Stem Cells in Acute Liver Failure
The potential use of stem cells as therapy for failing organ systems is being explored in diverse organ and tissue injury areas. It has been shown that bone marrow-derived stem cells can transdifferentiate into a variety of adult cell types, including hepatocytes [1–5]. Applications for hematopoietic stem cells and cytokines aimed at mobilizing stem cells in other organs have been assessed with benefit shown in myocardial ischemia [6,7] and acute kidney injury [8]. The aim of this review is to assess the emerging evidence for the role of stem cells in assisting the acutely failing liver.
Acute liver failure (ALF) occurs in approximately 2000 individuals each year within the United States and requires liver transplantation in around 400 cases per year [9]. Liver transplantation remains the gold standard for the treatment of irreversible fulminant hepatic failure. Without this therapy, survival rates range from 10% to 30% [10], with death caused by sepsis and cerebral edema [11]. In comparison, overall long-term survival with liver transplantation is 40% to 75%, superior to any other form of medical management. Yet a liver transplanted for fulminant hepatic failure has a lower chance of success than liver transplantation for other indications. Thus, a valuable resource is consumed with a greater rate of failure than is typically seen in other applications. Furthermore, the chance of spontaneous recovery in some of these cases also makes use of a limited resource less attractive. Nonsurgical strategies that increase rates of spontaneous recovery after liver injury would be greatly beneficial. Here the authors examine efforts to use exogenously provided or endogenously mobilized stem cells to assist in liver recovery.
Challenges presented by acute liver failure
In an attempt to determine which patients will require a liver transplant, predictive criteria have been analyzed and algorithms developed [9]. Prognosis for recovery from fulminant liver failure depends upon several factors. Etiology is important with spontaneous recovery rates from hepatitis A and acetaminophen toxicity being high [12,13], whereas recovery rates from other types of viral etiologies and drug reactions are lower [14]. A retrospective review of more than 200 patients with acute liver failure who underwent transplantation showed a 5-year survival of 66.9% with a 56.6% 5-year graft survival. Analysis showed that for graft survival, donor age, ethnicity and race, time from onset of jaundice to encephalopathy, need for veno-venous bypass, and intracranial pressure monitoring were important prognostic indicators. For patient survival, patients who were intubated had elevations in preoperative creatinine, bilirubin, and INR, and the time to onset of encephalopathy after jaundice was found to be associated with diminished survival. In general, after transplantation, patients who were sickest before surgical therapy fared the worst, suggesting the benefit of early intervention [9].
Transplantation for acute liver failure is also made more difficult by the potential for a more complicated postoperative course. In patients with acute liver failure, primary nonfunction occurs in up to 16% of transplantations as compared with those cases where transplantation is performed for other indications, thought to be closer to 5% [9]. Therapies that may aid the recovery of an acutely failing liver and improve outcomes or those that could successfully avoid the need for transplantation would be valuable. Stem cells have shown some potential in assisting the regenerating liver in recovery, and further understanding of the mechanism of action may prove useful in designing therapeutics.
Normal mechanisms of hepatic regeneration
The regenerative capacity of the liver is significant and occurs after surgical insult or other inflammatory causes. After surgical resection, the regenerative response in humans is proportional to the amount of liver removed, with liver mass being precisely regulated by physiologic stimuli [15]. After partial hepatectomy, proliferation of the existing mature cellular population, including hepatocytes, biliary epithelial cells, fenestrated epithelial cells, Kupffer cells, and cells of Ito, rebuilds the lost hepatic tissue. In contrast to other regenerating tissues, liver regeneration is not dependent on a small group of progenitor or stem cells [15]. Proliferation begins at periportal areas of the hepatic lobule and proceeds pericentrally within 36 to 48 hours [16]. Gradually, typical hepatic histology is restored [17]. The regenerative capacity of the hepatocyte itself is almost unlimited, with injected hepatocytes able to restore liver mass multiple times over [18].
Similar to other organs, the cellular lineage of the liver consists of stem cells, precursor cells, and mature hepatocytes. Mature cells respond to partial hepatectomy and centrilobular injury for example, as induced by carbon tetrachloride (CCl4). Ductular progenitor cells, fewer in number, respond to centrilobular injury when the proliferation of hepatocytes is inhibited. In rodents, these cells, termed oval cells, have been thought to exist within the terminal bile ductules, the canals of Hering, and have been termed bipolar because of their ability to differentiate into hepatocytes or ductular epithelium in vitro [19–21]. Examination of the livers of human fetuses has identified a population of CD34+ cells, termed side population cells, from which epithelial and hematopoietic cells arise and that can potentially contribute to hepatocyte generation. Side population cells in developing human liver may share a temporal relationship with oval/progenitor cells, responsible for liver regeneration after massive or chronic hepatic injury [22]. Finally, there are also rare cells of exogenous origin, hematopoietic stem cells originating in the bone marrow, supported by data showing that hepatocytes may express genetic markers of donor hematopoietic cells after bone marrow transplantation [2,4].
Much attention has been given to the triggers of regeneration. Hepatocyte growth factor (HGF) and its receptor c-Met are key factors and play an important role in liver growth and function [23]. HGF levels have been shown to increase substantially after a decrease in hepatic mass in humans, leading to changes in gene expression, termed immediate-early genes, within the hepatocyte [24].
In addition, the relationship of the hepatocyte to its surrounding matrix and the action of urokinase on the matrix and HGF are important determinants of regeneration. Other factors, including tumor necrosis factor-alpha and interleukin (IL)-6, are components of the early signaling pathways leading to regeneration, whereas growth factors, including epidermal growth factor, transforming growth factor, fibroblast growth factor, vascular endothelial growth factor, as well as norepinephrine and insulin, all have important effects, with the roles of some, like HGF, being essential, whereas others are facultative [19].
Involvement of hematopoietic stem cells in liver regeneration
Cells with stem cell properties may appear in large numbers when mature hepatocytes are inhibited from proliferation [15]. Bone marrow-derived hematopoietic stem cells (HSCs) participate in liver injury recovery under strong positive selection pressure when normal mechanisms of regeneration are either blocked or are inadequate [2]. After bone marrow transplantation from male rats into lethally irradiated syngeneic female rats, Petersen and colleagues [2] demonstrated the presence of the sry region of the donor male Y chromosome within the female recipients’ livers 13 days after injury. Similarly, after bone marrow transplant from dipeptidyl peptidase-IV-positive (DDPIV-IV+) male rats into females with a deletion of this enzyme, DDPIV-IV+ hepatocytes were found in the female recipients. An extrahepatic source for the repopulating liver cells was demonstrated when, after whole-liver transplantation of L21-6 antigen negative livers into rats expressing L21-6 antigen, cells expressing the antigen were detected in the ductal structures of the donor liver after injury. These results have been repeated by other investigators. However, in these experiments only a small amount of cells from the recipient populate the liver, with 2% of mouse hepatocytes of recipient origin and 4% to 40% of hepatocytes and cholangioles described as a recipient phenotype in human recipients of male bone marrow [5]. Therefore, repopulation of the regenerating liver with hematopoietic stem cells does occur, but only on a limited basis. It is unclear why the physiologic response to liver injury does not more completely use recruitment of endogenous marrow-derived cells.
Stem cells aid in liver recovery
The mechanisms by which stem cells exert their beneficial effects on the injured liver are uncertain. Hypotheses about the mechanism by which HSCs contribute to liver regeneration have included direct contribution of the bone marrow-derived stem cells to the recovering hepatocyte population through transdifferentiation into hepatocytes, cell fusion creating hepatocyte cell hybrids, and finally, paracrine effects promoting endogenous processes, in particular by enhancing angiogenesis [3,25,26]. Enhanced angiogenesis is thought to occur when hematopoietic stem cells commit to sinusoidal endothelial cells and consequently play a central role in coordinating angiogenesis [27].
In attempting to elucidate the role of the heterogeneous population of stem cells, multiple cell surface markers have been studied as candidates to uniquely identify the specific subset of stem cells responsible for influencing hepatic regeneration. Classic HSC surface antigens include CD34 and CD133 [28]. However, these cell surface markers represent a heterogeneous population of immature hematopoietic and endothelial cells with a continually and reversibly changing phenotype depending upon the state of activation. CD34 is a glycoprotein involved in cell-cell adhesion interactions that is also expressed on cells in the umbilical cord, mesenchymal stem cells, endothelial progenitor cells, and on mature endothelial cells [29]. Reports that levels of CD34+ cells increase in patients after hepatic resection exist [30], although other studies report data that conflicts with this and suggest differences between resection for benign versus malignant disease [31]. Other investigators have supported the hypothesis that mobilization of hematopoietic stem cells occurs after partial hepatectomy or the ischemic insult of orthotopic liver transplantation [32,33]. A highly heterogeneous population of stem cells was mobilized in living liver donors at 12 hours after resection. These cells were found to be rich in CD133 and coexpressed CD45 and CD 14. In addition, a small subset of mobilized cells expressed CD34. CD34+ HSCs were also found to be mobilized in patients after liver transplant by Lemoli and colleagues [33], however, they demonstrated that only ischemia/reperfusion injury associated with liver transplant resulted in the mobilization of bone marrow (BM) stem/progenitor cells.
Stem cell mobilization in hematologic disease
Mobilization of hematopoietic stem cells from the stem cell niche is already currently in use in certain clinical applications. HSCs reside in the marrow within a highly organized microenvironment consisting of marrow stromal cells, osteoblasts, osteoclasts, and their associated extracellular matrix proteins [34]. The in vivo regulatory microenvironment of the HSC has not been extensively explored.
Pharmacologic mobilization of HSCs from the stem cell niche has emerged as the standard of care for patients with hematologic disorders, including a variety of malignant and nonmalignant conditions, of which the most common are multiple myeloma [34,35] and non-Hodgkin lymphoma [36]. Treatment requires either autologous or allogeneic stem cell transplantation after ablative chemotherapy [35]. Currently, agents that achieve mobilization of BM-derived stem cells in patients with hematologic disorders include granulocyte-colony stimulating factor (G-CSF) (Neupogen) and plerixafor (Mozobil). In current protocols, G-CSF is administered for 5 days before plasmapheresis and cell collection [36]. G-CSF appears to mediate mobilization by downregulation of SDF-1 mRNA in osteoblasts and through the release of neutrophil-derived proteases, such as MMP-9 and neutrophil elastase. These proteases are released into the marrow space and cleave adhesion molecules that participate in tethering stem cells to their niche in the marrow [34]. Administration of G-CSF results in the release of CD34+ HSCs into the circulation after 4 to 5 days [36] and usually results in large numbers of CD34+ HSCs being mobilized successfully. However, in 5% to 30% of autologous donors, this agent fails to mobilize the minimum 2 million CD34+ stem cells [34] required for successful engraftment. With older age, chemotherapy, prior radiation, and extent of marrow disease, poor mobilization can be seen [34].
The use of plerixafor, a novel SDF-1/CXCR4 antagonist that is highly specific for the chemokine receptor CXCR4, has assisted in stem cell mobilization (Table 1). Plerixafor was initially developed as an anti-HIV drug. Despite low potency as an anti-HIV drug in vivo, it was noticed that in healthy volunteers plerixafor resulted in a massive mobilization of HSCs to the periphery. By inhibiting the SDF-1/CXCR4 interaction anchoring stem cells to their niche in the bone marrow [34], plerixafor produces mobilization of HSCs. This mobilization peaks at approximately 10 to 14 hours after the drug is administered [37].
Importantly, plerixafor is synergistic with G-CSF in the mobilization of bone marrow cells. In one randomized study, plerixafor resulted in more successful mobilization in combination with G-CSF as compared with G-CSF with placebo alone [34]. This finding was supported by Broxmeyer and colleagues [38] who showed a 4-fold increase in CD34 cells mobilized in healthy humans when a combination of G-CSF and plerixafor was used as opposed to mobilization with G-CSF alone. Furthermore, they demonstrated that the plerixafor-mediated HSC mobilizing capacity is not desensitized over time with repeat dosing.
Therapeutic mobilization of stem cells using different agents
It appears that differences exist between populations of stem cells mobilized by plerixafor and G-CSF, resulting in differences in engraftment capability and regenerative capacity. Larochelle and colleagues [39] demonstrated that plerixafor mobilizes a population of hematopoietic stem cells with intrinsic characteristics different from those of HSCs mobilized with G-CSF. In rhesus macaques, more plerixafor-mobilized CD34+ cells were in the G1 phase of the cell cycle (consistent with other studies [37]) and had higher levels of expression of both CXCR4 and the cell adhesion molecule VLA4 (a component of the adhesion receptor complex VCAM-1/VLA-4) compared with G-CSF-mobilized stem cells. Mozobil-mobilized cells also demonstrated a greater ability to migrate toward SDF1-alpha in vitro as compared with G-CSF-mobilized CD34+ cells [39].
In terms of HSCs ability to produce long-term durable engraftment, plerixafor has been demonstrated to mobilize true long-term repopulating stem cells that are capable of engrafting primary and secondary lethally irradiated mice [38]. Lapidot and Petit [40] propose a model wherein G-CSF stimulation induces downregulation of stromal derived factor-1 alpha (SDF-1 alpha) in bone marrow stroma and upregulates matrix-metalloproteinase-9, neutrophil elastase, and cathepsin G [40]. In the presence of proteases, cleavage of CXCR4 occurs, resulting in the mobilization of HSCs and progenitors into the peripheral blood. Interestingly, the combination of plerixafor and G-CSF result in increased engrafting into bone marrow. The highly engrafting nature of HSCs as seen in experiments of dual-mobilized cells provides strong evidence that the plerixafor mobilization process does not interfere with the functional capacity of the mobilized cells; this is consistent with the rapidly reversible effects of the drug [38].
Importance of the sdf1/cxcr4 axis in trafficking of HSCs
Stromal derived factor-1 alpha is a highly conserved chemokine that is constitutively expressed by murine and human bone marrow endothelial and endosteal bone lining stromal cells, both of which play an important role in the stem cell niche. The SDF-1/CXCR4 axis plays a major regulatory role in stem cell mobilization and is involved in signals transmitted to both hematopoietic and mesenchymal compartments. These tightly regulated signals, in addition to other mediators, determine cell-cycle status, motility, adhesion machinery, and proteolytic enzyme activity, and enable stem cell localization, migration, and development [41]. CD34+ HSCs are characterized by the presence of CXCR4 upon their cell surface. Stem cell mobilization depends crucially upon the interaction of the surface chemokine CXCR4 and its ligand, SDF-1. During injury, progenitor cells egress from the bone marrow to the peripheral circulation, with the SDF/CXCR4 axis being one of the principle regulators. Mobilization and homing occur in sequence as physiologic processes and with factors contributing to the attraction and engraftment of HSCs. The presence of an SDF-1 gradient contributes to chemoattraction of hematopoietic stem cells. It is interesting that the injured liver upregulates the hepatic production of SDF-1, resulting in potent chemoattraction of HSCs cells and provides a homing mechanism focused on the injured liver [42,43].
Additional factors also have been implicated in the control of hepatic migration of HSCs, including IL-8, hepatocyte growth factor, and matrix metalloprotein-9 with increased expression after hepatic injury [44], resulting in macrophage-led matrix remodeling. It also appears that SDF-1 plays an important role in adhesion of HSCs to the site of injury. Immobilized SDF-1 mediates activation of CD34+ adhesion molecules and cell surface integrins and results in firm arrest of CD34+ progenitors and lymphocytes under shear flow. This process initiates vascular extravasation in synergy with extracellular matrix components [41]. Knockout and overexpression experiments confirm the importance of the SDF-1/CXCR4 axis in cell trafficking and in the ability of HSCs to engraft within the bone marrow, suggesting that this relationship may also be an important determinant of successful engraftment within the liver.
Beneficial effects of stem cell mobilization in acute liver failure
Why physiologic mechanisms of recovery after massive liver insult do not make more use of HSCs is not clear. Patients with ALF have been shown to have markedly lower serum levels of several growth factors found to be important in stem cell mobilization, including stem cell factor and thrombopoietin. In patients with acute hepatitis and fulminant hepatitis, levels were shown to be lowest in the group that had the worst eventual outcomes [45]. HSC mobilization protocols exist and have been optimized in the setting of hematopoietic stem cell transplantation as previously described but have not yet been considered for treatment of acute liver injury where they may be of benefit. In patients with chronic liver disease, CD34+ HSCs can be given safely. It has also been shown that CD34+ HSCs can be mobilized effectively in this population while also providing a modest degree of clinical benefit [46–48].
G-CSF has significantly improved survival and histology in mice with chemically injured livers, predominantly by promoting endogenous repair mechanisms but also by encouraging engraftment of HSCs. In both an acute and chronic liver injury model, G-CSF administration ameliorated the histologic damage and accelerated the regeneration process. Quantitative analysis showed a higher percentage of BM-origin hepatocytes in the CCl4 and G-CSF group compared with the CCl4 group. However, the rate of engraftment still remained low. In this particular experiment, the recovery acceleration after chemical injury and G-CSF treatment was mainly mediated by increased proliferation of host hepatocytes with support from BM-origin cells. G-CSF treatment significantly improved survival and liver histology in chemically injured mice, predominantly by promoting endogenous repair mechanisms [49].
The potential role of stem cells in assisting endogenous repair mechanisms is also supported by results from mice with CCl4-induced chronic liver failure. In these experiments, levels of albumin, international normalized ratio, and bilirubin were restored after administration of endothelial progenitor cells. Administration of HSCs also increased levels of hepatocyte growth factor and transforming growth factor-alpha protein levels as well as the mRNA of these factors, leading to hepatocyte replication, showing that HSCs can assist in some recovery from chronic liver failure [50].
Other groups have used exogenously administered HSCs to improve the function of acutely damaged livers. Using a model in which the liver transplant undergoes portal cellular inflammatory changes, but survives without immunosuppression, Sun and colleagues [51] showed that regenerative stimuli were important in accelerating stem cell recruitment. By transplanting reduced-sized livers, the effect of a strong regenerative stimulus on the flux of host hematopoietic cells entering the liver was provided and found to be important in accelerating the process, ultimately leading to entire replacement of the allogeneic livers by host cells.
The regenerative and integrative potential of exogenous hematopoietic stem cells has been explored while achieving other aims, and applied to the acutely injured liver. In an effort to generate animal livers chimeric for human hepatocytes and provide a model for the study of human-specific disease, such as hepatitis-C, Locke and colleagues [52] showed that under conditions of liver injury, human embryoid body-derived cells migrate to and engraft in animal liver. In performing this experiment, severe combined immunodeficient mice and dark Agouti rats were studied, developing a model of acute liver failure. Injury was induced with CCl4 exposure or partial hepatectomy. Animals received intrasplenic injection of fluorescently labeled human stem cells that migrated to and engrafted in the injured animal liver. Stem cells were detectable at day 2 and were in abundance at 1 week following injection, persisting in the rodent liver long term (>1 month). Once engrafted, differentiation into functional human hepatocytes was found to have occurred as determined by the production of AFP and human albumin.
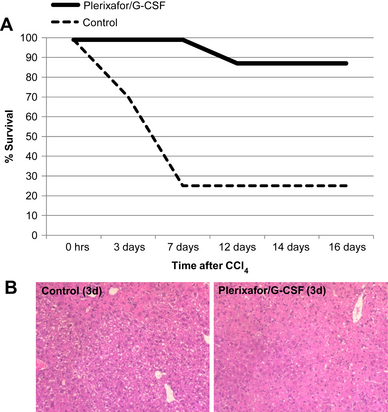
Mark and colleagues [53] further explored the role of HSCs in rodents with acute hepatic injury. Autologous hematopoietic stem cell mobilization was accomplished in the study group with plerixafor and G-CSF administered at 12 hours after the intraperitoneal injection of CCl4. This group was compared with the control group, which sustained injury and received saline injection as treatment. An elevation of serum leukocytes and CD34+ cells occurred in those animals that received treatment with plerixafor and G-CSF. Additionally, they demonstrated the rapid appearance of CD34+ cells in the livers of those animals that had undergone injury and mobilization of HSCs. Finally, results were striking with a mortality of 13% in the group that received G-CSF and plerixafor versus 74% in the control group (Fig. 1). The treatment group demonstrated less histologic injury as well as more infiltrating periportal CD34+ HSCs at 24 hours, suggesting a role for these cells in the treatment benefit but not necessarily a mechanism.
Summary
To be clinically relevant in scenarios of acute liver failure, stem cell mobilizing strategies would have to impact survival when administered well after injury. Applications in other settings may also prove useful. Limits to liver resection exist where the size of the future liver remnant governs the extent of resection possible. Preexisting functional impairment may be restrictive, and strategies involving stem cells may assist the future liver remnant in both normal and functionally impaired livers. Benefit has already been reported from treatment with G-CSF in other injured tissues, including the injured myocardium and acutely injured kidney [6,8,54]. However, as yet no clinical trial exists to assess the effects of stem cell mobilization in humans with acute liver failure. The familiarity in the use of and success demonstrated in the clinical and experimental use of plerixafor and G-CSF make exploration of hematopoietic stem cells as therapy in patients with acute liver failure appealing.
References
[13] W.M. Lee. Acute liver failure. N Engl J Med. 1993;329:1862-1872.
[15] G.K. Michalopoulos, M.C. DeFrances. Liver regeneration. Science. 1997;276:60-66.
[19] S. Sell. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738-750.
[23] A.E. Sirica. Ductular hepatocytes. Histol Histopathol. 1995;10:433-456.
Dr Cameron is a recipient of the American Surgical Association Foundation Fellowship Award as well as an extramural research grant from the Genzyme Corporation in Cambridge, Massachusetts.