Normal cell growth21
In order to function appropriately, cells and tissues need to maintain a steady state (homeostasis). Within defined limits, cells are capable of adapting to a variety of stimuli which may upset normality. Cellular adaptation is the state between a normal unstressed cell and the overstressed injured cell. By definition, an adaptive process is one which is potentially reversible. This chapter covers:
• normal cell growth and the cell cycle
• reversible adaptive responses.
3.1. Normal cell growth
Regeneration
Cells that are lost through death or injury need to be replaced. Furthermore, normal growth of tissues depends on a balance between the number of cells actively dividing and the number of cells dying. Therefore, tissues contain a population of cells capable of repeated mitotic division, called stem cells. Sometimes, differentiated cells can return to the cell cycle and divide to produce daughter cells. However, unless they take on the characteristics of stem cells, they cannot do so indefinitely. The reason is that the terminal ends of chromosomes called telomeres become slightly shorter with each cell division. Eventually, when the telomeres become too short, further mitotic division is not possible. In a sense, the telomeres act like a clock counting down the number of mitoses a cell is allowed. However, stem cells possess the enzyme telomerase, which reconstitutes the telomeres at each mitosis, allowing an infinite number of cell divisions. Most neoplastic cells (see Ch. 11) also possess telomerase, which explains why they too can divide indefinitely.
When a stem cell divides, it produces two daughter cells. One of these retains the characteristics of a stem cell so that it can divide again, but the other can differentiate and become a specialised cell of the tissue, such as an epithelial or connective tissue cell.
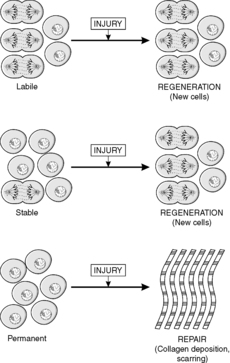
Traditionally, tissues have been divided into three types according to the nature of cell turnover they exhibit, namely: labile, stable and permanent. Labile tissues have a high cell turnover and show continuous mitotic activity, stable tissues contain cells with a longer lifespan and mitoses are rare or absent, and permanent tissues are incapable of any cell division. These concepts are useful when thinking about the way in which tissues respond to injury, and are defined in Box 3. (The term injury is used in a broad sense by pathologists to include any pathological alteration in the environment or integrity of a cell.)
Box 3
• Stable tissues. Although stable tissues also contain stem cells, the turnover is slow because the differentiated cells are long-lived. Therefore, a histological section of the tissue may not contain any mitoses. However, if the tissue is damaged and many cells are lost, the tissue can become highly active mitotically and regenerate itself. This can occur because the remaining stem cells are stimulated to divide, e.g. bone. Alternatively, in some tissues the differentiated cells can be stimulated to re-enter the cell cycle; an example is liver, in which it appears that liver cells can divide while retaining the characteristics of differentiated liver cells.
• Permanent tissues. These tissues only contain cells capable of division in fetal life. Therefore, cells lost after birth cannot be replaced. Examples of permanent cells are neurones and cardiac/skeletal muscle cells (although cardiac and skeletal muscle may be capable of limited proliferation under some circumstances).
In labile and stable tissues, stem cells can quickly replace dead cells within their population by cell division and replace them with cells of exactly the same type. This process is called regeneration (Figure 5). For example, removal of part of the liver triggers hepatocytes close to the area removed to enter the cell cycle, divide and replace the lost tissue. Successful regeneration of cell populations depends on an intact connective tissue matrix around the cells. If this matrix is destroyed, repair by fibrosis and scar formation is likely.
Repair
Permanent cells by definition cannot divide and replace lost cells by the same cell type. Instead, repair by fibrosis occurs (Figure 5). In this process, dead tissue is removed and scar tissue (collagen-rich fibrous tissue) fills the defect. The scar provides continuity and strength to the tissue but there is loss of the original specialised cell function. Repair, rather than regeneration, is also likely when labile or stable tissues show extensive injury that disrupts the normal architecture of the tissue or damages the connective tissue matrix. The mechanism of fibrosis is described in Chapter 10.
The cell cycle
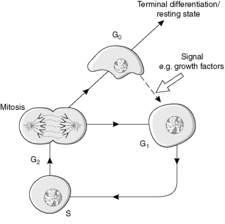
The four main stages of the cell cycle (Figure 6) are:
• M phase: mitosis when the cell divides (about 1 hour)
• G1 phase: gap 1, the preparation for S phase
• S phase: DNA synthesis
• G2 phase: gap 2, during which assembly of the apparatus for the distribution of chromosomes occurs.
 |
| Figure 6 |
In addition, a G0 phase of the cell cycle is recognised. This phase is non-proliferative and is known as growth arrest. Cells in G0 may re-enter the cell cycle at G1, thereby regaining the proliferative state. Locally active small-molecular-weight proteins called growth factors are important in stimulating this re-entry.
Stem cells are capable of going round the cell cycle indefinitely. In contrast, cells which undergo terminal differentiation cannot re-enter the cell cycle (Figure 6). In a permanent cell population, all the cells are terminally differentiated.
Control of the cell cycle
Growth factors
Growth factors are proteins of low molecular weight that have a similar mechanism of action to hormones. In general, the growth factor is produced by a cell, for example a macrophage, and acts either on the cell itself (autocrine action) or on a neighbouring cell (paracrine action) by linking to cell surface receptors. This interaction activates the receptor and triggers a series of cytoplasmic events usually involving phosphorylation-dephosphorylation of proteins. Ultimately, a signal reaches the nucleus where genes are switched on, new proteins produced and cell growth and division are initiated. Growth factors often have actions that go beyond effects on the cell cycle. They can also inhibit or promote apoptosis (programmed cell death, see Ch. 4), and can stimulate differentiation of cells.
Cyclins
The cyclins are a family of proteins which coordinate the journey of cells through the different phases of the cell cycle. They form complexes with a protein kinase (phosphorylating) enzyme. The cellular concentration and activity of different cyclins varies through the cell cycle.
3.2. Cellular adaptation
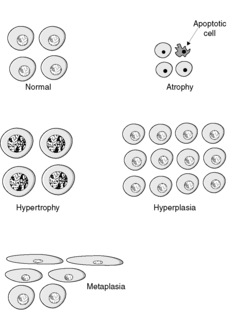
There are four main adaptive states (Figure 7):
• atrophy: shrinkage of an organ as a result of a decrease in cell size and/or number
• hypertrophy: enlargement of an organ as a result of increased cell size
• hyperplasia: enlargement of an organ through an increase in cell number
• metaplasia: change in tissue type as a result of replacement of one differentiated cell type by another.
These adaptations remain under the control of the complex web of genetic and environmental factors that control normal growth and development. In general, they are potentially reversible. Thus they differ from neoplasia, which is an irreversible process in which normal responses to control of growth and differentiation are lost.
Atrophy
Atrophy is a reduction in the mass of cells leading to a reduction in size of the tissue or organ. Two mechanisms can be involved: a reduction in the number of cells through apoptosis, and a reduction in the size of the cells.
Atrophy occurs in physiological circumstances, i.e. during normal growth and development, generally due to loss of endocrine stimulation. For example, the fall in circulating oestrogen after the menopause causes shrinkage of the endometrium, breast tissue, mucosal lining of the vagina, etc.
There are many ways in which atrophy occurs pathologically, i.e. as a result of a disease process:
• denervation, e.g. wasting of muscle caused by a lack of nerve stimulation, for example in poliomyelitis
• reduced blood supply, e.g. shrinkage of brain caused by atherosclerosis of carotid arteries
• inadequate nutrition, e.g. wasting of muscles and major organs in starvation
• decreased workload (disuse), e.g. wasting of muscles and bone (osteoporosis) after immobilisation of a limb in a plaster cast
• pressure, e.g. due to an adjacent tumour or cyst
• loss of endocrine stimulation, e.g. infarction of the pituitary gland results in atrophy of the tissues dependent on pituitary hormones, including the thyroid gland and adrenal gland.
Cell structural components are reduced in atrophy. There are:
• fewer mitochondria
• reduction in the amount of endoplasmic reticulum
• fewer cytoplasmic filaments.
The metabolic rate is reduced in atrophy. There is:
• less amino acid uptake
• less oxygen consumption
• less protein synthesis.
In atrophy, there is an increase in the number of autophagic vacuoles (intracellular dustbins) which contain fragments of intracellular debris such as organelles awaiting destruction. Lysosomes fuse with these vacuoles and discharge their digestive enzymes, which digest the material in the vacuole. Proteins destined for degradation are conjugated with the protein ubiquitin, which marks the protein for destruction in a specialised proteolytic organelle, called a proteasome.
Lipofuscin granules are yellow/brown in colour and represent non-digestible fragments of lipids and phospholipids combined with protein within autophagic vacuoles. They are commonly seen in ageing cells, particularly in the liver and myocardium.
Hypertrophy and hyperplasia
An organ or tissue can enlarge due to an increase in the number of constituent cells or to an increase in the size of the cells. The term hypertrophy is used to describe an increase in mass due to an increase in cell size, whereas hyperplasia is an increase in mass due to an increase in cell number. In practice, hypertrophy and hyperplasia commonly occur together. However, hyperplasia requires that the cells be capable of division. Therefore, it can only occur in labile and stable cell populations. Permanent cell populations can only enlarge by hypertrophy. Both hypertrophy and hyperplasia are reversible processes. If the cause is removed, the tissue can return towards normal.
In general, hypertrophy and hyperplasia are due to increased mechanical demand or to stimulation by hormones and/or growth factors. In the case of muscle cells, both types of signal promote hypertrophy, i.e. mechanical stress on the tissue, such as increased stretch, and the release of growth factors due to increased blood flow. As a result, the expression of genes is altered, causing protein synthesis to increase and protein degradation to decrease. Some of the changes shown by hypertrophic muscle cells include increased synthesis of membrane components and myofilaments, increased ATP synthesis and increased enzyme activity. In the skeletal muscles, athletic training will have these effects. In the heart, increased workload due to increased cardiac output or increased resistance to outflow causes hypertrophy. However, there is a limit beyond which the muscle cannot enlarge any further; the limiting factors for maximum muscle size are poorly understood but seem to involve the nutrient and blood supply available for oxidative phosphorylation.
The term compensatory hypertrophy or compensatory hyperplasia is used when tissue is lost through disease or surgical resection and the remaining tissue enlarges. An example is seen in the heart if part of the muscle is lost through myocardial infarction. The undamaged muscle cells become hypertrophic to compensate, at least partially, for the loss of muscle in the infarcted area. The term compensatory hyperplasia is analogous; it refers to hyperplasia in the remaining tissue after some of the tissue is lost. It is seen when the liver regenerates after partial hepatectomy.
Hypertrophy and hyperplasia can be physiological (normal) or pathological (abnormal).
Physiological
• Skeletal muscle hypertrophy in the bodybuilder or athlete.
• Hypertrophy and hyperplasia of myometrial smooth muscle causing enlargement of the pregnant uterus.
• Hypertrophy and hyperplasia of the breasts during lactation.
• Compensatory hypertrophy and hyperplasia of the remaining kidney after the other kidney is removed.
Pathological
• Cardiac muscle hypertrophy as a result of working against an increased peripheral resistance in hypertension (high blood pressure).
• Bladder smooth muscle hypertrophy and hyperplasia caused by increased resistance to outflow (e.g. due to an enlarged prostate gland).
• Hyperplasia of the adrenal gland due to excess secretion of adrenocorticotrophic hormone (ACTH) by a pituitary neoplasm.
• Hyperplasia of the gastric epithelium in response to chemical irritation.
• Hyperplasia of the prostate gland due to an abnormal response to androgens.
Although both hyperplastic and neoplastic tissues show an increase in cell numbers, it is important to distinguish these conditions from each other (see Ch. 10). Unlike neoplastic cells, hyperplastic cells respond normally to the regulatory factors that control cell growth, and hyperplasia is a potentially reversible process. Nevertheless, some types of pathological hyperplasia predispose to the development of neoplasia.
Endoplasmic reticulum
Hypertrophy of smooth endoplasmic reticulum, i.e. hypertrophy at the subcellular level, can also occur. Drugs such as phenobarbital cause an increase in the activity of the mixed function oxidase system of liver cells, which results in increased metabolism of other agents. In some instances, this is therapeutically beneficial, in others it can lead to poisoning of the liver cells by toxic metabolites.
Metaplasia
Metaplasia is the term used when one differentiated tissue is replaced by another. It is a potentially reversible change, in that if the cause of the metaplasia is removed the tissue may revert to normal. It is generally seen in epithelia, usually as a response to chronic physical or chemical irritation.
Metaplasia is a response to a change in the environment of the tissue, and is typically an adaptive response that produces a tissue more able to withstand an adverse environment. A common type of metaplasia is the replacement of glandular or transitional epithelium by stratified squamous epithelium, which is often better able to withstand mechanical or chemical irritation. For example:
• bronchial (pseudo-stratified ciliated columnar) epithelium changes to squamous epithelium in smokers
• transitional bladder epithelium changes to squamous epithelium in people with bladder stones and infection.
Sometimes stratified squamous epithelium changes into glandular epithelium. This occurs in the lower oesophagus in patients with gastro-oesophageal reflux. The reflux of the acidic gastric contents into the oesophagus injures the oesophageal squamous epithelium, which changes into a mucus-secreting glandular epithelium; the mucus protects the oesophageal lining from the acidic gastric juice.
Self-assessment: questions
One best answer questions
2. Which of the following is not a typical characteristic of stem cells?
a. can divide indefinitely
b. found in labile and stable tissues
c. permanently in G0 phase
d. respond to factors regulating cell growth
e. show telomerase activity
3. A researcher is using liver cells in tissue culture to investigate intracellular digestion. By blocking the action of substance X, she finds that proteins destined for destruction are not digested but simply accumulate in the cell. However, other cell functions continue as normal. What is the most likely identity of substance X?
a. ATP
b. cyclin
c. lipofuscin
d. potassium
e. ubiquitin
4. Which of the following is most likely to produce atrophy?
a. excess intake of food
b. increased blood flow through the tissue
c. increased functional demand
d. loss of innervation through nerve damage
e. reduced pressure on the tissue
True-false questions
1. The following are correctly paired:
a. hepatocytes – permanent cells
b. adult neuronal loss – neuronal regeneration
c. cell cycle – stem cells
d. renal epithelial cells – stable cells
e. hyperplasia – permanent cells
2. The following statements are correct:
a. atrophy involves oncosis of cells
b. smokers’ bronchial epithelium is usually atrophic
c. growth factors may inhibit cell growth or division
d. metaplasia inevitably leads to neoplasia
e. the lactating breast shows epithelial hyperplasia
3. A 58-year-old woman has abnormal vaginal bleeding due to endometrial hyperplasia. Which of the following statements is/are true?
a. the hyperplasia will persist indefinitely after the cause is removed
b. a likely cause is increased oestrogen production by an ovarian tumour
c. an ultrasound examination is likely to show the endometrium is thinner than normal
d. there is an increased risk of endometrial neoplasia
e. histological examination of the endometrium will show an increase in the number of cells
Extended matching items questions (EMIs)
EMI 1
Theme: Responses to cell injury
A. atrophy
B. hyperplasia
C. hypertrophy
D. metaplasia
E. neoplasia
F. repair
For each of the following scenarios, which of the above pathological processes is principally responsible?
1. An ophthalmologist examines the eye of a patient with vitamin A deficiency and finds the conjunctiva to be lined by keratinising squamous epithelium.
2. A patient with stenosis of the aortic valve dies. At autopsy, the heart is found to weigh 50% more than normal.
3. As a result of infarction of the pituitary gland, a patient has an abnormally low level of ACTH. An ultrasound examination of the adrenal glands shows they are both smaller than normal.
4. A man aged 70 years has urinary outflow obstruction, and part of the prostate gland is surgically resected and submitted for histopathological examination. The pathologist finds that the prostate cells are of normal size but increased in number.
Case history questions
Case history 1
A 4-year-old boy is treated for leukaemia (uncontrolled, neoplastic proliferation of bone marrow white cells) with cytotoxic drugs which block the cell cycle and destroy the leukaemic cells. Unfortunately, these drugs also affect the normally rapidly dividing cells of the body.
2. What symptoms and signs may the cytotoxic drugs cause in this patient?
3. Why are these drugs that block the cell cycle usually given in episodic doses (pulses) with a drug-free interval between treatments?
Case history 2
A 79-year-old woman falls over at home, injuring her left hip. She is taken to hospital, and on clinical examination and X-rays she is found to have fractured the neck of her left femur. Her bones appear less dense than normal on the X-ray and the radiologist suspects osteoporosis. She is otherwise reasonably fit and well for her age. Therefore she is operated on and the fracture fixed with internal screws and a plate. After the operation, she makes good recovery but is rather slow to mobilise. She requires extensive physiotherapy but eventually she can go home, walking with the aid of a stick.
1. What are the main risk factors for osteoporosis?
2. Why is it important for the patient to become mobile as quickly as possible postoperatively?
Self-assessment: answers
True-false answers
1.
a. False. Hepatocytes are a stable cell population. They can enter the cell cycle and divide in order to replace lost cell numbers.
b. False. Neurones are permanent cells (terminally differentiated) which do not enter the cell cycle. Lost neurones cannot be replaced by new ones in the adult central nervous system.
c. True. Stem cells go round the cell cycle indefinitely. At each division, one of the daughter cells can differentiate. The line of differentiation is controlled by interactions with cytokines, growth factors, hormones, the surrounding matrix and adjacent cells.
d. True. The renal tubular epithelial cells are good examples of stable cells. This is clinically relevant in acute renal failure due to renal tubular damage, because regeneration of the renal tubular cells will allow renal function to return to normal.
e. False. Permanent cells such as neurones and cardiac muscle cells do not divide. Hyperplasia can therefore only occur in labile or stable cell populations.
2.
a. False. Atrophy may involve death by apoptosis (individual programmed cell death) but oncosis does not occur (see Ch. 4).
b. False. Smoking leads to squamous metaplasia of the glandular bronchial epithelium. Metaplasia is the process where a differentiated epithelium is replaced by another differentiated cell type.
c. True. Although many growth factors are mitogenic, i.e. they stimulate cells to divide, others inhibit cell growth. Some growth factors can either promote or inhibit growth, depending on the presence of other factors involved in regulating growth and differentiation. It is the balance between the various types of growth factor that contributes to the stability of a cell population.
d. False. Metaplasia is a reversible adaptive response. Removal of the adverse stimulus will result in restitution of the normal tissue. In some cases, a persistent stimulus will lead to neoplastic change. In cigarette smokers, the metaplastic squamous bronchial epithelium becomes increasingly abnormal and eventually squamous cell carcinoma develops.
e. True. In pregnancy, there is a massive hormonal stimulus to the breast epithelium, which leads to physiological hyperplasia and milk production. When hormone levels return to normal, lactation ceases and the epithelium returns to normal. Hyperplasia is a reversible process. After completion of lactation, the superfluous cells will be removed by apoptosis.
3.
a. False. Hyperplasia is potentially reversible.
b. True. Excess oestrogen stimulation is the usual cause of endometrial hyperplasia. Ovarian tumours are a possible source of abnormal oestrogen secretion, and they are particularly likely to be responsible in post-menopausal women.
c. False. Hyperplasia causes an increase in mass of the tissue. An ultrasound of the uterus is likely to show a thickened endometrium, and this can be a useful diagnostic test.
d. True. There is an increased risk of endometrial adenocarcinoma.
e. True. This is the definition of hyperplasia.
EMI answers
EMI 1
Theme: Responses to cell injury
1. D. This is an example of metaplasia. The normal mucus-secreting epithelium of the conjunctiva has been replaced by another type of epithelium.
3. A. The loss of hormonal stimulation has caused atrophy of the adrenal glands.
4. B. The definition of hyperplasia is an increase in the mass of the tissue due to an increase in cell number.
Case history answers
Case history 1
1. The labile cells are most likely to be affected by the treatment. Cytotoxic agents are used in the treatment of some cancers. They act by blocking the cell cycle at various points, preventing cell division. It is not usually possible to target the drugs specifically at the cancer cells, and normal populations of labile cells, such as in the bone marrow, gut epithelium and skin, will also be killed.
2. This example of a child with acute leukaemia shows how knowledge of the cell cycle and understanding of cell turnover and proliferation is fundamental to clinical medicine. The effects of cytotoxic drugs are entirely predictable. Normal labile cells, as well as tumour cells, will be destroyed by the cytotoxic drugs. The child will lose his hair (labile hair root cells) and may have gastrointestinal upsets as a result of destruction of gut epithelium. Loss of bone marrow stem cells may lead to anaemia (insufficient red cells), bleeding tendency (insufficient platelets) and infection (insufficient white cells).
3. Cytotoxic drugs are often given in ‘pulses’ with drug-free intervals. The drug-free period between treatments allows some restoration of normal, labile cell numbers, particularly normal bone marrow stem cells. If cytotoxic drugs were given without a break the normal stem cells could be wiped out before the tumour cells were destroyed.
Case history 2
1. Comment: Osteoporosis is an extremely common and important condition in which there is reduction of total bone mass, i.e. bone atrophy, which causes weakening. It is most common in elderly women and predisposes to fractures. Osteoporosis may be localised to a single bone, e.g. after immobilisation in a plaster cast, or affect many bones in a generalised way. It is the latter form which is seen in the elderly. Post-menopausal women are especially at risk. In its advanced stages, osteoporosis may be seen on X-rays as pallor or thinning of the bones. The pathogenesis of the disease is not completely understood.
The main risk factors and associated conditions are:
• increasing age
• lack of oestrogen (post-menopausal women)
• immobilisation either after fracture or paralysis
• corticosteroid therapy, where increased bone resorption probably occurs.
2. It is important for this woman to become mobile as quickly as possible postoperatively to prevent further worsening of the osteoporotic process through disuse atrophy, which might lead to further fractures. The patient needs to develop muscle tone in her legs to prevent disuse atrophy of the muscles from immobilisation and the development of venous stasis and deep vein thrombosis. In addition, there are important social reasons for her to regain mobility and return home to an independent life.