5.2 The complement cascade38
5.3 Haemostasis40
5.4 Shock41
Self-assessment: questions43
Self-assessment: answers44
Cascade systems occur frequently in the body. They allow a rapid response to a stimulus because the inactive precursor molecules are already present. In addition, each step allows amplification of the response. The role of the caspase cascade in apoptosis has been discussed in Chapter 4; the complement and clotting cascades will be discussed in this chapter. Malfunction in any part of a cascade can lead to disease.
Shock is a complex series of changes which occur after severe and sudden diminution in the blood volume or cardiac output. The common feature of all causes of shock is insufficient circulating volume.
5.1. Principles of cascade systems
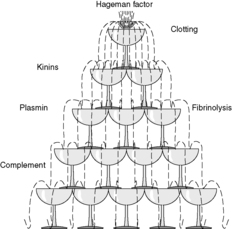
Enzyme cascades are involved in many processes in the body and are seen in immune, inflammatory and vascular events. They share the common underlying mechanism of cascade action in which the product of one reaction catalyses the subsequent reaction. For example, the blood plasma contains the components of four interlinked cascade systems that are involved in clotting and inflammation (see Table 5). Plasmin, a product of the fibrinolytic cascade, degrades the product of the clotting cascade and activates complement. Hageman factor, or factor XII, is a clotting factor which activates the complement, kinase and fibrinolytic systems (Figure 11). The caspase cascade has already been mentioned in Chapter 4.
| Activator(s) | Important end product(s) | Main functions | |
|---|---|---|---|
| Kinin system | Exposed collagen activates factor XII | Bradykinin | Vasodilatation/hypotension, increased vascular permeability, stimulates pain receptors |
| Complement system | Antigen-antibody complexes (classical pathway); microbial endotoxin (alternative and MBL pathways) | C3a/C5a Membrane attack complex (C5-C9) C3b |
Chemotaxis, increased vascular permeability, releases histamine from mast cells Cell lysis Opsonisation |
| Clotting system | Exposed collagen activates factor XII (intrinsic pathway); thromboplastin from damaged tissues activates factor VII (extrinsic pathway) | Thrombin | Production of fibrin clot, proinflammatory actions on leucocytes and other cells |
| Fibrinolytic system | Plasminogen activator from endothelium; activated factor XII | Plasmin | Lysis of fibrin clots by cleaving fibrin to form fibrin degradation products (FDPs), activation of complement cascade |
At each step of the cascade, an inactive precursor is activated. Thus, the biologically inert components of the cascade can be present in the body ready to be activated, in contrast with systems where the component molecules have to be synthesised by cells when they are required for action. Therefore, cascades allow a rapid response to a stimulus. Also, one activated enzyme can catalyse the activation of a large number of its substrate molecules, so amplification of the response occurs at each step. Furthermore, the activated components can have more than one action, allowing for a large number of possible final effects. In this way, a few molecules of an initiating substance can have huge consequences, as when activation of a small amount of caspase 8 by Fas ligand binding starts a series of reactions that kill the cell (see Ch. 4).
Another characteristic of cascades is that each step of the process can be promoted or inhibited by other factors, allowing for tight control of the cascade. Typically, the active constituents have a short half life and are rapidly broken down or inactivated, so the cascade can be rapidly ‘switched off’ if required.
In summary, the advantages of cascade systems are:
• amplification of the original stimulus
• rapid reaction
• a variety of different actions resulting from one initial stimulus
• modulation and control by a wide range of other factors.
5.2. The complement cascade
Complement is a system of soluble molecules that forms an important part of the body’s defence system against microbial infection. It is a cascade system and interacts with other components of the immune system (see Ch. 7).
Complement is activated via three pathways: the mannose-binding lectin (MBL, also known as mannan-binding lectin), classical and alternative pathways. The MBL and alternative pathways are triggered by microbial substances and do not require the participation of B cells or T cells. Therefore, these pathways represent part of innate immunity (see Ch. 7). The classical pathway is triggered by antigen–antibody binding. Figure 12 shows the main steps of the classical and alternative pathways leading to the formation of the membrane attack complex. The MBL pathway activates C4. Products of the fibrinolytic and kinin systems can also activate complement.
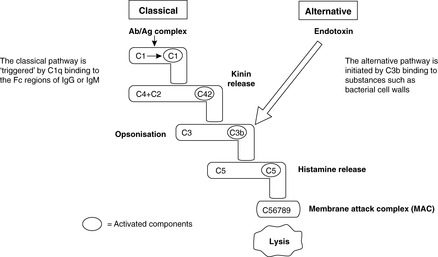 |
| Figure 12 |
There are many functional consequences of complement activation (Box 4, Figure 12). Formation of the membrane attack complex is the final common pathway of the different limbs of the cascade; it makes a pore in the membrane of the target cell that leads to cell lysis. The anaphylatoxins are fragments of activated complement components that have cytokine-like effects, activating inflammatory cells and acting as chemoattractants for them. They can also degranulate mast cells. Fragments that remain attached to the target, such as C3b, promote phagocytosis, i.e. they are opsonins.
Box 4
Classical pathway
• Triggered by antigen–antibody complexes involving IgG or IgM
Alternative pathway
• Activated without IgG or IgM being present, e.g.:
• bacterial endotoxin (surface lipopolysaccharide of Gram-negative bacteria)
• snake venom
• aggregated IgA
Complement assists in defence in three ways:
• Triggering acute inflammation: C3a, C5a, the anaphylatoxins, cause histamine-mediated vasodilatation and blood vessel leakage as well as acting as potent chemotaxins for neutrophils and monocytes
• Helping phagocytosis by coating foreign substances, e.g. bacterial cell walls, with protein (opsonisation)
• Direct killing of certain organisms: the membrane attack complex can kill some bacteria, e.g. Neisseria spp.
Complement activation can lead to extensive tissue damage and plays an important part in hypersensitivity reactions (see Ch. 8).
5.3. Haemostasis
You should:
• give an account of how the blood vessel wall, platelets and the clotting cascade contribute to haemostasis
• distinguish the intrinsic from the extrinsic pathway
• state the role of the fibrinolytic pathway
• discuss the pathophysiology of disseminated intravascular coagulation.
Blood clotting
The blood vessel wall, platelets and the clotting cascade are the three major components of normal blood clotting (haemostasis). A clot that forms in the vascular spaces during life is called a thrombus.
Blood vessel wall
Normal continuous endothelium inhibits intravascular clotting by preventing blood platelets and clotting factors from coming into contact with the collagen of the vessel wall. Endothelial cells also produce substances such as prostaglandins (PGI2), nitric oxide and plasminogen activator which inhibit platelet aggregation and promote fibrinolysis. When the endothelium is damaged and collagen is exposed to the blood, thrombogenic tissue factors will trigger the clotting cascade, and platelets will adhere to the exposed collagen. The aggregated platelets and strands of fibrin, together with entrapped blood cells, form a thrombus.
Platelets
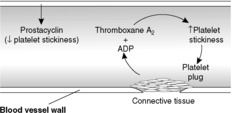
Platelets have a central role in clotting and form the initial plug at an area of endothelial damage. To do this they have to adhere to collagen exposed at the site of injury and then release stored products from granules rich in adenosine diphosphate (ADP), platelet-derived growth factor (PDGF), serotonin, calcium and fibrinogen, which trigger further platelet aggregation and the clotting cascade. Thromboxane A2 (TXA2), synthesised and released by activated platelets, plays a key role in platelet aggregation (Figure 13). The platelet plug is not a robust structure and fibrin is required to stabilise it.
 |
| Figure 13 |
The clotting (coagulation) cascade
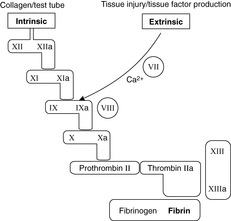
The clotting cascade is triggered by:
• exposure of blood to collagen (intrinsic pathway), activating factor XII; this pathway is also triggered if blood is placed in a glass test tube
In either case, the pathway leads to thrombin formation. Thrombin converts soluble fibrinogen to insoluble fibrin, the protein which ultimately stabilises the clot. The overall process is summarised in Figure 14.
 |
| Figure 14 |
Fibrinolysis
The fibrinolytic system is activated alongside the clotting system and results in the production of plasmin, which degrades fibrin and fibrin clots. Fibrinolysis counteracts and balances the haemostatic mechanisms; without fibrinolysis uncontrolled extension of a clot could occur. One of the ‘triggers’ involved in the activation of the fibrinolytic system is factor XII. Thus, factor XII initiates fibrinolysis at the same time as it promotes thrombosis. This is an example of the way in which these pathways are tightly interlinked in a complex web of relationships.
Disseminated intravascular coagulation (DIC)
This is an important condition where both clotting and haemorrhage occur together. The patient is usually very ill because of underlying diseases, such as:
• severe overwhelming infection (septicaemia)
• severe trauma or burns
• post-partum haemorrhage (massive bleeding after birth).
In all these conditions, there is activation of the coagulation system, either by the extrinsic or intrinsic pathways. Thus, endothelial cells may be damaged in burns and by immune complexes. Tissue factors may be released by the placenta. This mass activation of the clotting cascade leads to the consumption of vast amounts of platelets and fibrin. The formation of multiple platelet thrombi occurs in the microvasculature, causing small areas of infarction in many organs.
At the same time the fibrinolytic system is activated and thrombi are rapidly dissolved, producing large amounts of fibrin degradation products (FDPs). Supplies of clotting factors and platelets may become exhausted (consumption coagulopathy) so that a paradoxical situation occurs, where there is disseminated thrombosis with multi-organ infarcts together with uncontrolled haemorrhage. Disseminated intravascular coagulation is a serious life-threatening illness which needs to be managed by treating the underlying cause, such as antibiotics for infection, and by anticoagulants and replacement of clotting factors.
5.4. Shock
Shock is systemic hypoperfusion due to a reduction in either cardiac output or the effective circulating blood volume. It is associated with hypotension.
In response to the systemic hypoperfusion, the body reacts to preserve an adequate blood supply to essential organs such as the brain. In consequence, the effects of shock are usually manifested in other organs initially. The hypoxia causes reversible cell injury if relatively mild, but severe hypoperfusion will cause irreversible cell injury. Examples include subendocardial necrosis of the heart, acute tubular necrosis of the kidneys, and infarction of the intestine. When widespread tissue damage has occurred, the patient will continue to deteriorate despite therapy; this is irreversible or refractory shock.
Shock can be subdivided into various types depending on the pathogenesis. Hypovolaemic and cardiogenic shock are associated with a reduction in cardiac output. In contrast, the cardiac output in anaphylactic and neurogenic shock may be increased, but there is widespread vascular dilatation so the vessels cannot be adequately filled by the circulating blood volume; the consequence is hypotension and tissue hypoperfusion. In septic shock, there is both widespread vascular dilatation and a reduction in cardiac output.
Hypovolaemic shock
This occurs after severe haemorrhage, e.g. traumatic severing of a major artery, or loss of body fluids such as water from the colon in cholera or from the skin in burns cases. To preserve blood flow to the heart and brain, major vasoconstriction in other organs occurs. This vasoconstriction accounts for the cold, pale skin of patients in hypovolaemic shock.
Septic (endotoxic) shock
Circulatory failure occurs as the result of major bacterial infection, usually with Gram-negative bacteria such as Escherichia coli, Klebsiella or Pseudomonas spp. These bacteria release endotoxin (bacterial wall lipopolysaccharide), which activates macrophages and neutrophils and initiates the alternative complement pathway. In large amounts, endotoxin causes systemic vasodilatation and reduced myocardial contractility, resulting in shock. In addition, endotoxin has other effects, such as activation of the extrinsic coagulation pathway, which can complicate the clinical picture.
Anaphylactic shock
This occurs as a result of an acute systemic type 1 hypersensitivity reaction in individuals who are sensitised to the relevant antigen, e.g. penicillin or peanut allergy. It is a severe rapid reaction with bronchospasm, laryngeal oedema and hypotension. The hypotension is due to vasodilatation; severe hypotension causes shock.
Neurogenic shock
Damage to the spinal cord can cause loss of vascular tone, causing peripheral vasodilatation and pooling of blood. The blood volume cannot adequately fill the dilated vasculature and shock results.
Clinical features
In general, hypotension and tachycardia (fast heart rate) are common to all varieties of shock. In hypovolaemic and cardiogenic shock, there will also be a weak, thready pulse and cold, clammy skin due to peripheral vasoconstriction. In septic shock, the skin may be warm due to peripheral vasodilatation.
There is retention of salt and water by the kidneys, which acts to increase the circulating blood volume. Oliguria (reduced urine output) is thus a typical feature of shock. Ischaemic damage to the kidneys may reduce urine output further.
Self-assessment: questions
One best answer questions
2. A patient with extensive severe burns develops disseminated intravascular coagulation. Blood tests are performed. Which of the following is likely to be abnormally raised?
a. antithrombin III
b. blood volume
c. fibrin degradation products
d. fibrinogen
e. platelets
3. Which of the following is insoluble in plasma?
a. fibrin
b. fibrinogen
c. plasmin
d. prothrombin
e. thrombin
True-false questions
1. The following statements are true:
a. factor VIII deficiency (haemophilia A) leads to defective haemostasis
b. clotting factor VIII requires vitamin K for its synthesis
c. the intrinsic coagulation pathway is triggered by exposed collagen
d. platelet aggregation is promoted by thromboxane A2
e. activation of the kinin cascade in inflamed tissues is an important cause of pain
2. In the complement cascade:
a. amplification of the response occurs at each step
b. C5a causes lysis of cells
c. C3b is an opsonin
d. it takes at least 24 hours for the response to occur after the initial stimulus
e. plasmin can activate complement via the classical pathway
Case history questions
Case history 1
A 59-year-old male lorry driver was involved in a road traffic accident in which he sustained severe injuries to the head and lower limbs. On admission to hospital, he was unconscious and hypotensive (blood pressure 60/30mmHg). He was cold and clammy and had a pulse rate of 150 per minute.
1. What is the likely cause of shock in this patient?
The patient was transfused with 8 units of blood. Extensive surgery was required for bilateral fractured femurs and ruptured femoral blood vessels, after which his condition stabilised. Over the next few days, he regained consciousness, but it was noticed that he was not passing urine. Blood tests showed that he was in renal failure.
2. Why did the patient develop renal failure?
3. Is renal function likely to recover?
The patient then developed a wound infection and became pyrexial. Gram-negative bacteria were cultured from his blood, and he again became hypotensive, tachycardic and peripherally cyanosed.
4. Did this patient develop septicaemia or bacteraemia?
5. What is the pathogenesis of shock at this stage?
Self-assessment: answers
One best answer
2. c. Fibrin degradation products (FDPs) are increased in disseminated intravascular coagulation because they are produced by fibrinolysis from the large amounts of fibrin in the intravascular thrombi. Laboratories often test for a specific type of cross-linked FDP called D-dimer. Fibrinogen and platelets are consumed at a rate faster than they can be produced and their levels are low, hence the tendency to haemorrhage in this condition. Antithrombin III inactivates thrombin and other coagulation factors; it is also consumed in the process and is a useful clinical indicator of disease severity. Blood volume is likely to be depleted because of fluid loss from the burnt skin in this case.
3. a. Fibrin is an insoluble fibrillary protein produced by the clotting cascade during haemostasis. It binds the blood clot and seals blood vessel defects. It is produced from the soluble circulating precursor, fibrinogen.
True-false answers
1.
a. True. Haemophilia A is an X-linked genetic disorder, which means that it is mainly clinically apparent in males. Female carriers have approximately 50% of the normal levels of factor VIII and may occasionally show mild features of the condition. The lack of factor VIII causes inefficiency of the clotting cascade, and effective haemostasis cannot occur. Haemophilia B is caused by a genetic deficiency of factor IX; it is almost identical clinically to factor VIII deficiency. Individuals with haemophilia have haemorrhages into joints and tissues, often triggered by relatively minor trauma.
b. False. Most of the clotting factors are synthesised in the liver, but only factors II, VII, IX and X are vitamin K dependent. Patients with liver disease commonly have problems with haemostasis as their diseased liver cannot produce adequate amounts of clotting factors.
c. True. The intrinsic pathway is activated by exposed collagen, e.g. from vessel-wall trauma or atherosclerosis. It starts with factor XII and the cascade progresses quickly through to the formation of thrombin and fibrin.
d. True. Thromboxane A2 is a prostaglandin-like metabolite of arachidonic acid. It enhances platelet aggregation, which is an important step in the process of haemostasis.
e. True. Bradykinin stimulates pain receptors.
2.
a. True. This is a characteristic of cascades in general.
b. False. It is the membrane attack complex (C5–C9) that causes cell lysis; C5a has other effects.
c. True. When attached to bacteria, C3b promotes phagocytosis by binding to receptors on neutrophils and macrophages. Other opsonins include antibodies, lectins and C-reactive protein.
d. False. The complement cascade can be activated very rapidly; clinical effects may be observed in minutes or hours.
e. True. This is an important link between the clotting and complement systems.
Case history answers
Case history 1
1. This patient is in hypovolaemic shock as a result of massive blood loss from his fractured bones and torn blood vessels.
2. The patient has developed renal failure as a consequence of acute tubular necrosis. In severe shock with hypotension, the renal tubules become ischaemic, die and cease to function, leading to acute renal failure. The glomeruli are less susceptible to ischaemia and, therefore, would not be affected.
3. The epithelial cells of the renal tubules can regenerate from stem cells, provided that the basement membrane and matrix components of the tubules are intact. In clinical practice, as long as the condition is recognised and the patient can be supported with control of fluid, salt and acid–base balance, full recovery can occur.
4. Comment: distinction should be made between septicaemia, which is a life-threatening condition in which pathogenic organisms multiply and circulate in the bloodstream, and bacteraemia, in which non-harmful non-proliferating bacteria circulate in the blood but are easily removed by the body’s phagocytic cells. Bacteraemia can occur whenever there is potential for bacteria to enter the blood-stream, e.g. after a visit to the dentist, or after any invasive surgical procedure. In this case, the patient has septicaemia.
5. This patient now has septic shock associated with Gram-negative septicaemia. Endotoxin (bacterial wall lipopolysaccharides) in large amounts can directly trigger the alternative complement pathway and induce the production of excessive amounts of various cytokines by binding to toll-like receptors (see page 57, Pattern-recognition receptors). Consequences include fever, systemic vasodilatation, reduced contractility of the heart, and stimulation of inflammatory cells that release more pro-inflammatory cytokines. If the coagulation cascade is inappropriately triggered, disseminated intravascular coagulation may follow.