Chapter 595 Central Nervous System Infections
Infection of the CNS may be diffuse or focal. Meningitis and encephalitis are examples of diffuse infection. Meningitis implies primary involvement of the meninges, whereas encephalitis indicates brain parenchymal involvement. Because these anatomic boundaries are often not distinct, many patients have evidence of both meningeal and parenchymal involvement and should be considered to have meningoencephalitis. Brain abscess is the best example of a focal infection of the CNS. The neurologic expression of this infection is determined by the site and extent of the abscess(es) (Chapter 596).
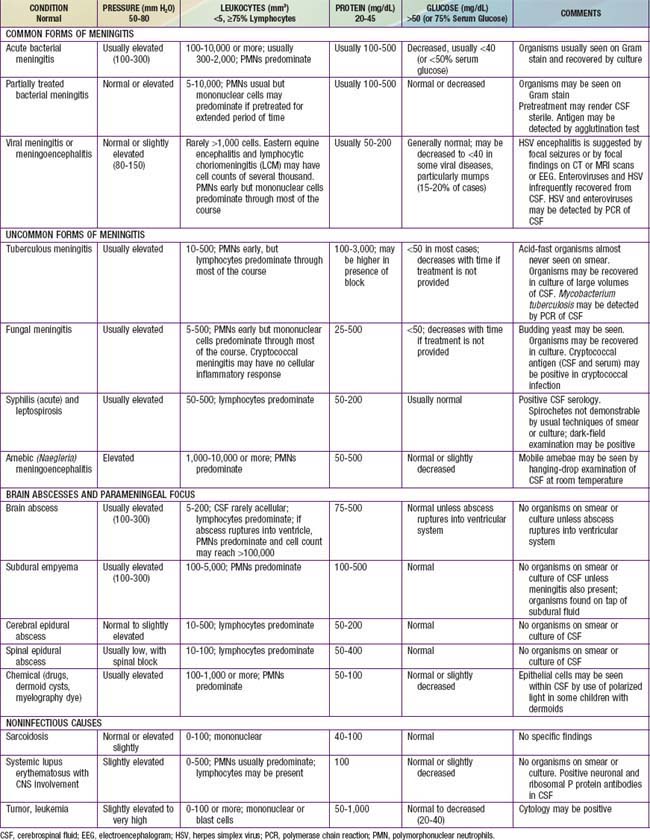
The diagnosis of diffuse CNS infections depends on examination of cerebrospinal fluid (CSF) obtained by lumbar puncture (LP). Table 595-1 provides an overview of the expected CSF abnormalities with various CNS disorders.
595.1 Acute Bacterial Meningitis Beyond the Neonatal Period
Pathology and Pathophysiology
Increased ICP is due to cell death (cytotoxic cerebral edema), cytokine-induced increased capillary vascular permeability (vasogenic cerebral edema), and, possibly, increased hydrostatic pressure (interstitial cerebral edema) after obstructed reabsorption of CSF in the arachnoid villus or obstruction of the flow of fluid from the ventricles. ICP may exceed 300 mm H2O; cerebral perfusion may be further compromised if the cerebral perfusion pressure (mean arterial pressure minus ICP) is <50 cm H2O due to systemic hypotension with reduced cerebral blood flow. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) may produce excessive water retention and potentially increase the risk of elevated ICP (Chapter 553). Hypotonicity of brain extracellular spaces may cause cytotoxic edema after cell swelling and lysis. Tentorial, falx, or cerebellar herniation does not usually occur because the increased ICP is transmitted to the entire subarachnoid space and there is little structural displacement. Furthermore, if the fontanels are still patent, increased ICP is not always dissipated.
Diagnosis
The diagnosis of acute pyogenic meningitis is confirmed by analysis of the CSF, which typically reveals microorganisms on Gram stain and culture, a neutrophilic pleocytosis, elevated protein, and reduced glucose concentrations (see Table 595-1). LP should be performed when bacterial meningitis is suspected. Contraindications for an immediate LP include (1) evidence of increased ICP (other than a bulging fontanel), such as 3rd or 6th cranial nerve palsy with a depressed level of consciousness, or hypertension and bradycardia with respiratory abnormalities (Chapter 584); (2) severe cardiopulmonary compromise requiring prompt resuscitative measures for shock or in patients in whom positioning for the LP would further compromise cardiopulmonary function; and (3) infection of the skin overlying the site of the LP. Thrombocytopenia is a relative contraindication for LP. If an LP is delayed, empirical antibiotic therapy should be initiated. CT scanning for evidence of a brain abscess or increased ICP should not delay therapy. LP may be performed after increased ICP has been treated or a brain abscess has been excluded.
Differential Diagnosis
In addition to S. pneumoniae, N. meningitidis, and H. influenzae type b, many other microorganisms can cause generalized infection of the CNS with similar clinical manifestations. These organisms include less typical bacteria, such as Mycobacterium tuberculosis, Nocardia spp., Treponema pallidum (syphilis), and Borrelia burgdorferi (Lyme disease); fungi, such as those endemic to specific geographic areas (Coccidioides, Histoplasma, and Blastomyces) and those responsible for infections in compromised hosts (Candida, Cryptococcus, and Aspergillus); parasites, such as Toxoplasma gondii and those that cause cysticercosis and, most frequently, viruses (Chapter 595.2) (Table 595-2). Focal infections of the CNS including brain abscess and parameningeal abscess (subdural empyema, cranial and spinal epidural abscess) may also be confused with meningitis. In addition, noninfectious illnesses can cause generalized inflammation of the CNS. Relative to infections, these disorders are uncommon and include malignancy, collagen vascular syndromes, and exposure to toxins (see Table 595-2).
Table 595-2 CLINICAL CONDITIONS AND INFECTIOUS AGENTS ASSOCIATED WITH ASEPTIC MENINGITIS
VIRUSES
BACTERIA
BACTERIAL PARAMENINGEAL FOCUS
FUNGI
PARASITES (EOSINOPHILIC)
PARASITES (NONEOSINOPHILIC)
POSTINFECTIOUS
SYSTEMIC OR IMMUNOLOGICALLY MEDIATED
MALIGNANCY
DRUGS
MISCELLANEOUS
Compiled from Cherry JD: Aseptic meningitis and viral meningitis. In Feigin RD, Cherry JD, editors: Textbook of pediatric infectious diseases, ed 4, Philadelphia, 1998, WB Saunders, p 450; Davis LE: Aseptic and viral meningitis. In Long SS, Pickering LK, Prober CG, editors: Principles and practice of pediatric infectious disease, New York, 1997, Churchill Livingstone, p 329; Kliegman RM, Greenbaum LA, Lye PS: Practical strategies in pediatric diagnosis therapy, ed 2, Philadelphia, 2004, Elsevier, p 961.
Acute viral meningoencephalitis is the most likely infection to be confused with bacterial meningitis (Tables 595-2 and 595-3). Although, in general, children with viral meningoencephalitis appear less ill than those with bacterial meningitis, both types of infection have a spectrum of severity. Some children with bacterial meningitis may have relatively mild signs and symptoms, whereas some with viral meningoencephalitis may be critically ill. Although classic CSF profiles associated with bacterial versus viral infection tend to be distinct (see Table 595-1), specific test results may have considerable overlap.
Table 595-3 CLASSIFICATION OF ENCEPHALITIS BY CAUSE AND SOURCE
CNS, central nervous system.
Modified from Behrman RE, editor: Nelson textbook of pediatrics, ed 14, Philadelphia, 1992, WB Saunders, p 667. From Kliegman RM, Greenbaum LA, Lye PS: Practical strategies in pediatric diagnosis and therapy, ed 2, Philadelphia, 2004, Elsevier, p 967.
Treatment
The therapeutic approach to patients with presumed bacterial meningitis depends on the nature of the initial manifestations of the illness. A child with rapidly progressing disease of less than 24 hr duration, in the absence of increased ICP, should receive antibiotics as soon as possible after an LP is performed. If there are signs of increased ICP or focal neurologic findings, antibiotics should be given without performing an LP and before obtaining a CT scan. Increased ICP should be treated simultaneously (Chapter 63). Immediate treatment of associated multiple organ system failure, shock (Chapter 64), and acute respiratory distress syndrome (Chapter 65) is also indicated.
Initial Antibiotic Therapy
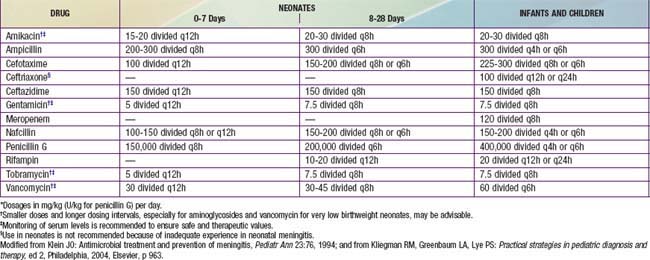
The initial (empirical) choice of therapy for meningitis in immunocompetent infants and children is primarily influenced by the antibiotic susceptibilities (Table 595-4) of S. pneumoniae. Selected antibiotics should achieve bactericidal levels in the CSF. Although there are substantial geographic differences in the frequency of resistance of S. pneumoniae to antibiotics, rates are increasing throughout the world. In the USA, 25-50% of strains of S. pneumoniae are currently resistant to penicillin; relative resistance (MIC = 0.1-1.0 µg/mL) is more common than high-level resistance (MIC = 2.0 µg/mL). Resistance to cefotaxime and ceftriaxone is also evident in up to 25% of isolates. In contrast, most strains of N. meningitidis are sensitive to penicillin and cephalosporins, although rare resistant isolates have been reported. Approximately 30-40% of isolates of H. influenzae type b produce β-lactamases and, therefore, are resistant to ampicillin. These β-lactamase–producing strains are sensitive to the extended-spectrum cephalosporins.
Based on the substantial rate of resistance of S. pneumoniae to β-lactam drugs, vancomycin (60 mg/kg/24 hr, given every 6 hr) is recommended as part of initial empirical therapy. Because of the efficacy of 3rd-generation cephalosporins in the therapy of meningitis caused by sensitive S. pneumoniae, N. meningitidis, and H. influenzae type b, cefotaxime (200 mg/kg/24 hr, given every 6 hr) or ceftriaxone (100 mg/kg/24 hr administered once per day or 50 mg/kg/dose, given every 12 hr) should also be used in initial empirical therapy. Patients allergic to β-lactam antibiotics and >1 mo of age can be treated with chloramphenicol, 100 mg/kg/24 hr, given every 6 hr. Alternatively, patients can be desensitized to the antibiotic (Chapter 146).
Duration of Antibiotic Therapy
Supportive Care
Neurologic complications include increased ICP with subsequent herniation, seizures, and an enlarging head circumference due to a subdural effusion or hydrocephalus. Signs of increased ICP should be treated emergently with endotracheal intubation and hyperventilation (to maintain the pCO2 at approximately 25 mm Hg). In addition, intravenous furosemide (Lasix, 1 mg/kg) and mannitol (0.5-1.0 g/kg) osmotherapy may reduce ICP (Chapter 63). Furosemide reduces brain swelling by venodilation and diuresis without increasing intracranial blood volume, whereas mannitol produces an osmolar gradient between the brain and plasma, thus shifting fluid from the CNS to the plasma, with subsequent excretion during an osmotic diuresis.
Complications
Collections of fluid in the subdural space develop in 10-30% of patients with meningitis and are asymptomatic in 85-90% of patients. Subdural effusions are especially common in infants. Symptomatic subdural effusions may result in a bulging fontanel, diastasis of sutures, enlarging head circumference, emesis, seizures, fever, and abnormal results of cranial transillumination. CT or MRI scanning confirms the presence of a subdural effusion. In the presence of increased ICP or a depressed level of consciousness, symptomatic subdural effusion should be treated by aspiration through the open fontanel (Chapters 63 and 584). Fever alone is not an indication for aspiration.
SIADH occurs in some patients with meningitis, resulting in hyponatremia and reduced serum osmolality. This may exacerbate cerebral edema or result in hyponatremic seizures (Chapter 52).
Prevention
Haemophilus influenzae Type B
The most striking advance in the prevention of childhood bacterial meningitis followed the development and licensure of conjugated vaccines against H. influenzae type b. Four conjugate vaccines are licensed in the USA. Although each vaccine elicits different profiles of antibody response in infants immunized at 2-6 mo of age, all result in protective levels of antibody with efficacy rates against invasive infections ranging from 70-100%. Efficacy is not as consistent in Native American populations, a group recognized as having an especially high incidence of disease. All children should be immunized with H. influenzae type b conjugate vaccine beginning at 2 mo of age (Chapter 165).
Aguilar J, Urday-Cornejo V, Donabedian S, et al. Staphylococcus aureus meningitis: case series and literature review. Medicine. 2010;89(2):117-125.
Assiri AM, Alasmari FA, Zimmerman VA, et al. Corticosteroid administration and outcome of adolescents and adults with acute bacterial meningitis: a meta-analysis. Mayo Clin Proc. 2009;84:403-409.
Blazer S, Berant M, Alon U. Bacterial meningitis: effect of antibiotic treatment on cerebrospinal fluid. J Clin Pathol. 1983;80:386-387.
Bonsu BK, Harper MB. Fever interval before diagnosis, prior antibiotic treatment, clinical outcome for young children with bacterial meningitis. Clin Infect Dis. 2001;32:566-572.
Brouwer MC, McIntyre P, de Gans J, et al: Corticosteroids for acute bacterial meningitis, Cochrane Database Syst Rev (9):CD004405, 2010.
Byingtom CL, Kendrick J, Sheng X. Normative cerebrospinal fluid profiles in febrile infants. J Pediatr. 2011;158:130-134.
Centers for Disease Control and Prevention. Invasive Haemophilus influenzae type b disease in five young children—Minnesota, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1-3.
Centers for Disease Control and Prevention. Pediatric bacterial meningitis surveillance—African region, 2002–2008. MMWR Morb Mortal Wkly Rep. 2009;58:493-497.
Chandran A, Herbert H, Misurski D, et al. Long-term sequelae of childhood bacterial meningitis. Pediatr Infect Dis J. 2011;30(1):3-6.
Curtis S, Stobart K, Vandermeer B, et al. Clinical features suggestive of meningitis in children: a systematic review of prospective data. Pediatrics. 2010;126:952-960.
De Gans J, Van De Beek D, for the Europena Dexamethasone in Adulthood Bacterial Meningitis Study Investigation. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-1556.
Dubos F, Korczowski B, Aygun DA, et al. Distinguishing between bacterial and aseptic meningitis in children: European comparison of two clinical decision rules. Arch Dis Child. 2010;95:963-967.
Enders A, Pannicke U, Berner R, et al. Two siblings with lethal pneumococcal meningitis in a family with a mutation in interleukin-1 receptor-associated kinase 4. J Pediatr. 2004;145:698-700.
Gessner BD, Sutanto A, Linehan M, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43-52.
Grimwood K, Anderson P, Anderson V, et al. Twelve year outcome following bacterial meningitis: further evidence for persisting effects. Arch Dis Child. 2000;83:111-116.
Healy CM, Baker CJ. The future of meningococcal vaccines. Pediatr Infect Dis J. 2005;24:175-176.
Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244-256.
Kestenbaum LA, Ebberson J, Zorc JJ, et al. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics. 2010;125:257-264.
Kilpi T, Anttila M, Kallio MJ, et al. Length of prediagnostic history related to the course and sequelae of childhood bacterial meningitis. Pediatr Infect Dis J. 1993;12:184-188.
Koster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57:449-454.
Kutz JW, Simon LM, Chennupati SK, et al. Clinical predictors for hearing loss in children with bacterial meningitis. Arch Otolaryngol Head Neck Surg. 2006;132:941-945.
Levy C, Taha MK, Olivier CW, et al. Association of meningococcal phenotypes and genotypes with clinical characteristics and mortality of meningitis in children. Pediatr Infect Dis J. 2010;29:618-623.
McIntyre PB, MacIntyre CR, Gilmour R, et al. A population based study of the impact of corticosteroid therapy and delayed diagnosis on the outcome of childhood pneumococcal meningitis. Arch Dis Child. 2005;90:391-396.
Mongelluzzo J, Mohamed Z, Ten Have TR, et al. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299:2048-2055.
Nigrovic LE, Kuppermann N, Macias CG, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;297:52-60.
Ninis N, Phillips C, Bailey L, et al. The role of healthcare delivery in the outcome of meningococcal disease in children: case-control study of fatal and non-fatal cases. Br Med J. 2005;330:1475-1478.
Peltola H, Roine I, Fernandez J, et al. Adjuvant glycerol and/or dexamethasone to improve the outcomes of childhood bacterial meningitis: a prospective, randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2007;45:1287-1289.
Peltola H, Roine I, Leinonen M, et al. Diagnosis of Streptococcus pneumoniae and Haemophilus influenzae type b meningitis by identifying DNA from cerebrospinal fluid-impregnated filter paper strips. Pediatr Infect Dis J. 2010;29:111-114.
Radetsky M. Duration of symptoms and outcome in bacterial meningitis: An analysis of causation and the implications of a delay in diagnosis. Pediatr Infect Dis J. 1992;11:694-698.
Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435-445.
Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007;44:250-255.
Roine I, Peltola H, Fernàndez J, et al. Influence of admission findings on death and neurological outcome from childhood bacterial meningitis. Clin Infect Dis. 2008;46:1248-1252.
Saha SK, Baqui AH, Darmstadt GL, et al. Invasive Haemophilus influenzae type B diseases in Bangladesh, with increased resistance to antibiotics. J Pediatr. 2005;146:227-233.
Schacham S, Kozer E, Bahat H, et al. Bulging fontanelle in febrile infants: is lumbar puncture mandatory? Arch Dis Child. 2009;94:690-692.
Scheld WM, Koedel U, Nathan B, et al. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis. 2002;186(Suppl 2):S225-S233.
Snape MD, Perrett KP, Ford KJ, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized control trial. JAMA. 2008;299:217-219.
Snape MD, Pollard AJ. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect Dis. 2005;5:21-30.
Swartz MN. Bacterial meningitis—a view of the past 90 years. N Engl J Med. 2004;351:1826-1828.
Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guideline for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
Van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-1859.
Van de Beek D, de Gans J. Dexamethasone in adults with community-acquired bacterial meningitis. Drugs. 2006;66:415-427.
Van de Beek D, de Gans J, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44-52.
Van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol. 2010;9:254-263.
Visintin C, Mugglestone MA, Fields EJ, et al. Management of bacterial meningitis and meningococcal septicemia in children and young people: summary of NICE guidance. BMJ. 2010;341:92-94.
Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495-1502.
595.2 Viral Meningoencephalitis
Etiology
Arboviruses are arthropod-borne agents, responsible for some cases of meningoencephalitis during summer months. Mosquitoes and ticks are the most common vectors, spreading disease to humans and other vertebrates, such as horses, after biting infected birds or small animals. Encephalitis in horses (“blind staggers”) may be the 1st indication of an incipient epidemic. Although rural exposure is most common, urban and suburban outbreaks also are frequent. The most common arboviruses responsible for CNS infection in the USA are West Nile virus (WNV) and St. Louis and California encephalitis viruses (Chapter 259). West Nile virus made its appearance in the Western hemisphere in 1999. It has gradually made its way from the east to the west coast over successive summers. Cumulatively, from 1999 through 2005, a total of 46 states reported roughly 19,000 human infections caused by WNV. WNV may also be transmitted by blood transfusion, organ transplantation, or vertically across the placenta. Most children with WNV are either asymptomatic or have a nonspecific viral-like illness. Approximately 1% develop CNS disease; adults are more severely affected than children.
Several members of the herpes family of viruses can cause meningoencephalitis. Herpes simplex virus type 1 (HSV-1) is an important cause of severe, sporadic encephalitis in children and adults. Brain involvement usually is focal; progression to coma and death occurs in 70% of cases without antiviral therapy. Severe encephalitis with diffuse brain involvement is caused by herpes simplex virus type 2 (HSV-2) in neonates who usually contract the virus from their mothers at delivery. A mild transient form of meningoencephalitis may accompany genital herpes infection in sexually active adolescents; most of these infections are caused by HSV-2. Varicella-zoster virus (VZV) may cause CNS infection in close temporal relationship with chickenpox. The most common manifestation of CNS involvement is cerebellar ataxia, and the most severe is acute encephalitis. After primary infection, VZV becomes latent in spinal and cranial nerve roots and ganglia, expressing itself later as herpes zoster, sometimes with accompanying mild meningoencephalitis. Cytomegalovirus (CMV) infection of the CNS may be part of congenital infection or disseminated disease in immunocompromised hosts, but it does not cause meningoencephalitis in normal infants and children. Epstein-Barr virus (EBV) has been associated with myriad CNS syndromes (Chapter 246). Human herpes virus 6 (HHV-6) can cause encephalitis, especially among immunocompromised hosts.
Diagnosis
The diagnosis of viral encephalitis is usually made on the basis of the clinical presentation of nonspecific prodrome followed by progressive CNS symptoms. The diagnosis is supported by examination of the CSF, which usually shows a mild mononuclear predominance (see Table 595-1). Other tests of potential value in the evaluation of patients with suspected viral meningoencephalitis include an electroencephalogram (EEG) and neuroimaging studies. The EEG typically shows diffuse slow-wave activity, usually without focal changes. Neuroimaging studies (CT or MRI) may show swelling of the brain parenchyma. Focal seizures or focal findings on EEG, CT, or MRI, especially involving the temporal lobes, suggest HSV encephalitis.
Differential Diagnosis
A number of clinical conditions that cause CNS inflammation mimic viral meningoencephalitis (see Table 595-2). The most important group of alternative infectious agents to consider is bacteria. Most children with acute bacterial meningitis appear more critically ill than those with CNS viral infection. Parameningeal bacterial infections, such as brain abscess or subdural or epidural empyema, may have features similar to viral CNS infections. Infections caused by M. tuberculosis, T. pallidum (syphilis), B. burgdorferi (Lyme disease), and Bartonella henselae, the bacillus associated with cat scratch disease, tend to result in indolent courses. Analysis of CSF and appropriate serologic tests are necessary to differentiate these various pathogens.
Treatment
With the exception of the use of acyclovir for HSV encephalitis (Chapter 244), treatment of viral meningoencephalitis is supportive. Treatment of mild disease may require only symptomatic relief. Headache and hyperesthesia are treated with rest, non–aspirin-containing analgesics, and a reduction in room light, noise, and visitors. Acetaminophen is recommended for fever. Codeine, morphine, and medications to reduce nausea may be useful, but if possible, their use in children should be minimized because they may induce misleading signs and symptoms. Intravenous fluids are occasionally necessary because of poor oral intake. More severe disease may require hospitalization and intensive care.
It is important to monitor patients with severe encephalitis closely for convulsions, cerebral edema, inadequate respiratory exchange, disturbed fluid and electrolyte balance, aspiration and asphyxia, and cardiac or respiratory arrest of central origin. In patients with evidence of increased ICP, placement of a pressure transducer in the epidural space may be indicated. The risks of cardiac and respiratory failure or arrest are high with severe disease. All fluids, electrolytes, and medications are initially given parenterally. In prolonged states of coma, parenteral alimentation is indicated. SIADH is common in acute CNS disorders; monitoring of serum sodium concentrations is required for early detection (Chapter 553). Normal blood levels of glucose, magnesium, and calcium must be maintained to minimize the likelihood of convulsions. If cerebral edema or seizures become evident, vigorous treatment should be instituted.
Amin R, Ford-Jones E, Richardson SE, et al. Acute childhood encephalitis and encephalopathy associated with influenza. Pediatr Infect Dis J. 2008;27:390-394.
Arnold JC, Singh KK, Milder E, et al. Human metapneumovirus associated with central nervous system infection in children. Pediatr Infect Dis J. 2009;28:1057-1060.
Bernit E, de Lamballerie X, Zandotti C, et al. Prospective investigation of a large outbreak of meningitis due to echovirus 30 during summer 2000 in Marseilles, France. Medicine. 2004;83:245-253.
Centers for Disease Control and Prevention. West Nile Virus update—United States, January 1-August 19, 2008. MMWR Morb Mortal Wkly Rep. 2008;57:899-900.
Centers for Disease Control and Prevention. Japanese encephalitis among three U.S. travelers returning from Asia, 2003–2008. MMWR Morb Mortal Wkly Rep. 2009;58:737-739.
Fowlkes AL, Honarmand S, Glaser C, et al. Enterovirus-associated encephalitis in the California Encephalitis Project, 1998–2005. J Infect Dis. 2008;198:1685-1691.
Glaser CA, Gilliam S, Schnurr D, et al. California Encephalitis Project, 1998–2000. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis. 2003;36:731-742.
Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565-1577.
Hayes EB, O’Leary DR. West Nile infection: a pediatric perspective. Pediatrics. 2004;113:1375-1381.
Kneen R, Jakka S, Mithyantha R, et al. The management of infants and children treated with acyclovir for suspected viral encephalitis. Arch Dis Child. 2010;95:100-106.
Lindsey NP, Hayes EB, Staples JE, et al. West Nile virus disease in children, United States, 1999–2007. Pediatrics. 2009;123:e1084-e1089.
Logan SAE, MacMahon E. Viral meningitis. BMJ. 2008;336:36-40.
Murray K, Walker C, Herrington E, et al. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2-4.
Rorabaugh ML, Berlin LE, Heldrich F, et al. Aseptic meningitis in infants younger than 2 years of age: acute illness and neurologic complications. Pediatrics. 1993;92:206-211.
Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370-378.
Tyler KL. West Nile virus encephalitis in America. N Engl J Med. 2001;344:1858-1859.
Verboon-Maciolek MA, Groenendaal F, Hahn CD, et al. Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol. 2008;64:266-273.
Yim R, Posfay-Barbe KM, Nolt D, et al. Spectrum of clinical manifestations of West Nile virus infection in children. Pediatrics. 2004;114:1673-1675.
595.3 Eosinophilic Meningitis
Etiology
Although any tissue-migrating helminth may cause eosinophilic meningitis, the most common cause is human infection with the rat lungworm, Angiostrongylus cantonensis (Chapter 289). Other parasites that can cause eosinophilic meningitis include Gnathostoma spinigerum (dog and cat roundworm) (Chapter 289), Baylisascaris procyonis (raccoon roundworm), Ascaris lumbricoides (human roundworm), Trichinella spiralis, Toxocara canis, T. gondii, Paragonimus westermani, Echinococcus granulosus, Schistosoma japonicum, Onchocerca volvulus, and Taenia solium. Eosinophilic meningitis may also occur as an unusual manifestation of more common viral, bacterial, or fungal infections of the CNS. Noninfectious causes of eosinophilic meningitis include multiple sclerosis, malignancy, hypereosinophilic syndrome, or a reaction to medications or a ventriculoperitoneal shunt.
Chotmongkol V, Sawanyawisuth K, Thavornpitak Y. Corticosteroids treatment of eosinophilic meningitis. Clin Infect Dis. 2000;31:660-662.
Hong DS, Bernstein M, Smith C, et al. Eosinophilic meningoencephalitis: psychiatric presentation and treatment. Intl J Psychiatry Med. 2008;38:287-295.
Hsu W, Chen J, Chien C, et al. Eosinophilic meningitis caused by Angiostrongylus cantonensis. Pediatr Infect Dis J. 1990;9:443-445.
Murray WJ, Kazacos KR. Raccoon roundworm encephalitis. Clin Infect Dis. 2004;39:1484-1492.
Ramirez-Avila L, Slome S, Schuster FL, et al. Eosinophilic meningitis due to Angiostrongylus and Gnathostoma species. Clin Infect Dis. 2009;48:322-327.
Weller PF. Eosinophilic meningitis. Am J Med. 1993;95:250-253.