Processes involved in cell injury30
Cell injury may be reversible (sublethal) or irreversible (lethal). Reversible injury may require cellular adaptation but the cell survives. Irreversible injury leads to death of the cell. When cell death occurs in the living body, the term necrosis is used. At the cellular level, there are many processes that can lead to necrosis. In most cases, the process can be classified as one or other of two main mechanisms: apoptosis and oncosis. In contrast, autolysis is used to imply cell death that has not occurred in a living body.
4.1. Processes involved in cell injury
Causes of cell injury
The causes of both reversible and irreversible cell injury are similar. Many of those listed below may result initially in reversible injury, from which the cell can recover if allowed time to repair itself. However, if the injury is of sufficient severity, the cell reaches a ‘point of no return’ and irreversible injury culminating in cell death will occur.
Possible causes of cell injury include:
• hypoxia (lack of oxygen), e.g. myocardial ischaemia (reduced blood flow to, and therefore oxygenation of, the heart) as a result of narrowing of the coronary arteries
• immunological mechanisms, e.g. thyroid damage caused by autoantibodies (antibodies produced by the body against its own tissues)
• infection by microorganisms, e.g. bacterial, viral, fungal infections (such as tuberculous infection of the lung or damage to respiratory mucosa by influenza virus)
• genetic abnormalities, e.g. Duchenne’s muscular dystrophy or sickle cell disease
• physical agents, e.g. radiation (such as sunburn due to UV light damage to the skin), trauma, heat, cold
• chemicals, e.g. damage to liver cells by alcohol.
Mechanisms of cell injury
The structure and metabolic functions of the cell are interdependent. Therefore, although an injurious agent may target a particular aspect of cell structure or function, this will rapidly lead to wide-ranging secondary effects. Recognised mechanisms of cell injury include:
• cell membrane damage
• complement-mediated lysis via the membrane attack complex (MAC)
• bacterial toxins
• free radicals
• mitochondrial damage leading to inadequate aerobic respiration
• hypoxia
• cyanide poisoning
• ribosomal damage leading to altered protein synthesis
• alcohol in liver cells
• antibiotics in bacterial cells
• nuclear damage
• viruses
• radiation
• free radicals.
Free radicals and cell membrane damage
Free radicals are highly reactive atoms or molecules which have an unpaired electron in an outer orbit. They can be produced in cells by a variety of processes, including normal metabolic oxidation reactions and drug metabolism. Radiation and many organic poisons induce free radicals. Most clinically important free radicals are derived from oxygen, e.g. superoxide and hydroxyl ions. Free radicals can injure cells by generating chain reactions, producing further free radicals, which cause cell membrane damage by cross-linking of proteins and by critical alterations of lipids.
Ion transporter function and intracellular calcium
Raised intracellular calcium can have a number of effects. It may initiate the caspase cascade (see below) causing apoptosis. It can also activate proteases and phospholipases, causing further damage to cell cytoskeleton and membranes and thus contribute to necrosis.
Consequences of cell injury
The consequences of cell injury depend on both the characteristics of the injured cell and the injurious agent.
Cell features
Certain features of cells make them more vulnerable to serious sequelae of cell injury. Specialised cells that are enzyme rich or have special organelles within the cytoplasm may be more vulnerable. The presence of specialised proteins within a cell may make it prone to certain types of injurious agent.
Cell state
Cells that have an inadequate supply of oxygen, hormones or growth factors or lack of essential nutrients may be more prone to injury.
Regenerative ability
The potential of a cell population to enter the cell cycle and divide is important in the response of tissues to injury. Damaged areas in tissues made of cells which can divide may be restored to normal, while populations of permanent cells will be incapable of regeneration.
Injury features
In addition, the character of the injury will also affect the severity of the damage.
Type of injury
The injury may be ischaemic, toxic, traumatic, etc. Some cells will be more susceptible to particular injurious agents than others. For example, hypoxia has a greater effect on heart muscle cells than connective tissue cells.
Intensity
The greater the intensity, the greater the probability of damage. For example, a bone can withstand a bending force up to a certain level, but if the force exceeds the strength of the bone then the bone will break. Likewise, a cell may survive partial hypoxia but not complete lack of oxygen (anoxia).
Exposure time
The length of time of exposure to a toxin or reduced oxygen concentration will affect the chance of a cell surviving the insult. Even relatively resistant cells will be damaged if the duration of exposure is prolonged.
Reversible cell injury
Within limits, cells can accommodate derangements in their metabolism through compensatory mechanisms. However, they may show morphological changes. Under the light microscope, cellular swelling and fatty change are associated with reversible cell injury. Cellular swelling, also known as ballooning or hydropic degeneration, is due to osmotic swelling of cells as they accumulate an excess of small molecules and ions in their cytoplasm. Fatty change is seen when the injured cell cannot process lipids normally and results in the accumulation of fat globules in the cytoplasm (discussed further in Ch. 13).
The ultrastructural changes associated with reversible cell injury can be seen under the electron microscope and include:
• distortion of microvilli and blebbing of the plasma membrane
• cytoplasmic vacuolation
• swelling of mitochondria and endoplasmic reticulum
• clumping of nuclear chromatin.
Irreversible cell injury
Irreversible injury implies that the cell cannot survive and will die by one of the mechanisms discussed in the next section. If the damage is not too severe, death is likely to occur by apoptosis via the intrinsic pathway, but more severe damage causes death by oncosis.
When does reversible injury become irreversible? The exact ‘point of no return’ is difficult to identify, although massive caspase activation and loss of mitochondrial transmembrane potential are among those that have been proposed. Irreversible injury is associated with an influx of calcium and the release of calcium sequestered in endoplasmic reticulum, which activates enzymes that further degrade the constituents of the cell.
The morphological changes of irreversible cell injury take time to develop. It may be 8–12 hours before the appearance of abnormalities that are recognisable at the light microscopic or macroscopic level.
4.2. Cell death
Autolysis
The term ‘autolysis’ has been used in different ways, but pathologists use it to describe the changes that occur in cells after death of an organism or after surgical removal. When an organism dies, the cells are degraded by the post-mortem release of digestive enzymes from lysosomes as the cell membranes break down. A similar process occurs when tissue is removed from the organism. Cells and tissues showing these changes are autolytic. Prompt preservation of tissues in a fixative such as formalin prevents autolysis after surgical removal.
Apoptosis
Apoptosis is also known as programmed cell death. It can occur in normal tissues, for example as a means of regulating the number of cells in a tissue or organ, and during embryological development. It is also seen in pathological processes. In pathological circumstances, apoptosis follows irreversible cell injury that is not sufficiently severe to damage cell membranes. If cell membrane damage does occur, death is likely to be by oncosis (see next section).
Examples of physiological apoptosis
• Embryogenesis: e.g. formation of digits from the limb buds.
• Menstrual cycle: endometrial cell loss during menstruation.
• Breastfeeding: reversal of changes in the lactating breast once breastfeeding is finished.
• Immune cell development: deletion of lymphocytes that may react with the body’s own tissues.
Examples of pathological apoptosis
• Any of the injurious agents that can cause necrosis will cause apoptosis if the injury is insufficient to cause cell death by oncosis. For example, severe loss of oxygen in the heart muscle causes oncosis, but lesser degrees of hypoxia cause death by apoptosis.
• Viral illness: infected cells can die through apoptosis, e.g. in viral hepatitis, histological sections of the liver show apoptosis of hepatocytes.
• Acquired immune deficiency syndrome (AIDS): loss of lymphocytes occurs by apoptosis.
In addition, in neoplasms the balance between apoptosis and cell proliferation is disturbed, so that cell proliferation exceeds apoptosis. Thus, in a sense tumours suffer from a lack of apoptosis.
Apoptosis is triggered through one of two main pathways, both of which activate the caspases, a cascade of proteolytic enzymes responsible for the processes of apoptosis. The first is the intrinsic (mitochondrial) pathway, in which pro-apoptotic molecules are released from mitochondria into the cytoplasm. The second is the extrinsic (death receptor) pathway, in which transmembrane receptors bind to their ligand and thereby initiate cell signalling mechanisms. An example of the extrinsic pathway is seen in cells killed by cytotoxic T cells. The T cells express Fas ligand on their cell membranes; when this ligand binds to the Fas receptor on a cell it initiates apoptosis.
The cell signalling pathways and enzyme-induced events involved in apoptosis are complex, but the caspases are central to the execution phase of the process. As seen in other enzyme cascade reactions, such as the complement system (see Ch. 5), this process serves to amplify the initial apoptotic signal. Some caspases activate other enzymes, while others have a direct effect on the structure of the cell by breaking down components of the cytoskeleton. Endonucleases break down DNA into regular fragments at internucleosomal sites, and phospholipases change the configuration of cell membranes.
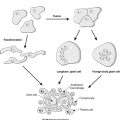
Morphologically, apoptotic cells shrink and the nucleus condenses. The organelles and nucleus break up, and then the cell breaks into fragments called apoptotic bodies. These express ligands on their surface membranes that are recognised by other cells, which bind to the apoptotic bodies and engulf them by phagocytosis. It is not just macrophages and neutrophils that can phagocytose apoptotic bodies – cells of many different types are able to do so (Figure 8).
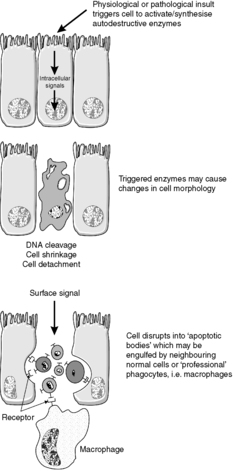 |
| Figure 8 |
In contrast with oncosis, the cell membrane pumps remain viable and continue to function until the terminal stages of the process. Apoptosis does not provoke an inflammatory response.
The control of apoptosis is crucial in the process of neoplasia. Some genes involved in cancer formation (e.g. the bcl-2 oncogene) switch off apoptosis, thus allowing the neoplastic cells to live indefinitely.
Oncosis
Oncosis is a term that has been introduced relatively recently to describe the passive cell death of necrosis. It derives its name from the characteristic cellular swelling that differentiates the process morphologically from apoptosis, in which cells tend to shrink. It is a useful term that distinguishes between the concept of passive death and programmed (i.e. apoptotic) death at the cellular level. Unfortunately, the word is rather similar to ‘oncogenesis’ and ‘oncotic’, both of which mean completely different things (see Glossary).
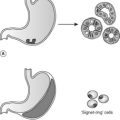
Oncosis is characterised by cellular swelling and the breakdown of the cell membrane. It is always pathological. In oncosis, death occurs of a large number of cells in one area, as opposed to the selective cell death of apoptosis (Figure 9). These changes occur because of digestion and denaturation of cellular proteins, largely by release of hydrolytic enzymes from damaged lysosomes. The appearance of necrotic tissue depends in part on the balance between digestion and denaturation.
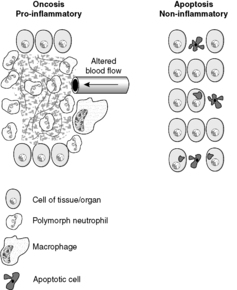 |
| Figure 9 |
The principal differences between apoptosis and oncosis are summarised in Table 4. In many pathological circumstances, both processes are involved. For example, in myocardial infarction the damage at the centre of the infarct is mainly due to oncosis, but at the periphery (where the hypoxia is less severe) apoptosis is usually more important.
| Apoptosis | Oncosis |
|---|---|
| Membrane integrity preserved | Membranes breached |
| No inflammation | Inflammatory response |
| Single cells | Contiguous cells |
| Active process; requires protein synthesis and consumes ATP | Passive process |
Necrosis
The consequences of necrosis include:
• cessation of function of a tissue or organ
• release of cellular components through damaged cell membranes; these can sometimes be detected in the blood and used as markers of the extent or timing of damage to a particular organ, e.g. cardiac enzymes and troponins after myocardial infarction
• initiation of the inflammatory response by areas of oncosis.
Types of necrosis
There are five main types of necrosis (Figure 10):
• coagulative
• caseous
• liquefaction
• fat
• gangrenous.
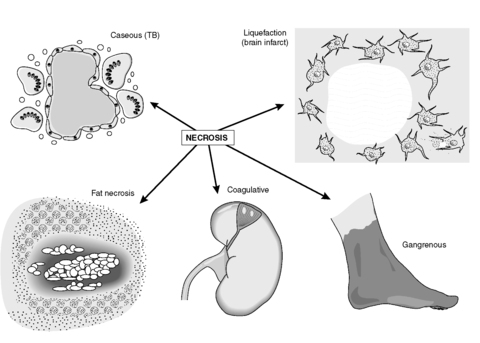 |
| Figure 10 |
Caseous necrosis
This cell death is characteristic of tuberculosis (TB) and is seen only rarely otherwise. The creamy white appearance of the dead tissue resembles cheese and is probably a result of the accumulation of the partly digested waxy lipid cell wall components of the tuberculous organisms. The tissue architecture is completely destroyed.
Liquefaction (colliquative) necrosis
This is characterised by tissue softening with destruction of architecture. The result is an accumulation of semi-fluid tissue. It is usually seen in the brain and spinal cord.
Fat necrosis
The necrosis of fat is distinctive because it is characterised by the presence of large numbers of foamy macrophages. They form when the dead adipocytes rupture and release fat, which is taken up by macrophages. The oily material in the macrophage cytoplasm gives the histological appearance of foam. The macrophages commonly form multinucleate giant cells (see Ch. 9). Sometimes, the liberated fat combines with calcium to produce soapy material in a process called saponification. Fat necrosis is encountered in the female breast as a result of trauma, or in the adipose tissue surrounding the pancreas in pancreatitis due to enzymes release from the diseased pancreas.
Gangrenous necrosis (gangrene)
This life-threatening condition occurs when coagulative necrosis of tissues is associated with superadded infection by putrefactive bacteria. Bacteria grow readily in necrotic tissue, so if tissues that have a resident bacterial population such as the intestine become necrotic, gangrene is likely to follow. Some bacteria are able to colonise living tissues and cause gangrene that way; an example is the contamination of a limb wound by Clostridium spp. derived from the soil. Gangrenous tissue is foul smelling and black. The bacteria produce toxins which destroy collagen and enable the infection to spread rapidly; it can reach the bloodstream and cause systemic infection and multi-organ failure. If fermentation occurs, gas gangrene ensues, characterised by the collection of gas within the gangrenous tissue.
Sometimes, the word gangrene is used to describe the necrotic death of part of a limb when there is little or no infection. In this case, the term ‘dry gangrene’ is used and the process resembles mummification.
Self-assessment: questions
One best answer questions
2. A lymphocyte induces apoptosis in a cell infected with a virus. Which of the following is likely to occur first in this process?
a. binding of Fas to Fas ligand
b. cleavage of DNA by endonucleases
c. initiation of the caspase cascade
d. phagocytosis of apoptotic bodies
e. widespread destruction of cell membranes
3. In which one of the following organs 1week after an infarct is a pathologist most likely to observe complete loss of tissue architecture histologically?
a. brain
b. kidney
c. liver
d. lung
e. spleen
True-false questions
1. The following statements are correct:
a. caseous necrosis is characteristically caused by ischaemia
b. carbon tetrachloride causes cell injury through free radicals
c. ion pumps often fail in hypoxic cells
d. the lung is a common site for gangrenous necrosis
e. testicular torsion can cause venous infarction
2. Apoptotic cells:
a. exclude vital dyes that enter cells across damaged plasma membranes
b. provoke an acute inflammatory reaction
c. may be phagocytosed by neighbouring cells
d. contain enzymatically degraded nuclear fragments
e. may occur in conjunction with oncosis
Case history questions
Case history 1
A 59-year-old man, a heavy smoker for much of his adult life, was brought into casualty at 4am with a 3-hour history of crushing, band-like chest pain. He was seen immediately but died while being examined. His medical history included diet-controlled (type 2) diabetes mellitus, hypertension and intermittent claudication. He had had several attacks of chest pain in the 5years before his death. A post-mortem examination was performed.
1. What is the likely cause of death?
2. At post mortem, the pathologist found no morphological evidence of acute myocardial infarction. Why was this?
3. What changes might have been present in the heart muscle and coronary arteries?
4. How do the medical and social history relate to this terminal event?
Essay question
1. Discuss the different types of necrosis that can be observed macroscopically. How does necrosis differ from autolysis?
Viva questions
1. Define infarction. How long does it take before morphological changes of infarction can be observed by a pathologist?
2. What are free radicals? How do they cause cell injury?
Self-assessment: answers
One best answer
2. a. The sequence of events is a, c, b, d. Widespread destruction of cell membranes is a feature of oncosis, not apoptosis.
3. a. The kidney, liver, lung and spleen generally undergo coagulative necrosis, unless there are unusual factors such as infection with tuberculosis causing caseation. Coagulative necrosis is characterised by relative preservation of tissue architecture, whereas liquefactive necrosis, which occurs typically in the brain, is characterised by complete loss of tissue architecture.
True-false answers
1.
a. False. Caseous necrosis characteristically occurs as a response to Mycobacterium infection, e.g. TB.
b. True. Carbon tetrachloride poisoning is one of the prototypic examples of free-radical-induced injury.
c. True. This is an important factor contributing to hypoxic cell injury.
d. False. Gangrenous necrosis usually occurs when a tissue/organ that has a resident population of bacteria dies. The organisms can then invade the tissues. The lung is normally sterile, so gangrene does not occur.
e. True. Torsion (twisting) of the testis on its cord leads to obstruction to the venous drainage. The reduction in perfusion leads to infarction, which is markedly haemorrhagic due to the vascular congestion (see Ch. 6). Infarction of the testis can be prevented if the cord can be untwisted in time. Venous infarction can also be seen in the ovary and intestine.
2.
a. True. One of the essential differences between an oncotic and apoptotic cell is that the latter remains ‘alive’ until late in the process. Therefore, dyes which enter dead cells across their damaged membranes are excluded by apoptotic cells.
b. False. Apoptotic cells are disposed of by neighbouring cells or macrophages. No acute inflammation occurs; therefore there is no tissue damage.
c. True. See answer (b).
d. True. Soon after apoptosis is triggered, nuclear material in the apoptotic cell is chopped up by endonucleases.
e. True. The processes of oncosis and apoptosis are not mutually exclusive and usually occur together in necrotic tissue. For example, in infarcts the apoptotic cells are typically found in areas where the hypoxia is less severe around the margins of the infarct. In these zones, the cells retain the ability to initiate apoptotic mechanisms when injury becomes irreversible. In contrast, the more severe hypoxia in the centre of the infarct causes significant cell membrane damage and thus oncosis.
Case history answer
Case history 1
1. The likely cause of death in this man is ischaemic heart disease caused by coronary artery atherosclerosis. The chest pain in this patient is quite characteristic of pain owing to an ischaemic myocardium (heart muscle deprived of blood supply and thus oxygen). Severe atherosclerosis of the coronary arteries would account for this.
2. The macroscopic (naked eye) and microscopic changes that characterise all forms of necrosis take time to develop. There is a significant lag, in this and in any case, between the onset of ischaemia and any changes in the myocardium that can be seen by the naked eye. This is not an unusual scenario at autopsy. A patient who has died instantly from myocardial ischaemia (clinically causing a lethal abnormal heart rhythm such as ventricular fibrillation) because of complete blockage of a coronary artery will have no visible evidence of acute infarction. If, however, the patient had lived for at least 8–12 hours after this event and then died, changes of myocardial necrosis would be seen (see Ch. 14).
3. The history of previous attacks of chest pain suggest past ischaemic events and the pathologist may find fibrosis (scarring) of the myocardium (cardiac muscle cells are permanent cells and regeneration cannot occur). The patient suffered from hypertension (high blood pressure); therefore hypertrophy of the myocardium is also likely. The normal heart weighs about 250–350g, but in severe hypertension the weight can double as a consequence of hypertrophy of the left ventricular myocardium. Myocardium that has become pathologically hypertrophic in this way is more susceptible to ischaemia than a normal heart. The coronary arteries will be atherosclerotic (see answers 2 and 4).
Essay answer
Comment: It is always worth starting with a brief definition of the matter in hand, i.e. necrosis is the death of cells within the living body. It would be appropriate to indicate that apoptosis and oncosis can contribute to necrotic lesions. However, the question does not ask for a description of cellular mechanisms, so details of oncosis and apoptosis at the cellular level would attract no marks. Likewise, a general discussion of irreversible cell injury would be a waste of time.
Then describe the five main types of necrosis, each under its own heading, describing the macroscopic (naked eye) appearances and giving examples of the causes:
• coagulative: denaturation of intracellular protein; commonest type of necrosis; wide range of causes, including ischaemia, physical causes and chemicals; typically there is preservation of cell outlines
• caseous: tissue architecture destroyed with a characteristic vital reaction around, typically including multinucleate giant cells; cell outlines are not apparent; prototype is infection with mycobacteria (although other conditions such as histoplasmosis can also cause caseation)
• liquefactive: accumulation of semi-fluid tissue typical of necrosis in the central nervous system; loss of cell outlines
• fat: adipocytes rupture releasing fats that are broken down; the oily material is ingested by macrophages to give a foreign body giant cell reaction or combine with calcium; can result from direct trauma, e.g. in the breast, or from pancreatic diseases, e.g. acute pancreatitis
• gangrenous: infection of necrotic tissue by putrefactive bacteria; characteristic smell and colour; toxins destroy collagen; fermentation produces gases; systemic infection can follow.
Diagrams to show you know what the different lesions look like would be appropriate.
Finally, autolysis is the death of cells and tissues after death or removal from the body. It differs from necrosis because there is no inflammation associated with it (inflammation requires a blood supply).
Viva answers
1. Infarction is the death of tissue within the living body due to ischaemia (lack of oxygen supply). It takes several hours before changes occur that can be recognised as irreversible cell injury either with the naked eye or the light microscope. In general, the tissue appears normal for about 8–12 hours.
2. Free radicals are highly reactive atoms or molecules which have an unpaired election. They can injure cells by generating a chain reaction of free radical production which causes cell membrane damage by cross-linking of proteins and alterations to membrane lipids.