11 Causes of Premature Aging of the Spine
KEY POINTS
Introduction
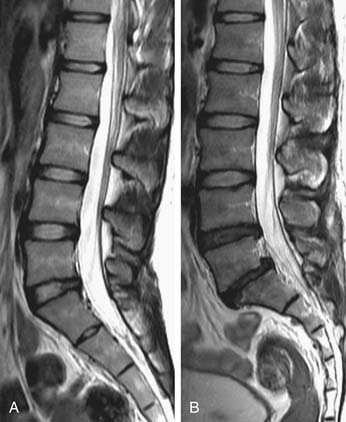
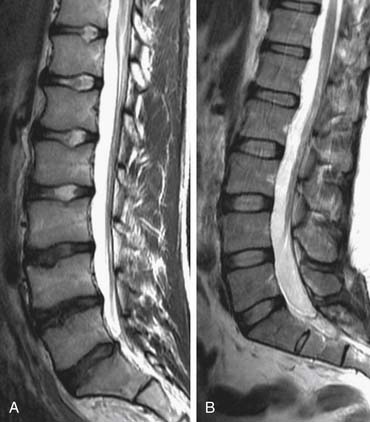
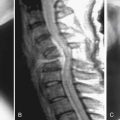
Premature aging of the spine is a salient concern, in that if it achieves clinical relevance associated with symptoms and impaired function, it has the potential to incur severe socioeconomic consequences. To identify such degenerative changes, advanced imaging, such as magnetic resonance imaging (MRI), has been a popular mainstay in the armamentarium of the physician for diagnostic and therapeutic interventions. However, numerous studies have also documented that the severity of radiological changes is not always associated with clinical symptoms (Figure 11-1).1 Nonetheless, it is essential to determine whether degenerative changes of the spine are a part of the natural evolution of the spine because of age, or if they result from a disease process (Figure 11-2) heralded by risk factors, possibly preventable, that prematurely change the spine. In this chapter, the authors will discuss the numerous factors that may contribute to premature aging of the spine.
Premature Aging Factors
Biochemical
In humans, the notochordal cells in the nucleus pulposus (NP) dramatically decrease after birth, and they eventually disappear, probably through apoptosis, and are replaced by chondrocyte-like cells by the first decade. The reduction in notochordal cell population with maturity could decrease proteoglycan production and contribute to the degenerative process. Furthermore, the naturally occurring cellular senescence, via telomere shortening, plays a role in disc aging as well as degeneration. However, degenerated discs are prone to increased cell senescence as the exposure to various factors, such as interleukin-1 (IL-1), reactive oxygen species, and mechanical load, further accelerate disc degeneration. Therefore exposure to such factors could induce premature senescence of the disc.2
In addition, degenerated discs have been shown to have higher concentrations and activities of degradative enzymes than normal discs, which could be due to the phenotypic changes of disc cells in response to various stimuli such as chemical mediators and mechanical loading. The alteration in disc cell phenotype leads to a cascade of biochemical changes that include the following: (1) decrease in matrix synthesis (e.g., aggrecan, decorin, type II and type IX collagens); (2) downregulation in the expression of growth factors and their receptors, which impairs the regenerative processes; and (3) upregulation of the catabolic metabolism through the increase in concentration and activity of matrix metalloproteinases (MMPs), reduction in tissue inhibitors of metalloproteinases (TIMP) levels, as well as the increase in proinflammatory cytokines and their receptor levels. Among all the cytokines, interleukin-1β in particular seems to play a central role, because it suppresses matrix synthesis and also stimulates the production of other inflammatory mediators, which further enhances matrix catabolism.2,3
Biomechanical
Clinically, disc degeneration is generally more prevalent and severe in the lower lumbar discs, suggesting that higher mechanical loading in this region may be a strong causative factor. Mechanical insults to the disc could induce fatigue failures in the endplates or annulus, which accelerate the catabolic cascade. However, mechanical loading is not a deleterious factor in itself as loading within physiological range stimulates disc matrix turnover and enhances anabolic factors, such as proteoglycan synthesis and TIMP production, whereas loading outside this range (less or more than optimal) is detrimental to disc metabolism. In vivo animal studies showed that high magnitudes or frequencies of dynamic and static compression induced cell apoptosis, structural failure and increased catabolism, whereas downregulation of anabolic gene expressions has been shown in the discs exposed to immobilization.4–7
A cadaveric investigation by Videman et al8 reported that history of occupational physical loading was related to disc degeneration and pathological changes of the lumbar spine, but a clear linear dose-dependent relationship was not established. In former elite athletes, more spinal degenerative findings were presented in those who engaged in heavier loading exercises versus light exercises, yet it only accounted for less than 10% of variability of MRI findings, despite the extreme difference in loading conditions.9 In fact, heavier lifetime physical loading, involving both occupational and leisure time activities, accounted only for small amounts of variance in disc degeneration in MRI, being 7% over T12-L4 and 2% over L4-S1.10 Moreover, when accounting for individual anthropometric parameters, such as body weight, lifting strength, and axial disc area in relation to disc degeneration, although all with modest effect, the lifelong continuous loading associated with these parameters was more influential than extrinsic physical loading related to occupation and leisure activities.11
Atherosclerosis
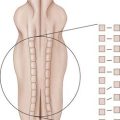
The blood supply to the lumbar spine is derived from the abdominal aorta, which gives off branches to supply regional vertebral segments (Figure 11-3).12 The nutritional supply to the intervertebral disc (IVD) depends on the diffusion potential through the endplates of the vertebral bodies. Therefore disc nutrition could be impeded by factors that diminish blood flow to the vertebrae, by defects or calcification of the endplates, or a combination of the three. An autopsy study by Kauppila et al.13 determined that the severity of disc degeneration was significantly associated with the grade of stenosis of the segmental arteries supplying the disc, and this association was stronger in the upper three lumbar levels than the lower two levels. Moreover, the degree of disc degeneration also increased in line with the complexity of the atherosclerotic lesions in the abdominal aorta. In accordance to the cadaveric studies by Kauppila et al.13 in vivo MRI studies have confirmed the association between impaired lumbar artery blood flow and diminished disc diffusion in both healthy subjects14 and patients with low back symptoms.15 It is generally thought that the reduced disc diffusion could in turn cause disc degeneration, but no definite answer to this hypothesis is available. Furthermore, clinical low back symptoms were also found to be associated with the presence of lumbar arterial stenosis.16–18 An atherosclerotic contribution to back symptoms is further substantiated in a long-term prospective study by Leino-Arjas et al.19 in which high baseline serum total cholesterol and triglyceride levels were associated with incident radiating low back pain among Finnish industrial employees. The increased risk of abnormal levels of cholesterol and triglycerides on radiating pain was independent of other potential risk factors, such as age, gender, occupational class, work history, exercise habits, smoking, and body mass index (BMI). The mechanism through which atherosclerosis and occlusion of arteries affect disc degeneration may be directly related to diminished blood flow, and hence decreased nutritional supply to the IVD, and also by the systemic inflammatory effect associated with atherosclerosis.
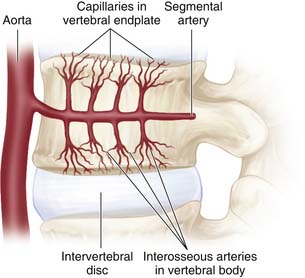
FIGURE 11-3 The segmental artery provides blood supply to the vertebral body.
(Adapted from Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract 2008;8:18-44.)
In a long-term follow-up study of 98,407 female nurses, cardiovascular risk factors, such as smoking, diabetes, hypertension, high cholesterol, obesity, and family history of myocardial infarction before the age of 60 years, were significantly associated with an increased risk of lumbar disc herniation. Moreover, after adjustment for other cardiovascular risk factors, the increased risk of symptomatic disc herniation exhibited a dose-dependent relationship with smoking (p = .003) and overweight (p = .01).20 However, in an observational study of 270 Japanese elderly with a mean age of 68.4 years, high-levels of LDL cholesterol was only associated with increased risk of disc degeneration at L4/5 (adjusted odds ratio = 2.65; 95% confidence interval = 1.33-5.52), but not at other levels. In the Japanese study, increased disc degeneration was not associated with their atherosclerosis index or with hyperglycemia.21 However, the presence or severity of atherosclerosis at lumbar arteries was not properly assessed, and therefore the general interpretation of “atherosclerosis” in this study could be problematic. Nevertheless, the cited studies highlight the complexity of cardiovascular risk factors and their role upon disc degeneration.
Lifestyle Factors
Disc degeneration had been previously regarded as an accelerated aging process caused by mechanical insults, such as encountered in physically demanding occupations or sports. However, lifestyle factors, such as smoking and obesity are regarded nowadays as risk factors of low back pain on the basis of meta-analyses.22 In recent decades, a series of exposure-discordant twin studies using MRI to assess the association between disc degeneration and environmental risk factors exposure has been conducted. By comparing the differences of interest between twin siblings in these studies, the effects of genetics and early childhood exposures between subjects were better understood, providing a new perspective regarding disc degeneration.23 On the basis of these twin studies, detrimental lifestyle factors may have negligible effect upon disc degeneration.
Smoking
Battie and associates24 assessed the effect of smoking on disc degeneration in 20 pairs of monozygotic (MZ) male twins and found that the disc degeneration score was 18% higher in smokers than in nonsmoking siblings. Additionally, smoking seemed to exert a systemic effect, because both upper and lower lumbar discs had higher disc degeneration scores, and the smokers had significantly more carotid atherosclerotic changes than nonsmoking co-twins. It was suggested that atherosclerotic changes associated with smoking could decrease the blood supply to vertebral vessels and structures, and hence could reduce disc nutrition and promote degeneration. However, despite the extraordinary discordance in smoking exposure with a mean difference of 31.6 smoking years between co-twins in this study, the effect of smoking was very trivial because it only explained 2% of the total amount of variance in disc degeneration scores.24 In addition, in their later exposure-discordant study in 115 pairs of male MZ twins, smoking failed to demonstrate a significant association with disc degeneration.10 The contradicting results were regarded to be due to the large differences in the mean smoking discordance between co-twins recruited in these two studies and the overall minor effect of smoking itself.23 Nevertheless, more recent studies showed that smoking (nicotine) could, indeed, directly mediate the disc cells metabolism by inhibiting cell proliferation and their synthetic activity of extracellular matrix in vitro,25 as well as increase the local production and release of inflammatory cytokines26 and downregulate the expression of collagen genes in vivo.27 These cellular responses of smoking provide evidence for the association of smoking with the degenerative process of the intervertebral discs. In clinical perspective, a recent prospective study among adolescence also demonstrated that regular smoking was associated with low back pain with an exposure–response relationship among young females.28
Obesity
In a recent study, a BMI above 25 kg/m2 was found to be significantly associated with lumbar disc degeneration at the lower four discs levels among elderly Japanese subjects.21 The importance of obesity was also reported by Liuke et al,29 who investigated the progression of disc degeneration in 129 middle-aged males. They found that persistent overweight (BMI above 25 kg/m2 at both 25 and 40 to 45 years) significantly increased the risk of disc degeneration by 4.3-fold, whereas other lifestyle variables, such as smoking, occupation, driving, and the presence of historical back injuries, were not associated with disc degeneration. Moreover, obesity at young age was particularly detrimental, because subjects with BMI of 24 to 25 kg/m2 and above 25 kg/m2 at the age of 25 years had 3.5- and 4.5-fold increased risk of disc degeneration, respectively.29 Furthermore, data of this chapter’s authors (Samartzis et al, unpublished) identified that obesity was the strongest risk factor associated with juvenile disc degeneration in a population-based cohort of Southern Chinese, because adolescents with BMI over 25 kg/m2 had an 18-fold increased risk associated with disc degeneration. In addition, in a study assessing the association of BMI and lumbar disc degeneration in 2252 Southern Chinese adults, Samartzis and colleagues (unpublished observation) further noted that an increased BMI was associated with the presence and severity of disc degeneration, noting a significant linear dose–response association. In fact, the authors further noted that a positive linear relation with an increase in BMI to that of the number of degenerated disc levels.
The exact mechanism by which obesity or overweight causes lumbar disc degeneration is yet to be elucidated, but it could partly be due to the excess mechanical load acting directly on the intervertebral discs, and indirectly through systemic risk factors associated with obesity.29 Obesity has been regarded as a low-grade systemic inflammatory condition, in which serum levels of C-reactive protein, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were shown to be increased in obese individuals.30 The increased levels of proinflammatory cytokines in obese subjects can be explained by the observation that both macrophages residing in adipose tissue and adipocytes are metabolically active, secreting proinflammatory cytokines, such as IL-6 and TNF-α, and adipocytokines (leptin, adiponectin, resistin).31 Cytokines, in particular IL-1, not only enhance degradation of the disc matrix3 but also are implicated in the pathophysiology of atherosclerosis.32 Therefore, disc dsegeneration could be accelerated in obese subjects through inflammatory pathways. In fact, it has been shown that gene polymorphisms could have an interaction with obesity to increase the risk of disc degeneration.33
Genetic Factors
The association between lifestyle factors and disc degeneration has been rather weak and even somewhat controversial as previously discussed, which suggest that other factors may potentially be more influential. Genetic determinants related to disc degeneration have received considerable attention in recent years. However, an accurate phenotype definition of disc degeneration is an absolute prerequisite for studies that evaluate the association between genetic factors and degenerative changes.34 Nonetheless, it is not surprising that evidence from twin studies suggests a key role for familial aggregation, which includes the influences by both genetic factors and early environmental exposure. Overall, the reported heredity estimate ranges from 50% to 74%.23 In an MRI study on monozygotic male twins, Battie et al10 reported the disproportion of variance in disc degeneration explained by familial aggregation to be 61% and 34% in the upper versus lower lumbar spine, respectively. The combined effect of age and physical loading only accounted for less than 16% of the total variance. The disproportional variance across spinal levels was suggested to be due to the interactions between genetic influences and mechanical loading with spinal anthropometrics. Yet in a study of predominantly female British and Australian twins, heritability prevalence was almost identical in the lumbar (74%) and cervical spines (73%). However, heritability estimates varied for different degenerative phenotypes, such as lumbar osteophytes (54%), disc bulging (65%), and disc height narrowing (79%).35
Heredity may determine the development of the spinal structures, therefore, affecting their mechanical strength and properties. It could also affect the biochemical properties by influencing the homeostasis between matrix synthesis and degradation. In turn, the structural integrity of the tissue and the vulnerability of the spinal structures to withstand external loading could be compromised with genetic defects, and predispose the spinal structures to accelerated degeneration. To date, several genes associated with disc degeneration as well as endplate changes have been identified, which entail genes coding for collagens (e.g., COL1A1 gene for collagen I, and COL9A2 and COL9A3 genes for collagen IX),36–42 aggrecan,43–45 cartilage intermediate layer protein (CILP),46–48 vitamin D receptor (VDR),49–51 inflammatory mediators such as IL-1,43,52–56 and degradative enzymes such as MMPs.43,47,54,57–60 Although the association of disc degeneration with gene polymorphisms was evident, inconsistent results between studies were reported even when accounting for the same gene (e.g., COL9A2 and COL9A3 among Finns, Chinese, Japanese, and Greek). This could be due to the disparities in allelic frequencies according to ethnicity. Nevertheless, the identification of genetic predisposition was undoubtedly an important breakthrough in understanding the cause in affecting the premature aging of the spine.
Discussion
Aging is an inevitable process that affects all spinal structures; disc degeneration has been described as an age-dependent, cell-mediated matrix degradation process that results in progressive structural failure.61 It is a complex condition that is determined by multiple biomechanical, environmental, lifestyle, and genetic factors and the interactions between these factors.
Numerous environmental factors have been suggested to be related to disc degeneration, as mentioned in previous sections, yet inconsistent results have been reported. On the contrary, the importance of genetic predisposition has been put forward by the twin and gene association studies in the recent decade.23 In the aforementioned genetic studies, it is evident that disc degeneration is associated with multiple gene polymorphisms with substantial ethnicity variance, yet the difference in study methodologies, particularly the difference in phenotypic definition, between these studies make it difficult to appreciate which genes and the interactions between them would exert a dominant role in specific degenerative changes. Solovieva et al62 provided evidence of gene–gene interaction, with an effect of a particular gene polymorphism on disc degeneration that would be modified by the presence of another gene polymorphism. It was found that an eightfold increased risk of higher disc degeneration score was associated with polymorphisms of COL9A3 in the absence of interleukin IL-1βT(3594), but no association was found with their joint occurrence. The gene programs the disc matrix turnover by coding for particular matrix synthesis and controlling its degradation. The effect of gene polymorphism should therefore, in theory, act systemically as long as the same gene polymorphism is coding for the same component, and producing the abnormal matrix identically in every disc across the whole spine. However, regional variations in disc degeneration are evident, with lower lumbar discs involved more commonly than the upper lumbar, suggestive of a gene–environment interaction affecting the vulnerability of a disc to degenerate. In fact, the notion of regional differences and gene–environment interactions have been confirmed by a recent twin study by Battie et al,63 which noted that considerable differences were found in the genetic and environmental influences for upper versus lower lumbar levels. Moreover, three common unique environmental factors and two common genetic factors were found to influence the disc signal intensity across the lumbar levels. Interestingly, of the two identified genetic factors, it seemed that the first factor influenced all lumbar levels, but the second factor appeared to be specific for L4-S1 only. Besides that, approximately two thirds of the genetic influences were found to be shared among disc degeneration phenotypes studied (disc signal intensity, and disc height narrowing and bulging), suggesting that they have a common pathogenesis pathway in association with disc degeneration.
In a study among middle-aged occupationally active Finnish men by Solovieva et al,33 the authors noted that 45% to 71% of the individuals with disc degeneration also presented with COL9A3 polymorphism and persistent obesity. The copresence of these two previously identified risk factors acted synergistically to increase the risk of having multilevel posterior disc bulge and multilevel decreased disc height, threefold and fourfold, respectively. In addition, they also found that the deleterious effect of occupational physical loading could be modified by the presence of IL-1 gene polymorphism.55 Additionally, recent study by Virtanen et al64 further substantiates the notion of gene–environment interactions, whereby a whole-body vibration was found to exert additive effect with all the polymorphisms analyzed (COL9A2, COL9A3, COL11A2, IL-1α, IL-1β, IL-6, MMP-3, and VDR) to increase the risk for symptomatic disc degeneration.
Histological evidence has illustrated that diminished blood supply to the disc is detrimental, appearing to initiate disc matrix degradation as early as in the first decade of life.65 Moreover, the effect of various gene mutations on disc degeneration, such as the Trp2 allele of collagen IX,37,38 D allele of the MMP-1 gene,59 and the t allele of vitamin D receptor gene,49 were also shown to exhibit an age-dependent effect.
1. Urban J.P., Winlove C.P. Pathophysiology of the intervertebral disc and the challenges for MRI. J. Magn. Reson. Imaging. 2007;25:419-432.
2. Zhao C.Q., Wang L.M., Jiang L.S., et al. The cell biology of intervertebral disc aging and degeneration. Ageing Res. Rev.. 2007;6:247-261.
3. Hadjipavlou A.G., Tzermiadianos M.N., Bogduk N., et al. The pathophysiology of disc degeneration: a critical review. J. Bone Joint Surg. Br.. 2008;90:1261-1270.
4. Stokes I.A., Iatridis J.C. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29:2724-2732.
5. Elfervig M.K., Minchew J.T., Francke E., et al. IL-1beta sensitizes intervertebral disc annulus cells to fluid-induced shear stress. J. Cell Biochem.. 2001;82:290-298.
6. Miyamoto H., Doita M., Nishida K., et al. Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine. 2006;31:4-9.
7. Ulrich J.A., Liebenberg E.C., Thuillier D.U., et al. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine. 2007;32:2812-2819.
8. Videman T., Nurminen M., Troup J.D. 1990 Volvo Award in clinical sciences. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation, and physical loading. Spine. 1990;15:728-740.
9. Videman T., Sarna S., Battie M.C., et al. The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine. 1995;20:699-709.
10. Battie M.C., Videman T., Gibbons L.E., et al. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601-2612.
11. Videman T., Levalahti E., Battie M.C. The effects of anthropometrics, lifting strength, and physical activities in disc degeneration. Spine. 2007;32:1406-1413.
12. Raj P.P. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18-44.
13. Kauppila L.I., Penttila A., Karhunen P.J., et al. Lumbar disc degeneration and atherosclerosis of the abdominal aorta. Spine. 1994;19:923-929.
14. Kurunlahti M., Kerttula L., Jauhiainen J., et al. Correlation of diffusion in lumbar intervertebral discs with occlusion of lumbar arteries: a study in adult volunteers. Radiology. 2001;221:779-786.
15. Tokuda O., Okada M., Fujita T., et al. Correlation between diffusion in lumbar intervertebral discs and lumbar artery status: evaluation with fresh blood imaging technique. J. Magn. Reson. Imaging. 2007;25:185-191.
16. Korkiakoski A., Niinimaki J., Karppinen J., et al. Association of lumbar arterial stenosis with low back symptoms: a cross-sectional study using two-dimensional time-of-flight magnetic resonance angiography. Acta. Radiol.. 2009;50:48-54.
17. Kauppila L.I., Tallroth K. Postmortem angiographic findings for arteries supplying the lumbar spine: their relationship to low-back symptoms. J. Spinal Disord.. 1993;6:124-129.
18. Kurunlahti M., Karppinen J., Haapea M., et al. Three-year follow-up of lumbar artery occlusion with magnetic resonance angiography in patients with sciatica: associations between occlusion and patient-reported symptoms. Spine. 2004;29:1804-1808.
19. Leino-Arjas P., Kaila-Kangas L., Solovieva S., et al. Serum lipids and low back pain: an association? A follow-up study of a working population sample. Spine. 2006;31:1032-1037.
20. Jhawar B.S., Fuchs C.S., Colditz G.A., et al. Cardiovascular risk factors for physician-diagnosed lumbar disc herniation. Spine J.. 2006;6:684-691.
21. Hangai M., Kaneoka K., Kuno S., et al. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J.. 2008;8:732-740.
22. R. Shiri, J. Karppinen, P. Leino-Arjas, et al., The association between smoking and low back pain: a meta-analysis. Am. J. Med. 123 (2010) 87.e7–e35.
23. Battie M.C., Videman T., Kaprio J., et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J.. 2009;9:47-59.
24. Battie M.C., Videman T., Gill K., et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine. 1991;16:1015-1021.
25. Akmal M., Kesani A., Anand B., et al. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine. 2004;29:568-575.
26. Oda H., Matsuzaki H., Tokuhashi Y., et al. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. J. Orthop. Sci.. 2004;9:135-141.
27. Uei H., Matsuzaki H., Oda H., et al. Gene expression changes in an early stage of intervertebral disc degeneration induced by passive cigarette smoking. Spine. 2006;31:510-514.
28. Mikkonen P., Leino-Arjas P., Remes J., et al. Is smoking a risk factor for low back pain in adolescents? A prospective cohort study. Spine. 2008;33:527-532.
29. Liuke M., Solovieva S., Lamminen A., et al. Disc degeneration of the lumbar spine in relation to overweight. Int. J. Obes. (Lond). 2005;29:903-908.
30. Das U.N. Is obesity an inflammatory condition? Nutrition. 2001;17:953-966.
31. Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol.. 2006;6:772-783.
32. Girn H.R., Orsi N.M., Homer-Vanniasinkam S. An overview of cytokine interactions in atherosclerosis and implications for peripheral arterial disease. Vasc. Med.. 2007;12:299-309.
33. Solovieva S., Lohiniva J., Leino-Arjas P., et al. COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene-environment interaction. Spine. 2002;27:2691-2696.
34. Chan D., Song Y., Sham P., et al. Genetics of disc degeneration. Eur. Spine J.. 2006;15(Suppl. 3):S317-S325.
35. Sambrook P.N., MacGregor A.J., Spector T.D. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366-372.
36. Annunen S., Paassilta P., Lohiniva J., et al. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285:409-412.
37. Higashino K., Matsui Y., Yagi S., et al. The alpha2 type IX collagen tryptophan polymorphism is associated with the severity of disc degeneration in younger patients with herniated nucleus pulposus of the lumbar spine. Int. Orthop.. 2007;31:107-111.
38. Jim J.J., Noponen-Hietala N., Cheung K.M., et al. The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine. 2005;30:2735-2742.
39. Kales S.N., Linos A., Chatzis C., et al. The role of collagen IX tryptophan polymorphisms in symptomatic intervertebral disc disease in Southern European patients. Spine. 2004;29:1266-1270.
40. Karppinen J., Paakko E., Raina S., et al. Magnetic resonance imaging findings in relation to the COL9A2 tryptophan allele among patients with sciatica. Spine. 2002;27:78-83.
41. Paassilta P., Lohiniva J., Goring H.H., et al. Identification of a novel common genetic risk factor for lumbar disc disease. JAMA. 2001;285:1843-1849.
42. Seki S., Kawaguchi Y., Mori M., et al. Association study of COL9A2 with lumbar disc disease in the Japanese population. J. Hum. Genet.. 2006;51:1063-1067.
43. Karppinen J., Daavittila I., Solovieva S., et al. Genetic factors are associated with modic changes in endplates of lumbar vertebral bodies. Spine. 2008;33:1236-1241.
44. Roughley P., Martens D., Rantakokko J., et al. The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur. Cell Mater.. 2006;11:1-7.
45. Solovieva S., Noponen N., Mannikko M., et al. Association between the aggrecan gene variable number of tandem repeats polymorphism and intervertebral disc degeneration. Spine. 2007;32:1700-1705.
46. Seki S., Kawaguchi Y., Chiba K., et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat. Genet.. 2005;37:607-612.
47. Valdes A.M., Hassett G., Hart D.J., et al. Radiographic progression of lumbar spine disc degeneration is influenced by variation at inflammatory genes: a candidate SNP association study in the Chingford cohort. Spine. 2005;30:2445-2451.
48. Virtanen I.M., Song Y.Q., Cheung K.M., et al. Phenotypic and population differences in the association between CILP and lumbar disc disease. J. Med. Genet.. 2007;44:285-288.
49. Cheung K.M., Chan D., Karppinen J., et al. Association of the Taq I allele in vitamin Dreceptor with degenerative disc disease and disc bulge in a Chinese population. Spine. 2006;31:1143-1148.
50. Kawaguchi Y., Kanamori M., Ishihara H., et al. The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J. Bone Joint Surg. Am.. 2002;84-A:2022-2028.
51. Videman T., Leppavuori J., Kaprio J., et al. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine. 1998;23:2477-2485.
52. Le Maitre C.L., Freemont A.J., Hoyland J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther.. 2005;7:R732-R745.
53. Le Maitre C.L., Hoyland J.A., Freemont A.J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res. Ther.. 2007;9:R77.
54. Podichetty V.K. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol. Biol. (Noisy-le-grand). 2007;53:4-18.
55. Solovieva S., Kouhia S., Leino-Arjas P., et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15:626-633.
56. Solovieva S., Leino-Arjas P., Saarela J., et al. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain. 2004;109:8-19.
57. Dong D.M., Yao M., Liu B., et al. Association between the -1306C/T polymorphism of matrix metalloproteinase-2 gene and lumbar disc disease in Chinese young adults. Eur. Spine. J.. 2007;16:1958-1961.
58. Goupille P., Jayson M.I., Valat J.P., et al. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612-1626.
59. Song Y.Q., Ho D.W., Karppinen J., et al. Association between promoter -1607 polymorphism of MMP1 and lumbar disc disease in Southern Chinese. BMC Med. Genet.. 2008;9:38.
60. Takahashi M., Haro H., Wakabayashi Y., et al. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J. Bone Joint Surg. Br.. 2001;83:491-495.
61. Adams M.A., Roughley P.J. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151-2161.
62. Solovieva S., Lohiniva J., Leino-Arjas P., et al. Intervertebral disc degeneration in relation to the COL9A3 and the IL-1ss gene polymorphisms. Eur. Spine J.. 2006;15:613-619.
63. Battie M.C., Videman T., Levalahti E., et al. Genetic and environmental effects on disc degeneration by phenotype and spinal level: a multivariate twin study. Spine. 2008;33:2801-2808.
64. Virtanen I.M., Karppinen J., Taimela S., et al. Occupational and genetic risk factors associated with intervertebral disc disease. Spine. 2007;32:1129-1134.
65. Boos N., Weissbach S., Rohrbach H., et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631-2644.