Chapter 105 Cauda Equina Syndrome
Cauda equina syndrome (CES) is a complex of symptoms and signs, including low back pain, unilateral or bilateral radiculopathy, lower extremity motor weakness, sensory disturbance including saddle anesthesia, and loss of visceral function (i.e., bladder and bowel incompetence ranging from frequency to bladder and anal sphincter paralysis, and erectile dysfunction), that results from either acute or chronic cauda equina compression (Box 105-1). This syndrome is characterized by a variable clinical presentation that depends on the anatomic location (lumbar, sacral, or coccygeal/focal central or complete compression), rapidity, and duration of compression of the cauda equina. Motor weakness involving the lumbar, sacral, and coccygeal roots in isolation or in combination is often present. Hypesthesia or anesthesia is often present in the dermatomal distribution of L3 to Coc1, inclusive. Radicular signs and symptoms may be either unilateral or bilateral. Bowel or bladder dysfunction is common and is the source of the hallmark signs and symptoms of CES. The knee and ankle jerk may be absent. There are typically no upper motor neuron findings, and the Babinski sign is absent. CES, particularly if unrecognized and untreated, often results in paraplegia, severe paraparesis, permanent bladder and bowel incontinence, or sexual dysfunction.
Pathophysiology
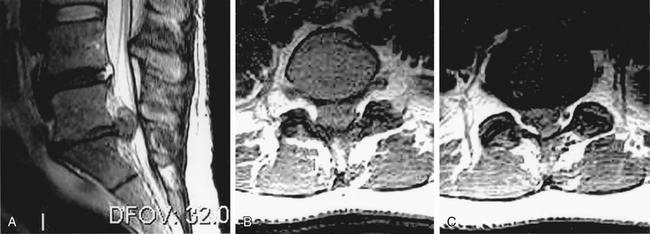
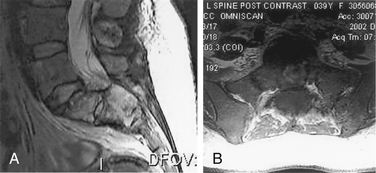
Spinal nerve root compression commonly occurs in conditions such as acute herniated disc, spinal stenosis, trauma (e.g., burst fractures), metastatic or primary tumors of the spine, or spinal infections (e.g., epidural abscess) (Box 105-2). Acute CES most commonly presents secondary to lumbosacral intervertebral disc prolapse (Fig. 105-1). However, the pathophysiology of the symptoms and signs related to spinal nerve root compression remains poorly defined.
Several experimental studies have assessed the pathophysiologic mechanism of CES. Delamarter et al.1,2 developed an animal model of CES, subjecting 30 beagle dogs to L6-7 laminectomy and cauda equina compression. Neurologic recovery was assessed in animals undergoing 75% constriction of the cauda equina followed by immediate, early, or delayed decompression. The first group was constricted and immediately decompressed. The remaining groups were constricted for 1 hour, 6 hours, 24 hours, and 1 week, respectively, before being decompressed. Evoked potentials were measured before and after surgery, before and after decompression, and 6 weeks after decompression. Six weeks after decompression, all dogs were killed, and the neural elements were analyzed histologically. After compression, all 30 dogs had significant lower extremity weakness, tail paralysis, and urinary incontinence. All dogs recovered significant motor function by 6 weeks after decompression. The dogs with immediate decompression typically recovered neurologic function within 2 to 5 days. The dogs receiving 1- and 6-hour compression recovered within 5 to 7 days. Dogs receiving 24 hours of compression remained paraparetic for 5 to 7 days, with bladder dysfunction persisting for 7 to 10 days and tail dysfunction for up to 4 weeks. The dogs with compression for 1 week were paraparetic and incontinent during the duration of cauda equina compression. They recovered the ability to walk by 1 week and regained bladder and tail control by the time of euthanasia. Immediately after compression, all five groups demonstrated at least 50% deterioration of the posterior tibial evoked potential amplitudes.2 Delamarter et al.2 demonstrated axoplasmic flow blockade and wallerian degeneration of the motor nerve roots distal to the constriction and of the sensory roots proximal to the site of constriction, as well as dorsal column degeneration. Severe arterial narrowing occurred at the level of the constriction with venous congestion of the roots and dorsal root ganglia of the seventh lumbar and first sacral nerves.1 Evoked potentials were the most sensitive predictor of neural compression, revealing neurologic abnormalities before the appearance of neurologic signs and symptoms.1 Cystometrograms were not sensitive until severe compression was achieved. Bladder dysfunction was correlated with axoplasmic flow blockade and early sensory changes during neurovenous congestion.
Olmarker et al.3–7 developed an experimental model of acute, graded compression of the cauda equina in pigs that accurately mimics the neural and vascular anatomy of the human cauda equina. There were structural and vascular differences between spinal nerve roots and peripheral nerves that could contribute to differences in compression susceptibility between these two parts of the nervous system. Pressure transmission from the balloon to the nerve roots permitted determination of occlusion pressures for the arterioles, capillaries, and venules of the cauda equina.7 Arteriolar blood flow ceased when the applied pressure approached the mean arterial blood pressure. Capillary blood flow was dependent on flow in connected venules, and the blood flow in some venules ceased at 5 to 10 mm Hg despite venous occlusion pressures ranging from 5 to 60 mm Hg. Compression up to 200 mm Hg for 2 hours did not induce a no-reflow phenomenon upon compression release. However, transient hyperemia was noted at all pressure-time relations studied, indicating nutritional deficit in the compressed segment during compression. Signs of edema were observed in nerve roots exposed to compression for 2 hours at either 50 or 200 mm Hg. The nutritional supply to the cauda equina was impaired at low pressure levels (<10 mm Hg).4,5 Thus diffusion from adjacent tissues with a better nutritional supply, including the cerebrospinal fluid, could not compensate completely for compression-induced effects on the transport of nutrients. However, a certain nutritional supply to the compressed segment was present even at 200 mm Hg compression. A rapid compression rate resulted in more pronounced effects on the nutritional supply than did a slow compression rate. Nutritional impairment was observed both within and outside the compressed nerve segment. An increase in vascular permeability was induced by compression at 50 mm Hg for 2 minutes.6 The magnitude of this permeability increase was dependent on both the magnitude and the duration of compression. The permeability increase was more pronounced for the rapid compression onset rate than for the slow compression onset rate at all pressure-time relations studied. Reduction of muscle action-potential amplitude in tail muscles, after stimulation cranial to the compression zone, was induced by compression at 100 and 200 mm Hg for 2 hours.4,6
Pedowitz et al.8 and Rydevik et al.9,10 presented an experimental model of compression-induced functional changes of the porcine cauda equina that permits electrophysiologic investigation of the neurophysiologic changes induced by nerve root deformation. In several studies, they compared the effects of various pressures and durations of acute compression on spinal nerve root conduction in the pig cauda equina. Changes in both afferent (compound nerve action potentials) and efferent (compound motor action potentials) conduction were induced at an acute pressure threshold of 50 to 75 mm Hg. Higher compression pressures produced a differential recovery in afferent and efferent conduction.9,10 Efferent conduction and afferent conduction were monitored during compression for 2 or 4 hours with compression pressures of 0 (sham treatment), 50, 100, or 200 mm Hg. Recovery was monitored for 1.5 hours. No significant deficits in spinal nerve root conduction were observed with 0 or 50 mm Hg compression, whereas significant deficits were induced by 100 and 200 mm Hg compression. Variance analysis demonstrated significant effects of compression pressure and duration on conduction, with a significant difference between efferent and afferent conduction at the end of the recovery period, suggesting a synergistic interaction between biomechanical and microvascular mechanisms in the production of nerve root conduction deficits.8
Compression of the spinal nerve roots often occurs at multiple levels simultaneously; however, the basic pathophysiology of multilevel compression is poorly defined. Using a thermal diffusion technique, Takahashi et al.11 quantitated intraneural blood flow in the uncompressed segment between two compressive balloons in the porcine cauda equina. At 10 mm Hg compression, there was a 64% reduction of total blood flow in the uncompressed segment compared with precompression values. Total ischemia occurred at pressures 10 to 20 mm Hg less than the mean arterial blood pressure. After two-level compression at 200 mm Hg for 10 minutes, there was a gradual recovery of the intraneural blood flow toward the baseline. Recovery was less rapid and less complete after 2 hours of compression. Double-level compression of the cauda equina induced blood flow impairment, not only at the sites of compression but also in the intermediate nerve segments located between two compression sites, even at very low pressures.
Cauda Equina Syndrome Secondary to Disc Herniation
Epidemiology
CES secondary to a large central disc herniation is a relatively uncommon entity, but its clinical importance far exceeds its rarity (see Fig. 105-1). CES secondary to lumbar disc herniation is an absolute indication for surgical intervention.12
The incidence of CES has been estimated to range from 1.2% to 6%. In 1970, Raaf13 reported an incidence of 2% in 624 patients with protruded discs. In 1972, Spangfort14 reported a 1.2% incidence in 2504 cases, and his review of the literature found a total incidence of approximately 2.4%. In 1986, Kostuik et al.15 reported a 2.2% incidence of CES in patients admitted for lumbar laminectomy. In 1990, Gleave and MacFarlane16 reported cauda equina paralysis secondary to lumbar disc prolapse in 3.2% of cases; this probably overestimated the true incidence because they did not consider nonoperatively treated patients.
Clinical Syndrome
Some reports contend that bilateral sciatica is a necessary component of CES, but a number of large series refute this notion. In a review of 31 patients by Kostuik et al.15 in 1986, sciatica was bilateral in 14 patients and unilateral in 17. Severe saddle anesthesia was indicative of a poor prognosis, particularly for return of bladder and bowel function.15 However, any correlation of factors such as severity of somatic signs and symptoms, symptom duration before surgical decompression, and the size and location of disc protrusion with recovery of bowel and bladder function was unclear. In a review of 58 patients by Doman et al. in 2009, urinary retention of more than 500 mL alone or in combination with two or more of the following symptoms—bilateral sciatica, urinary retention, or rectal incontinence—were the most important predictors of MRI-confirmed cauda compressions.17
Clinical Course
Tay and Chacha18 reported eight cases observed over a 5-year period that fell into three clinical groups. The first group of patients noted sudden onset of symptoms without previous back problems. The second group noted recurrent episodes of backache and sciatica, with the most recent episode resulting in cauda equina involvement. The final group of patients had slowly evolving backache and sciatica that progressed to cauda equina paralysis. Disc prolapse occurred between the L5 and S1 vertebrae in 50% of the patients, most of whom had no limitation in straight-leg raising. Urgent myelography and disc removal within 2 weeks of symptom onset resulted in substantial recovery of motor and bladder function within 5 months after surgery. Sensory and sexual function recovery was incomplete for as long as 4 years postoperatively.18 Patients at the preclinical and early stages have better functional recovery than patients in later stages after surgical decompression.19
Choudhury and Taylor20 reported on 42 patients with lumbar disc disease and herniation who presented with CES. Simple disc herniation accounted for the syndrome in only five cases. Associated structural lesions were noted in the remaining 37 cases, and operative manipulation and trauma during disc removal through an interlaminar approach was reported in two patients.20
Lafuente et al.21 noted sacral sparing and preservation of sphincter control in 8 of 14 cases of cauda equina compression from central lumbar disc herniation and postulated that the triangular shape of the lumbar spinal canal may be one factor for this constellation of findings, because the increase in linear strain on the stretched roots of the cauda equina is least in the more centrally placed lower sacral roots. Kostuik et al.15 identified two distinct modes of presentation. The first was an acute mode (in 10 patients) in which there was an abrupt onset of severe symptoms and signs and a slightly poorer prognosis after decompression, especially for the return of bladder function. The second mode of presentation (in 21 patients) had a more protracted onset, characterized by prior symptoms for varying time intervals before the gradual onset of CES. All patients reported preoperative urinary retention. Bladder function was the most seriously affected function preoperatively and remained so postoperatively. The prognosis for return of motor function was good. Of 30 patients receiving surgery, 27 regained normal motor function.15
Diagnostic Imaging
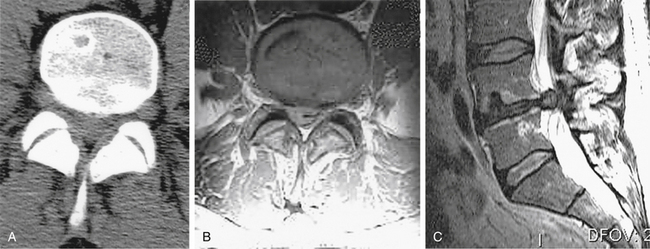
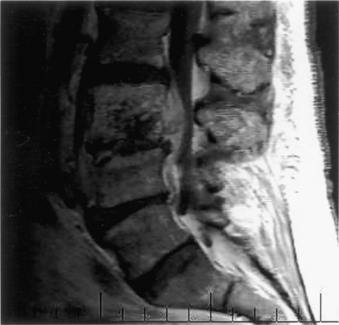
Diagnosis and treatment are often delayed because of lack of recognition of the condition and failure to appreciate the surgical imperative for its treatment. Once cauda equina compression is recognized or suspected, MRI is the investigation method of choice. If an MRI is contraindicated, of poor quality because of motion artifact (patients with CES are often in severe pain), or unavailable, CT-myelography is recommended. CT alone can be misleading in cases of complete canal occlusion (Fig. 105-2).
Surgical Therapy
Urgent surgical intervention should commence after diagnosis. Choudhury and Taylor20 advocated wide laminectomy with excision of the overhanging facet joints and adequate visualization of the lumbar nerve roots. This yielded good or excellent results in 95% of patients and fair results in the remainder. No postoperative spinal instability or significant morbidity was reported.20
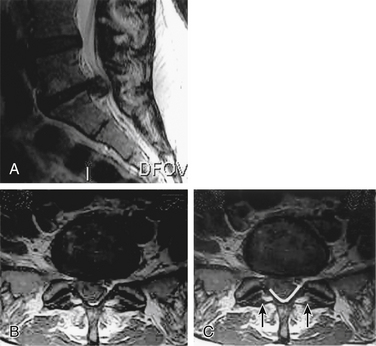
A routine microdiscectomy interlaminar approach necessitates retraction on already severely compromised nerve roots. Furthermore, because of the extent of compression, these roots often have no available canal space; thus, retraction not only increases traction but also may cause direct compression (against the disc or the overlying lamina). For the same reason, the use of large punches with a thick foot plate is also not recommended (compresses underlying roots against the herniated disc) (Fig. 105-3). Consequently, the authors advocate a wide bilateral decompression (laminectomy and medial third facetectomy). To avoid iatrogenic compression of the cauda equina, the surgeon can perform the laminectomy using a high-speed bur to thin out the lamina and then curettes and small punches lateral to the area of maximal compression to release the medial portion of the lamina (see Fig. 105-3). Once the lamina is released, it can be safely lifted away from the cauda equina. The decompression should be adequate enough to allow access lateral to the thecal sac and traversing roots at the level of the affected disc space and/or the sequestered fragment. The surgeon should carefully perform retrieval of the herniated fragment to avoid creating a further mass effect and therefore increased traction on the less mobile central sacral roots. This can be accomplished by performing a lateral anulotomy and discectomy and then pushing the central fragment back into the disc space with a reverse-angle curette. If the fragment is sequestered, it should be manipulated in a lateral direction using a nerve hook or angled curette and then retrieved. Before closure, the surgeon should confirm adequate decompression.
Timing of Surgical Intervention
Controversy persists regarding the definition, cause, diagnosis, and timing of surgical intervention for CES resulting from lumbar disc herniation. Conventional wisdom has been that early detection of CES is essential to maximize the probability of neurologic recovery after decompressive laminectomy and discectomy.22 Some investigators have gone so far as to advocate the necessity for decompression within 6 hours of presentation.23 However, data supporting immediate intervention are far from clear.
Kostuik et al.15 reported 31 patients with CES secondary to a central disc lesion. The average time to surgical decompression after initial presentation ranged from 1.1 days for the group with more acute lesions to 3.3 days for the second group. There was no correlation between these times and return of function. The authors recommended early surgery but noted that decompression did not have to be immediate.15
Shapiro24 studied 14 patients with acute CES from herniated lumbar discs who all presented with bilateral sciatica and leg weakness; 93% had bladder or bowel dysfunction. All patients were emergently studied with CT, myelography, or MRI. Nine patients had large or massive herniations, and five had smaller herniations superimposed on preexisting stenoses. The time to surgery ranged from less than 24 hours to more than 30 days. Postoperatively, six patients (43%) were normal, four patients (28.5%) had chronic pain and numbness, and four patients (28.5%) had persistent incontinence and weakness. Of the 10 patients without postoperative incontinence, 7 underwent surgery within 48 hours of onset. Of the four patients with persistent incontinence, all underwent surgery 48 hours or more after presentation.24
The onset of bladder paralysis is an important indicator for urgent surgery. Dinning and Schaeffer25 reported a significant difference in the outcome of patients operated on within 24 hours of bladder paralysis compared with those operated on after this period. Thus it would appear that urgent decompression is superior to delayed surgery with regard to the recovery of neurologic function, but the necessity to perform immediate surgery (within 6 hours of presentation) is less clear. Hussain et al.26 recently reported on 20 patients with CES from herniated lumbar disc. Nine patients underwent emergent decompression (<5 hours from time of presentation to the surgical unit), and the remainder had their surgery the next day but within 24 hours. At a mean of 16 months follow-up, there was no difference in urologic or quality of life outcome between the emergent and delayed groups.26 Qureshi and Sell conducted a prospective longitudinal inception cohort study of 33 patients undergoing surgery for CES. They reported a significantly better outcome in patients who were continent of urine at presentation compared with those who were incontinent.27
Cauda Equina Syndrome Resulting from Intradural Disc Rupture
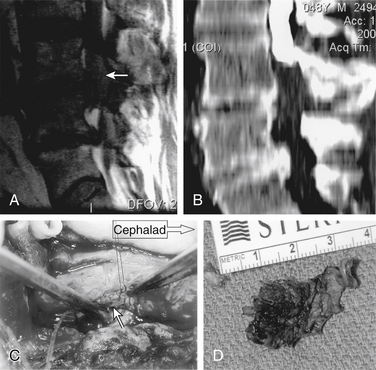
There is a strong association between the presence of intradural disc rupture and the development of CES. Dinning and Schaeffer25 reported that intradural sequestration of disc fragments occurs in 7.5% of cases, although in most other series the rate is less than 1%. Spangfort14 detected no differences in age or sex distribution. Lower lumbar discs were the most commonly affected levels; however, there was a significantly larger number of high lumbar herniations leading to this problem than to other disc syndromes. Myelography typically demonstrates complete block, and at surgery, intradural fragments of sequestrated disc material can be found (Fig. 105-4). Intradural exploration or transdural sequestrectomy avoids traction on already compromised nerve roots and is often safer than extradural sequestrectomy.
Cauda Equina Syndrome Secondary to Trauma
Patients with sacral fracture or dislocation and CES have been reported. Schnaid et al.28 reported CES complicating acute lumbosacral fracture or dislocation 3 weeks postinjury. Isolated transverse sacral fractures are rare, but extensive neurologic deficits may accompany these injuries. Extradural hemorrhage can accompany these fractures and produce a serious neurologic deficit requiring urgent sacral laminectomy.29
Hilibrand et al.30 reported CES associated with acute spondylolytic spondylolisthesis after major trauma. The deformity progressed from grade III to grade V (spondyloptosis), and the patient developed CES. The patient was treated with dorsal reduction and arthrodesis followed by an anterior arthrodesis, and the neurologic deficits resolved. Although minor or repetitive trauma is often associated with spondylolysis, high-energy trauma may produce a more severe form of spondylolysis with spondylolisthesis. These deformities are more unstable, with instability similar to that of a fracture-dislocation, and they have a greater propensity to progress than does the usual form of spondylolytic spondylolisthesis.30
Dorsal dural lacerations associated with lumbar burst fractures can occur and are produced by dural impaction within a fractured lamina. Neural elements can be extruded outside the dura mater and be trapped in a lamina fracture. Preoperative appreciation of this diagnosis is imperative and is based on clinical presentation, fracture pattern, and radiographic findings. Denis and Burkus31 recommended extraction of entrapped neural elements from the fractured lamina by laminoplasty of the dorsal neural arch. Neurologically impaired patients with lumbar burst fractures and radiographic evidence of dorsal displacement of the neural elements in the lamina fracture should undergo dorsal exploration of the spinal canal, extraction of cauda equina neural elements, and repair of the dural laceration before any spinal reduction maneuver is attempted.31
Intradural lumbar arachnoid diverticulas are rare but can cause mild compression of the cauda equina and produce debilitating symptoms. A chronic posttraumatic lumbar intradural arachnoid cyst causing CES has been reported.32
Cauda Equina Syndrome Secondary to Primary or Secondary Neoplasms
Primary extradural or intradural tumors may rarely present with acute CES.23 For example, myxopapillary ependymomas of the conus or filum may present acutely with subarachnoid hemorrhage and cauda equina dysfunction.33,34 There are reported cases of paraganglioma of the cauda equina causing compression requiring urgent treatment.35
Metastatic tumors to the lumbosacral spine can create CES by one of two means: (1) cauda equina compression by epidural tumor or (2) vertebral instability resulting in canal compromise and subsequent cauda equina compression (Fig. 105-5). The most common clinical manifestation is pain with or without concomitant nerve root signs. Regardless of the cause, the major goal of treatment is prompt decompression of the neural elements with stabilization or, at a minimum, avoidance of destabilization of the spine.
Spiegelmann et al.36 reported a patient with acute myelogenous leukemia with CES as the presenting symptom. Staff et al. reported two patients with metastatic lobular breast adenocarcinoma presenting as CES.37 There have been eight reported cases in the literature of intradural spinal metastasis from renal cell carcinoma causing CES.38 Woo et al.39 reported 97 Chinese patients with multiple myeloma, of whom 23.7% had cauda equina or spinal cord compression. Predictive features for spinal cord compression include paraprotein type, hemoglobin level, and extent of bone lesion at initial hematologic diagnosis. They concluded that vertebral cortex involvement predisposes the patient to spinal cord compression.39 Rarely, patients with gynecologic tumors may present with acute CES.23There have been two reported cases of CES due to leptomeningeal carcinomatosis of the ovary.40
Cauda Equina Syndrome Secondary to Ankylosing Spondylitis
CES may occur as a neurologic complication of long-standing ankylosing spondylitis. The syndrome is uncommon, and its pathophysiology is poorly defined. There are several case reports of slowly progressive CES in patients with long-standing ankylosing spondylitis, with loss of lower spinal motor and sensory root function. The pathogenesis of CES in ankylosing spondylitis may be demyelination, postirradiation ischemia, or compression from spinal arachnoiditis, or it may arise in association with massive dural ectasia.41–43
Symmetrical neurologic deficits typically progress slowly and occur late in the evolution of spondylitis, long after the onset of the condition and well after the rheumatologic symptoms have abated. The initial symptom is typically disturbance of sphincter control with subsequent changes in ankle reflexes and cutaneous sensation, particularly in the sacral dermatomes. Variable lower nerve root involvement has been reported. Typical findings include cutaneous sensory impairment of the lower limbs and perineum with sphincter disturbances. Motor impairment occurs less commonly, and associated pain is inconsistent. Eventually, patients experience cutaneous sensory loss in the fifth lumbar and sacral dermatomes and develop a lower motor neuron urinary sphincter disturbance with loss of rectal sphincter tone and with incontinence or severe constipation. Some patients have mild to moderate weakness in the lumbosacral myotomes or pain in the rectum or lower limbs.44
A cauda equina–like syndrome with neuropathic bladder is a rare but well-described complication of long-standing ankylosing spondylitis. Diverticulas from the subarachnoid space may erode into the lumbosacral vertebrae. These spinal diverticulas can be demonstrated by CT.45 Electromyographic (EMG) abnormalities are typically consistent with multiple lumbosacral radiculopathies.44 In the setting of ankylosing spondylitis, MRI is valuable in excluding other spinal lesions because it excludes the presence of extrinsic compression of the cauda equina.46
The clinical diagnosis of CES can be adequately confirmed with plain radiology, EMG, and CT. CT and MRI represent noninvasive means of establishing the diagnosis of CES in patients with ankylosing spondylitis.43 MRI of the CES in patients with ankylosing spondylitis aids in the diagnosis and may provide valuable insight into the pathophysiology of this condition.47 The features observed on MRI are pathognomonic, allowing accurate noninvasive diagnosis of the disorder.48
CT-myelography of the lumbosacral spine typically shows dilation of the spinal canal and lumbar sac with multiple dorsal arachnoid diverticulas eroding the laminae and spinous processes. Young et al.49 found no compressive lesions with myelography, and foraminal encroachment was not seen. The pathogenesis of these erosions may be related to arachnoiditis in the early phase of the spondylitis.50 Recognition of this syndrome, coupled with CT of the lower spinal canal, allows the clinician to avoid myelography, a procedure that is often difficult to perform in these patients because of associated spine abnormalities.
Early investigators concluded that surgery for CES secondary to ankylosing spondylitis was neither beneficial nor indicated and that it should be avoided.43,44 However, surgery may be indicated in some patients, particularly when there is nerve root compression by the arachnoid cysts and when the patient is treated before irreversible damage to the cauda equina has occurred.51
Cauda Equina Syndrome Secondary to Arteriovenous Malformation or Hemorrhage
Spinal subdural hematoma is a rare cause of cauda equina compression that can occur in patients with a bleeding diathesis. Johnson et al.52 reported a case of subacute lumbar subdural hematoma that was demonstrated by MRI. Laus et al.53 reported three cases of CES resulting from epidural hematoma secondary to warfarin (Coumadin)-induced coagulopathy. They recommended anticoagulant correction with appropriate medications and hemoderivatives, with surgical treatment for the severe cases. Schmidt et al.54 presented a patient with acute CES from a ruptured aneurysm in the sacral canal. The lesion was associated with pathologic enlargement of the lateral sacral arteries bilaterally, which occurred to provide cross-pelvic collateral flow in response to the diversion of the right internal iliac artery for renal transplantation. The patient showed signs and symptoms of spontaneous spinal epidural hemorrhage. In addition to angiography and partial embolization of the vascular supply, contrast-enhanced, high-resolution CT was essential in the diagnosis and treatment of this aneurysm.54 Brown and Stambough reported a case of CES caused by an epidural hematoma secondary to a rupture of a synovial cyst.55 In the literature it is reported that patients who have undergone exploration and evacuation of spinal epidural hematoma experienced the most extensive neurologic recovery within 6 hours of diagnosis. Patients who were surgically treated within 8 hours of diagnosis demonstrated less recovery.56,57
CES caused by massive spontaneous subarachnoid hemorrhage from an intradural angioblastic meningioma of the filum terminale has been reported.58 Kulali et al.58 reviewed six cases of spontaneous spinal subarachnoid hematoma caused by subarachnoid hemorrhage from a spinal tumor with acute compression of the adjoining nervous structures. Likewise, Malbrain et al.34 reported a patient with acute CES resulting from intratumoral and spinal subarachnoid hemorrhage from a filum terminale ependymoma after therapy with oral anticoagulants. Only 13 cases of spinal subarachnoid hemorrhage caused by cauda equina ependymoma have been reported.
Cauda Equina Syndrome Secondary to Pregnancy
CES is rarely associated with pregnancy, with few cases reported in literature. Curtin and Rice reported a case of CES in a 37-year-old woman in the first trimester of pregnancy, due to a prolapsed lumbar disc.59 Chow et al. reported a case of CES immediately following caesarean section.60 We recommend that vigilance be maintained in the neurologic surveillance of pregnant women with known disc disease.
Iatrogenic Cauda Equina Syndrome
Postsurgical Cauda Equina Syndrome
Laus et al.61 analyzed the complications resulting from the surgical treatment of lumbar stenosis and reported 4 cases of CES in 96 patients undergoing multiple bilateral laminectomies. Maurice-Williams and Marsh62 reported four cases of cauda equina lesions after dorsal surgery for severe dysplastic spondylolisthesis: three cases after in situ fusion and one case after decompressive laminectomy. The mechanism of nerve root injury was postulated to be mechanical, occurring during decortication before bone grafting.62
Schoenecker et al.63 identified 12 patients who developed CES after in situ bilateral dorsolateral arthrodesis for grades III or IV lumbosacral spondylolisthesis. No mention of direct cauda equina injury was noted in the operative reports. Five of the 12 patients eventually recovered completely. The remaining seven patients had a permanent residual neurologic deficit, manifested by complete or partial inability to control the bowel and bladder. Schoenecker et al.63 postulated that preoperative sacral nerve root dysfunction in a patient with lumbosacral spondylolisthesis is an indication for cauda equina decompression concomitant with the arthrodesis. They recommended immediate decompression, including resection of the dorsal rostral rim of the dome of the sacrum and the adjacent intervertebral disc for acute CES after in situ arthrodesis for spondylolisthesis.
McLaren and Bailey64 reported six cases of acute CES after lumbar discectomy in a series of 2842 lumbar discectomies. Five patients had coexisting bony spinal stenosis at the level of the disc protrusion that was not decompressed at the time of surgery. Inadequate decompression played a role in the postoperative neurologic deterioration. Bowel and bladder recovery was good when the cauda equina was decompressed early, sensory recovery was universally good, and motor recovery was poor if a severe deficit developed before decompression. McLaren and Bailey64 advised urgent decompression of postoperative CES if cauda equina compression is radiographically confirmed. Bowen and Ferrer65 reported a case of CES that resulted from displacement of a hook plate into the canal at L5, eroding the dura and pressing on the nerve roots. Removal of the hook and fusion of L5 and S1 relieved the symptoms. Mulder et al. reported a case of cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy. The dural sealant was used to stop an iatrogenic cerebrospinal fluid leak at L4-5. Postoperative imaging and operative findings confirmed swelling and migration of the material.66 Iatrogenic CES following lumbar puncture is rare, but can cause significant morbidity.67
The overall incidence of neurologic deficit after lumbar spine surgery is approximately 0.2%. The development of postoperative CES in patients undergoing lumbar discectomy is a well-documented complication. Dimopoulos et al. presented two cases of postoperative CES in patients undergoing single-level lumbar microdiscectomy. They found that intraoperative somatosensory evoked potential monitoring may be useful in predicting the development of CES in patients undergoing this operation.68 We use electrophysiologic monitoring, including somatosensory evoked potentials, as an intraoperative adjunct when decompressing lesions involving the cauda equina. During resection of large tumors involving nerve roots or during surgery for congenital malformations such as tethered cord, intraoperative stimulation of nerve roots and recording of EMG activity from selected muscles including the tibialis anterior, gastrocnemius, and perianal sphincter are performed.69,70
Cauda Equina Syndrome after Intradiscal Therapy
Automated percutaneous lumbar discectomy to manage contained herniated lumbar discs has a low associated morbidity. Epstein71 reported cauda equina injury after a left-sided L5-S1 automated percutaneous discectomy. Despite delayed surgery, which included excision of a sequestrated L5-S1 disc and intradural exploration, S1 radiculopathy, sacral numbness, and overflow incontinence persisted. Onik et al.72 reported CES secondary to a Nucleotome probe (Clarus Medical, Minneapolis, MN) placed in the thecal sac, reviewed the landmarks for the thecal sac, and emphasized the preventable nature of this complication.
Hedtmann et al.73 reported two patients who developed CES after intradiscal collagenase therapy to treat large extruded disc fragments. Smith et al.74 similarly reported three cases of acute disc herniation causing cauda equina compression syndrome after chemonucleolysis. All three patients had myelographic blocks and, despite emergency decompression procedures, were left with residual neurologic deficits.
Postchiropractic Cauda Equina Syndrome
CES has been implicated as a potential complication of spinal manipulation. Multiple cases of CES have been reported to result from chiropractic lumbar spinal manipulation.75–77 Haldeman and Rubinstein78 identified 10 reported cases of CES in patients who underwent manipulation between 1911 and 1989 and presented 3 new cases in which cauda equina symptoms occurred after lumbar manipulation. Kostuik et al.15 reported three patients who developed CES after chiropractic manipulation of the spine. Solheim et al. reported a case of a72-year-old man receiving anticoagulation therapy for chronic atrial fibrillation who developed CES due to a spinal epidural hematoma immediately following a chiropractic manipulation. Caution should be shown in patients seeking chiropractic treatment while receiving antithrombotic therapy79.
Cauda Equina Syndrome Secondary to Continuous Spinal Anesthesia
CES may occur after continuous spinal anesthesia.80 In the described cases, there was evidence of a focal sensory block and, to obtain adequate analgesia, the patients were given a dose of local anesthetic that was greater than the dose usually administered with a single-injection technique. The combination of maldistribution and a relatively high dose of local anesthetic was postulated to result in neurotoxic injury. The increased incidence of CES associated with the use of microcatheters and continuous spinal anesthesia prompted the U.S. Food and Drug Administration to issue a safety alert.80
Cauda Equina Syndrome Caused by Infection
CES can manifest in association with lumbar spinal infections that result in an epidural abscess or pathologic fracture (Fig. 105-6). CES typically results from direct compression of the cauda equina, but may also occur secondary to subarachnoid vascular injury or thrombosis.81 Management consists of early detection, antibiotic and surgical decompression, and debridement with or without spinal stabilization.82
Arend et al.83 described a patient who developed toxic shock syndrome, meningitis, and CES several days after lumbar laminectomy. Enterotoxin-producing Staphylococcus aureus was cultured from the surgical wound and the cerebrospinal fluid. The patient recovered from toxic shock syndrome but remained partially paralyzed. CES was postulated to result from the neurotoxic effects of the intrathecally produced S. aureus exotoxins.83
Crawfurd et al.84 presented five patients who were HIV positive who had symptoms initially thought to be indicative of lumbar disc lesions. Signs of nerve root or cauda equina compression were noted in all five patients, but lumbar imaging studies demonstrated no evidence of compressive lesions. Therefore, physicians must exercise caution in diagnosing nerve root compression in HIV-positive patients. Likewise, Zeman and Donaghy85 reported a case of CES in the context of acute infection with HIV and emphasized the importance of considering the possibility of primary HIV infection in a wide range of self-limiting neurologic disorders.
Diagnostic Pitfalls
Tullberg and Isacson86 reported on 19 patients with normal lumbar myelograms and symptoms consistent with CES. Two patients had thoracic lesions—one with a tumor and the other with a herniated disc—who were discovered on a second myelogram. One patient was considered to have hysterical paresis. In three other patients, the symptoms were attributed to diseases not related to the spine. Thirteen patients recovered spontaneously. The authors concluded that the prognosis for patients with signs of compression of the cauda equina is favorable, provided the myelography results are normal and surgical exploration is not indicated. However, if severe unexplained symptoms persist and the CT-myelogram is normal, the authors recommend myelography of the thoracic spine.86
A large number of patients present to neurosurgical units with symptoms suggestive of CES without radiologic evidence of pathology. In these cases, a high level of suspicion should be maintained. If lumbosacral compression is not demonstrated, then imaging studies should be pursued to rule out a proximal lesion.87
Prognosis for Recovery of Bladder Dysfunction
Gleave and MacFarlane16 reviewed 932 patients who underwent surgery for prolapsed lumbar intervertebral disc and identified a group of 33 with acute urinary retention. No identifiable factor predisposed this group of patients to CES. The mean duration of bladder paralysis before operation was 3.6 days. Ultimately, 79% of patients claimed full recovery of bladder function, but only 22% were left without sensory deficits in the limbs or perineum. There was no correlation between recovery and the duration of bladder paralysis before surgery, except in three patients in whom there was no sciatica and in whom the correct diagnosis was delayed for many days. Retention developing less than 48 hours after acute prolapse was associated with a worse prognosis.
Hellstrom et al.88 urodynamically evaluated bladder function after surgery in 17 patients with CES caused by lumbar intervertebral disc herniation. At the 3-year follow-up, 10 patients (59%) reported normal bladder function, whereas 7 patients (41%) noted obstruction or incontinence. Urodynamic function was normal in four patients (24%), three patients (18%) had no detrusor contraction, two patients (12%) used the detrusor but strained during voiding, and three patients (18%) had an unstable detrusor; the remaining five patients (29%) had either an increased bladder capacity or a decreased maximal flow rate. Neurologic findings were normal in two patients (12%). Bladder function is often substantially disturbed in patients with asymptomatic CES, and therefore urodynamic testing is warranted in all patients with CES. Urgent surgical intervention may improve long-term bladder function. Regeneration of the autonomic nervous supply to the bladder and genitals often requires several months or even years.
Nielsen et al.89 reported that among 1972 patients undergoing surgery for lumbar disc herniation in a 7-year period (from 1971 to 1978), CES was diagnosed in 26 patients. Twenty-two patients had follow-up visits 4 to 72 months postoperatively (average, 37 months). At follow-up, half of the patients had a history of normal micturition, and only six patients had a serious complaint of micturition. One third of the patients were free from lumbar or sciatic pain, whereas two thirds complained of distressing pain and neurologic symptoms. The urodynamic investigation revealed complete bladder emptying in all but one patient. In half of the patients, normal detrusor contraction was demonstrated, whereas one third of the patients voided by straining only. Preoperative micturition symptoms exceeded 2 days in almost all patients voiding by straining. This finding indicates a connection of early diagnosis and treatment with the degree of detrusor damage.
Summary
Patients with CES should undergo rapid radiologic evaluation and surgical intervention. It is prudent to adhere to a few simple principles during evaluation of patients with acute back pain or lumbosacral trauma (Box 105-3). It is important to maintain a high index of suspicion for the diagnosis of CES and its implications both diagnostically and prognostically. It is essential that these patients are specifically asked about urinary incontinence and retention and that their bladder and bowel functions are monitored vigilantly. If the clinician is suspicious, he or she should obtain a postvoid residual urine volume. In addition, trauma patients often receive an indwelling catheter at the time of admission to the emergency department. This catheter should be removed and checked for postvoid residual urine volume as quickly as possible. A rectal examination and evaluation of perianal sensation is mandatory when assessing any patient with acute onset of severe back pain or significant lumbosacral trauma. Imaging studies to determine the nature of the lesion (e.g., disc herniation, fracture, hematoma, tumor) must be performed expeditiously. If one imaging study (e.g., MRI) is inadequate, a complementary study (e.g., CT-myelography) should be obtained. If surgical management is indicated, meticulous surgical technique is critical so as to avoid additional nerve root traction or compression.
Domen P.M., Hofman P.A., van Santbrink H., et al. Predictive value of clinical characteristics in patients with suspected cauda equina syndrome. Eur J Neurol. 2009;16(3):416-419.
Gleave J.R., MacFarlane R. Prognosis for recovery of bladder function following lumbar central disc prolapse. Br J Neurosurg. 1990;4:205-209.
Pedowitz R.A., Garfin S.R., Massie J.B., et al. Effects of magnitude and duration of compression on spinal nerve root conduction. Spine (Phila Pa 1976). 1992;17:194-199.
Rooney A., Statham P.F., Stone J. Cauda equina syndrome with normal MR imaging. J Neurol. 2009;256(5):721-725.
Shi J., Jia L., Yuan W., et al. Clinical classification of cauda equina syndrome for proper treatment. Acta Orthop. 2010;81(3):391-395.
Tullberg T., Isacson J. Cauda equina syndrome with normal lumbar myelography. Acta Orthop Scand. 1989;60:265-267.
1. Delamarter R.B., Bohlman H.H., Dodge L.D., Biro C. Experimental lumbar spinal stenosis. Analysis of the cortical evoked potentials, microvasculature, and histopathology. J Bone Joint Surg [Am]. 1990;72:110-120.
2. Delamarter R.B., Sherman J.E., Carr J.B. Cauda equina syndrome: neurologic recovery following immediate, early, or late decompression. Spine (Phila Pa 1976). 1991;16:1022-1029.
3. Olmarker K., Holm S., Rosenqvist A.L., Rydevik B. Experimental nerve root compression: a model of acute, graded compression of the porcine cauda equina and an analysis of neural and vascular anatomy. Spine (Phila Pa 1976). 1991;16:61-69.
4. Olmarker K., Holm S., Rydevik B. Importance of compression onset rate for the degree of impairment of impulse propagation in experimental compression injury of the porcine cauda equina. Spine (Phila Pa 1976). 1990;15:416-419.
5. Olmarker K., Rydevik B., Hansson T., Holm S. Compression-induced changes of the nutritional supply to the porcine cauda equina. J Spinal Disord. 1990;3:25-29.
6. Olmarker K., Rydevik B., Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine (Phila Pa 1976). 1989;14:569-573.
7. Olmarker K., Rydevik B., Holm S., Bagge U. Effects of experimental graded compression on blood flow in spinal nerve roots: a vital microscopic study on the porcine cauda equina. J Orthop Res. 1989;7:817-823.
8. Pedowitz R.A., Garfin S.R., Massie J.B., et al. Effects of magnitude and duration of compression on spinal nerve root conduction. Spine (Phila Pa 1976). 1992;17:194-199.
9. Rydevik B. Neurophysiology of cauda equina compression. Acta Orthop Scand Suppl. 1993;251:52-55.
10. Rydevik B.L., Pedowitz R.A., Hargens A.R., et al. Effects of acute, graded compression on spinal nerve root function and structure. An experimental study of the pig cauda equina. Spine (Phila Pa 1976). 1991;16:487-493.
11. Takahashi K., Olmarker K., Holm S., et al. Double-level cauda equina compression: an experimental study with continuous monitoring of intraneural blood flow in the porcine cauda equina. J Orthop Res. 1993;11:104-109.
12. Olivero W.C., Wang H., Hanigan W.C., et al. Cauda equina syndrome (CES) from lumbar disc herniations. J Spinal Disord Tech. 2009;22(3):202-206.
13. Raaf J. Removal of protruded lumbar intervertebral discs. J Neurosurg. 1970;32:604-611.
14. Spangfort E.V. The lumbar disc herniation: a computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
15. Kostuik J.P., Harrington I., Alexander D., et al. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg [Am]. 1986;68:386-391.
16. Gleave J.R., MacFarlane R. Prognosis for recovery of bladder function following lumbar central disc prolapse. Br J Neurosurg. 1990;4:205-209.
17. Domen P.M., Hofman P.A., van Santbrink H., Weber W.E. Predictive value of clinical characteristics in patients with suspected cauda equina syndrome. Eur J Neurol. 2009;16(3):416-419.
18. Tay E.C., Chacha P.B. Midline prolapse of a lumbar intervertebral disc with compression of the cauda equina. J Bone Joint Surg [Br]. 1979;61:43-46.
19. Shi J., Jia L., Yuan W., et al. Clinical classification of cauda equina syndrome for proper treatment. Acta Orthop. 2010;81(3):391-395.
20. Choudhury A.R., Taylor J.C. Cauda equina syndrome in lumbar disc disease. Acta Orthop Scand. 1980;51:493-499.
21. Lafuente D.J., Andrew J., Joy A. Sacral sparing with cauda equina compression from central lumbar intervertebral disc prolapse. J Neurol Neurosurg Psychiatr. 1985;48:579-581.
22. Floman Y., Wiesel S.W., Rothman R.H. Cauda equina syndrome presenting as a herniated lumbar disk. Clin Orthop Relat Res. 1980;147:234.
23. den Boon J., Avezaat C.J., van der Gaast A., et al. Conus-cauda syndrome as a presenting symptom of endodermal sinus tumor. Gynecol Oncol. 1995;57:121-125.
24. Shapiro S. Cauda equina syndrome secondary to lumbar disc herniation. Neurosurgery. 1993;32:743-746.
25. Dinning T.A., Schaeffer H.R. Discogenic compression of the cauda equina: a surgical emergency. Aust NZ J Surg. 1993;63:927-934.
26. Hussain S.A., Gullan R.W., Chitnavis B.P. Cauda equina syndrome: outcome and implications for management. Br J Neurosurg. 2003;17(2):164-167.
27. Qureshi A., Sell P. Cauda equina syndrome treated by surgical decompression: the influence of timing on surgical outcome. Eur Spine J. 2007;16(12):2143-2151.
28. Schnaid E., Eisenstein S.M., Drummond W.J. Delayed post-traumatic cauda equina compression syndrome. J Trauma. 1985;25:1099-1101.
29. Fisher R.G. Sacral fracture with compression of cauda equina: surgical treatment. J Trauma. 1988;28:1678.
30. Hilibrand A.S., Urquhart A.G., Graziano G.P., Hensinger R.N. Acute spondylolytic spondylolisthesis: risk of progression and neurological complications. J Bone Joint Surg [Am]. 1995;77:190-196.
31. Denis F., Burkus J.K. Diagnosis and treatment of cauda equina entrapment in the vertical lamina fracture of lumbar burst fractures. Spine (Phila Pa 1976). 1991;16:S433-S439.
32. Zuccarello M., Powers G., Tobler W.D., et al. Chronic posttraumatic lumbar intradural arachnoid cyst with cauda equina compression: case report. Neurosurgery. 1987;20:636-638.
33. Admiraal P., Hazenberg G.J., Algra P.R., et al. Spinal subarachnoid hemorrhage due to a filum terminale ependymoma. Clin Neurol Neurosurg. 1992;94:61-72.
34. Malbrain M.L., Kamper A.M., Lambrecht G.L., et al. Filum terminale ependymoma revealed by acute cauda equina compression syndrome following intratumoral and spinal subarachnoid hemorrhage in a patient on oral anticoagulants. Acta Neurol Belg. 1994;94:35-43.
35. Iliadis C., Kirgiannis K., Wozniak G., et al. Paraganglioma of cauda equina. Neurol Neurochir Pol. 2008;42(5):463-466.
36. Spiegelmann R., Ram Z., Findler G., et al. Spinal cord involvement as the presenting symptom of acute monocytic leukemia. Surg Neurol. 1988;29:145-148.
37. Staff N.P., Bosch E.P., Engelstad J., et al. Metastatic lobular breast adenocarcinoma presenting as cauda equina syndrome. J Peripher Nerv Syst. 2010;15(1):75-78.
38. Kim D.Y., Lee J.K., Moon S.J., et al. Intradural spinal metastasis to the cauda equina in renal cell carcinoma: a case report and review of the literature. Spine (Phila Pa 1976). 2009;34(24):E892-E895.
39. Woo E., Yu Y.L., Ng M., et al. Spinal cord compression in multiple myeloma: who gets it? Aust NZ J Med. 1986;16:671-675.
40. Baek W.S., Kubba S.V. Cauda equina syndrome due to leptomeningeal carcinomatosis of the ovary. Gynecol Oncol. 2008;111(3):544-545.
41. Koenigsberg R.A., Klahr J., Zital L., et al. Magnetic resonance imaging of the cauda equina syndrome in ankylosing spondylitis. J Neuroimaging. 1995;5:46-48.
42. Mitchell M.J., Sartoris D.J., Moody D., Resnick D. Cauda equina syndrome complicating ankylosing spondylitis. Radiology. 1990;175:521-525.
43. Soeur M., Monseu G., Baleriaux W.D., et al. Cauda equina syndrome in ankylosing spondylitis: anatomical, diagnostic, and therapeutic considerations. Acta Neurochir (Wien). 1981;55:303-315.
44. Bartleson J.D., Cohen M.D., Harrington T.M., et al. Cauda equina syndrome secondary to long-standing ankylosing spondylitis. Am Neurol. 1983;14:662-669.
45. Haddad F.S., Sachdev J.S., Bellapravalu M. Neuropathic bladder in ankylosing spondylitis with spinal diverticula. Urology. 1990;35:313-316.
46. Arslanoglu A., Aygun N. Magnetic resonance imaging of cauda equina syndrome in long-standing ankylosing spondylitis. Australas Radiol. 2007;51(4):375-377.
47. Tullous M.W., Skerhut H.E., Story J.L., et al. Cauda equina syndrome of long-standing ankylosing spondylitis. Case report and review of the literature. J Neurosurg. 1990;73:441-477.
48. Normand J.P., Dufour M., Lang J.Y., et al. Radiographic features of cauda equina syndrome complicating ankylosing spondylitis. Can Assoc Radiol J. 1994;45:58-61.
49. Young A., Dixon A., Getty J., et al. Cauda equina syndrome complicating ankylosing spondylitis: use of electromyography and computerised tomography in diagnosis. Ann Rheum Dis. 1981;40:317-322.
50. Grosman H., Gray R., St Louis E.L. CT of long-standing ankylosing spondylitis with cauda equina syndrome. Am J Neuroradiol. 1983;4:1077-1080.
51. Shaw P.J., Allcutt D.A., Bates D., Crawford P.J. Cauda equina syndrome associated with multiple lumbar arachnoid cysts in ankylosing spondylitis: improvement following surgical therapy. J Neurol Neurosurg Psychiatry. 1990;53:1076-1079.
52. Johnson P.J., Hahn F., McConnell J., et al. The importance of MRI findings for the diagnosis of nontraumatic lumbar subacute subdural haematomas. Acta Neurochir (Wien). 1991;113:186-188.
53. Laus M., Alfonso C., Giunti A. Lumbosciatic pain and coagulopathies. Chir Organi Mov. 1991;76:229-236.
54. Schmidt R.H., Grady M.S., Cohen W., et al. Acute cauda equina syndrome from a ruptured aneurysm in the sacral canal. Case report. J Neurosurg. 1992;77:945-948.
55. Brown C., Stambough J.L. Epidural hematoma secondary to a rupture of a synovial cyst. Spine (Phila Pa 1976. 2005;5(4):446-450.
56. Kaner T., Sasani M., Oktenoglu T., et al. Postoperative spinal epidural hematoma resulting in cauda equina syndrome: a case report and review of the literature. Cases J. 2009;2:8584.
57. Kebaish K.M., Awad J.N. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus. 2004;16(6):1-4.
58. Kulali A., von Wild K., Hobik H.P. Subarachnoid haemorrhage with acute cauda symptom due to spinal tumour. Neurochirurgia (Stuttg). 1989;32:87-90.
59. Curtin P., Rice J. Cauda equina syndrome in early pregnancy: a case report. Acta Obstet Gynecol Scand. 86(6), 2008. 758–759
60. Chow J., Chen K., Sen R., et al. Cauda equina syndrome post-caesarean section. Aust N Z J Obstet Gynaecol. 2008;48(2):218-220.
61. Laus M., Pignatti G., Alfonso C., et al. Complications in the surgical treatment of lumbar stenosis. Chir Organi Mov. 1992;77:65-71.
62. Maurice-Williams R.S., Marsh H.T. Priapism as a feature of claudication of the cauda equina. Surg Neurol. 1985;23:626-628.
63. Schoenecker P.L., Cole H.O., Herring J.A., et al. Cauda equina syndrome after in situ arthrodesis for severe spondylolisthesis at the lumbosacral junction. J Bone Joint Surg [Am]. 1990;72:369-377.
64. McLaren A.C., Bailey S.I. Cauda equina syndrome: a complication of lumbar discectomy. Clin Orthop Relat Res. 1986;204:143-149.
65. Bowen J.R., Ferrer J. Spinal stenosis caused by a Harrington hook in neuromuscular disease. A case report. Clin Orthop Relat Res. 1983;80:179-181.
66. Mulder M., Crosier J., Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: case report. Spine (Phila Pa 1976). 2009;34(4):E144-E148.
67. Sinclair A.J., Carroll C., Davies B. Cauda equina syndrome following a lumbar puncture. J Clin Neurosci. 2009;16(5):714-716.
68. Dimopoulos V., Fountas K.N., Machinis T.G., et al. Postoperative cauda equina syndrome in patients undergoing single-level lumbar microdiscectomy. Report of two cases. Neurosurg Focus. 2005;19(2):E11.
69. Herdmann J., Dvorak J., Vohanka S. Neurophysiological evaluation of disorders and procedures affecting the spinal cord and the cauda equina. Curr Opin Neurol Neurosurg. 1992;5:544-548.
70. Kothbauer K., Schmid U.D., Seiler R.W., Eisner W. Intraoperative motor and sensory monitoring of the cauda equina. Neurosurgery. 1994;34:702-707.
71. Epstein N.E. Surgically confirmed cauda equina and nerve root injury following percutaneous discectomy at an outside institution: a case report. J Spinal Disord. 1990;3:380-382.
72. Onik G., Maroon J.C., Jackson R. Cauda equina syndrome secondary to an improperly placed nucleotome probe. Neurosurgery. 1992;30:412-414.
73. Hedtmann A., Steffen R., Kramer J. Prospective comparative study of intradiscal high-dose and low-dose collagenase versus chymopapain. Spine (Phila Pa 1976). 1987;12:388-392.
74. Smith S., Leibrock L.G., Gelber B.R., Pierson E.W. Acute herniated nucleus pulposus with cauda equina compression syndrome following chemonucleolysis: report of three cases. J Neurosurg. 1987;66:614-617.
75. Dan N.G., Saccasan P.A. Serious complications of lumbar spinal manipulation. Med J Aust. 1983;2:672-673.
76. Gallinaro P., Cartesegna M. Three cases of lumbar disc rupture and one of cauda equina associated with spinal manipulation (chiropraxis). Lancet. 1983;1(8321):411.
77. Malmivaara A., Pohjola R. Cauda equina syndrome caused by chiropraxis on a patient previously free of lumbar spine symptoms. Lancet. 1982;2:986-987.
78. Haldeman S., Rubinstein S.M. Cauda equina syndrome in patients undergoing manipulation of the lumbar spine. Spine (Phila Pa 1976). 1992;17:1469-1473.
79. Solheim O., Jorgensen J.V., Nygaard O.P. Lumbar epidural hematoma after chiropractic manipulation for lower-back pain: case report. Neurosurgery. 2007;61(1):E170-E171.
80. Griffith R.W. Complications of continuous spinal anesthesia. CRNA. 1992;3:164-169.
81. Currier B.L., Eismont F.J. Infections of the spine. In Rothman R.H., Simeone F.A., editors: The spine, ed 3, Philadelphia: WB Saunders, 1992.
82. Lenehan B., Sullivan P., Street J., Dudeney S. Epidural abscess causing cauda equina syndrome. Ir J Med Sci. 2005;174(3):88-91.
83. Arend S.M., Steenmeyer A.V., Mosmans P.C., et al. Postoperative cauda syndrome caused by. Staphylococcus aureus. Infection. 1993;21:248-250.
84. Crawfurd E.J., Baird P.R., Clark A.L. Cauda equina and lumbar nerve root compression in patients with AIDS. J Bone Joint Surg [Br]. 1987;69:36-37.
85. Zeman A., Donaghy M. Acute infection with human immunodeficiency virus presenting with neurogenic urinary retention. Genitourin Med. 1991;67:345-347.
86. Tullberg T., Isacson J. Cauda equina syndrome with normal lumbar myelography. Acta Orthop Scand. 1989;60:265-267.
87. Rooney A., Statham P.F., Stone J. Cauda equina syndrome with normal MR imaging. J Neurol. 2009;256(5):721-725.
88. Hellstrom P., Kortelainen P., Kontturi M. Late urodynamic findings after surgery for cauda equina syndrome caused by a prolapsed lumbar intervertebral disk. J Urol. 1986;135:308-312.
89. Nielsen B., de Nully M., Schmidt K., Hansen R.I. A urodynamic study of cauda equina syndrome due to lumbar disc herniation. Urol Int. 1980;35:167-170.