Chapter 2 Cells, Tissues, and Organs of the Immune System
• Most cells of the immune system derive from hemopoietic stem cells. The primary lymphoid organs in mammals are the thymus and bone marrow, where lymphocyte differentiation occurs.
• Phagocytic cells are found in the circulation as monocytes and granulocytes. Monocytes differentiate into macrophages that reside in tissues (e.g. Kupffer cells in the liver). Neutrophils are short-lived phagocytes present in high numbers in the blood and at sites of acute inflammation.
• Eosinophils, basophils, mast cells, and platelets, together with cytokines and complement, take part in the inflammatory response.
• NK cells recognize and kill virus-infected cells and certain tumor cells by inducing apoptosis.
• Antigen-presenting cells link the innate and adaptive immune systems and are required by T cells to enable them to respond to antigens.
• Lymphocytes are heterogeneous phenotypically, functionally, and morphologically.
• B lymphocytes and T lymphocytes express specific antigen receptors called the B cell receptor (BCR) and T cell receptor (TCR) respectively.
• There are three major subpopulations of T cells which have helper, cytotoxic and regulatory activities (TH, TC and Treg).
• B cells can differentiate into antibody-secreting plasma cells and memory cells.
• T cells developing in the thymus are subject to positive and negative selection processes.
• Mammalian B cells develop mainly in the fetal liver and from birth onwards in the bone marrow. This process continues throughout life. B cells also undergo a negative selection process at the site of B cell generation.
• Lymphocytes migrate to, and function in, the secondary lymphoid organs and tissues.
• Secondary lymphoid organs and tissue protect different body sites – the spleen responds to blood borne organisms; the lymph nodes respond to lymph-borne antigens; and the mucosa-associated lymphoid tissue (MALT) protects the mucosal surfaces.
• Most lymphocytes recirculate around the body; there is continuous lymphocyte traffic from the blood stream into lymphoid tissues and back again into the blood via the thoracic duct and right lymphatic duct.
Cells of the immune system
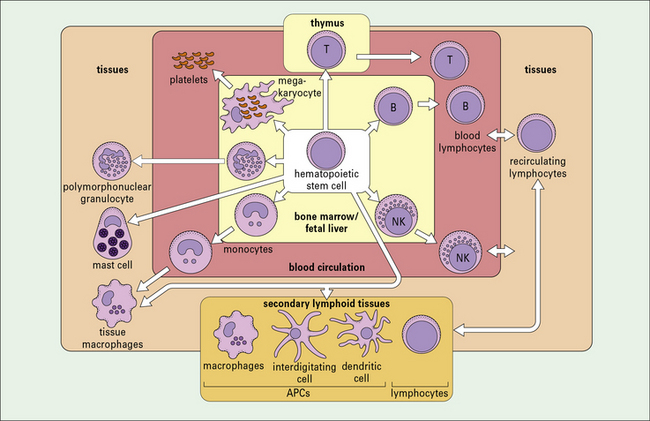
There is great heterogeneity in the cells of the immune system, most of which originate from hematopoietic stem cells in the fetal liver and in the postnatal bone marrow – mainly in the vertebrae, sternum, ribs, femur and tibia (Fig. 2.1). This morphological heterogeneity reflects the fact that cells of the immune system are called on to provide a wide variety of functions including:
Cells of the innate immune system include monocytes/macrophages, polymorphonuclear granulocytes, NK cells, mast cells, and platelets
Phagocytic cells of the innate immune system belong to the myeloid lineage and include:
• the monocytes: circulating blood cells;
• the macrophages: differentiated from monocytes and residing in various tissues;
• the polymorphonuclear granulocytes (polymorphonuclear neutrophils [PMNs], basophils, and eosinophils): circulating blood cells.
All phagocytic cells are mainly involved in defense from extracellular microbes.
Mast cells and platelets are pivotal in inducing and maintaining inflammation.
Microbes express various cell surface and intracellular molecules called pathogen-associated molecular patterns (PAMPs). Cells of the innate system recognize microbes through their receptors to PAMPs called pattern recognition receptors (PRR). PRR have broad specificity and a non-clonal distribution, features which distinguish them from the specific antigen receptors of the adaptive immune system (see Chapter 6).
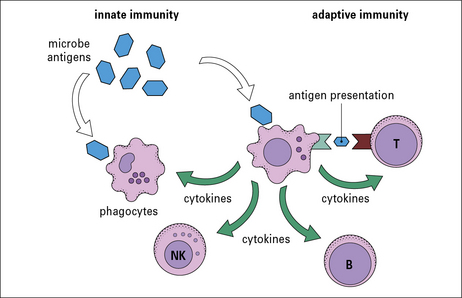
Antigen-presenting cells (APCs) link the innate and adaptive immune systems
A specialized group of cells termed antigen-presenting cells (APCs) link the innate and adaptive immune systems by taking up and processing antigens so they can be recognized by T cells, and by producing cytokines. APCs enhance innate immune cell function and they are essential for activation of T cells (Fig. 2.2).
Adaptive immune system cells are lymphocytes
Lymphocytes (T and B cells) recognize antigens through clonally expressed, highly specific antigen receptors (see Chapters 3 and Chapter 5). T cells are produced in the thymus (see Fig. 2.1) and require antigen to be processed and presented to them by specialized APCs.
• the thymus – the site of T cell development;
• the fetal liver and postnatal bone marrow – the sites of B cell development.
Myeloid cells
Mononuclear phagocytes and polymorphonuclear granulocytes are the two major phagocyte lineages
• the neutrophils, also called polymorphonuclear neutrophils (PMNs), are most numerous and constitute the majority of leukocytes (white blood cells) in the blood stream (around 60–70% in adults);
• the primary actions of eosinophils and basophils, which can both function as phagocytes, involve granule release (exocytosis).
The mononuclear phagocytes and polymorphonuclear granulocytes develop from a common precursor.
Mononuclear phagocytes are widely distributed throughout the body
Cells of the mononuclear phagocytic system are found in virtually all organs of the body where the local microenvironment determines their morphology and functional characteristics, e.g. in the lung as alveolar macrophages, in kidney as glomerular mesangial cells, and in the liver as Kupffer cells (Fig. 2.3 and see Fig. 1.2).
• is large (10–18 μm in diameter) relative to the lymphocyte;
• has a horseshoe-shaped nucleus;
• contains primary azurophilic (blue-staining) granules; and
• possesses ruffled membranes, a well-developed Golgi complex, and many intracytoplasmic lysosomes (Fig. 2.4).
Microbial adherence occurs through pattern recognition receptors (see Chapters 6 and 7), followed by phagocytosis. Coating microbes with complement components and/or antibodies (opsonization) enhances phagocytosis by monocytes/macrophages and is mediated by specialized complement receptors and antibody receptors expressed by the phagocytic cells (see Chapters 3 and 4).
There are three different types of polymorphonuclear granulocyte
• are released from the bone marrow at a rate of around 7 million per minute;
• are short-lived (2–3 days) relative to monocytes/macrophages, which may live for months or years.
Like monocytes, PMNs marginate (adhere to endothelial cells lining the blood vessels) and extravasate by squeezing between the endothelial cells to leave the circulation (see Fig. 1.17) to reach the site of infection in tissues. This process is known as diapedesis. Adhesion is mediated by receptors on the granulocytes and ligands on the endothelial cells, and is promoted by chemo-attractants (chemokines) such as interleukin-8 (IL-8) (see Chapter 6).
The importance of granulocytes is evident from the observation of individuals who have a reduced number of white cells or who have rare genetic defects that prevent polymorph extravasation in response to chemotactic stimuli (see Chapter 16). These individuals have a markedly increased susceptibility to bacterial and fungal infection.
Neutrophils comprise over 95% of the circulating granulocytes
Neutrophils have a characteristic multilobed nucleus and are 10–20 μm in diameter (Fig. 2.5). Chemotactic agents attracting neutrophils to the site of infection include:
• protein fragments released when complement is activated (e.g. C5a);
• factors derived from the fibrinolytic and kinin systems;
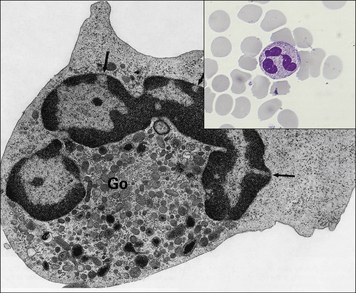
Fig. 2.5 Morphology of the neutrophil
(From Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
• the primary (azurophilic) granules are lysosomes containing acid hydrolases, myeloperoxidase, and muramidase (lysozyme); they also contain the antimicrobial proteins including defensins, seprocidins, cathelicidins, and bacterial permeability inducing (BPI) protein; and
• the secondary granules (specific to neutrophils) contain lactoferrin and lysozyme (see Fig. 2.5).
Neutrophils can also release granules and cytotoxic substances extracellularly when they are activated by immune complexes (antibodies bound to their specific antigen molecules) through their Fc receptors. This is an important example of collaboration between the innate and adaptive immune systems, and may be an important pathogenetic mechanism in immune complex diseases (type III hypersensitivity, see Chapter 25).
Granulocytes and mononuclear phagocytes develop from a common precursor
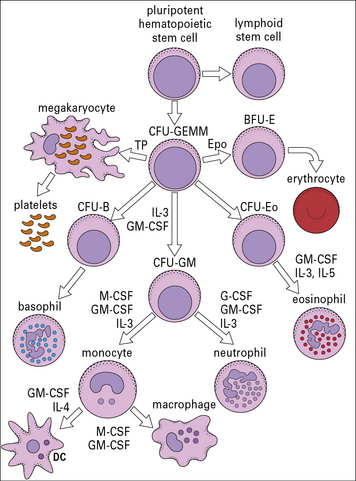
Studies in which colonies have been grown in vitro from individual stems cells have shown that the progenitor of the myeloid lineage (CFU-GEMM) can give rise to granulocytes, monocytes and megakaryocytes (Fig. 2.6). Monocytes and neutrophils develop from a common precursor cell, the CFU-granulocyte macrophage cells (CFU-GMs) (see Fig. 2.6). Myelopoiesis (the development of myeloid cells) commences in the liver of the human fetus at about 6 weeks of gestation.
CFU-GEMMs mature under the influence of colony-stimulating factors (CSFs) and several interleukins (see Fig. 2.6). These factors, which are relevant for the positive regulation of hemopoiesis, are:
• derived mainly from stromal cells (connective tissue cells) in the bone marrow;
• also produced by mature forms of differentiated myeloid and lymphoid cells.
Neutrophils express adhesion molecules and receptors involved in phagocytosis
Other surface molecules acquired during the differentiation process include:
• adhesion molecules (e.g. the leukocyte integrins CD11a, b, and c, associated with CD18 β2 chains); and
• receptors involved in phagocytosis including complement and antibody Fc receptors.
Neutrophils constitutively express FcγRIII and FcγRII, and FcγRI is induced on activation.
Activation of neutrophils by cytokines and chemokines is also a prerequisite for their migration into tissues (see Chapter 9).
Eosinophils, basophils, mast cells and platelets in inflammation
Eosinophils are thought to play a role in immunity to parasitic worms
Eosinophils comprise 2–5% of blood leukocytes in healthy, non-allergic individuals. Human blood eosinophils usually have a bilobed nucleus and many cytoplasmic granules, which stain with acidic dyes such as eosin (Fig. 2.7). Although not their primary function, eosinophils appear to be capable of phagocytosing and killing ingested microorganisms.
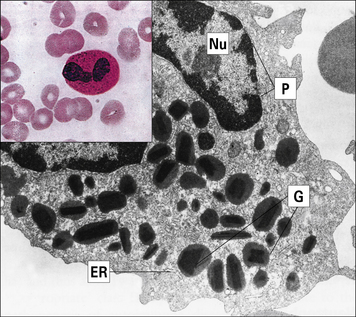
Fig. 2.7 Morphology of the eosinophil
(From Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
The granules in mature eosinophils are membrane-bound organelles with crystalloid cores that differ in electron density from the surrounding matrix (see Fig. 2.7). The crystalloid core contains the major basic protein (MBP), which:
• is a potent toxin for helminth worms;
• induces histamine release from mast cells;
Other proteins with similar effects are found in the granule matrix, for example:
Release of the granules on eosinophil activation is the only way in which eosinophils can kill large pathogens (e.g. schistosomula), which cannot be phagocytosed. Eosinophils are therefore thought to play a specialized role in immunity to parasitic worms using this mechanism (see Fig. 15.13).
Basophils and mast cells play a role in immunity against parasites
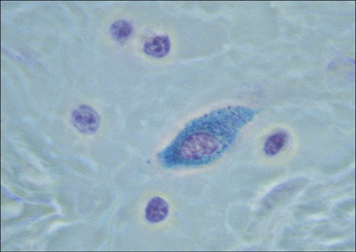
Basophils are found in very small numbers in the circulation and account for less than 0.2% of leukocytes (Fig. 2.8).
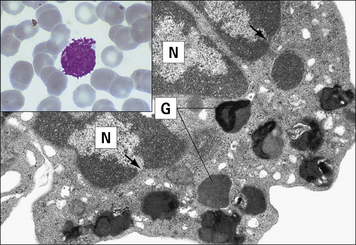
Fig. 2.8 Morphology of the basophil
(Adapted from Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
The mast cell (Fig. 2.9), which is present in tissues and not in the circulation, is indistinguishable from the basophil in a number of its characteristics, but displays some distinctive morphological features (Fig. 2.10). Their shared functions may indicate a convergent differentiation pathway.
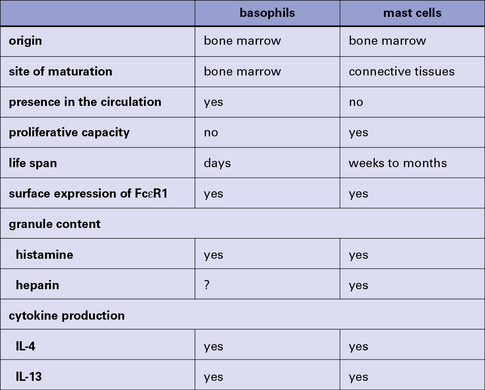
Fig. 2.10 Some distinctive and common characteristics of basophils and mast cells
Some distinctive and common characteristics of basophils and mast cells.
The stimulus for mast cell or basophil degranulation is often an allergen (i.e. an antigen causing an allergic reaction). To be effective, an allergen must cross-link IgE molecules bound to the surface of the mast cell or basophil via its high-affinity Fc receptors for IgE (FcεRI). Degranulation of a basophil or mast cell results in all contents of the granules being released very rapidly. This occurs by intracytoplasmic fusion of the granules, followed by discharge of their contents (Fig. 2.11).
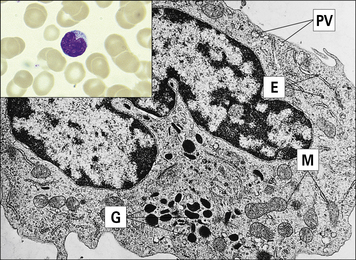
Platelets have a role in clotting and inflammation
Blood platelets (Fig. 2.12) are not cells, but cell fragments derived from megakaryocytes in the bone marrow. They contain granules, microtubules, and actin/myosin filaments, which are involved in clot contraction. Platelets also participate in immune responses, especially in inflammation.
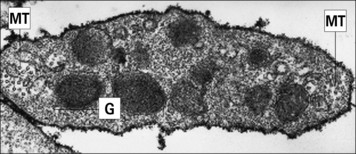
Fig. 2.12 Ultrastructure of a platelet
(Adapted from Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
• receptors for clotting factors (e.g. factor VIII); and
• other molecules important for their function, such as the GpIIb/IIIa complex (CD41) responsible for binding to fibrinogen, fibronectin, vitronectin (tissue matrix), and von Willebrand factor (another clotting factor).
Both receptors and adhesion molecules are important in the activation of platelets.
NK cells
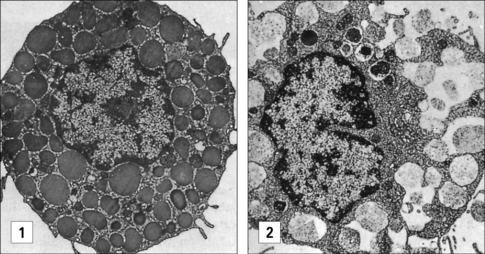
NK cells account for up to 15% of blood lymphocytes and express neither T cell nor B cell antigen receptors. They are derived from the bone marrow and morphologically have the appearance of large granular lymphocytes (see Fig. 2.19).
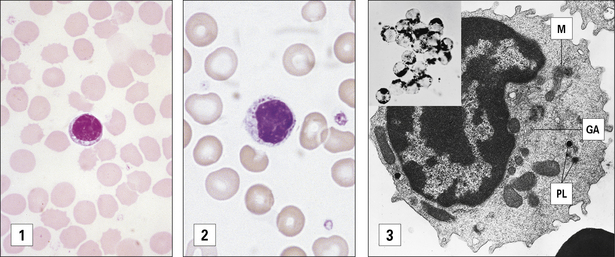
Fig. 2.19 Morphological heterogeneity of lymphocytes
((1) Adapted from Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003. (2) Courtesy of Dr A Stevens and Professor J Lowe.)
Nevertheless many surface markers are shared with T cells, monocytes/macrophages or neutrophils.
CD16 and CD56 are important markers of NK cells
Resting NK cells also express the β chain of the IL-2 receptor, and the signal transducing common γ chain of IL-2 and other cytokine receptors (see Fig. 8.18). Therefore, direct stimulation with IL-2 activates NK cells.
The function of NK cells is to recognize and kill virus-infected cells (Fig. 2.13) and certain tumor cells by mechanisms described in chapter 10.
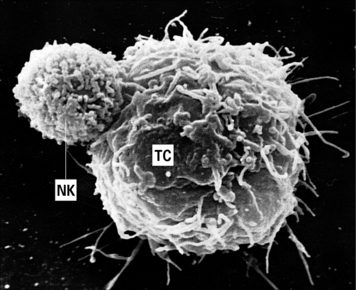
Fig. 2.13 An NK cell attached to a target cell
An NK cell (NK) attached to a target cell (TC). × 4500.
(Courtesy of Dr G Arancia and W Malorni, Rome.)
Classical and non-classical MHC class I molecules (see Fig. 5.15) are ligands for inhibitory receptors on the NK cells which prevent killing and this explains why normal body cells (all of which normally express MHC class I molecules) are not targeted by NK cells.
Antigen presenting cells
APCs are a heterogeneous population of leukocytes that are important in innate immunity (see Fig. 2.2) and play a pivotal role in the induction of functional activity of T helper (TH) cells.
Both macrophages and B cells are rich in membrane MHC class II molecules, especially after activation, and are thus able to process and present specific antigens to (activated) T cells (see Chapter 8).
Somatic cells other than immune cells do not normally express class II MHC molecules, but cytokines such as IFNγ and tumor necrosis factor-α (TNFα) can induce the expression of class II molecules on some cell types, and thus allow them to present antigen (non-professional APCs). This induction of ‘inappropriate’ class II expression might contribute to the pathogenesis of autoimmune diseases and to prolonged inflammation (see Chapter 20).
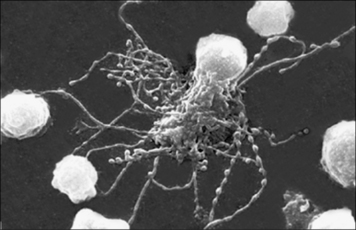
Dendritic cells are derived from several different lineages
Functionally, dendritic cells (DC) are divided into those that both process and present foreign protein antigens to T cells – ‘classical’ dendritic cells (DCs) – and a separate type that passively presents foreign antigen in the form of immune complexes to B cells in lymphoid follicles – follicular dendritic cells (FDCs; Fig. 2.14).
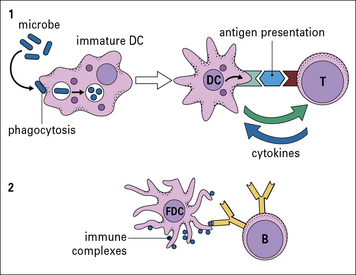
Fig. 2.14 Different kinds of antigen-presenting cells (APCs)
There are two main types of dendritic cells – classical DC and follicular dendritic cells (FDCs). (1) Immature DCs are derived from bone marrow and interact mainly with T cells. They are highly phagocytic, take up microbes, process the foreign microbial antigens into small peptides, and become mature APCs carrying the processed antigen (a peptide) on their surface with specialized MHC molecules. Specific T cells recognize the displayed peptide in a complex with MHC and, in the presence of cytokines produced by the mature DC, proliferate and also produce cytokines. (2) FDCs are not bone marrow derived and interact with B cells. In the B cell follicles of lymphoid organs and tissues they bind small immune complexes (IC, called iccosomes). Antigen contained within the IC is presented to specific B cells in the lymphoid follicles. This protects the B cell from cell death. The B cell then proliferates and with T cell help can leave the follicle and become a plasma cell or a memory cell (see Fig. 2.48).
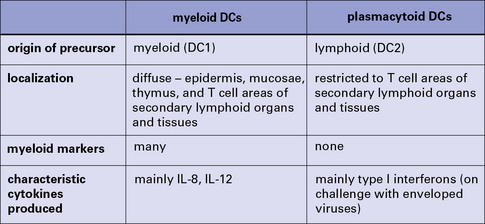
Most DCs derive from one of two precursors:
• a myeloid progenitor (DC1) that gives rise to myeloid DCs, otherwise called bone-marrow derived or bm-DCs; and
• a lymphoid progenitor (DC2) that develops into plasmacytoid DCs (pDCs).
A summary of the main properties of myeloid and plasmacytoid dendritic cells is shown in Figure 2.15.
BM-DCs express various receptors that are involved in antigen uptake:
• C-type lectin receptors – for glycosyl groups, e.g. macrophage mannose receptor (MMR) family;
• receptors for heat shock protein–peptide complexes;
• receptors for apoptotic corpses;
Before DCs take up antigen (become loaded) they are called immature DCs and express various markers characteristic for this, resting, stage, the most important being chemokine receptors CCR1, CCR5 and CCR6. DCs are attracted to the infection site by chemokines through these receptors (see Chapter 6).
Mature DCs loaded with antigen down-regulate expression of CCR1, 5, 6 and up-regulate CCR7. This encourages their migration from various tissues into peripheral lymphatics, where CCR7 interacts with secondary lymphoid tissue chemokine SLC (CCL21) expressed on vascular endothelium (see Fig. 6.15).
Langerhans’ cells and interdigitating dendritic cells are rich in MHC class II molecules
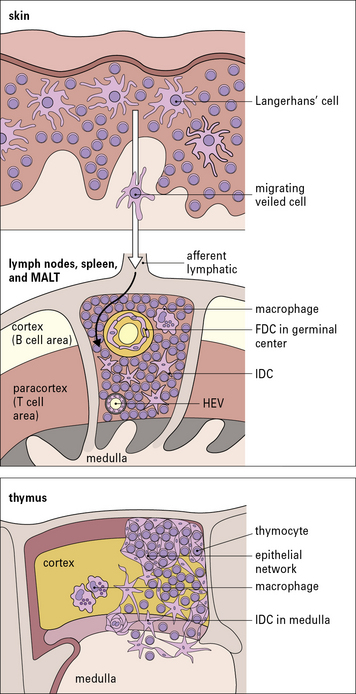
Langerhans’ cells in the epidermis and in other squamous epithelia migrate via the afferent lymphatics into the paracortex of the draining lymph nodes (Fig. 2.16). Here, they interact with T cells and are termed interdigitating cells (IDCs, Fig. 2.17). These DCs are rich in class II MHC molecules, which are important for presenting antigen to helper T cells.
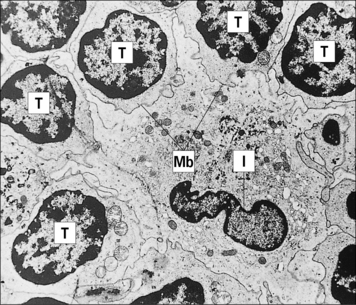
Fig. 2.17 Ultrastructure of an interdigitating dendritic cell (IDC) in the T cell area of a rat lymph node
(Courtesy of Dr BH Balfour.)
BM-DCs are also present within the germinal centers (GCs) of secondary lymphoid follicles (i.e. they are the MHC class II molecule-positive germinal center DCs [GCDCs]). In contrast to FDCs, they are migrating cells, which on arrival in the GC interact with germinal center T cells and are probably involved in antibody class switching (see Chapter 9).
The thymus is of crucial importance in the development and maturation of T cells. In thymus there are cortical DCs and IDCs which are especially abundant in the medulla (see Fig. 2.16). They participate in two important stages in T cell maturation/differentiation in thymus positive and negative selection respectively (see below).
FDCs lack class II MHC molecules and are found in B cell areas
Unlike the APCs that actively process and present protein antigens to T cells, FDCs have a passive role in presenting antigen in the form of immune complexes to B cells. They are therefore found in the primary and secondary follicles of the B cell areas of the lymph nodes, spleen, and MALT (see Fig. 2.16). They are a non-migratory population of cells and form a stable network (a kind of web) by establishing strong intercellular connections via desmosomes.
FDCs lack class II MHC molecules, but bind antigen via complement receptors (CD21 and CD35), which attach to complement associated with immune complexes (iccosomes; Fig. 2.18). They also express Fc receptors. The FDCs produce chemokines that are important in homing of B cells to the follicular areas in lymphoid tissues. They are not bone marrow derived, but are of mesenchymal origin.
Lymphocytes
Lymphocytes are phenotypically and functionally heterogeneous
Many mature lymphoid cells are long-lived, and persist as memory cells for many years.
Lymphocytes are morphologically heterogeneous
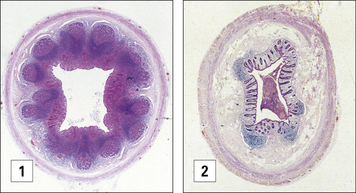
Two distinct morphological types of lymphocyte are seen in the circulation as determined by light microscopy and a hematological stain such as Giemsa (Fig. 2.19):
• the first type is relatively small, is typically agranular and has a high nuclear to cytoplasmic (N:C) ratio (Fig. 2.19[1]);
• the second type is larger, has a lower N:C ratio, contains cytoplasmic azurophilic granules, and is known as the large granular lymphocyte (LGL).
Most T helper (TH) cells (approximately 95%) and a proportion (approximately 50%) of cytotoxic T cells (TC or CTL) have the morphology shown in Figure 2.19(1).
The LGL morphological pattern displayed in Figure 2.19(2) is shown by less than 5% of TH cells and by about 30–50% of TC cells. These cells display LGL morphology with primary lysosomes dispersed in the cytoplasm and a well-developed Golgi apparatus, as shown in Figure 2.19(3).
Most B cells, when resting, have a morphology similar to that seen in Figure 2.19(1) under light microscopy.
Lymphocytes express characteristic surface and cytoplasmic markers
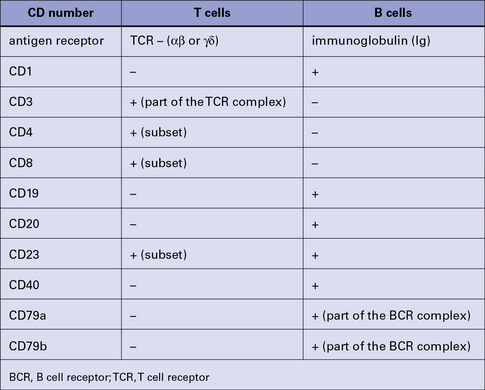
Lymphocytes (and other leukocytes) express a large number of different functionally important molecules mostly on their surfaces but also in their cytoplasm, which can be used to distinguish (‘mark’) cell subsets. Many of these cell markers can be identified by specific monoclonal antibodies (mAb) and can be used to distinguish T cells from B cells (Fig. 2.20).
• one integrin subfamily (the β2-integrins) uses CD18 as the β chain, which can be associated with CD11a, CD11b, CD11c, or αd – these combinations make up the lymphocyte function antigens LFA-1, Mac-1 (CR3), p150, 95, and αdβ2 surface molecules respectively – and are commonly found on leukocytes;
• a second subfamily (the β1-integrins) has CD29 as the β chain, which again is associated with various other peptides and includes the VLA (very late activation) markers.
• the tumor necrosis factor (TNF) and nerve growth factor (NGF) receptor superfamily;
• the C-type lectin superfamily;
• the family of receptors with seven transmembrane segments (tm7); and
• the tetraspanins, a superfamily with four membrane-spanning segments (tm4), for example CD20.
Identification of lymphocyte subsets
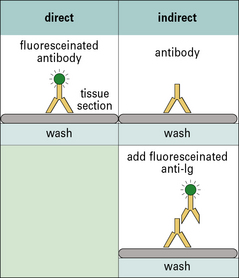
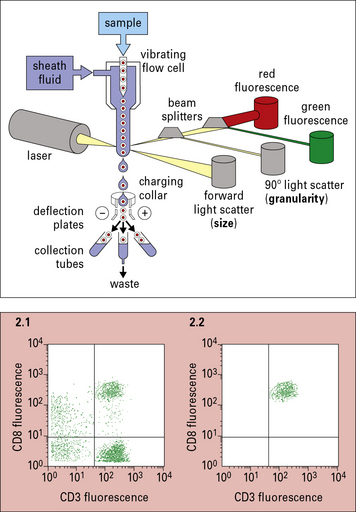
Human lymphocytes can be identified in tissues and their function can be measured as separated populations. Immunofluorescence techniques to identify leukocytes are shown in Method box 2.1.
Method box 2.1 Identification of cell populations
Immunofluorescence
Indirect complement amplified – an elaboration of indirect immunofluorescence
This is an elaboration of the indirect immunofluorescence for the detection of complement-fixing primary antibody. In the second step fresh complement is added, which becomes fixed around the site of antibody binding. Due to the amplification steps in the classical complement pathway (see Chapter 4) one antibody molecule can cause many C3b molecules to bind to the section – these are then visualized with fluoresceinated anti-C3 secondary antibody.
Marker molecules allow lymphocytes to be isolated from each other
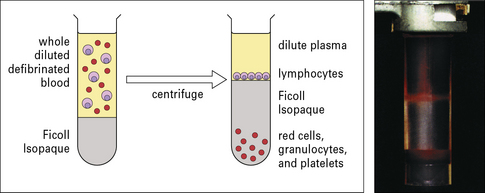
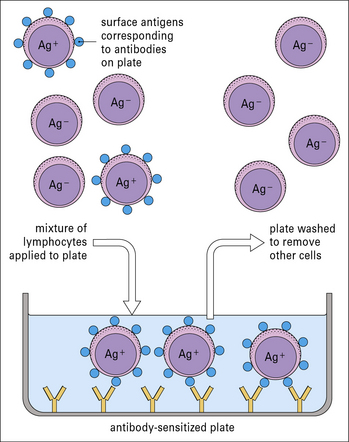
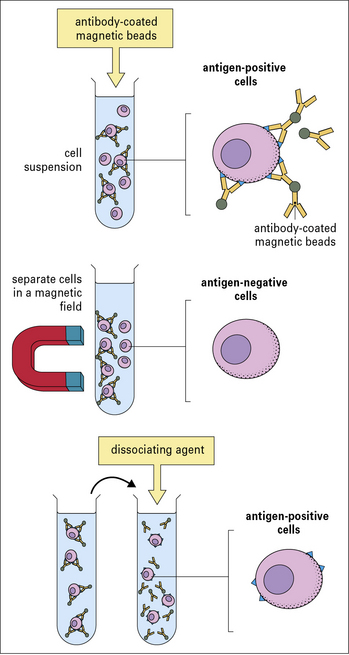
The presence of characteristic surface molecules expressed by cell populations allows them to be identified using fluorescent antibodies as probes. These can be applied to tissue sections to identify cell populations or be used in flow cytometry to enumerate and separate cells in suspension on the basis of their size and fluorescent staining (see Method box 2.1). These techniques together with the expression of surface molecules allowing the cell populations to be isolated from each other using cell panning and immunomagnetic beads (Method box 2.2) have allowed a detailed dissection of lymphoid cell populations.
Method box 2.2 Isolating cell populations
Isolation of lymphocyte subpopulations – panning
Cell separation by immunomagnetic beads
Direct method (shown in Fig. MB2.2.3)
The beads are coated with a monoclonal antibody to the cellular antigen of interest, by either:
Cell sorting
Cells can be isolated by their surface markers using a flow cytometer with a sorting function (cell sorter). The sorter will direct the cells fluorescing due to a specific fluoresceinated antibody attached to them into a collection vessel. Using the example discussed in Method box 2.1, cells with attached FITC-conjugated antibody would be directed to one collection vessel for green cells and cells with attached PE-labeled (orange) antibody – to separate collection vessel for orange cells.
T cells can be distinguished by their different antigen receptors
• a heterodimer of two disulfide-linked polypeptides (α and β);
• a structurally similar heterodimer consisting of γ and δ polypeptides.
Both receptors are associated with a set of five polypeptides (the CD3 complex) and together form the TCR complex (TCR–CD3 complex; see Chapter 5).
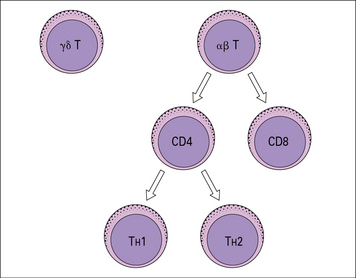
There are three major subpopulations of αβ T cells
• Helper T cells (TH) that express the CD4 marker (CD4+ T cells), and mainly ‘helps’ or ‘induces’ immune responses, divided into two main subsets (TH1 and TH2).
• Regulatory T cells (Tregs) that express the CD4 marker (CD4+T cells) and regulate immune responses.
• Cytotoxic T cells (TC) that express the CD8 marker (CD8+ T cells) – also called cytotoxic T lymphocytes (CTLs).
CD4+ T cells recognize their specific antigens in association with MHC class II molecules, whereas CD8+ T cells recognize antigens in association with MHC class I molecules (see Chapter 7). Thus, the presence of CD4 or CD8 limits (restricts) the type of cell with which the T cell can interact (Fig. 2.21).
T helper subsets are distinguished by their cytokine profiles
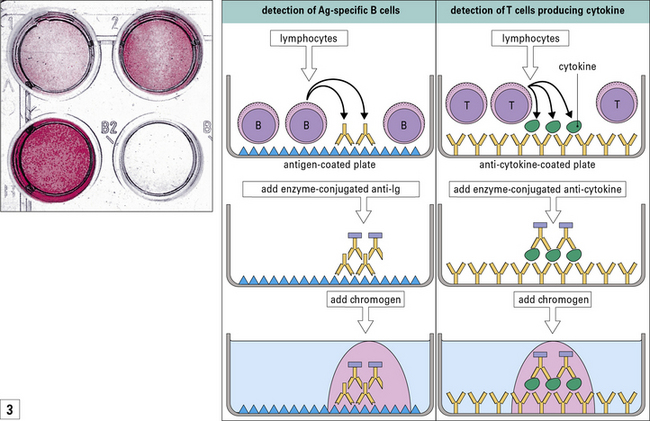
The number of cells producing a given cytokine can be measured using flow cytometry and antibodies that are allowed to penetrate the cells following permeabilization (![]() see Method box 2.1). The same technique can be used to determine the number of B cells producing a particular antibody.
see Method box 2.1). The same technique can be used to determine the number of B cells producing a particular antibody.
The measurement of single cells secreting a particular cytokine or antibody can be achieved using an enzyme-linked method, namely ELISPOT (![]() see Method box 2.1).
see Method box 2.1).
Other T cell subsets include γδ T cells and NKT cells
γδ T cells may protect the mucosal surfaces of the body
γδ T cells have a specific repertoire of TCRs biased towards certain bacterial/viral antigens (superantigens, see Fig. 14.16). Human blood γδ T cells have specificity for low molecular mass mycobacterial products (e.g. ethylamine and isopentenyl pyrophosphate).
γδ T cells display LGL characteristics (see Fig. 2.19) and some have a dendritic morphology in lymphoid tissues (Fig. 2.22). They appear to have a broader specificity for recognition of unconventional antigens such as heat shock proteins, phospholipids and phosphoproteins. Unlike αβ T cells, they do not generally recognize antigens in association with classical MHC class I and II molecules. There is evidence that γδ T cells show cytotoxicity and regulatory functions and subsets of them appear to have specific tissue locations.
NKT cells may initiate T cell responses
NKT cells have T markers and also some NK cell markers: they express CD3 and have a unique αβ TCR (expressing an invariant Vα and Vβ11, see Chapter 5).
NKT cells are thought to recognize glycolipid antigens presented by CD1d molecules (see Chapter 5), but not conventional MHC molecules. In response to antigen they are capable of producing large amounts of IFNγ and IL-4.
B cells recognize antigen using the B cell receptor complex
Most human B cells in peripheral blood express two immunoglobulin isotypes on their surface:
On any B cell, the antigen-binding sites of these IgM and IgD isotypes are identical.
The heterodimers interact with the transmembrane segments of the immunoglobulin receptor (see Fig. 3.1), and, like the separate molecular components of the TCR/CD3 complex (see Fig. 5.2), are involved in cellular activation. Intracellular domains of CD79a/b have immunoreceptor tyrosine-based activation motifs (ITAMs). BCR interaction with specific antigen triggers ITAM phosphorylation and this initiates a downstream cascade of intracellular events leading to the activation-related changes in gene expression.
Other B cell markers include MHC class II antigens and complement and Fc receptors
Most B cells carry MHC class II antigens, which are important for cooperative (cognate) interactions with T cells (see Fig. 5.18).
Fc receptors for exogenous IgG (FcγRII, CD32) are also present on B cells and play a role in negative signaling to the B cell (see Chapter 11).
CD40 is an important molecule on B cells and is involved in cognate interactions between T and B cells (see Fig. 9.6) with T cells expressing CD40 ligand (CD40L).
CD5+ B-1 cells and marginal zone B cells produce natural antibodies
CD5+ B-1 cells have a variety of roles
CD5+ B-1 cells express their immunoglobulins from unmutated or minimally mutated germline genes (see Chapter 3) and produce mostly IgM, but also some IgG and IgA. These so-called natural antibodies are of low avidity, but, unusually, they are polyreactive and are found at high concentration in the adult serum. CD5+ B-1 cells:
• respond well to TI (T-independent) antigens (i.e. antigens that can directly stimulate B cells without T cell help);
• may be involved in antigen processing and antigen presentation to T cells; and
• probably play a role in both tolerance and antibody responses.
Functions proposed for natural antibodies include:
• the first line of defense against microorganisms;
• clearance of damaged self components; and
• regulatory ‘idiotype network’ interactions within the immune system.
Characteristically, natural antibodies react against autoantigens including:
B cells can differentiate into antibody-secreting plasma cells
Under light microscopy, the cytoplasm of the plasma cells is basophilic due to the large amount of RNA being used for antibody synthesis in the rough endoplasmic reticulum. At the ultrastructural level, the rough endoplasmic reticulum can often be seen in parallel arrays (Fig. 2.23).
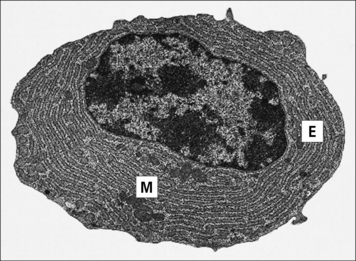
Fig. 2.23 Ultrastructure of the plasma cell
(Adapted from Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
Antibodies produced by a single plasma cell are of one specificity (idiotype) and immunoglobulin class (isotype and allotype; see Chapter 3).
Immunoglobulins can be visualized in the plasma cell cytoplasm by staining with fluorochrome-labeled specific antibodies (Fig. 2.24).
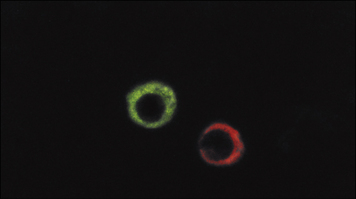
Fig. 2.24 Immunofluorescent staining of intracytoplasmic immunoglobulin in plasma cells
(Adapted from Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
Many plasma cells have a short life span, surviving for a few days and dying by apoptosis (Fig. 2.25). However, a subset of plasma cells with a long life span (months) has recently been described in the bone marrow that might be important in giving rise to sustained antibody responses.
Lymphocyte development
These other cell types are termed accessory cells and include:
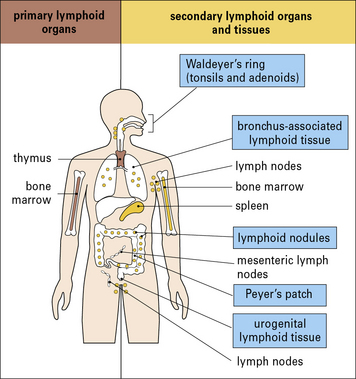
The lymphoid system is arranged into either discrete encapsulated organs or accumulations of diffuse lymphoid tissue, which are classified into primary (central) and secondary (peripheral) organs or tissues (Fig. 2.26).
• are produced, mature, and are selected in primary lymphoid organs; and
• exert their effector functions in the secondary lymphoid organs and tissues.
Lymphoid stem cells develop and mature within primary lymphoid organs
In the primary lymphoid organs, lymphocytes (B and T cells):
In mammals, T cells mature in the thymus and B cells mature in the fetal liver and postnatal bone marrow (see Chapter 9). Birds have a specialized site of B cell generation, the bursa of Fabricius.
In the primary lymphoid organs:
• lymphocytes acquire their repertoire of specific antigen receptors to cope with the antigenic challenges that individuals encounter during their lifetime;
• cells with receptors for autoantigens are mostly eliminated; and
• in the thymus, T cells also ‘learn’ to recognize appropriate self MHC molecules.
There is evidence that some lymphocyte development might occur outside primary lymphoid organs.
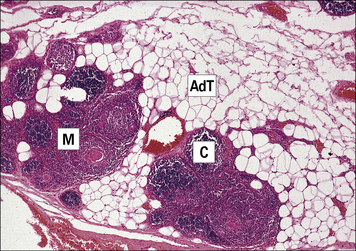
T cells develop in the thymus
Within each lobule, the lymphoid cells (thymocytes) are arranged into:
• an outer tightly packed cortex, which contains the majority of relatively immature proliferating thymocytes; and
• an inner medulla containing more mature cells, implying a differentiation gradient from cortex to medulla (Fig. 2.27).
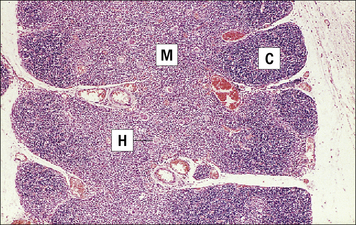
Fig. 2.27 Thymus section showing the lobular organization
(Courtesy of Dr A Stevens and Professor J Lowe.)
The main blood vessels that regulate cell traffic in the thymus are high endothelial venules (HEVs; see Fig. 2.29) at the corticomedullary junction of thymic lobules. It is through these veins that T cell progenitors formed in the fetal liver and bone marrow enter the epithelial anlage and migrate towards the cortex.
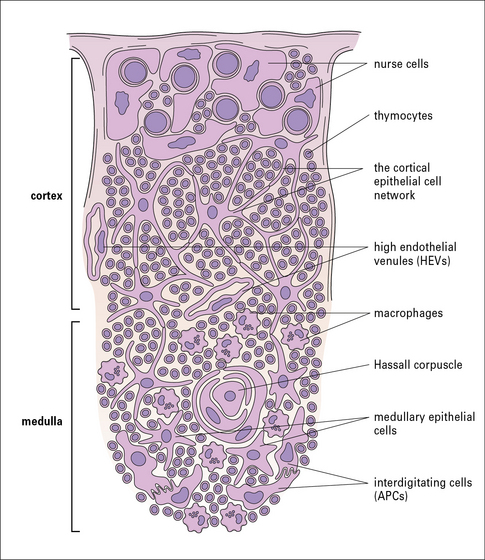
Fig. 2.29 Schematic structure of the thymus
(From Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
The mature T cells probably leave the thymus through the same PCVs, at the corticomedullary junction from which the T cell progenitors entered (Fig. 2.28).
Three types of thymic epithelial cell have important roles in T cell production
• the epithelial nurse cells are in the outer cortex;
• the cortical thymic epithelial cells (TECs) form an epithelial network; and
• the medullary TECs are mostly organized into clusters (Fig. 2.29).
• nurse cells in the outer cortex sustain the proliferation of progenitor T cells, mainly through cytokine production (e.g. IL-7);
• cortical TECs are responsible for the positive selection of maturing thymocytes, allowing survival of cells that recognize MHC class I and II molecules with associated peptides via TCRs of intermediate affinity; and
• medullary TECs display a large variety of organ-specific self peptides through transcription factors such as AIRE (autoimmune regulator).
Hassall’s corpuscles (see Fig. 2.27) are found in the thymic medulla. Their function is unknown, but they appear to contain degenerating epithelial cells rich in high molecular weight cytokeratins.
The mammalian thymus involutes with age (Fig. 2.30). In humans, atrophy begins at puberty and continues throughout life. Thymic involution begins within the cortex and this region may disappear completely, whereas medullary remnants persist.
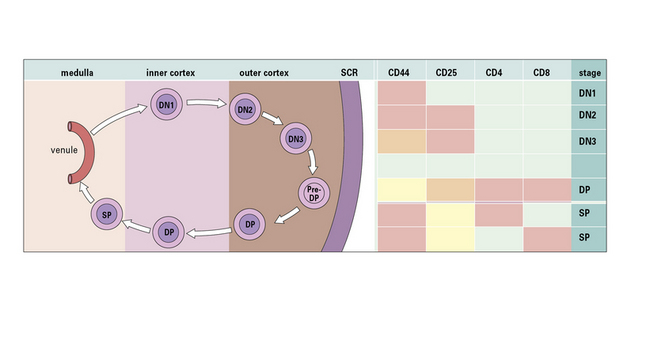
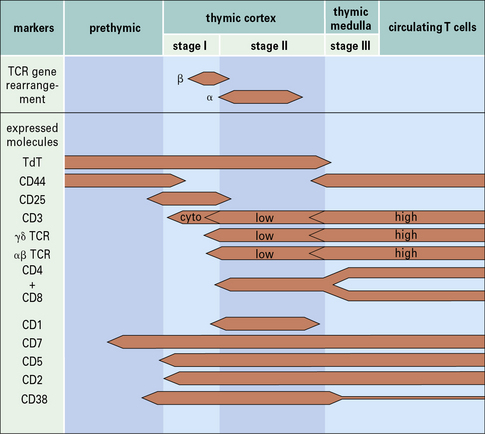
T cells change their phenotype during maturation
Phenotypic analyses have shown sequential changes in surface membrane antigens during T cell maturation (Fig. 2.w1). The phenotypic variations can be simplified into a three-stage model.
Stage I thymocytes are CD4–, CD8–
Stage III thymocytes become either CD4+ or CD8+
Stage III (mature) thymocytes show major phenotypic changes, namely:
• cell surface CD3 associated with the αβ TCR expressed at a higher density;
• the distinction of two subsets of cells expressing either CD4 or CD8 (i.e. single positives).
The T cell receptor is generated during development in the thymus
TCR gene recombination takes place within the subcapsular and outer cortex of the thymus, where there is active cell proliferation. Through a random assortment of different gene segments, a large number of different TCRs are made and thymocytes that fail to make a functional receptor die. The TCRs associate with peptides of the CD3 complex, which transduces activating signals to the cell (see Chapter 5).
Positive and negative selection of developing T cells takes place in the thymus
The processes involved in the education of T cells are shown in Figure 2.31, and self tolerance is discussed fully in Chapter 11. Positive selection ensures only TCRs with an intermediate affinity for self MHC develop further.
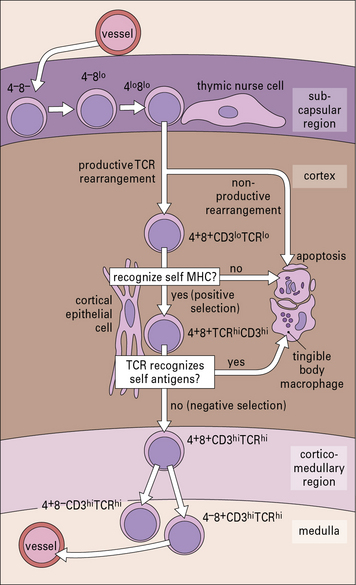
Fig. 2.31 T cell differentiation within the thymus
(Adapted from D Zucker-Franklin, CE Grossi, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
• recognize antigenic peptides only when presented by self MHC molecules on APCs; and
• show ‘dual recognition’ of both the antigenic peptides and the polymorphic part of the MHC molecules.
Only T cells that fail to recognize self antigen are allowed to proceed in their development. The rest undergo apoptosis and are destroyed. These, and all the other apoptotic cells generated in the thymus, are phagocytosed by (tingible body) macrophages (see Fig. 2.45) in the deep cortex.
Less than 5% of thymocytes leave the thymus as mature T cells. The rest die as the result of:
Negative selection may also occur outside the thymus in peripheral lymphoid tissues
Given the survival of some self-reacting T cells, a separate mechanism is required to prevent them attacking the body. Experiments with transgenic mice have suggested that peripheral inactivation of self-reactive T cells (peripheral tolerance, see Chapter 19) could occur via several mechanisms as follows:
Regulatory T cells are involved in peripheral tolerance
• constitutively express CD25 (the α chain of the receptor for IL-2);
• constitute about 5–10% of the peripheral CD4+ T cells;
• express the unique transcription factor FoxP3;
• constitutively express the marker CTLA4;
• do not proliferate in response to antigenic challenge;
• are thought to produce their suppressive effects through cell contact (e.g. with APCs, TH1 or TH2 cells).
B cells develop mainly in the fetal liver and bone marrow
Unlike birds, which have a discrete organ for the generation of B cells (the bursa of Fabricius), in mammals B cells develop directly from lymphoid stem cells in the hematopoietic tissue of the fetal liver (Fig. 2.32). This occurs at 8–9 weeks of gestation in humans, and by about 14 days in the mouse. Later, the site of B cell production moves from the liver to the bone marrow, where it continues through adult life. This fetal liver-bone marrow migration of stem cells is also true for cells of other hematopoietic lineages such as erythrocytes, granulocytes, monocytes, and platelets. The B cell lineage specifier is the transcription factor PAX5 which is expressed initially in B cell precursors but then at all stages of B cell development
B cell production in the bone marrow does not occur in distinct domains
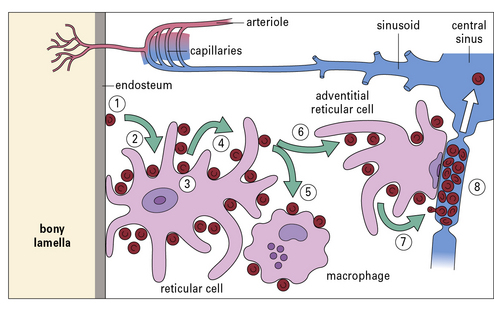
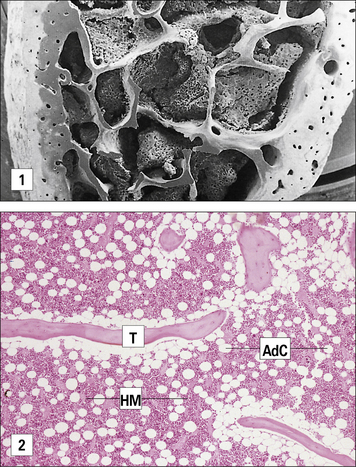
B cell progenitors in the bone marrow are seen adjacent to the endosteum of the bone lamellae (Fig. 2.33). Each B cell progenitor, at the stage of immunoglobulin gene rearrangement may produce up to 64 progeny. The progeny migrate towards the center of each cavity of the spongy bone and reach the lumen of a venous sinusoid (Fig. 2.34).
B cells are subject to selection processes
Many self-reactive B cells are also eliminated through negative selection in the bone marrow.
Immunoglobulins are the definitive B cell lineage markers
Lymphoid stem cells expressing terminal deoxynucleotidyl transferase (TdT) proliferate, differentiate, and undergo immunoglobulin gene rearrangements to emerge as pre-B cells (see Chapters 3 and 9). A sequence of immunoglobulin gene rearrangements and phenotypic changes takes place during B cell ontogeny (see Chapter 8), similar to that described above for T cells.
B cells migrate to and function in the secondary lymphoid tissues
Some CD5+ B cells are also found in the marginal zone of the spleen and mantle zone of secondary follicles in adult lymph nodes (see Fig. 2.42).
Lymphoid organs
Lymphoid organs and tissues protect different body sites
The systemic lymphoid organs and the mucosal system have different functions in immunity:
• the spleen is responsive to blood-borne antigens, and patients who have had their spleen removed are much more susceptible to pathogens that reach the blood stream;
• the lymph nodes protect the body from antigens that come from skin or from internal surfaces and are transported via the lymphatic vessels;
• the intestinal tract – gut-associated lymphoid tissue (GALT);
• the respiratory tract – bronchus-associated lymphoid tissue (BALT); and
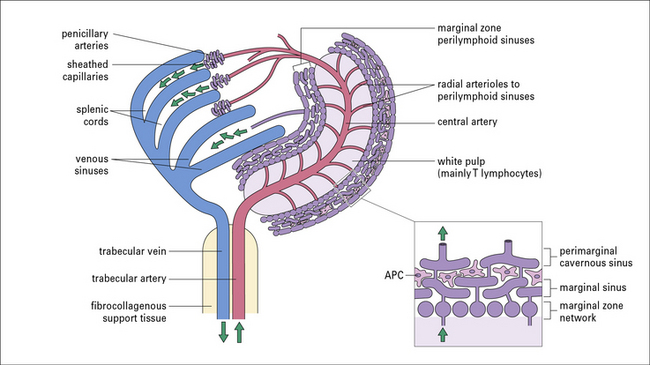
The spleen is made up of white pulp, red pulp, and a marginal zone
A third compartment, the marginal zone, is located at the outer limit of the white pulp.
The white pulp consists of lymphoid tissue
The white pulp of the spleen consists of lymphoid tissue, the bulk of which is arranged around a central arteriole to form the periarteriolar lymphoid sheaths (PALS; Fig. 2.35). PALS are composed of T and B cell areas:
• the T cells are found around the central arteriole;
• the B cells may be organized into either primary ‘unstimulated’ follicles (aggregates of virgin B cells) or secondary ‘stimulated’ follicles (which possess a germinal center with memory cells).
The red pulp consists of venous sinuses and cellular cords
The venous sinuses and cellular cords of the red pulp contain:
The functions of the spleen are made possible by its vascular organization (Fig. 2.36). Central arteries surrounded by PALS end with arterial capillaries, which open freely into the red pulp cords. Circulating cells can therefore reach these cords and become trapped. Aged platelets and erythrocytes are recognized and phagocytosed by macrophages.
The marginal zone contains B cells, macrophages, and dendritic cells
The marginal zone surrounds the white pulp and exhibits two major features, namely:
• a characteristic vascular organization; and
• unique subsets of resident cells (B cells, macrophages, and dendritic cells).
The blood vessels of the marginal zone form a system of communicating sinuses, which receive blood from branches of the central artery (see Fig. 2.36).
Cells residing in the marginal zone comprise:
• various types of APC – metallophilic macrophages, marginal zone macrophages, dendritic cells;
• a subset of B cells with distinctive phenotype and function – they express high levels of IgM and low or absent IgD and in humans are long-lived recirculating cells;
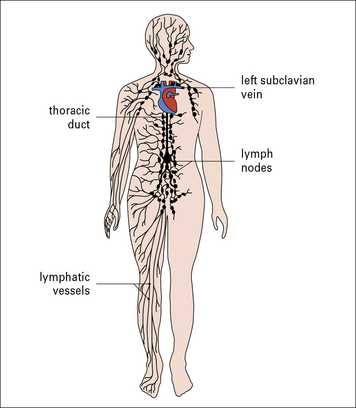
Lymph nodes filter antigens from the interstitial tissue fluid and lymph
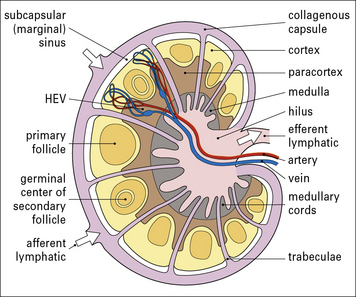
The lymph nodes form part of a network that filters antigens from the interstitial tissue fluid and lymph during its passage from the periphery to the thoracic duct and the other major collecting ducts (Fig. 2.37).
Lymph nodes consist of B and T cell areas and a medulla
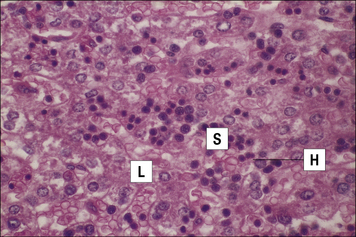
• a T cell area (paracortex); and
• a central medulla, consisting of cellular cords containing T cells, B cells, abundant plasma cells, and macrophages (Figs 2.38–2.40).
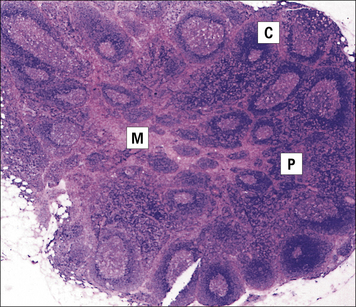
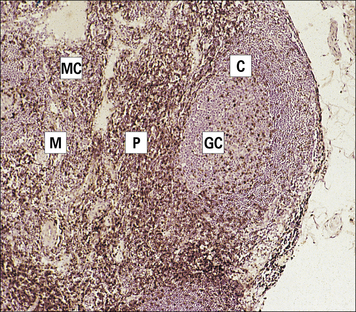
Fig. 2.38 Lymph node section
(Adapted from Zucker-Franklin D, Grossi CE, eds. Atlas of blood cells: function and pathology, 3rd edn. Milan: Edi Ermes; 2003.)
The paracortex contains many APCs (interdigitating cells), which express high levels of MHC class II surface molecules. These are cells migrating from the skin (Langerhans’ cells) or from mucosae (dendritic cells), which transport processed antigens into the lymph nodes from the external and internal surfaces of the body (Fig. 2.41). The bulk of the lymphoid tissue is found in the cortex and paracortex.
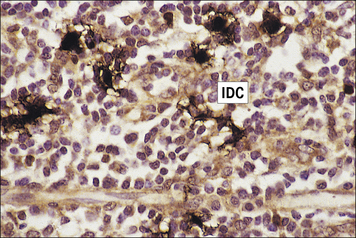
Fig. 2.41 Intedigitating cells in the lymph node paracortex
Interdigitating dendritic cells (IDC; stained dark brown) form contacts with each other and with paracortical T cells (see also Fig. 2.16).
(Courtesy of Dr A Stevens and Professor J Lowe.)
The medulla is organized into cords separated by lymph (medullary) sinuses, which drain into a terminal sinus – the origin of the efferent lymphatic vessel (see Fig. 2.40).
The cortex contains aggregates of B cells in the form of primary or secondary follicles.
Secondary follicles are made up of a germinal center and a mantle zone
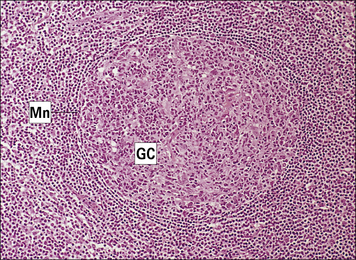
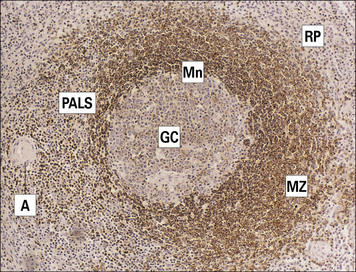
Germinal centers are surrounded by a mantle zone of lymphocytes (Fig. 2.42). Mantle zone B cells (Fig. 2.43) co-express surface IgM, IgD, and CD44. This is taken as evidence that they are naive, actively recirculating B cells.
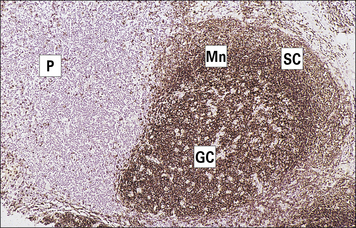
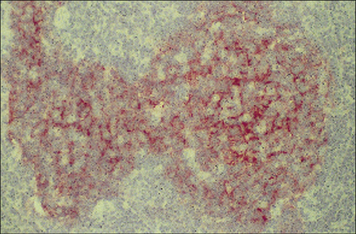
Fig. 2.43 Distribution of B cells in the lymph node cortex
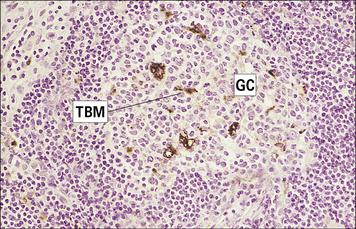
Immunohistochemical staining of B cells for surface immunoglobulin shows that they are concentrated largely in the secondary follicle, germinal centre (GC), mantle zone (Mn), and between the capsule and the follicle – the subcapsular zone (SC). A few B cells are seen in the paracortex (P), which contains mainly T cells (see also Fig. 2.41).
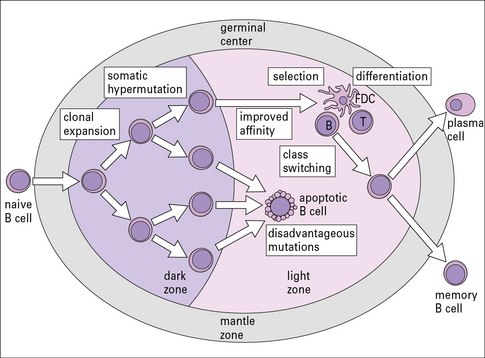
In the germinal centers B cells proliferate, are selected, and differentiate into memory cells plasma cell precursors
The germinal center consists of a dark zone and a light zone:
• the dark zone is the site where one or a few B cells enter the primary lymphoid follicle and undergo active proliferation leading to clonal expansion – these B cells are termed centroblasts. Their immunoglobulin genes undergo a process of somatic hypermutation, which leads to the generation of cells with a wide range of affinities for antigen;
• in the light zone, B cells (centrocytes) encounter the antigen on the surface of FDCs (see Fig. 2.14) and only those cells with higher affinity for antigen survive.
Selected centrocytes interact with germinal center CD4+ TH cells, and their BCRs undergo class switching (i.e. replacement of their originally expressed immunoglobulin heavy chain constant region genes by another class – for instance IgM to IgG or IgA, see Chapter 9).
The selected germinal center B cells differentiate into memory B cells or plasma cell precursors and leave the germinal center (Fig. 2.46).
MALT includes all lymphoid tissues associated with mucosa
Aggregates of encapsulated and non-encapsulated lymphoid tissue are found especially in the lamina propria and submucosal areas of the gastrointestinal, respiratory, and genitourinary tracts (see Fig. 2.26).
Aggregates of lymphoid tissue are also seen lining the bronchi and along the genitourinary tract.
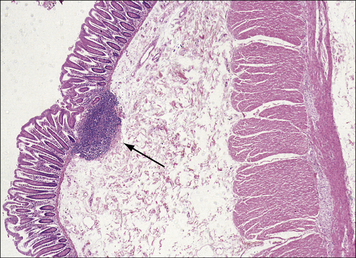
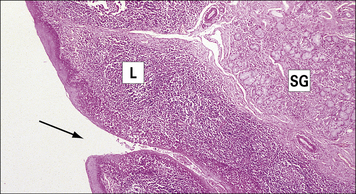
Fig. 2.48 A solitary lymphoid nodule in the large intestine
This nodule is localized in the mucosa and submucosa of the intestinal wall (arrow).
(Courtesy of Dr A Stevens and Professor J Lowe.)
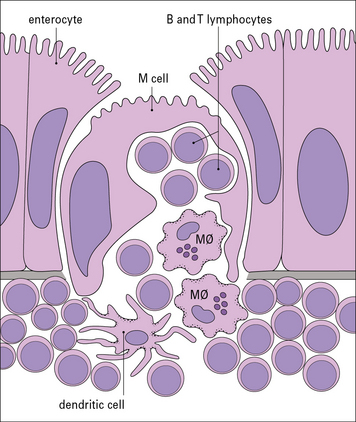
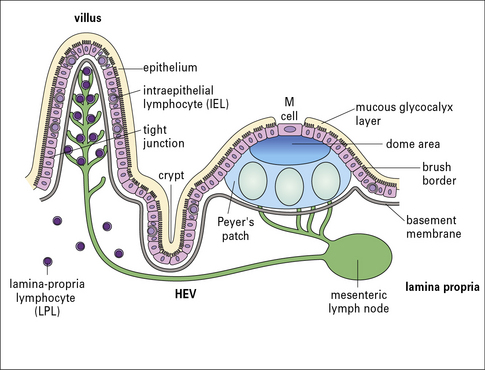
Follicle-associated epithelium is specialized to transport pathogens into the lymphoid tissue
M cells contain deep invaginations in their basolateral plasma membrane, which form pockets containing B and T lymphocytes, dendritic cells, and macrophages (Fig. 2.50). Antigens and microorganisms are transcytosed into the pocket and to the organized mucosal lymphoid tissue under the epithelium (Fig. 2.51) and taken up by the dendritic cells.
Q. The major defense at mucosal surfaces is antibody of the IgA isotype. What characteristics of this antibody would be critical in this respect?
A. The IgA isotype antibody produced at the mucosal level is a specific secretory form that can traverse epithelial membranes and helps prevent the entry of infectious microorganisms. Resistance to digestion by enzymes in the gut would also be an important feature of secretory IgA in GALT. Transport of IgA across mucosal epithelium is described in detail in Chapter 3 (see Fig. 3.8).
Lamina propria and intraepithelial lymphocytes are found in mucosa
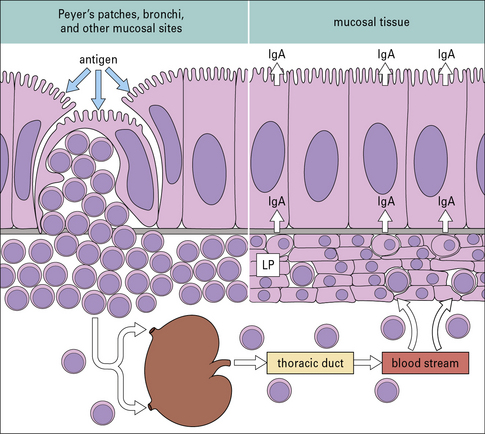
• lamina propria lymphocytes (LPLs) are predominantly activated T cells, but numerous activated B cells and plasma cells are also detected – these plasma cells secrete mainly IgA, which is transported across the epithelial cells and released into the lumen;
• intraepithelial lymphocytes (IELs) are mostly T cells – the population is different from the LPLs because it includes a high proportion of γδ T cells (10–40%) and CD8+ cells (70%).
Lymphocyte recirculation
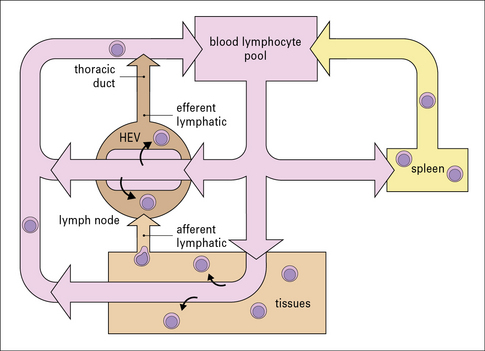
Once in the secondary tissues the lymphocytes do not simply remain there; many move from one lymphoid organ to another via the blood and lymph (Fig. 2.52).
Lymphocytes leave the blood via high endothelial venules
Although some lymphocytes leave the blood through non-specialized venules, the main exit route in mammals is through a specialized section known as high endothelial venules (HEVs; Figs 2.53 and 2.54). In the lymph nodes these are mainly in the paracortex, with fewer in the cortex and none in the medulla.
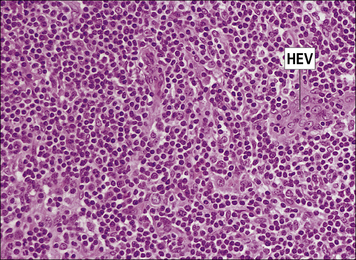
Fig. 2.53 Lymph node paracortex showing high endothelial venules (HEVs)
Lymphocytes leave the circulation through HEVs and enter the node. H&E. × 200.
(Courtesy of Dr A Stevens and Professor J Lowe.)
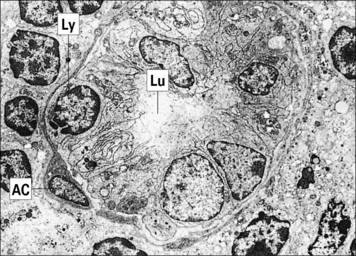
Fig. 2.54 Electron micrograph showing a high endothelial venule (HEV) in the paracortex of a lymph node
Besides lymph nodes, HEVs are also found in MALT and in the thymus (see Fig. 2.29).
The movement of lymphocytes across endothelium is controlled by adhesion molecules and chemokines (see Chapters 6 and 12). For example:
• the adhesion molecule MadCAM-1 is expressed on endothelial cells in intestinal tissues;
• VCAM-1 is present on endothelial cells in the lung and skin.
Homing molecules on lymphocytes selectively direct lymphocytes to particular organs by interaction with these adhesion molecules (see Chapter 6). In the case of the intestine, a critical role is played by α4β7-integrins, which mediate adherence of lymphocytes to HEVs of Peyer’s patches that express MadCAM-1.
Lymphocyte trafficking exposes antigen to a large number of lymphocytes
Antigen stimulation at one mucosal area elicits an antibody response largely restricted to MALT
One reason for considering MALT as a system distinct from the systemic lymphoid organs is that mucosa-associated lymphoid cells mainly recirculate within the mucosal lymphoid system. Thus, lymphoid cells stimulated in Peyer’s patches pass via regional lymph nodes to the blood stream and then ‘home’ back into the intestinal lamina propria (Fig. 2.55 and see Fig. 2.51).
Critical thinking: Development of the immune system (see p. 433 for explanations)
1. What effect would you expect this defect to have on numbers and types of lymphocytes in the blood? How would this affect the structure of the lymph nodes? What effect would this have on the ability of the mice to fight infections?
2. What effect would you expect adult thymectomy to have on the ability of such patients to fight infections?
3. RAG-1? (RAG-1 and RAG-2 genes are involved in the recombination processes that generate antigen receptors on B and T cells.)
Kindt T.J., Osborne B.A., Goldsby R.A. Kuby Immunology, 6th edn. Oxford: WH Freeman; 2006.
Mestecky J, Bienenstock J. Lamm M.E., Strober W., McGhee J.R., Mayer L. Mucosal Immunology. San Diego: Academic Press, 2005.
Steinman R.M., Odoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17.
Lydyard P.M., Whelan A., Fanger M.W. Instant Notes in Immunology, 2nd edn. London: Garland Science/Bios Scientific Publishing; 2004.
Playfair J.H.L., Chain B.M. Immunology at a Glance, 9th edn. Wiley-Blackwell; 2009.
Reddy K.V., Yedery R.D., Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24:536–547.
Roitt I.M., Delves P. Essential Immunology, 10th edn. Oxford: Blackwell Scientific Publications; 2001.
Shevach E. Regulatory/suppressor T cells in health and disease. Arthritis Rheum. 2004;50:2721–2724.
Von Andrian U.H., Mempel T.R. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878.