al. Clinical outcome after 450 revascularization procedures for moyamoya disease. J Neurosurg. 2009;111:927-935.
14 Hallemeier C.L., Rich K.M., Grubb R.L.Jr, et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke. 2006;37:1490-1496.
15 Yilmaz E.Y., Pritz M.B., Bruno A., et al. Moyamoya: Indiana University Medical Center experience. Arch Neurol. 2001;58:1274-1278.
16 Suzuki J., Kodama N. Moyamoya disease—a review. Stroke. 1983;14:104-109.
17 Chiu D., Shedden P., Bratina P., et al. Clinical features of moyamoya disease in the United States. Stroke. 1998;29:1347-1351.
18 Olesen J., Friberg L., Olsen T.S., et al. Ischaemia-induced (symptomatic) migraine attacks may be more frequent than migraine-induced ischaemic insults. Brain. 1993;116(Pt 1):187-202.
19 Seol H.J., Wang K.C., Kim S.K., et al. Headache in pediatric moyamoya disease: review of 204 consecutive cases. J Neurosurg. 2005;103:439-442.
20 Ikeda H., Sasaki T., Yoshimoto T., et al. Mapping of a familial moyamoya disease gene to chromosome 3p24.2-p26. Am J Hum Genet. 1999;64:533-537.
21 Inoue T.K., Ikezaki K., Sasazuki T., et al. Linkage analysis of moyamoya disease on chromosome 6. J Child Neurol. 2000;15:179-182.
22 Sakurai K., Horiuchi Y., Ikeda H., et al. A novel susceptibility locus for moyamoya disease on chromosome 8q23. J HumGenet. 2004;49:278-281.
23 Mineharu Y., Liu W., Inoue K., et al. Autosomal dominant moyamoya disease maps to chromosome 17q25.3. Neurology. 2008;70:2357-2363.
24 Guo D.C., Papke C.L., Tran-Fadulu V., et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617-627.
25 Imaizumi T., Hayashi K., Saito K., et al. Long-term outcomes of pediatric moyamoya disease monitored to adulthood. Pediatr Neurol. 1998;18:321-325.
26 Kuroda S., Ishikawa T., Houkin K., et al. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 2005;36:2148-2153.
27 Kuroda S., Hashimoto N., Yoshimoto T., et al. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. 2007;38:1430-1435.
28 Lee M., Zaharchuk G., Guzman R., et al. Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg Focus. 2009;26:E5.
29 Veeravagu A., Guzman R., Patil C.G., et al. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurg Focus. 2008;24:E16.
30 Mesiwala A.H., Sviri G., Fatemi N., et al. Long-term outcome of superficial temporal artery–middle cerebral artery bypass for patients with moyamoya disease in the US. Neurosurg Focus. 2008;24:E15.
31 Scott R.M., Smith J.L., Robertson R.L., et al. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100:142-149.
32 Starke R.M., Komotar R.J., Hickman Z.L., et al. Clinical features, surgical treatment, and long-term outcome in adult patients with moyamoya disease. Clinical article. J Neurosurg. 2009;111:936-942.
33 Steinberg G.K., Ogilvy C.S., Shuer L.M., et al. Comparison of endovascular and surface cooling during unruptured cerebral aneurysm repair. Neurosurgery. 2004;55:307-314.