Chapter 33 Carotid Endarterectomy
Historical Background
For many years, the relationship between carotid artery disease and stroke was overlooked because autopsy protocol did not include harvesting the cervical vessels. Therefore, the only pathology observed in hemispheric stroke was thrombus in the intracranial vessels, most commonly the middle cerebral artery (MCA).One of the earliest observations of the relationship of cervical carotid artery disease with stroke was made in 1856 by Savory, who described a patient with left monocular blindness, right hemiplegia, right dysesthesia, and an occluded left internal carotid artery (ICA) at the carotid bifurcation.1 A similar observation was made by Gowers in 1875.2 It was not until 1914 that Hunt described the relationship between carotid artery disease, transient ischemic attacks (TIAs), and stroke.3 The ability to identify carotid bifurcation disease prior to death awaited the work of Moniz, who in 1927 reported on the technique of carotid angiography. This, for the first time, provided the opportunity to identify carotid artery occlusive disease in the living patient.4 In spite of this, clinicians solely used cerebral angiography to evaluate the intracranial circulation. In 1951, Fisher reemphasized the importance of the cervical carotid artery, pointing out that prior to occlusion, a stenosis was present that might be amenable to surgical correction.5
The surgical phase for treating carotid artery disease began in 1951. Carrea et al. from Buenos Aires resected the diseased segment of an ICA and restored flow by anastomosing the external carotid artery (ECA) to the distal ICA. They waited until 1955 for sufficient follow-up before reporting the case.6 Probably the first successful carotid endarterectomy (CEA) was performed by DeBakey et al. in 1953, but it was not reported until 1959, with a long-term follow-up reported in 1975.7 The publication that led to rapid incorporation of carotid artery surgery into clinical practice was the operation reported by Eastcott et al. in 1954. They described a case of a woman who was experiencing hemispheric TIAs, with an associated stenosis of the bulb of the carotid artery. They resected the carotid artery bifurcation and restored flow by a direct anastomosis between the common and distal ICAs with a successful outcome and cessation of symptoms. This report led to an explosion of interest in the treatment of carotid bifurcation disease.8
Pathology of Carotid Bifurcation Disease
Pathogenetic Mechanisms of Stroke and Transient Ischemic Events
The pathogenetic mechanism for ischemic events is primarily thrombotic or atheroembolic (also see Chapter 30). If the occlusive plaque in the carotid bulb progresses to critical flow reduction, the ICA will proceed to thrombotic occlusion. The pace of the occlusive process is important. If this process occurs slowly, collateral circulation may develop from the contralateral carotid and vertebral arteries. In addition, the ipsilateral ECA can be a source of collateral blood flow by flow reversal through the ophthalmic artery to the siphon of the ICA. Under these circumstances, thrombosis of the ICA may be a silent event. On the other hand, if plaque expansion occurs rapidly or if collateral circulation is inadequate, there will be thrombotic propagation beyond the ophthalmic branch of the ICA into the middle cerebral artery, causing hemisphere infarction and neurological deficit.
Clinical Evaluation
Clinical evaluation of patients with cerebrovascular disease (carotid artery disease) is discussed in Chapter 30.
Preoperative Imaging
The noninvasive examination of choice for patients with suspected carotid artery disease is a carotid duplex ultrasound scan using modern equipment in a validated vascular laboratory (also see Chapter 12). This study identifies lesions in the carotid artery, classifies the severity of stenosis, and provides information regarding plaque consistency. The opportunity to examine flow velocity and pulse wave velocity analysis provides information about other portions of the circulation, including proximal lesions at the level of the aortic arch and distal intracranial lesions. In many centers, the duplex scan serves as the definitive preoperative study.9 Additional studies such as magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), computed tomographic angiography (CTA), or catheter-based contrast angiography are reserved for special circumstances.9 Use of MRA or CTA in conjunction with duplex ultrasound of the carotid artery is considered when the results of duplex ultrasonography are difficult to interpret or inconsistent with the clinical presentation.
Magnetic resonance angiography is used frequently because it does not require ionizing radiation. When contrast is added, the images are often clear and resemble those obtained via catheter-based intraarterial angiography, providing a sensitivity and specificity of 88% and 84%, respectively, for diagnosing a 70% to 99% stenosis.9 Magnetic resonance angiography permits imaging of the intracranial vascular anatomy, and when combined with MRI, can identify areas of cerebral infarction or other intracranial pathology. The major limitation of MRA is that it tends to overestimate percent stenosis of lesions in the carotid bifurcation (also see Chapter 13). This phenomenon occurs because turbulent blood blow, such as occurs at carotid bulb stenosis, results in signal dropout and void, giving the impression of a high-grade carotid stenosis.
Computed tomography (CT) and CTA are also quite helpful in identifying intracranial lesions. Computed tomography angiography is accurate in identifying and quantifying intracranial and extracranial carotid stenosis (also see Chapter 14). The sensitivity and specificity of CTA for determination of carotid artery stenosis are 95% and 99%, respectively.10 The major drawbacks of CT scanning are exposure to ionizing radiation and the requirement for a large volume of iodinated contrast material, which can be nephrotoxic or cause allergic reaction in patients sensitive to iodine.
Intraarterial contrast angiography is considered the gold standard for identifying and quantifying arterial stenoses. Major disadvantages of this invasive procedure include arterial injury, occlusion, and embolization resulting in cerebral infarction. In the Asymptomatic Carotid Artery Study (ACAS), angiography was associated with a 1% stroke rate.11 In addition, it requires ionizing radiation and iodinated contrast material. This technique is now rarely indicated prior to carotid endarterectomies. It does have a role for preprocedure imaging as a part of carotid angioplasty/stenting.
Techniques of Carotid Endarterectomy
There are several acceptable methods for monitoring the adequacy of cerebral perfusion. The first method that was described was measurement of ICA backpressure in patients undergoing CEA under local anesthesia. With clamping of the common carotid artery (CCA) and ECA, the residual pressure in the carotid artery determines the perfusion in the middle cerebral artery. One study found that the conscious response to clamping correlated with the ICA backpressure.12 The minimum pressure associated with no neurological deficit was 25 mmHg. This observation was subsequently validated in patients undergoing operation with general anesthesia.13,14 The next method, and one that is most commonly used today, is continuous electroencephalographic (EEG) monitoring. This method is sensitive, easy to use, and has the advantage of providing continuous monitoring rather than a single observation. Other methods used to monitor cerebral perfusion include assessment of the somatosensory evoked response and transcranial Doppler (TCD) interrogation.
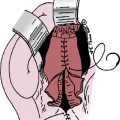
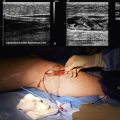
The arteriotomy is closed with a patch (typically Dacron) to prevent stenosis and accommodate any restriction from intimal hyperplasia that might occur with primary closure. Patch closure is associated with a lower incidence of postoperative thromboembolic complication and recurrent carotid stenosis.15 Before the arteriotomy is completely closed, the vessels should be “backbled” and flushed to remove any debris. Upon completion of closure, flow is restored first to the ECA and then the ICA. At this time, it is important to verify the quality of the endarterectomy by intraoperative duplex ultrasound scanning or a completion angiogram. The latter permits accurate visualization of the carotid artery bifurcation and the intracranial portion of the repaired ICA to detect any anatomical problems or residual stenosis. If an intimal flap is seen in the ICA, the patch is removed and the arteriotomy extended beyond the flap, thereby reducing the risk of postoperative thromboembolism. A new patch is used to close the arteriotomy. More commonly, an endpoint problem is found in the ECA, which can lead to postoperative thrombosis of the ECA and then in retrograde propagation of thrombus to the ICA, embolism, and stroke.16 When a problem is identified, the ECA is clamped both at its origin and distally. A transverse arteriotomy is made just distal to the residual lesion, and the remaining plaque is removed. The transverse arteriotomy is closed with interrupted polypropylene sutures, and flow is restored. The neck incision is then closed. Closure consists of a running absorbable suture in the platysma layer and a running absorbable subcuticular suture for cosmetic skin closure.
Complications of Carotid Endarterectomy
The mortality rate reported in large surgical trials of CEA is approximately 1% and is usually cardiovascular in nature.17 This mortality rate is similar to that observed in a general vascular surgical practice.18 The risk of stroke is approximately 2.6%.19 The principal etiology of postoperative stroke is embolism of thrombus or atheromatous debris.20
Cranial nerve injury occurs in up to 7% of patients, although permanent nerve palsy is noted in 1%.21 Several cranial nerves lie within the operative field, including the vagus (X), hypoglossal (XII), and (less commonly) glossopharyngeal (IX). The mandibular branch of the facial nerve (VII) may be at risk if the surgical incision is made anterior to the ear instead of on a line connecting the mastoid process and the suprasternal notch.
Clinical Trials of Carotid Endarterectomy
Prospective randomized trials comparing CEA to medical management involve either symptomatic or asymptomatic patients. They serve as the basis for current CEA recommendations in appropriately selected patients.21
Symptomatic Carotid Endarterectomy Trials
The North American Symptomatic Carotid Endarterectomy Trial (NASCET) was a multicenter prospective randomized trial carried out in the United States and Canada. The trial was divided into two cohorts; one involved patients with carotid artery stenosis of 70% to 99%, and the other involved patients with stenosis of 50% to 69%. The high-grade (70%-99%) stenosis component of the study was stopped after only 18 months because stroke morbidity and mortality for CEA was 9%, compared with 26% for best medical management.22 The second part of the study, for patients with moderate stenosis, went to completion. It demonstrated a 5-year event rate after CEA of 15.7%, vs. 22.2% for medical management.23 The European Carotid Endarterectomy Trial (ECST) was conducted during the same time as NASCET. It recruited a population similar to the high-grade stenosis portion of NASCET, with similar findings. Stroke morbidity and mortality after CEA was 10.3%, vs. 16.8% for medical management.24 The Veterans Administration Symptomatic Trial was started after the other two trials were ongoing. It was stopped after 189 patients were entered as the results of the North American and European trials were reported. Despite the small number of patients, the rate of stroke and crescendo TIA after only 12 months’ follow-up was 7.7% for surgery and 19.4% for medical management.25 Based on these trials, CEA is recommended for symptomatic patients with greater than 50% stenosis.21
Asymptomatic Carotid Endarterectomy Trials
The Veterans Affairs Asymptomatic Carotid Endarterectomy Trial enrolled 444 asymptomatic men with carotid artery stenoses greater than 50% as documented by angiography. These were randomized to CEA plus best medical management, or best medical management alone. At the end of 5 years, the primary event rate of TIA, stroke, and death was 8% for surgery, vs. 20.6% for medical management.26 The ACAS trial was a multicenter prospective randomized trial that compared CEA plus best medical management to best medical management alone. At the end of 5 years, the primary event rate of stroke or death was 5.1% for CEA and 11% for medical management.11 The Asymptomatic Carotid Stenosis Trial (ACST) randomized 1560 patients to immediate CEA and 1560 patients to medical management. At the end of 5 years, the primary event rate for immediate CEA was 6.4%, compared with 11.8% in patients randomized to medical management.19 The American College of Cardiology/American Heart Association (ACC/AHA) guidelines give a class IIa recommendation for CEA for asymptomatic patients with carotid artery stenosis greater than 70%.
Carotid Endarterectomy Compared to Carotid Angioplasty/Stenting
Carotid artery angiography and stenting is described in detail in Chapter 32. Several clinical trials have compared CEA with carotid artery stenting (CAS).
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial was a prospective multicenter randomized trial of symptomatic and asymptomatic patients considered high risk for surgery due to coexisting medical morbidity or a hostile neck; 156 patients were randomized to CAS, and 151 were randomized to CEA. The primary composite endpoint included death, stroke, and myocardial infarction (MI). The 30-day rates of death and stroke were no different between the two procedures, but with MI added to death and stroke, the composite endpoint rate for CAS was 5.8%, vs. 12.6% for CEA. These differences persisted for 1 year, but by 4 years, there was no difference between the two groups regarding event-free survival.27,28
The Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3 S) trial was a prospective multicenter trial carried out in France. A total of 527 patients with greater than 60% stenosis were randomized to CEA (262) or CAS (265). The study was stopped early because the death and stroke rate at 30 days was 3.9% for CEA and 9.6% for CAS. The study patients were followed for 4 years, at which time the death and stroke rates were 6.2% for those treated with CEA and 11.1% for those treated with CAS.29,30
The Stent-Protected Angioplasty versus Carotid Endarterectomy for Symptomatic Stenosis (SPACE) trial, carried out in Germany, Austria, and Switzerland, was a prospective multicenter randomized trial of symptomatic patients with high-grade carotid stenoses. The hypothesis was that CAS was not inferior to CEA. Twelve hundred patients were randomized, but the study was stopped because a futility analysis was performed indicating that the primary hypothesis could not be proved. At the time the study was stopped, the death and stroke rate for CAS was 7.7%, vs. 6.5% for CEA. Of note, by 2 years, the recurrent stenosis rate was 10.7% for CAS and 4.6% for CEA.31
The International Carotid Artery Stenting Study (ICSS) compared CEA with CAS. It involved 50 academic centers in the United Kingdom, Europe, Australia, New Zealand, and Canada. A total of 1713 patients with high-grade carotid stenoses were randomly allocated to CEA (858) or CAS (855). The primary outcomes were stroke, death, or procedural MI analyzed at 120 days following the procedure. The incidence of the combined endpoints of death, stroke, and MI in the CAS group was 8.5%, vs. 5.2% for CEA. Subgroup analyses of the patients who underwent pre- and postprocedure MRI found that 50% of the CAS group had at least one new area of cerebral infarction, vs. 17% of the CEA group.32
The Carotid Revascularization Endarterectomy versus Stenting (CREST) trial was a multicenter prospective randomized trial of symptomatic and asymptomatic patients with hemodynamically significant carotid stenoses; it was carried out in 108 centers in the United States and Canada. Between the years 2000 and 2008, 2502 patients were randomized; 47% were asymptomatic, and 53% were symptomatic. The initial analysis occurred after the last group of patients had at least 1 year of follow-up, and median follow-up was 2.5 years. The primary composite endpoint was periprocedural death, stroke in any distribution, and MI, which occurred in 4.5% of patients randomized to CEA and 5.2% randomized to CAS. The differences were not significant. The combined rate of death and stroke was 2.3% for CEA and 4.4% for CAS. There was a higher nonfatal MI rate in the CEA group. The study identified age as an important differentiating factor between CEA and CAS. Older patients did better with CEA, and younger patients did better with CAS. The inflection point occurred at age 70.33 Based on these studies, the ACC/AHA guidelines give a class IIa recommendation for CEA over CAS for older patients.
1 Savory W.S. Case of a young woman in whom the main arteries of both upper extremities and of the left side of the neck were throughout completely obliterated. Med Chir Trans. 1856;39:205–219.
2 Gowers W. On a case of simultaneous embolism of central retinal and middle cerebral arteries. Lancet. 1875;2:794.
3 Hunt J. The role of the carotid arteries in the causation of vascular lesions of the brain, with remarks on certain special features of symptomatology. Am J Med Sci. 1914;147:704–713.
4 Moniz E. L’encephalographie arterielle: Son importance dans la localization des tumeurs cerebrale. Rev Neurol (Paris). 1927;2:72–90.
5 Fisher M. Occlusion of the internal carotid artery. AMA Arch Neurol Psychiatry. 1951;65:346–377.
6 Carrea R., Molins M., Murphy G. Surgical treatment of spontaneous thrombosis of the internal carotid artery in the neck: carotid-carotidal anastomosis. Report of a case. Acta Neurol Latinoam. 1955;1:71–78.
7 DeBakey M.E. Successful carotid endarterectomy for cerebrovascular insufficiency. Nineteen-year follow-up. JAMA. 1975;233:1083–1085.
8 Eastcott H.H., Pickering G.W., Rob C.G. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954;267:994–996.
9 Ricotta J.J., Aburahma A., Ascher E., et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–e31.
10 Anzidei M., Napoli A., Zaccagna F., et al. Diagnostic accuracy of colour Doppler ultrasonography, CT angiography and blood-pool-enhanced MR angiography in assessing carotid stenosis: a comparative study with DSA in 170 patients. Radiol Med (Torino). 2011;117:54–71.
11 Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428.
12 Naylor A.R., Whyman M., Wildsmith J.A., et al. Immediate effects of carotid clamp release on middle cerebral artery blood flow velocity during carotid endarterectomy. Eur J Vasc Surg. 1993;7:308–316.
13 Moore W.S., Hall A.D. Carotid artery back pressure: a test of cerebral tolerance to temporary carotid occlusion. Arch Surg. 1969;99:702–710.
14 Moore W.S., Yee J.M., Hall A.D. Collateral cerebral blood pressure. An index of tolerance to temporary carotid occlusion. Arch Surg. 1973;106:521–523.
15 AbuRahma A.F., Robinson P.A., Saiedy S., et al. Prospective randomized trial of bilateral carotid endarterectomies: primary closure versus patching. Stroke. 1999;30:1185–1189.
16 Moore W.S., Martello J.Y., Quinones-Baldrich W.J., et al. Etiologic importance of the intimal flap of the external carotid artery in the development of post carotid endarterectomy stroke. Stroke. 1990;21:1497–1502.
17 Halliday A., Harrison M., Hayter E., et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376:1074–1084.
18 Finks J.F., Osborne N.H., Birkmeyer J.D. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137.
19 Halliday A., Mansfield A., Marro J., et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502.
20 Spencer M.P. Transcranial Doppler monitoring and causes of stroke from carotid endarterectomy. Stroke. 1997;28:685–691.
21 Brott T.G., Halperin J.L., Abbara S., et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:e54–e130.
22 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453.
23 Barnett H.J., Taylor D.W., Eliasziw M., et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425.
24 European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70%-99%) or with mild (0%-29%) carotid stenosis. Lancet. 1991;337:1235–1243.
25 Mayberg M.R., Wilson S.E., Yatsu F., et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA. 1991;266:3289–3294.
26 Hobson R.W.2nd, Fields W.S., Weiss D.G., et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–227.
27 Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501.
28 Gurm H.S., Yadav J.S., Fayad P., et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579.
29 Mas J.L., Chatellier G., Beyssen B., et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671.
30 Mas J.L., Trinquart L., Leys D., et al. Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892.
31 Ringleb P.A., Allenberg J., Bruckmann H., et al. 30 day results from the SPACE trial of Stent-Protected Angioplasty versus Carotid Endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247.
32 Bonati L.H., Jongen L.M., Haller S., et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol. 2010;9:353–362.
33 Brott T.G., Hobson R.W.2nd, Howard G., et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23.