CHAPTER 390 Carotid-Cavernous Fistulas
The term carotid-cavernous fistula (CCF) refers to an abnormal communication between the internal carotid artery (ICA) or one of its branches or the external carotid artery (ECA) and the cavernous sinus (CS). Over the years, numerous classifications have been applied to CCFs.1 They are based on angiographic features (high-flow and low-flow fistulas), mechanism of onset (spontaneous and traumatic), morphology, and angioarchitecture (direct and indirect fistulas). Based on arterial supply, Barrow and colleagues classically divided CCFs into four types.2 A type A fistula is a direct communications between the ICA and the CS; type B fistulas are supplied exclusively by dural branches of the ICA and are uncommon. Supply to type C fistulas is provided by dural branches of both the ICA and the ECA, whereas type D fistulas are supplied exclusively by dural branches of the ECA. The Barrow classification has been widely adopted. However, from a practical, etiopathogenetic, clinical, and therapeutic point of view, the simplest and most useful classification divides CCFs into direct and indirect. These two types of fistulas are considered separately in this chapter.
Anatomy
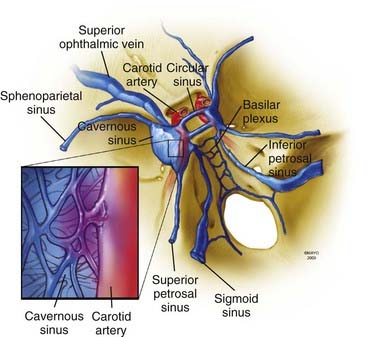
A brief overview of the general, arterial, and venous anatomy of the CS is important for the understanding of CCFs and their treatment. The CS or, more properly, the lateral sellar compartment is an anatomic extradural space in direct continuity through the clivus and basiocciput with the epidural space surrounding the spine.3 The CS is an extradural space contained between the two layers of dura laterally and superiorly and the periosteum covering the lateral portion of the sphenoid sinus and the sphenoid bone inferiorly and medially. This extradural space contains the ICA with its typical S shape, as well as nerves, fat, and a plexus of veins.3 Therefore, the CS is not, as classically described in older anatomic textbooks, a single venous space through which the ICA and the cranial nerves course. The plexiform arrangement of the veins in the CS was apparent to Dwight Parkinson when he entered this space to directly obliterate a long-standing CCF.3 He remarked that “the engorged and thickened ‘arterialized’ veins were readily noted to be neither cavernous nor a dural sinus but a plexus of veins.”3 This observation explains “the compartmentalization” of the CS often noted during endovascular approaches to CCFs.
The ICA gives origin to a variable number of small arterial branches during its course in the CS. The most consistent one is the meningohypophysial trunk arising before the apex of the first curve of the ICA. This vessel provides arterial supply to the dura of the tentorium (tentorial artery or artery of Bernasconi-Cassinari) and the hypophysis (inferior hypophysial artery). Other fairly constant branches are the inferolateral trunk, which supplies the segment of the cranial nerves running into the lateral sellar compartment, and the capsular artery of McDonnel. There are constant anastomoses between branches of the ICA and the ECA. Although not always angiographically visible, these anastomoses are anatomically constant. Knowledge of these anastomotic channels is very important to prevent complications during endovascular approaches to indirect CCFs.4
Understanding of the venous channels connected to CS veins is key to interpretation of the angiographic anatomy of CCFs and to plan their treatment (Fig. 390-1). The anterior portion of the CS receives the superior and inferior ophthalmic veins and the sphenoparietal sinus. The two CSs communicate through the anterior and posterior intercavernous sinus forming the circular sinus. Posterior drainage is through the basilar plexus and the superior and inferior petrosal sinuses. Inferolaterally, connections exist through dural veins draining into the pterygoid plexus. Intermittently, the cavernous sinus can receive drainage from the inferomedial surface of the brain.
Clinical Findings
The clinical findings and initial symptoms and signs are primarily related to the pattern of drainage of the fistula and its rapidity of development. As a general rule, direct fistulas have a more dramatic manifestation and quite often tend to exhibit the “classic” triad of exophthalmos, chemosis, and visual loss. In a large series of patients with direct CCFs, the most common signs and symptoms at initial evaluation were an orbital bruit (80%), proptosis (72%), chemosis (55%), cranial nerve VI palsy (49%), complete ophthalmoplegia (24%), and visual loss (18%).5 Indirect fistulas often have a more subtle clinical course, and not unusually patients have few symptoms, often transient. In the setting of major facial and head trauma, a CCF can easily be overlooked, so it is good practice to auscultate over the eyelid to rule it out. Diplopia is common and related to ischemic dysfunction of cranial nerves, mechanical compression of the nerves, and restricted movement secondary to venous engorgement of the orbital contents. In patients with direct fistulas, cranial nerve VI is more commonly involved because of the contiguity of the carotid artery to this nerve. The pattern of double vision created by engorgement of the orbital contents and restricted movement may not fit with a particular cranial neuropathy. Venous drainage is also dependent on the anatomic location of the fistula. Anteriorly placed fistulas drain through the superior ophthalmic vein and have a significant exophthalmic component.6 Posterior fistulas often have contralateral involvement. Epistaxis, even fatal epistaxis, is not uncommon with a traumatic direct CCF and can occur in delayed fashion, weeks after the original trauma.7 Intracranial hemorrhage can also occur with both direct and indirect fistulas associated with retrograde cortical venous drainage. Intracranial hemorrhage in the setting of a direct CCF frequently portends a poor prognosis with a high risk for short-term rehemorrhage. Thus, treatment should be considered on an urgent basis in such a situation.
Imaging Studies
Axial imaging studies such as computed tomography and magnetic resonance imaging can show indirect signs such as fullness in the area of the CS, proptosis, or pathologically enlarged venous channels. However, despite the sophistication of modern imaging techniques, catheter-based angiography remains the “gold standard” for diagnosing and defining CCFs. A cerebral angiogram must include the internal and external carotid supply, as well as study of the contralateral side and posterior circulation. Selective injection of the external carotid system is important to define the supply and angioarchitecture of indirect CCFs. With direct fistulas, it can be difficult to exactly define the location and size of the fistulous tract because of high flow. Vertebral angiography with compression of the carotid artery in the neck usually localizes the fistula by demonstrating slow filling though the posterior communicating artery (Huber’s maneuver).8 Similarly, selective injection of the ICA while gently compressing the common carotid artery may help localize the fistula (Mehringer-Hieshima maneuver). On rare occasion, the ICA proximal to the fistula may have been dissected by the injury, and this situation may compromise the ability to safely catheterize the vessel.
Direct Fistulas
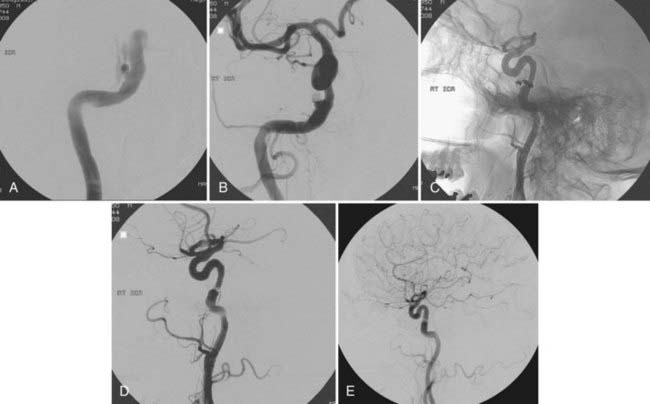
Direct CCFs can be divided into traumatic and spontaneous. In large series, 80% to 90% of direct CCFs are traumatic,6 the result of direct or indirect trauma involving the creation of a pathologic communication between the cavernous ICA or less commonly one of its branches and CS veins. The propensity of this segment of the ICA to rupture is probably related to tethering of the intracavernous ICA by dural rings at its entrance into and its exit from the CS. Iatrogenic fistulas have been reported after various surgical procedures, including transsphenoidal resection of pituitary tumors, attempts at reopening an occluded ICA with a Fogarty balloon, and endoscopic procedures. Much less common are spontaneous CCFs occurring as a result of rupture of an intracavernous aneurysm (Fig. 390-2) or as part of congenital connective tissue defects, such as Ehlers-Danlos type IV syndrome.
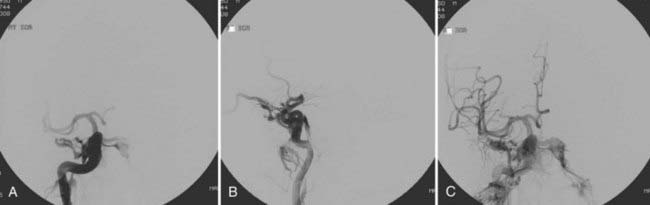
FIGURE 390-2 Selective right internal carotid artery injection. Anteroposterior (AP) (A) and lateral (B) early arterial phase images show a direct carotid-cavernous fistula with anterior and posterior drainage. An AP late arterial phase image (C) shows opacification of the contralateral cavernous sinus through the intercavernous sinus. This 60-year-old man had an abrupt onset of disabling bruit secondary to spontaneous rupture of a small intracavernous internal carotid artery aneurysm (see Fig. 390-3).
Treatment of Direct Carotid-Cavernous Fistulas
Detachable balloons were introduced for the treatment of CCFs by Serbinenko in 1974 and have provided a valid solution to the majority of direct CCFs for the past 3 decades.9 Balloons can be flow directed through the orifices of the fistula, inflated in the venous side up to a size larger than the original communication to prevent herniation into the ICA, and then detached. With a detachable balloon, closure of the fistula with preservation of the ICA is possible in approximately 75% to 80% of cases.5,6 Reconstruction of the parent vessel with this technique is not always perfect, and an irregularity at the level of the ICA tear with protrusion into the CS can be seen in up to 50% of patients after successful treatment with detachable balloons.6 Long-term follow-up of these wall irregularities is important because enlargement has been observed in up to 30% of patients.10 Limitations of detachable balloons for the treatment of CCFs include premature detachment with the danger of distal embolism, delayed balloon deflation with recurrence of the fistula, and rupture of the balloon because of osseous spicula in the presence of traumatic fractures with subsequent deflation and recurrence of the fistula. In some cases it can be difficult to navigate the balloon through a small communication; similarly, in the presence of a large communication between the artery and the venous side, balloons may be inadequate to close the fistulous tract and the ICA may need to be sacrificed. After detachable balloon treatment, up to a third of patients can experience worsening of preexisting or new extraocular motility deficits. These deficits often involve the sixth cranial nerve running between the ICA and the lateral wall of the CS. Postoperative deficits are usually transient but can be permanent in about 15% of patients.6 Ischemic complications have been reported in 7% of patients (3% transient and 4% permanent).5 Since 2003, detachable balloons have not been available in the United States.
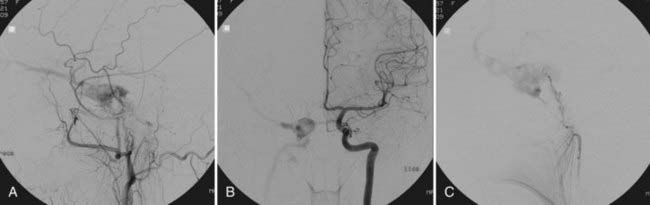
With the availability of detachable coils beginning in the early 1990s, these devices have commonly been used for transarterial or transvenous closure of direct CCFs. Detachable coils overcome some of the problems encountered with detachable balloons. Moreover, with withdrawal of detachable balloons from the U.S. market, detachable coils have become the mainstay of treatment of direct CCFs in the United States (Fig. 390-3). The main advantage of detachable coils is their ability to be retrieved in the event of inadequate placement. Coils are available in different sizes and shapes and can be easily adapted to the individual characteristics of the specific patient. From a historical point of view, the first clinical case in which Guglielmi Detachable Coils (GDCs, Boston Scientific, Natick, MA) were used involved a patient with a CCF.11 HydroCoil (MicroVention, Inc., Tustin, CA), which is a coil coated with a gel that “swells” when in contact with blood, has recently also been used successfully for the obliteration of direct CCFs.12 Because of the properties of these coils and the ability of the coil mass to “expand” after deployment, a smaller number of coils are required to achieve occlusion. When deploying coils, if retrograde cortical venous drainage exists, it is important to obliterate this portion of the fistula first with coils to avoid redirecting residual flow preferentially to this portion of the fistulous communication and incur the risk of intracranial venous engorgement and hemorrhage.
With the availability of Onyx, another alternative embolic material for the treatment of both direct and indirect CCFs has become available. Unlike coils, which may further compartmentalize the CS, Onyx tends to more fully obliterate the fistulous connection. This embolic material is also easier to control than n-butyl-2-cyanoacrylate (NBCA), and because it is not adhesive, it can be delivered slowly without incurring the risk of “gluing” the microcatheter. A higher Onyx viscosity mixture, HD 500, available for embolization of aneurysms, can also be used. During delivery of Onyx into direct CCFs, a balloon is inflated across the fistulous communication to prevent reflux into the ICA. Disadvantages of Onyx include very slow injection rates and high penetration, which increases the risk of obliterating the supply to cranial nerves with subsequent ischemic neuropathies. Onyx can be used in combination with coils to occlude the small residual fistula that sometimes persists after coil embolization.13,14
More recently, covered stents, originally approved for application in the coronary arteries as bailout in the event of vessel rupture, have been used for the treatment of direct CCFs. Covered stents can be navigated to the site of the CCF and, under fluoroscopic control, placed precisely across the orifice and detached. Ideally, covered stents should be able to immediately seal the defect in the artery wall. In reality, there is often a mismatch between the size of the stent and the diameter of the vessel, so blood can still “find its way” between the vessel wall and stent into the fistula and create an “endoleak” that results in angiographic persistence of the fistula.15,16 This can usually be repaired by reinflating the balloon at higher pressure. Drawbacks of the current covered stents include the stiffness of these systems, which makes neuronavigation difficult with resultant vasospasm, and problems with endoleaks. In addition, the need for dual antiplatelet therapy makes their use impractical in patients with recent major trauma who are at risk for systemic bleeding complications. It is plausible that with further advances in endovascular techniques and refinement of new devices, covered stents will eventually become the treatment of choice for direct CCFs.
Indirect Fistulas
Indirect fistulas are abnormal dural communications between branches of the ICA or the ECA (or both) and the CS. These defects are true dural arteriovenous fistulas (AVFs) and are often referred to as dural CCFs. Their pathogenesis is considered to be similar to that of other intracranial dural AVFs. Indirect CCFs are much more frequent in women and are commonly encountered in the sixth and seventh decades of life (mean age, 66.8 years).17 Like their intracranial counterparts involving the dural sinuses, indirect CCFs may be the consequence of preceding CS thrombosis. The CS is the second most common location for intracranial dural AVFs after the transverse-sigmoid sinus. Indirect fistulas often have a more benign clinical course than direct CCFs and not infrequently can regress spontaneously. Up to 19% of patients with indirect CCFs have bilateral involvement.18 In large endovascular series, the clinical signs and symptoms at initial evaluation include chemosis (94%), proptosis (87%), cranial neuropathy involving the extraocular motility nerves or less commonly the trigeminal nerve (54%), increased intraocular pressure (60%), and compromised visual acuity (28%).17 Retrograde venous drainage toward the orbit is usually associated with prominent ocular findings, whereas preferential drainage toward the inferior petrosal sinus (IPS), or the sphenoparietal sinus, may cause focal neurological symptoms, seizures, and frank hemorrhage. Indications for treatment include increased intraocular pressure, progressive or disabling symptoms, retrograde cortical venous drainage (present in more than a third of patients requiring treatment),17 frank intracranial hemorrhagic manifestation, and cosmetic deformity.
Definitive diagnosis is based on catheter angiography, which must include selective injections of both ICAs and ECAs and at least one vertebral artery. Low-flow indirect CCFs may not be visualized on common carotid injections. Venous drainage involves the superior ophthalmic vein in more than 80% of patients, the IPS in 42%, and the contralateral cavernous sinus in 23%.17 In absence of significant symptoms or retrograde cortical venous drainage, conservative management is a valid option and should be pursued because spontaneous thrombosis is not that uncommon.2 Intermittent ipsilateral carotid compression has been recommended in the past in patients with minor symptoms and without “dangerous” angioarchitecture features. Whether intermittent carotid compression is indeed successful or the results observed are a mere consequence of the tendency for spontaneous thrombosis in some of these patients is unknown.
Treatment of Indirect Carotid-Cavernous Fistulas
Endovascular treatment is the primary modality for managing indirect CCFs (Fig. 390-4). Surgical treatment, indicated in patients with cortical venous drainage if an effective endovascular route is not feasible, is rarely necessary. Stereotactic radiosurgery also has a role in the management of these lesions. With advancements in endovascular techniques, numerous routes of access and different occlusion methods are available.19 Therefore, the approach to an individual indirect CCF is dictated by a variety of factors, including the operator’s preference for a specific embolic agent, the angioarchitecture of the fistula, clinical symptoms, and the routes of access available.
A transarterial approach to indirect CCFs can be used when adequate supply from the external carotid branches exists. A transarterial approach with particles is easy and safe (especially with particles larger than 250 µm). It is generally used as a palliative maneuver to decrease symptoms in patients with fistulas without dangerous features and as an adjunct to Gamma Knife radiosurgery.20 Particulate transarterial embolization has been abandoned as a primary therapeutic approach because of very high recanalization rates. Cure of indirect CCFs has been reported with the use of NBCA.21 This approach requires distal arterial catheterization in a “wedge” position to allow distal penetration of the glue. The main problems with NBCA embolization are related to its quick polymerization, which requires very short periods of injection. Moreover, NBCA is not cohesive and consequently is associated with a risk for microemboli. Because of these significant limitations, NBCA is very operator dependent and requires a high degree of skill and experience with this material for safe and effective transarterial treatment of indirect CCFs.
With the availability of Onyx as a new embolic agent, there has been a resurgence of interest in transarterial approaches to indirect CCFs. Careful study of the anatomy of the fistula and continuous radiographic monitoring during injection are of paramount importance to prevent penetration of Onyx into small collaterals and incurring a risk for cranial nerve palsies, bradycardia (probably caused by the trigeminocardiac reflex), and arterial stroke from embolization of the ICA. Creative approaches with different endovascular devices in combination have been reported. For example, placement of a covered stent to occlude the ICA supply followed by transarterial Onyx injection to occlude the ECA supply has been reported to be successful in obliterating an indirect CCF with leptomeningeal drainage and venous varices compressing the brainstem.22
Traditionally, until Onyx became widely available, a transvenous approach to indirect CCFs had been the preferred route for endovascular obliteration of these lesions. Various access routes to the CS are available, and selection of the route also depends on the type of preferential drainage of the fistula. Access to the CS through the ipsilateral IPS is usually the shortest and most direct route because of the direct connection of the IPS to the internal jugular vein through the petro-occipital fissure. Thrombosis of the IPS can be a major impediment to direct catheterization of the CS. However, catheterization of the IPS is possible even when this sinus is not angiographically visible and even in the presence of thrombosis.17 Based on the complex pattern of drainage of indirect CCFs, alternative venous pathways for accessing the CS have included the contralateral IPS through the intercavernous sinus,18 the clival venous plexus,23 the cortical venous drainage,24 the pterygoid plexus,25 the inferior ophthalmic vein,26 and the superior ophthalmic vein through the angular or retromandibular veins.18,27 The choice of one of these alternative pathways depends on the individual characteristics of the fistula’s venous drainage and the anatomic position of the fistula itself in relation to the CS. When these routes are not immediately available, percutaneous access though the superior ophthalmic vein via surgical cutdown is a well-established procedure. Direct percutaneous puncture of the CS is also feasible and avoids the need for surgical cutdown. However, intrinsic to direct CS puncture is the risk for intraorbital hematoma because no local pressure can be applied on needle withdrawal.
Various embolic agents alone or in combination have been used for transvenous obliteration of indirect CCFs. Detachable or fibered coils are generally preferred, although liquid embolic agents, often in combination with coils, have also been used.28 Excellent immediate and long-term angiographic and clinical results have been reported with the transvenous approach. Complete obliteration of the fistula can be achieved in 84% of patients with a single endovascular procedure.17 Symptoms related to increased intraocular pressure are often the first to improve after successful obliteration of the fistula. Cranial neuropathies improve in the majority of patients but may fail to improve in up to 11%.17 Paradoxical aggravation of the ocular symptoms and signs is uncommon (3%) and probably related to the effects of CS thrombosis or mass effect from the embolizing material. Frequently, these new postoperative deficits are transient and improve over a 1- to 2-month period after the procedure.18 Overall, transvenous embolization of indirect CCFs is safe in experienced hands with extremely low rates of severe permanent morbidity and virtually no mortality.
Radiosurgery
Radiosurgery is an effective treatment of low-flow indirect CCFs. The drawback of radiosurgery is the latency time during which patients continue to be exposed to the symptoms and the potential for intracranial hemorrhage in those with retrograde cortical venous drainage. To obviate this limitation of radiosurgery, Pollock and coworkers advocated a staged approach consisting of Gamma Knife radiosurgery followed by embolization.20 The rationale for performing embolization after radiosurgery is to allow adequate coverage of the fistulous nidus without having it obscured by the embolization. After radiosurgery, transarterial particle embolization can be performed with the goal of achieving rapid improvement in symptoms. In this situation, particle embolization can be also used with the goal of decreasing flow to the lesion while radiosurgery takes effect. Because indirect CCFs often involve the inferior or the posterior portions of the CS, the radiation dose to the optic apparatus can be limited to less than 10 Gy.20 With this combined radiosurgery-embolization strategy, chemosis and proptosis were reported to improve in 94% of patients. Of those with decreased visual acuity or visual field defects, 88% experienced resolution of their symptoms and 77% of those with diplopia noticed improvement or resolution.20 Eighty percent to 91% of patients undergoing follow-up cerebral angiography after Gamma Knife radiosurgery for indirect CCFs were reported to have complete angiographic obliteration.20,29,30
Archondakis E, Pero G, Valvassori L, et al. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007;28:342-347.
Argo A, Bono G, Zerbo S, et al. Post-traumatic lethal carotid-cavernous fistula. J Forensic Legal Med. 2008;15:266-268.
Barcia-Salorio JL, Soler F, Barcia JA, et al. Stereotactic radiosurgery for the treatment of low-flow carotid-cavernous fistulae: results in a series of 25 cases. Stereotact Funct Neurosurg. 1994;63:266-270.
Barrow DC, Spector RH, Braun IF, et al. Classification and treatment of carotid cavernous fistulas. J Neurosurg. 1985;62:248-256.
Bellon RJ, Liu AY, Adler JRJr, et al. Percutaneous transfemoral embolization of an indirect carotid-cavernous fistula with cortical venous access to the cavernous sinus. J Neurosurg. 1999;90:959-963.
Cheng KM, Chan CM, Cheung YL. Transvenous embolization of dural carotid-cavernous fistulas by multiple venous routes: a series of 27 cases. Acta Neurochir (Wien). 2003;145:17-25.
Debrun GM, Vinuela F, Fox AJ, et al. Indications for treatment and classification of 132 carotid-cavernous fistulas. Neurosurgery. 1988;22:285-289.
Geibprasert S, Pongpech S, Armstrong D, et al. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009;30:1459-1468.
Gemmete JJ, Ansari SA, Gandhi DM. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuroophthalmol. 2009;29:62-71.
Guglielmi G, Vinuela F, Briganti F, et al. Carotid-cavernous fistula caused by a ruptured intracavernous aneurysm. Endovascular treatment by electrothrombosis with detachable coils. J Neurosurg. 1992;31:591-596.
Guo WY, Pan DHC, Wu HM, et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 1998;19:1081-1087.
Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the cavernous sinus. AJNR Am J Neuroradiol. 1989;10:377-383.
He HW, Jiang CH, Wu ZX, et al. Transvenous embolization with a combination of detachable coils and Onyx for a complicated cavernous dural arteriovenous fistula. Chin Med. 2008;121:1651-1655.
Huber P. A technical contribution to the exact angiographic localization of carotid cavernous fistulas. Neuroradiology. 1976;10:239-241.
Jahan R, Gobin YP, Glenn B, et al. Transvenous embolization of a dural arteriovenous fistula of the cavernous sinus through the contralateral pterygoid plexus. Neuroradiology. 1998;40:189-193.
Kirsch M, Henkes H, Liebig T, et al. Endovascular management of dural carotid-cavernous fistulas in 141 patients. Neuroradiology. 2006;48:486-490.
Lewis AI, Tomsick TA, Tew JMJr. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:239-244.
Li MH, Tan HQ, Fang C, et al. Trans-arterial embolization therapy of dural carotid-cavernous fistulae using low concentration n-butyl-cyanoacrylate. Acta Neurochir (Wien). 2008;150:1149-1156.
Michels KS, Ng JD, Falardeau J, et al. Transvenous embolization of a dural carotid-cavernous sinus fistula vis the inferior ophthalmic vein. Ophthal Plast Reconstr Surg. 2007;23:480-495.
Moret J. Comment to: Lewis AI, Tomsick TA, Tew JM Jr: Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:244.
Parkinson D. Lateral sellar compartment O.T. (cavernous sinus): history, anatomy, terminology. Anat Rec. 1998;251:480-490.
Pollock BE, Nichols DA, Garrity GA, et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45:459-466.
Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: anatomy, classification, and treatment. Neurosurg Clin N Am. 2005;16:279-295.
Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125-145.
Shi ZS, Qi TW, Gonzalez NR, et al. Combined covered stent and Onyx treatment for complex dural arteriovenous fistula involving the clivus and cavernous sinus. Surg Neurol. 2009;72:169-174.
Suzuki S, Lee DW, Jahan R, et al. Transvenous treatment of spontaneous dural carotid-cavernous fistula using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol. 2006;27:1346-1349.
Wahkloo AK, Perlow A, Linfante I, et al. Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulas: technical considerations and clinical outcome. AJNR Am J Neuroradiol. 2005;26:1888-1897.
Wang C, Xie X, You C, et al. Placement of covered stents for the treatment of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 2009;30:1342-1346.
Yu SC, Cheng HK, Wang GK, et al. Transvenous embolization of dural carotid-cavernous fistulae with transfacial catheterization through the superior ophthalmic vein. Neurosurgery. 2007;60:1032-1037.
Zhi-Gang W, Xuan D, Ji-Qing Z, et al. HydroCoil occlusion for treatment of traumatic carotid-cavernous fistula: preliminary experience. Eur J Radiol. 2009;71:456-460.
1 Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: anatomy, classification, and treatment. Neurosurg Clin N Am. 2005;16:279-295.
2 Barrow DC, Spector RH, Braun IF, et al. Classification and treatment of carotid cavernous fistulas. J Neurosurg. 1985;62:248-256.
3 Parkinson D. Lateral sellar compartment O.T. (cavernous sinus): history, anatomy, terminology. Anat Rec. 1998;251:480-490.
4 Geibprasert S, Pongpech S, Armstrong D, et al. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009;30:1459-1468.
5 Lewis AI, Tomsick TA, Tew JMJr. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:239-244.
6 Debrun GM, Vinuela F, Fox AJ, et al. Indications for treatment and classification of 132 carotid-cavernous fistulas. Neurosurgery. 1988;22:285-289.
7 Argo A, Bono G, Zerbo S, et al. Post-traumatic lethal carotid-cavernous fistula. J Forensic Legal Med. 2008;15:266-268.
8 Huber P. A technical contribution to the exact angiographic localization of carotid cavernous fistulas. Neuroradiology. 1976;10:239-241.
9 Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125-145.
10 Moret J. Comment to: Lewis AI, Tomsick TA, Tew JM Jr: Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:244.
11 Guglielmi G, Vinuela F, Briganti F, et al. Carotid-cavernous fistula caused by a ruptured intracavernous aneurysm. Endovascular treatment by electrothrombosis with detachable coils. J Neurosurg. 1992;31:591-596.
12 Zhi-Gang W, Xuan D, Ji-Qing Z, et al. HydroCoil occlusion for treatment of traumatic carotid-cavernous fistula: preliminary experience. Eur J Radiol. 2009;71:456-460.
13 He HW, Jiang CH, Wu ZX, et al. Transvenous embolization with a combination of detachable coils and Onyx for a complicated cavernous dural arteriovenous fistula. Chin Med. 2008;121:1651-1655.
14 Suzuki S, Lee DW, Jahan R, et al. Transvenous treatment of spontaneous dural carotid-cavernous fistula using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol. 2006;27:1346-1349.
15 Archondakis E, Pero G, Valvassori L, et al. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007;28:342-347.
16 Wang C, Xie X, You C, et al. Placement of covered stents for the treatment of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 2009;30:1342-1346.
17 Kirsch M, Henkes H, Liebig T, et al. Endovascular management of dural carotid-cavernous fistulas in 141 patients. Neuroradiology. 2006;48:486-490.
18 Yu SC, Cheng HK, Wang GK, et al. Transvenous embolization of dural carotid-cavernous fistulae with transfacial catheterization through the superior ophthalmic vein. Neurosurgery. 2007;60:1032-1037.
19 Gemmete JJ, Ansari SA, Gandhi DM. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuroophthalmol. 2009;29:62-71.
20 Pollock BE, Nichols DA, Garrity GA, et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45:459-466.
21 Li MH, Tan HQ, Fang C, et al. Trans-arterial embolization therapy of dural carotid-cavernous fistulae using low concentration n-butyl-cyanoacrylate. Acta Neurochir (Wien). 2008;150:1149-1156.
22 Shi ZS, Qi TW, Gonzalez NR, et al. Combined covered stent and Onyx treatment for complex dural arteriovenous fistula involving the clivus and cavernous sinus. Surg Neurol. 2009;72:169-174.
23 Cheng KM, Chan CM, Cheung YL. Transvenous embolization of dural carotid-cavernous fistulas by multiple venous routes: a series of 27 cases. Acta Neurochir (Wien). 2003;145:17-25.
24 Bellon RJ, Liu AY, Adler JRJr, et al. Percutaneous transfemoral embolization of an indirect carotid-cavernous fistula with cortical venous access to the cavernous sinus. J Neurosurg. 1999;90:959-963.
25 Jahan R, Gobin YP, Glenn B, et al. Transvenous embolization of a dural arteriovenous fistula of the cavernous sinus through the contralateral pterygoid plexus. Neuroradiology. 1998;40:189-193.
26 Michels KS, Ng JD, Falardeau J, et al. Transvenous embolization of a dural carotid-cavernous sinus fistula vis the inferior ophthalmic vein. Ophthal Plast Reconstr Surg. 2007;23:480-495.
27 Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the cavernous sinus. AJNR Am J Neuroradiol. 1989;10:377-383.
28 Wahkloo AK, Perlow A, Linfante I, et al. Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulas: technical considerations and clinical outcome. AJNR Am J Neuroradiol. 2005;26:1888-1897.
29 Barcia-Salorio JL, Soler F, Barcia JA, et al. Stereotactic radiosurgery for the treatment of low-flow carotid-cavernous fistulae: results in a series of 25 cases. Stereotact Funct Neurosurg. 1994;63:266-270.
30 Guo WY, Pan DHC, Wu HM, et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 1998;19:1081-1087.