Chapter 103
Carotid Artery
Aneurysms
Marvin D. Atkins, Ruth L. Bush
Aneurysms of the extracranial carotid arteries can occur as a result of atherosclerotic degeneration, traumatic injury, dissection, or local infection, or as a complication after carotid endarterectomy (CEA). Extracranial carotid artery aneurysm (ECAA) is an uncommon but important clinical entity. Carotid aneurysms are extremely rare in comparison with atherosclerotic occlusive disease of the same location. These aneurysms are also rare in comparison with aneurysms involving the intracranial carotid arteries and their branches. The reported incidence of incidental intracranial aneurysms discovered in autopsy studies ranges from 0.8% to 18%.1,2 The incidence of ECAA is largely unknown, but it represents only 1% to 1.5% of procedures performed for extracranial cerebrovascular disease at major referral centers.3–5 The true incidence of ECAA is well less than 1% of all carotid diseases.
Experience with endovascular carotid interventions for occlusive disease has produced technologies that have increasingly been used as minimally invasive alternatives to conventional surgical treatment of ECAAs. This chapter updates the contemporary management of ECAAs, including both open surgery and the evolving experience with endovascular therapy.
Definition
The normal carotid bifurcation is typically 40% greater in diameter than the more distal internal carotid artery (ICA). The accepted definition of most arterial aneurysms is “an artery having at least a 50% increase in diameter compared to the expected normal diameter of the artery.”6 Given this definition, it does not require much dilatation of the carotid bulb to reach this threshold, a fact that has led to disagreement about what constitutes an ECAA. deJong et al7 proposed that ECAA be defined as bulb dilatation greater than 200% of the diameter of the ICA or than 150% of the diameter of the common carotid artery. This strict definition is used in many of the contemporary reports of ECAA and is helpful, given the normal physiologic dilatation of the carotid bulb.
Historical Review
Sir Astley Cooper8 is credited with the first both unsuccessful and successful operations for ECAA in London in 1806 and 1808, respectively. Because direct surgical reconstruction was not possible, ligation of the common carotid artery was the sole treatment. Winslow9 reported an exhaustive review of 124 reported cases through 1925. In that review, 82 patients treated by carotid ligation were reported to have a mortality rate of 28%. By the 1970s, direct arterial reconstruction or autogenous vein grafting (or both) had supplanted carotid ligation. With the introduction of endovascular therapy for carotid occlusive disease, this strategy was first applied to ECAAs beginning in the 1990s.
Epidemiology
Population Affected
The population affected and age at diagnosis are directly related to the cause of the aneurysm. The relative frequency of the various potential causes of ECAA has changed over the years. Syphilis, tuberculosis, and middle ear and tonsillar infections were the most common causes of carotid artery aneurysms before the advent of antibiotics. In Winslow’s review, the majority of these early cases were pseudoaneurysms related to trauma or “erosions” from middle ear infections and tonsillitis rather than true atherosclerotic aneurysms.9 Therefore, the majority of patients were between 20 and 40 years of age, and the surgical morbidity and mortality associated with treatment were excessive. Although mycotic ECAAs were thought to be a problem of the past, a modern series reported from Durban, South Africa found 22 patients presenting with ECAAs to have human immunodeficiency virus (HIV) and/or tuberculosis, suggesting that ECAAs are more common than documented in the immunocompromised patient population.10
In modern practice, atherosclerotic degeneration, dissection, trauma, and previous carotid surgery have supplanted infection as the most frequent causes of ECAA, so the age at initial evaluation tends to mirror that of patients with carotid occlusive disease (Table 103-1).3,11–23 Increasing use of antibiotics for head and neck infections has significantly reduced the incidence of mycotic arterial infections involving the carotid artery from local extension of a septic process. ECAAs have also occurred in patients who have undergone extensive surgery and radiation therapy for head and neck cancer.24
Table 103-1
Data from the 14 Largest Single-Center Experiences with Extracranial Carotid Artery Aneurysms Since 1950*
| Total number of carotid procedures | 17,854 |
| Cases of extracranial carotid artery aneurysm (ECAA): | |
| Number | 434 |
| % of total | 2.4 |
| Indications for treatment (% of ECAA cases): | |
| Atherosclerotic origin | 42 |
| Pseudoaneurysm | 21 |
| Trauma | 16 |
| Dissection | 10 |
| Fibromuscular dysplasia | 7 |
| Infection | 2 |
| Other | 3 |
| Bilateral aneurysms | 6% of ECAA cases |
* Data from references 11 through 23.
Adapted from El-Sabrout R, et al: Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg 31:702-712, 2000.
Blunt trauma, dissection, and penetrating injury to the neck can result in a carotid pseudoaneurysm. Such lesions are typically encountered in a younger population. False aneurysms after CEA performed for occlusive disease usually affect individuals in their sixth or seventh decades of life.
True degenerative carotid aneurysms affect men twice as often as women,14,25 and there does not seem to be a predilection for the right or left side. Most patients are older than 60 years, but true degenerative carotid artery aneurysms have been reported in children.26 There does not seem to be a specific racial distribution of true degenerative carotid aneurysms. Earlier reports suggested that the incidence of ECAAs coexisting with other aneurysmal disease ranged from 14% to 25%.9,12 Most modern series, however, have not reported similar findings. There have been several reports of ECAAs in patients with connective tissue disorders, including Ehlers-Danlos syndrome, neurofibromatosis, and the vasculitis of Behçet’s disease. It should be noted that in such patients, it is unwise to use autologous vein for arterial reconstruction because there has been late aneurysmal degeneration of the repairs using autologous vein. Synthetic conduits to replace the ECAA appear to be the best option at this point in this patient population.
Incidence/Prevalence
A 1979 report of a search of the world’s literature from 1687 to 1977 found only 853 ECAAs.4,5 Pooled data from the 13 largest single-center series from 1960 to 1995 demonstrated 392 aneurysms involving the extracranial carotid arteries. Including only series that reported CEA volume during that same period, 17,854 carotid procedures were performed, 276 of which were for ECAA, for a relative incidence of 1.54%. The largest single-center series reported, that by El-Sabrout et al3 from the Texas Heart Institute, included 67 ECAAs treated between 1960 and 1995. During the same period, 7394 peripheral aneurysm and 4991 carotid operations were performed at the same institution. This 1.31% relative incidence is mirrored in several other published series.13,27 These single-center series are from large referral centers, so the true incidence of ECAAs is probably less than 1% of all carotid disorders. Because these aneurysms are rare, it is impossible to define their true incidence or to determine whether their frequency is increasing. However, advances in vascular and soft tissue imaging have contributed to their greater recognition, especially in victims of trauma.28
Pathogenesis
Etiology
Degenerative/Atherosclerotic
Currently, degenerative (or atherosclerotic) is the most frequently reported pathology associated with ECAAs (40% to 70% of cases). These are “true aneurysms” (see Chapter 129). The histologic features are typically atherosclerotic (often termed degenerative), with disruption of the internal elastic lamina and thinning of the media. Although most true carotid aneurysms exhibit arteriosclerosis, it is considered a secondary event rather than a primary etiologic factor. Grossly, these aneurysms tend to be fusiform rather than saccular and are most commonly located at the bifurcation of the common carotid artery or the proximal ICA, where atherosclerotic plaque is common. Atherosclerotic aneurysms that do not involve the carotid bifurcation are frequently saccular and occur in patients with severe arterial hypertension. Most bilateral, nontraumatic ECAAs are of the saccular type.
Posttraumatic Causes
Penetrating Injury.
Penetrating injuries involving the extracranial carotid arteries can have two important vascular sequelae: arteriovenous fistula and false aneurysm formation. The incidence of carotid artery injury in civilian trauma series ranges from 12% to 17% of the total penetrating neck injuries. The jugular vein appears to be more frequently injured in most reports. Unlike military carotid injuries, civilian carotid injuries tend to be caused by blunt or stabbing mechanisms. Data from the Vietnam vascular registry suggest that most penetrating carotid injuries involve the common carotid artery.29 The incidence of false aneurysm development after penetrating carotid injury is not known. The vast majority of injuries require immediate surgical intervention. Delays in treatment can result in the development of a false aneurysm. What is not known, however, is the number of penetrating injuries that eventually heal without sequelae.
Iatrogenic injury to the carotid artery during attempted placement of a catheter in the internal jugular vein is another frequent cause of false aneurysm, the wall of which is composed of the surrounding fascial and soft tissue structures (see Chapter 129).
Blunt Cervical Injury and Carotid Dissection.
Blunt cervical carotid injury, though rare, can be a devastating injury. Blunt carotid injuries typically involve the distal cervical segment of the ICA at the skull base. These injuries present a unique set of challenges because they often occur in the setting of multisystem trauma, particularly head injuries, and symptoms are frequently attributed to traumatic brain injury. Unfortunately, because the vast majority of blunt cervical carotid injuries are diagnosed only after the development of symptoms from central nervous system ischemia, neurologic morbidity rates of up to 80% and associated mortality of up to 40% have been reported. Blunt injury to the carotid or vertebral vessels is diagnosed in approximately 1 in 1000 (0.1%) patients hospitalized for trauma in the United States. When asymptomatic patients are screened for blunt cerebrovascular injury, the incidence rises to 1% of all blunt trauma patients.
Blunt trauma to the cervical carotid arteries produces a spectrum of injury that includes vasospasm, intimal and medial tears, thrombosis, and partial or complete transection of the artery. Of the four main mechanisms associated with blunt cervical carotid injury, the most common involves hyperextension and rotation of the head and neck. The lateral articular processes and pedicles of the upper three cervical vertebrae project more anteriorly than C4 through C7, so the distal cervical ICA is prone to stretch injury during hyperextension. The styloid process has also been implicated in the pathophysiology of these injuries because it rotates independently with the skull on the dens, whereas the artery moves with the cervical spine.30 Other mechanisms of injury include a direct blow to the artery and compression between the mandible and vertebral body associated with severe hyperflexion. Basilar skull fractures involving the sphenoid or petrous bones can lacerate the artery from sharp fragments. In addition, intraoral trauma can directly injure the artery and lead to a traumatic pseudoaneurysm.
Blunt traumatic injuries to the carotid arteries can also lead to dissection and intramural hematoma, which can cause various degrees of luminal obstruction. Carotid dissection can occur spontaneously or as consequence of “minor trauma” such as chiropractic manipulation, shaving, sneezing, vomiting, and a host of other innocuous activities. Biffl et al31 proposed an injury grading scale to classify blunt carotid and vertebral artery injuries (Box 103-1). Injury of grade III refers to traumatic pseudoaneurysms and represents disruption of the continuity of the arterial wall with the development of a false aneurysm. A periarterial hematoma contained by the fascial planes and surrounding soft tissue structures is formed. This cavity, which contains blood and laminated thrombus, is in continuity with the arterial lumen and has the potential for embolization as well as expansion and rupture.
Fabian et al32 reported a large single-center experience with traumatic carotid artery injuries. Heparin was the only factor independently associated with improved neurologic outcome. When heparin anticoagulation is possible in a traumatized patient, it is the initial treatment of choice. In patients unable to tolerate anticoagulation, antiplatelet therapy is recommended. Endovascular treatment with a carotid stent in an attempt to tack down the intima, exclude the pseudoaneurysm from the circulation, and prevent distal embolization and rupture is gaining acceptance as the treatment of choice in patients with a persistent traumatic pseudoaneurysm. Some writers have suggested that a traumatic pseudoaneurysm present beyond a week warrants intervention.33 The management of symptomatic carotid and vertebral dissections not associated with pseudoaneurysms, but with significant luminal narrowing, remains controversial. There is a growing body of literature suggesting that in patients undergoing anticoagulation for carotid and vertebral dissection who remain symptomatic, endovascular stenting is a reasonable alternative treatment34,35 (see Chapter 102).
Postendarterectomy Aneurysms
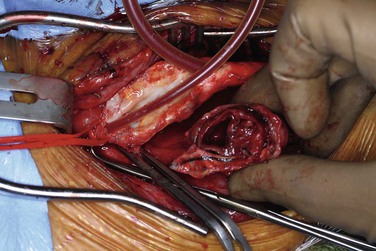
CEA-related pseudoaneurysms are some of the most frequently reported aneurysms of the extracranial carotid arteries. El-Sabrout and Cooley3 demonstrated that 57% of their 67 cases were a result of previous CEA. Zhou et al,23 in a later series from the Baylor College of Medicine, found post-CEA pseudoaneurysm to be the principal etiology in 15 (36%) of 42 cases. The development of post-CEA pseudoaneurysm is related to either suture line failure or infection. El-Sabrout and Cooley3 reported seven patients in whom the silk sutures used for patch angioplasty before the advent of monofilament sutures degenerated. Infection complicates approximately a third of all post-CEA pseudoaneurysms. The patients typically have local signs and symptoms of infection, including pain and erythema at the operative site or draining neck sinuses. Infection of the synthetic patch is identified at the time of removal, with Staphylococcus species being the most commonly cultured causative organism (Figures 103-1 and 103-2).
Arterial Dysplasia
Arterial dysplasia, usually a fibromuscular variant, was the most frequent pathologic cause of ECAAs reported in the series by Faggioli et al19 and also by several others.27,36 However, this finding has not been the experience documented in most other reported large series. The arteries of patients with fibromuscular dysplasia typically display a beaded appearance (alternating stenotic webs and dilatations). These dysplastic lesions may lead to ICA dissection and false aneurysm formation.
Pathology
Most primary ECAAs are secondary to a degenerative process that causes true aneurysms. Although ECAAs are frequently associated with atherosclerotic pathologic changes, the pathologic process is much more complex (see Chapter 9). There is an enormous disparity between the two types of aneurysms in the incidence of carotid occlusive disease and aneurysmal disease in the extracranial carotid artery. The rarity of primary carotid artery aneurysm in comparison with the hundreds of thousands of patients with carotid occlusive disease makes it difficult to accept atherosclerosis as the sole cause. Histologic study of carotid artery aneurysms, however, does reveal many of the findings seen in atherosclerotic specimens: fragmentation of the elastic lamina, lipid-laden foam cells, extracellular accumulation of cholesterol, deposition of hemosiderin, degeneration of the media, and neoangiogenesis. Thinning of the media and fragmentation of the internal elastic lamina are also seen, as in aging arteries. Just as many authorities propose for abdominal aortic aneurysms, atherosclerosis is a coexisting finding but may not be the primary cause.37,38
The location of aneurysms involving the extracranial carotid artery depends on the underlying pathology. True atherosclerotic aneurysms typically involve the carotid bifurcation (Fig. 103-3). Penetrating traumatic injuries usually involve the common carotid artery, and blunt traumatic injuries the distal ICA. No predisposition exists for right- or left-sided involvement in patients with unilateral aneurysms.
Clinical Findings
Physical Features/Symptoms
Pulsatile Mass
The symptoms of ECAAs vary according to their location, size, and etiology. The most common symptom is a pulsatile neck mass, which was the initial symptom in 93% of patients in the series reported by Zhou et al.23 Small internal carotid aneurysms may be asymptomatic, but most cervical carotid aneurysms are identified by the finding of a pulsating mass in the neck just below the angle of the mandible. These aneurysms may be painful, tender, or asymptomatic. Tenderness and overlying erythema, especially if associated with fever, should raise suspicion for an infected aneurysm.
ICA aneurysm is occasionally recognized as a pulsating mass in the tonsillar fossa or pharynx with little or no manifestation of its presence externally in the neck. The classic analytic study by Shipley et al39 emphasized that aneurysms of the ICA are directed inward into the throat, whereas those of the common carotid artery are directed outward into the neck. The absence of cervical swelling in the former is attributed to the dense, deep cervical fascia and muscles attached to the styloid process anteriorly and the cervical vertebrae posteriorly, which crowd the gradually dilating aneurysm inward toward the tonsillar fossa, where the thin superior pharyngeal constrictor muscle and mucous membrane offer only minimal resistance to inward protrusion. The level at which the common carotid artery bifurcates also influences the point of appearance. When the carotid bifurcation is low, an internal carotid aneurysm can be visible and palpable externally in the neck.
Aneurysms that arise at or proximal to the carotid bifurcation are readily palpable and usually pose no diagnostic difficulty. Those arising from the ICA near the base of the skull can and do cause diagnostic problems. A chronic unilateral swelling of the posterior pharynx should raise the level of suspicion, especially when other physical signs are lacking, bizarre, or atypical. Otolaryngologists are often the first to see these lesions. A high index of suspicion usually leads to computed tomography angiography (CTA), magnetic resonance angiography (MRA), or catheter-based angiography, any of which is nearly always diagnostic when an aneurysm is present.
Neurologic Symptoms
Many series report hemispheric neurologic events as the initial symptom of carotid artery aneurysms. In the Texas Heart Institute series, 28 of the 65 patients (43%) had neurologic symptoms, including amaurosis fugax and transient hemispheric ischemic attacks.3 Three of the 28 patients suffered a stroke preoperatively. Zhou et al23 reported six patients (14%) with transient ischemic attack, stroke, or Horner’s syndrome. Most neurologic events are secondary to embolization of thrombotic material from within the aneurysm wall, but some could be potentially related to diminished flow and compression of the ICA from the mass effect of large aneurysms. Transient ischemic attacks appear to occur twice as often as completed strokes.12,14,40
Cranial Nerve Dysfunction
Distal ICA aneurysms are more frequently associated with cranial nerve dysfunction than are aneurysms located more proximally, but clearly nerve injury can occur with large proximal carotid aneurysms as well. The ICA enters the cranium through the foramen lacerum and traverses the carotid canal in the petrous portion of the temporal bone. Accompanying the ICA are the sympathetic nerve fibers of the carotid plexus. Compression of these fibers can result in Horner’s syndrome, which consists of ptosis, miosis, anhidrosis, enophthalmos, and vasodilatation affecting the facial and cervical skin. Aneurysms located more proximally can result in hoarseness from compression of the vagus or recurrent laryngeal nerve. Compression of the facial nerve can cause severe facial pain. Compression of the fifth (trigeminal) and sixth (abducens) cranial nerves has been reported as well.
Dysphagia
Occasionally, the mass of a large aneurysm can cause difficulty swallowing. Protrusion of the aneurysm into the pharyngeal constrictor muscles can produce the sensation of dysphagia as well as compression of the nerves involved in the swallowing mechanism. On occasion these aneurysms are discovered during evaluation for dysphagia.
Hemorrhage and Rupture
Fortunately, hemorrhage and rupture are now infrequent manifestations of carotid artery aneurysms. There have been descriptions of “herald bleeds” or multiple smaller bleeding episodes before massive rupture. These episodes are similar to the bleeding associated with aortoenteric fistula. When these aneurysms do rupture into the oropharynx, the bleeding is profound and death is usually due to suffocation and aspiration. Mycotic aneurysms are especially susceptible to rupture and bleeding, but with the advent of antibiotics, they are exceedingly rare.
Another group of patients at risk for the so-called carotid blowout syndrome24 consist of those who have received extensive head and neck radiation therapy and those undergoing extensive surgery for head and neck cancer. Lesley et al24 reported their experience with 16 actual or impending carotid ruptures in 12 patients. Ten of these patients had undergone extensive treatment of head and neck cancer. Risk factors identified for the development of carotid blowout as a complication of treatment of head and neck cancer included thrombosis of the vasa vasorum secondary to wound infection, direct exposure and desiccation of the carotid artery, stripping of the carotid sheath, exposure of the artery to saliva, adjacent tissue necrosis, pharyngeal fistula formation, and previous irradiation.
Differential Diagnosis
The differential diagnosis for a pulsatile neck mass is extensive. The most common cause is a tortuous, kinked, or coiled carotid artery. Duplex ultrasound and occasionally CTA are required to help differentiate this finding from an ECAA (see Chapter 104). Other entities in the differential diagnosis include a prominent carotid bifurcation in a thin neck, cervical lymph nodes overlying the carotid bifurcation, carotid body tumors, glomus jugulare tumors, cervical metastatic disease, branchial cleft cysts, and cystic hygromas.
Diagnostic Evaluation
Duplex ultrasound is the initial diagnostic imaging modality of choice for the evaluation of ECAAs emanating low enough in the neck to be evaluated by this modality. Aneurysms located high in the distal ICA, such as those related to blunt cervical carotid dissection, are notoriously missed by ultrasound. Such aneurysms require further imaging with CTA or MRA. MRA has the advantage of being able to distinguish old from recent thrombus, a differentiation that is particularly helpful in cases of carotid dissection. Knowledge of the strengths and weaknesses of the imaging modalities at one’s institution should direct the next noninvasive imaging study chosen (see Chapter 98).
CTA has the benefit of visualizing the relationships of bony anatomic landmarks, which are critical in deciding whether a lesion is considered “surgically inaccessible” and requires an endovascular intervention (Fig. 103-4). MRA, when obtained in conjunction with head and brain imaging, provides indispensable information regarding the circle of Willis and collateral cerebral circulation. CTA can also provide similar intracranial imaging views, depending on the institution performing it. We have found it useful to obtain both studies at our institution to provide such complementary information.
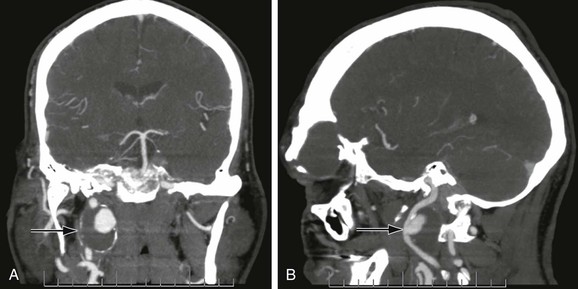
Figure 103-4 Anterior (A) and lateral (B) CT arteriogram reconstructions of a 4-cm distal internal carotid artery aneurysm (arrow). This aneurysm was treated with a covered stent graft with good result. (Courtesy Steven Oweida, MD, and John Parp Jones, MD.)
Catheter-based angiography was previously considered mandatory in the evaluation of ECAAs to obtain the detailed vascular anatomy information necessary to planning surgical treatment.41 We currently find this practice unnecessary and reserve catheter-based angiography for endovascular interventions because of the potential stroke risk associated with this invasive diagnostic procedure.
The utility of diagnostic angiography in the current management of ECAAs involves the rare case in which open or endovascular reconstruction is not considered an option and carotid ligation may be necessary. Combined preprocedure noninvasive imaging of the circle of Willis anatomy and a balloon occlusion test of the ipsilateral ICA have been recommended before ligation. The latter study involves a period of occlusion with an end-hole balloon occlusion catheter in patients who are awake, anticoagulated, and at baseline blood pressure. The end-hole catheter also allows measurement of carotid artery “stump” pressure or back-pressure. A stump pressure greater than 50% of mean systemic pressure indicates adequate cerebral blood flow during the carotid balloon occlusion test.42 Occlusion of the ICA is typically performed for 30 minutes, and the awake patient is assessed for neurologic changes. Blood pressure is also pharmacologically lowered to assess tolerance of hypotension. Several reports have detailed the inadequacy of the balloon occlusion test to accurately predict tolerance of carotid occlusion in 10% to 20% of patients.43,44 In patients in whom ipsilateral hemispheric neurologic events developed after carotid occlusion, thromboembolic events secondary to disturbed flow were thought to be the cause. These reports stress the importance of anticoagulation with warfarin for 6 weeks to 3 months after carotid ligation (see later discussions of ligation and morbidity under “Open Surgical Repair”).
Natural History
The natural history of ECAAs managed by observation is poorly defined. No single institution has a large clinical experience. The largest reported series is 67 aneurysms in 65 patients from the Texas Heart Institute.12 Only estimates of natural history can be made because they are based on multiple case reports, small series, and collected reviews. These series typically include aneurysms of all types and causes, and because the numbers are small, it is difficult to correlate results with specific aneurysm etiology. Typically, only aneurysms requiring medical attention are reported in the literature. Therefore, it is impossible to determine the number and clinical outcome of asymptomatic, incidentally found ECAAs that are managed by observation. Although routine autopsy studies suggest that the incidence of ECAA is low, the available literature would suggest that the natural history of these lesions is unfavorable. Winslow’s9 1926 report showed a 71% mortality rate from rupture, thrombosis, or distal embolization in 35 untreated patients, but this and other early reported series had a preponderance of mycotic aneurysms, which are associated with a higher risk for rupture; accordingly, the natural history of degenerative lesions is likely more benign. However, in a more contemporary report from the University of Michigan, 13 of the 19 patients with carotid aneurysms had amaurosis fugax, transient ischemic attack, stroke, or vague neurologic symptoms.15 Given the likelihood of symptoms and risk of permanent adverse neurologic events, a conservative approach to ECAAs cannot be justified in the vast majority of cases.
Treatment
Treatment of ECAAs has evolved with the specialty of vascular surgery. The primary objective in the treatment of such aneurysms is to prevent the permanent neurologic deficits that can arise from atheroembolism and thromboembolism. This objective is best accomplished by exclusion of the aneurysm from the arterial circulation and restoration of antegrade flow. The choice of therapy must be tailored to the individual and based on the location, size, and cause of the aneurysm as well as the overall condition of the patient.
Open Surgical Repair
Ligation
History.
In 1552, Ambroise Paré published the first account of operative ligation of the common carotid artery to control hemorrhage caused by a laceration of the artery. Unfortunately, this procedure resulted in aphasia and contralateral hemiplegia.45 Sir Astley Cooper performed the first common carotid ligation for the treatment of an aneurysm in 1806. Hemiplegia resulted on the eighth postoperative day, and the patient died 13 days later. Two years later he successfully ligated a carotid artery with good clinical outcome. Proximal ligation, coupled occasionally with distal ligation and resection, was the mainstay of treatment of carotid artery aneurysms until Matas developed the technique of endoaneurysmorrhaphy.
The subsequent development of modern reconstructive vascular techniques has eliminated ligation as a standard therapy for ECAAs. Ligation was performed in just 1 of the 65 patients with carotid aneurysms treated at the Texas Heart Institute over a 35-year period.12 Aneurysms extending to the skull base, once thought to be nonreconstructible because of an inability to achieve distal control, have been treated by special maneuvers to improve distal exposure, such as drilling away portions of the petrous and mastoid bones.27 Ligation of the carotid artery may still be necessary in emergency situations of arterial rupture, especially if infection is the cause and the artery is deemed unreconstructable.
Morbidity.
Ligation typically results in thrombosis from the level of interruption up to the first major intracranial arterial branch, usually the ophthalmic artery. As shown by Cooper’s first attempt in 1806, the risk for major stroke is significant, and stroke is estimated to occur in 30% to 60% of patients, half of whom die as a result.46,47 Stroke in these patients is due to acute cerebrovascular insufficiency secondary to inadequate collateral circulation or clot propagation and distal embolization. The fact that many strokes occur in a delayed fashion after carotid ligation adds evidence in support of the latter mechanism. It is recommended that patients undergoing carotid ligation be started on heparin therapy and then switched to warfarin anticoagulation for a period to prevent distal embolization because the ICA progressively fills with thrombus, with the risk of stump embolization. This approach is analogous to management of carotid artery trauma that might require ligation of the ICA. The duration of such anticoagulation is not standardized, but several groups have recommended a 2-week to 3-month course of therapy.48,49
Balloon Occlusion Test.
If a carotid ligation procedure is being considered, preoperative evaluation with the carotid balloon occlusion test and stump pressure measurement, as described earlier, is necessary. Ligation may be the only reasonable option for a nonreconstructible ECAA after extensive neck irradiation and radical neck resection in a patient with head and neck cancer. An endovascular alternative to open surgical ligation may have utility in the patient with a hostile surgical field. Coil embolization and detachable permanent balloon occlusion are useful endovascular techniques for this difficult situation (see “Endovascular Treatment”).
Adjunctive Measures.
In patients in whom the balloon occlusion test has a negative result and in whom carotid ligation is required, consideration should be given to extracranial-to-intracranial (EC-IC) bypass. This procedure is seldom performed, however, because of results of the EC-IC Bypass Study, which did not demonstrate better outcome with bypass surgery for internal carotid occlusion or intracranial occlusive lesions.50 However, it may be useful in selected patients, even though the technique is limited in modern neurosurgical practice. Candon et al51 described the novel technique of a saphenous vein bypass graft tunneled through the lumen of the distal ICA aneurysm and anastomosed to the petrous portion of the ICA for the treatment of a very distal ICA aneurysm.
Other historical attempts at aneurysm repair, such as wrapping of the aneurysm with fascia lata or prosthetic material, are mentioned only to be condemned. Wrapping may control the growth of the aneurysm and limit the risk of rupture but does nothing to reduce the more significant risk of distal embolization or thrombosis.
Resection and Reconstruction
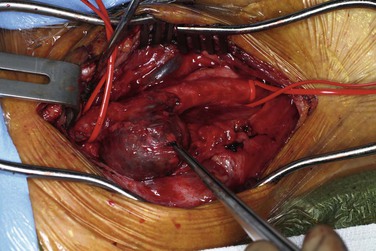
Resection of the aneurysm with restoration of antegrade flow has been the conventional standard for treatment of ECAAs in contemporary practice (Fig. 103-5). This surgical option is applicable to lesions involving the common carotid artery and proximal third of the ICA. Aneurysms involving distal portions of the ICA require further adjuncts to gain distal exposure and control (see discussion of open surgical repair under “Treatment Technique”).27,52 Aneurysms involving the external carotid artery alone are typically ligated without reconstruction.
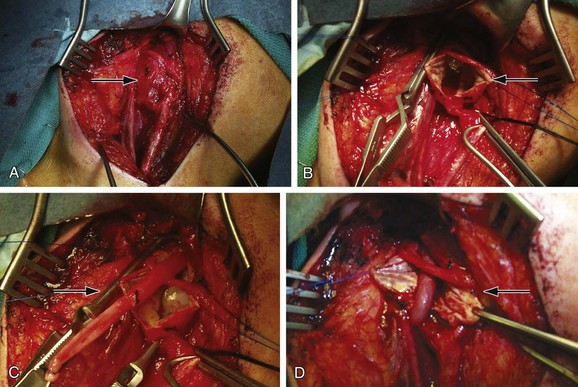
Figure 103-5 A through D, Open repair of the extracranial carotid artery aneurysm seen in Figure 103-3 via reversed–saphenous vein interposition.
History.
The first report of resection of a carotid aneurysm with primary anastomosis was described by Shea in 1955.52a The first successful procedure of this type was performed by Dimitza in 1952.53 When inadequate length of vessels precludes primary anastomosis, an interposition graft must be used. Beall et al54 performed the first prosthetic graft replacement for this lesion in 1959. Prosthetic and autogenous (artery or vein) grafts have been used with equally good result; however, an autogenous conduit is preferred whenever the possibility of infection exists.
Morbidity.
Complete excision of large carotid aneurysms risks injury to the cranial nerves, including the facial, vagus, spinal accessory, hypoglossal, and glossopharyngeal nerves. Profound disturbances in swallowing can occur as a result of injury to the pharyngeal muscular branches arising from the vagus, superior laryngeal, and glossopharyngeal nerves. Although these deficits are usually temporary, they cause considerable morbidity and concern for the patient. To minimize such problems, the surgeon must use extreme care when dissecting these structures. Use of the bipolar cautery for hemostasis has been advocated by some. The surgeon should always handle the aneurysm gently to prevent dislodgement and distal embolization of a mural thrombus.
Reconstruction Options.
After resection of a carotid artery aneurysm, several reconstruction options are available. Small saccular aneurysms with narrow necks can be resected, and the artery closed primarily or with a patch. Mobilization of a tortuous carotid artery can occasionally allow resection with primary end-to-end anastomosis. Another option that has previously been described for penetrating injuries of the ICA involves transposition of the external carotid artery after branch vessel ligation.
Pseudoaneurysm Repair after Previous Carotid Endarterectomy.
Patch angioplasty during CEA has become routine in an effort to decrease the restenosis rates seen after primary closure of the arteriotomy. Materials currently used for patch angioplasty include Dacron, polytetrafluoroethylene, and bovine pericardium. Callow55 reported his early experience with 22 patients in whom pseudoaneurysms developed after patch closure, including 12 in whom external jugular vein was used. He also described similar experience with patches made of saphenous vein harvested at the ankle. These vein patches lack the tensile strength to withstand pulsatile arterial pressure and are prone to aneurysmal degeneration. If an autologous conduit is to be used, as in cases of infection, saphenous vein from the groin should be used whenever possible. In current practice, patch pseudoaneurysm after CEA is more frequently related to infection than to degeneration of the patch.
Clinical Experience
Primary repair by direct closure, patch angioplasty, or resection and end-to-end primary anastomosis without grafting was used in 50 of the 67 aneurysms treated at the Texas Heart Institute.12 Most of the patients (n = 38, 58%) had pseudoaneurysms related to Dacron patches that had been applied at the time of CEA, including 7 in which silk sutures had been used and another 13 that were infected. Hertzer,56 in his invited review of the Texas Heart Institute experience, noted the researchers’ preference for partial aneurysm excision and patch angioplasty, even in cases of true atherosclerotic aneurysms (9 of 23, 39%). One would suspect that leaving residual aneurysmal tissue behind could predispose to the formation of a recurrent aneurysm. However, follow-up in 20 of the 23 patients (at an average of 6 years) revealed no further pseudoaneurysms. Follow-up in this study consisted of duplex ultrasound at 3 months and then when clinically indicated. In other words, it is possible that recurrent aneurysms might have been missed, particularly if they were very small.
In cases of true ECAA, it would seem intuitive to replace the entire aneurysmal segment to prevent the risk of further aneurysmal degeneration with partial excision. In the treatment of patch pseudoaneurysm, resection back to normal arterial wall plus patch angioplasty with autologous conduit is an acceptable alternative to interposition grafting.
Adjunctive Measures
Methods of cerebral monitoring and protection during carotid cross-clamping are the same as those used during conventional CEA and include electroencephalographic waveform analysis, selective shunting based on carotid stump pressure, and routine shunting (see Chapter 100). General anesthesia is usually recommended over cervical block regional anesthesia because of the difficult exposure and longer operative times for aneurysm repair than for CEA. As part of our usual practice, we routinely use shunting during CEA in patients under general anesthesia. This approach is recommended during carotid aneurysm repair as well. Painter et al25 recommended the use of an in-line straight shunt, as opposed to the Javid type, during vein interposition to tailor the vein graft to the appropriate length so that kinking could be avoided. In such cases, the vein interposition graft is telescoped over a straight shunt. After the distal anastomosis is performed, the vein graft is pulled to length over the shunt and trimmed accordingly. The shunt is then removed just before completion of the proximal anastomosis, and flushing maneuvers are performed in the usual fashion. We routinely use intraoperative duplex ultrasound to evaluate carotid reconstructions.
Endovascular Treatment
Endovascular management of ECAAs offers the advantages of avoiding a potentially difficult dissection and eliminating the need for high cervical exposure, thus reducing the risk for cranial nerve injuries and other procedure-related complications. Although most cranial nerve dysfunction is temporary, the incidence of such injuries is significant, reaching 20% in some series.23
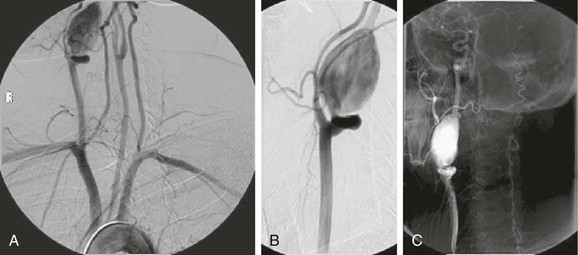
Several endovascular techniques for the treatment of ECAAs have been reported, including bare-metal stents with and without trans-stent coiling (Fig. 103-6),57 placement of double stents,58 autogenous vein graft–covered stents,59 endovascular coil or balloon occlusion,60 and placement of covered stent-grafts (Fig. 103-7).61
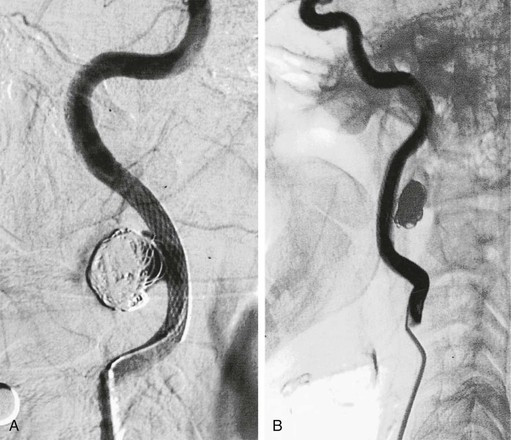
Figure 103-6 After placement of a self-expanding stent across the neck of the aneurysm, a microcatheter is used to introduce coils into the aneurysm sac. A, Completion arteriogram documented a patent internal carotid artery (ICA) with aneurysm exclusion. B, Arteriography after 5 months showed excellent flow through the ICA with exclusion of the pseudoaneurysm. (From Bush RL, et al: Endoluminal stent placement and coil embolization for the management of carotid artery pseudoaneurysms. J Endovasc Ther 8:53-61, 2001.)
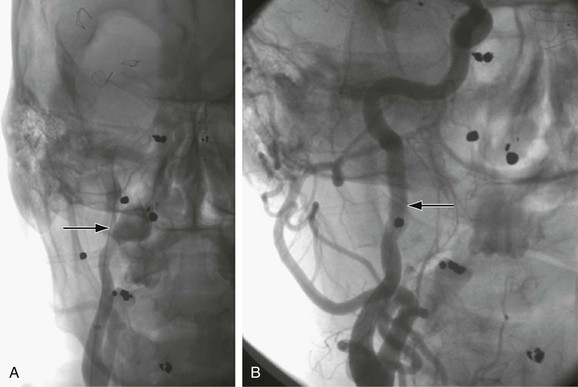
Figure 103-7 A, Selective right carotid arteriogram revealing the distal right internal carotid artery aneurysm demonstrated in Figure 103-2. B, Placement of a 5-mm × 2.5-cm Gore Viabahn (W.L. Gore and Associates, Inc., Flagstaff, Ariz) covered stent-graft for treatment of the distal right internal carotid artery aneurysm seen in A and Figure 103-4.
Percutaneous injection of thrombin under ultrasound guidance has become the treatment of choice for traumatic pseudoaneurysms of the common femoral artery. Holder et al62 reported successful thrombosis of a traumatic carotid pseudoaneurysm by balloon occlusion of the neck of the aneurysm followed by percutaneous injection of thrombin after inadvertent central venous catheter puncture. We have had no personal experience with this technique and have concerns about the risk for embolization once the balloon is deflated.
In 2006, the Baylor College of Medicine group reported their development in the treatment of such aneurysms, including 14 patients managed by endovascular means.23 Stent-grafts were used in 7 patients, carotid stenting with trans-stent coiling in 6, and endovascular balloon occlusion in 1. In most series of endovascular treatment of carotid artery aneurysms a variety of endovascular therapies have been used, and thus the small numbers in each group have precluded any meaningful comparison of the various treatments.
In patients with prohibitive operative risk, endovascular therapy is preferable to observational therapy, given the high risk associated with the natural history of such aneurysms.
Suspicion of infection or the presence of a known mycotic aneurysm is generally a contraindication to endovascular therapy. Although there has been a report of successful endovascular treatment of an infected carotid aneurysm combined with suppressive antibiotic therapy,63 this approach cannot be recommended except in the most extenuating circumstances.
Selection of Treatment
The location and size of a carotid aneurysm also play a critical role in determining which therapy to offer. Large aneurysms and those involving the distal ICA, because of the difficult surgical exposure and significant morbidity, are probably best managed with endovascular techniques. On the other hand, the presence of unstable-appearing thrombus within an aneurysm or pseudoaneurysm may be considered a relative contraindication to endovascular repair. Aneurysms in very tortuous carotid arteries are also a relative contraindication to endovascular therapy because of the difficulty of stent tracking and conformability to the artery wall. In fact, tortuous arteries lend themselves to aneurysm excision and primary end-to-end repair of the artery.
Treatment Technique
Open Surgical Repair
Large aneurysms and aneurysms extending to the most distal ICA are technically challenging. Several techniques can be used to improve distal operative exposure, including the one described here. Nasotracheal (rather than orotracheal) intubation should be used because it allows complete closure of the jaw which opens the space around the distal ICA.
2. Divide the ansa cervicalis to allow gentle retraction on the hypoglossal nerve.
3. Divide the posterior belly of the digastric muscle.
4. Divide the occipital artery and adjacent venous branches.
5. Divide the ascending pharyngeal artery.
Once proximal and distal control is obtained, the decision must be made as to complete aneurysm exclusion or partial resection. With a true atherosclerotic aneurysm, we typically replace the entire aneurysm with a saphenous vein interposition graft harvested from the groin (see “Pseudoaneurysm Repair after Previous Carotid Endarterectomy”). We typically use an endoaneurysmorrhaphy technique so that the cranial nerves are not injured during excision of the aneurysm wall. As with repair of abdominal aortic aneurysms, the sac of the aneurysm is used to cover the interposition graft. Use of a prosthetic graft in this situation has been reported. No large series have compared use of the two conduits, but a prosthetic graft is a reasonable alternative.
In situations of prosthetic patch pseudoaneurysm, El-Sabrout and Cooley3 have reported good results with resection of the pseudoaneurysm back to normal arterial wall and repeat patch angioplasty. In such circumstances we would also recommend autologous material to patch the artery because infection is frequently encountered in this situation.
We recommend the use of an in-line straight shunt, as opposed to the Javid type, during vein interposition to tailor the vein graft to the appropriate length to avoid kinking. In such cases, the vein interposition graft is telescoped over a straight shunt. The distal anastomosis is performed, and the vein graft is pulled to length over the shunt and trimmed accordingly. The shunt is then removed just before completion of the proximal anastomosis, and flushing maneuvers are performed in the usual fashion. We routinely use intraoperative duplex ultrasound to evaluate carotid reconstructions.
Endovascular Therapy
Applications of noninvasive endovascular treatment of carotid occlusive disease and improvements in technology specific for the carotid circulation have been noteworthy. Long hydrophilic sheaths designed for carotid stenting procedures have decreased in size since their original introduction. We currently use a 6 Fr Flexor Shuttle Select Sheath (Cook Medical, Inc., Bloomington, Ind) in the majority of carotid interventions performed from a femoral approach. If one is contemplating the use of a covered stent, such as the Gore Viabahn Endoprosthesis (W. L. Gore and Associates, Inc., Flagstaff, Ariz) or Atrium iCAST (Atrium Medical Corporation, Hudson, NH), larger sheaths may be needed (Table 103-2). We typically use a 0.014-inch wire to cross the aneurysm and have used a distal embolic protection device as long as there is reasonable length to land such a device above the aneurysm neck. In a circumstance of difficult transfemoral access, a cervical direct common carotid cutdown can be utilized.
Table 103-2
Endovascular Stent-Grafts Available in the United States
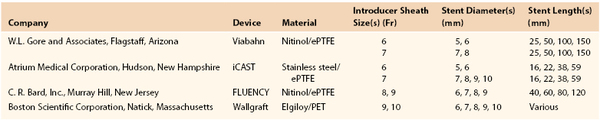
ePTFE, Expanded polytetrafluoroethylene; PET, polyethylene terephthalate.
Trans-stent Coil Embolization
The technique of trans-stent coiling involves initially crossing the neck of a saccular aneurysm with a self-expanding stent. If a distal embolic protection device is deployed on the 0.014-inch wire, there is adequate room to introduce a second wire (“buddy wire”) to track a 3 Fr RAPIDTRANSIT Microcatheter (Cordis Endovascular) into the aneurysm sac between the stent interstices. Coil embolization of the entire aneurysm sac is performed with detachable or platinum coils. The stent prevents migration of the coils into the distal carotid circulation.
Stent-Graft Coverage
In patient who has a fusiform aneurysm without a discrete neck, a stent-graft prosthesis is a better treatment option as long as there is adequate length of artery for proximal and distal sealing. How much proximal and distal seal zone is considered adequate, however, is unknown. Typically, a larger introducer sheath is required for a stent-graft prosthesis (see “Endovascular Therapy”). The stents are oversized to the artery per the instructions for use of the individual device. The stent-graft with the shortest length that will have adequate seal is chosen so that a distal kink is not created in the carotid artery. We angioplasty only within the stent graft and avoid angioplasty of the normal adjacent proximal and distal intimal of the carotid artery.
Hori et al58 described a novel double-stent technique in which overlapping uncovered stents are placed within each other. The greater surface area coverage of the two stents is thought to increase the chance of immediate aneurysmal thrombosis through decreased flow into the sac. We have no personal experience with this technique but are concerned that if aneurysm sac flow is persistent, it may be impossible to track a catheter across the double latticework of stents to place coils.
Treatment Outcome
Early Results
Open Surgical Repair
With modern vascular surgical techniques, correction of most ECAAs should be possible with a high rate of success and an acceptable rate of neurologic complications. Results vary widely depending on the type, size, and location of the aneurysm. Carotid ligation was previously associated with a 30% to 60% risk of stroke, and half of such patients died after stroke.9,46,47 In a 2000 review of the 13 largest single-center series since 1950, El-Sabrout and Cooley3 found that the combined stroke/death rate associated with carotid ligation had decreased to 12%. This change probably represents improvements in anesthetic technique and medical management as well as an understanding of the importance of anticoagulation after carotid ligation. Carotid ligation is reserved in current surgical practice for the unusual circumstance of a nonreconstructible carotid artery.
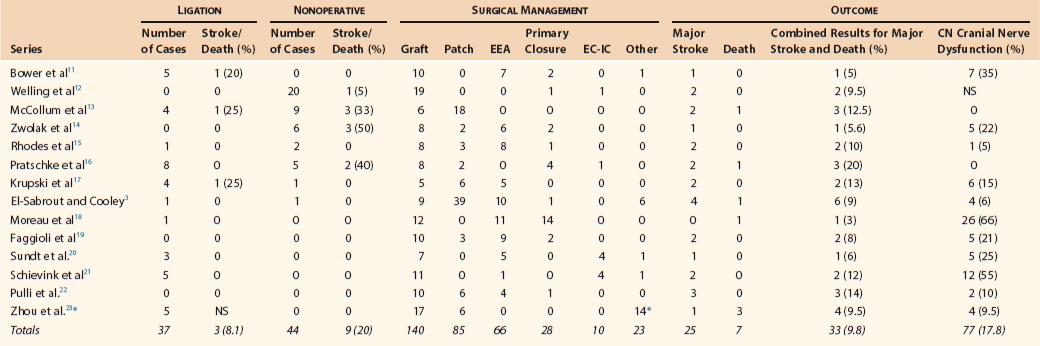
In general, surgical reconstruction is associated with a combined stroke and mortality rate of about 10%.3 This rate is obviously higher than that associated with the treatment of occlusive carotid bifurcation atherosclerosis. Transient cranial nerve dysfunction occurs in about 20% of patients.3 Pooled data reported by El-Sabrout and Cooley,3 including 392 cases of ECAA, showed a combined stroke and death rate of 21% in those managed nonoperatively, 12% in those treated by carotid ligation, and 9% in those treated by surgical reconstruction (Table 103-3).11–23
Endovascular Therapy
The early results of endovascular repair of ECAAs appear favorable in comparison with those of open surgical repair. Most of the small case series report no strokes associated with the procedure. As one would expect, endovascular therapy does not lead to cranial nerve dysfunction. There may, however, be selection bias in the published literature concerning ECAAs because negative results are not frequently reported; nevertheless, the data available are promising. Multiple successful single case reports abound, but there are limited series with more than a few patients.
Saatci et al64 reported using stent-grafts to treat 25 distal ICA pseudoaneurysms, the majority of which were post-traumatic. Endoleak, which occurred in 2 patients, resolved spontaneously in one and required placement of a bare-metal stent in the other. Twenty-three aneurysms were immediately excluded from the circulation after stent-graft placement. No technical adverse events—vessel dissection, vessel perforation, or thromboembolism—occurred. No mortality or morbidity developed during or after the procedure or during the follow-up period. Follow-up angiography in 21 patients showed reconstruction of the ICA with no aneurysm recanalization. All symptoms resolved after treatment in patients who initially had mass effect complications.
Coldwell et al33 reported their results in 14 patients with traumatic pseudoaneurysms after blunt cerebrovascular injury. In their protocol, all patients with evidence of blunt carotid dissection underwent anticoagulation with heparin for 7 days, followed by repeat arteriography. Those with flow-limiting dissections or pseudoaneurysm formation were treated with self-expanding WALLSTENT endoprostheses (Boston Scientific Corporation, Natick, Mass) and warfarin anticoagulation. In the follow-up period of 2.5 years, no strokes occurred and arteriography showed all lesions to have healed by 4 months.
Zhou et al23 reported the Baylor College of Medicine series and compared two different treatment periods: 22 cases all treated by open repair before 1995 and 20 cases treated after 1995. Of the 20 later cases, 14 were treated with endovascular therapy by a variety of techniques as listed earlier. The researchers found that in the second treatment period, hospital length of stay was significantly shorter, the rate of cranial nerve injury was diminished, and 30-day combined stroke/death rates were lower (14% vs. 5%, P < .004). No strokes occurred in the endovascular group at 30 days. At a mean follow-up of 4.6 years, 11 of 16 deaths were thought be related to cardiovascular causes, and continued aneurysm exclusion was confirmed in all patients.
Treatment of Carotid Blowout
Lesley et al24 reported a series of 16 carotid blowout events occurring in 12 patients, the majority of whom had undergone radiation therapy or surgery (or both) for head and neck cancer. All the patients were deemed to be at high risk for cerebral ischemic complications because of a negative balloon occlusion test result or known incomplete circle of Willis. These patients were managed with a variety of stent devices and techniques. Adjunctive embolization of carotid pseudoaneurysms with platinum coils or acrylic glue was performed in 5 patients. Hemostasis was achieved in all cases, although 1 patient with traumatic carotid blowout and 3 patients with aggressive head and neck cancer–related carotid blowout syndrome required retreatment with endovascular therapy. Rates of recurrent carotid blowout were similar to those reported in other studies using percutaneous balloon occlusion. Overall, no treatment-related strokes or deaths occurred.
Li et al65 systematically reviewed all available published data of the endovascular treatment of ECAA during 1995 through 2010. There were 113 studies comprising 224 patients undergoing treatment for ECAA. Procedural success was high, at 92.8%. Endoleaks occurred in 8.1%. The periprocedural stroke and death rates were very low, at 1.8% and 4.1%, respectively. The rate of cranial nerve injury, the major cause of morbidity during open surgical reconstruction for ECAA, was very low as expected, at 0.5%. Follow-up of these patients averaged 15 months, and the stent patency rate was reported to be 93.2%. The investigators concluded that these intermediate results suggest that low complication and high success rates make endovascular treatment of ECAAs favorable. As with all meta-analyses, selection bias may contribute to the favorable results reported.
Late Results
Open Surgical Repair
The long-term results of open surgical reconstruction for ECAAs are generally very good. Recurrent true aneurysms or pseudoaneurysms are rare after operative repair. There have been reports of delayed infection complicating the repair of carotid artery aneurysms. For example, six patients had such a complication in El-Sabrout and Cooley’s series.3 They were managed with a variety of techniques, including carotid ligation (one patient), autologous patch closure (one patient), and prosthetic patch closure (three patients) with reportedly good long-term results. Use of prosthetic patch material in the presence of an infected previous repair cannot generally be recommended except in extenuating circumstances.
Endovascular Therapy
The midterm results of endovascular therapy for ECAAs show that it is a feasible and durable alternative to conventional open repair. On the basis of available evidence from small case series of endovascular repair for carotid artery aneurysms, the combined stroke and death rates appear to be at least equivalent to those reported for open surgery. Obviously, there is probably significant publication bias in the limited small case series, but such promising results warrant further investigation. Long-term follow-up is necessary if the results of endovascular therapy are to be compared with those of open surgical repair. To date no sizable series of endovascular ECAA repair with long-term results has been reported.
Medical Management
Antithrombotic Therapy
Medical management after open repair or endovascular treatment of carotid artery aneurysms has not been standardized. After carotid ligation there is consensus for anticoagulation with warfarin to prevent distal embolization of the distal ICA thrombosis up to its first intracranial branch. There are no specific data to help guide the length of treatment, but most writers have recommended 2 to 12 weeks.48,49 Most patients who have undergone open reconstruction with primary repair, patch angioplasty, or interposition grafting have been treated with aspirin alone.
Patients being considered for elective endovascular therapy are typically started on clopidogrel therapy at least 5 days preoperatively. In urgent or emergency situations, therapeutic levels of clopidogrel can be reached by loading patients with 300 mg of Plavix after endovascular therapy. After endovascular repair, dual-antiplatelet therapy with aspirin and clopidogrel (Plavix, 75 mg/day) has been suggested by many to facilitate re-endothelialization of the treated surface.66 The duration of administration is not standardized, but we have typically kept patients who underwent stent placement in the carotid circulation on a regimen of clopidogrel for 6 weeks and aspirin for life. Based on lack of evidence, no additional recommendation can be made for additional treatment in those undergoing endovascular stent-grafting as opposed to stenting alone.
Statins
A series from the Johns Hopkins Hospital examined the use of statin therapy perioperatively after CEA.67 The researchers found that perioperative use of statins decreased the risk for stroke threefold, the risk for death fivefold, and the length of hospitalization by a day. In another study, Kennedy et al68 reviewed 3360 CEAs performed throughout western Canada and found a 75% reduction (odds ratio [OR], 0.25; 95% confidence interval [CI], 0.07 to 0.9) in the odds for death and a 45% reduction (OR, 0.55; 95% CI, 0.32 to 0.95) in the odds for ischemic stroke or death in symptomatic patients. Groschel et al69 reported a series of 180 patients undergoing carotid artery stenting for high-grade symptomatic carotid artery stenoses and examined the use of statins. As seen with CEA, statin use significantly decreased the rates of stroke, myocardial infarction, and death.
These data suggest that statins have an acute neuroprotective benefit during the perioperative period, the mechanism of which is not entirely clear. Proposed mechanisms include anti-inflammatory effects, plaque stabilization, and effects on thrombosis and coagulation. Given these data, it would seem reasonable to extrapolate that perioperative administration of statins is justified in the management of carotid artery aneurysms, although this statement is purely speculative.
Mycotic Aneurysms
Medical management of suspected or known mycotic carotid aneurysms involves the perioperative administration of antibiotics specific for the organism or organisms responsible. Staphylococcus aureus and Staphylococcus epidermidis are the most frequently encountered organisms. Gram-positive “coverage” with either vancomycin or linezolid is recommended until definitive culture and susceptibility results are available. Escherichia coli, Klebsiella species, Corynebacterium species, Proteus mirabilis, and Yersinia enterocolitica have also been reported. Therefore, initial broad gram-negative coverage is warranted as well. Once the standards for treating vascular infection have been completed, including graft removal, autologous reconstruction, débridement of perigraft tissue, muscle flap coverage, and drainage, a course of parenteral antibiotics is recommended. There are no clinical trial data on which to base recommendations for the length of antibiotic therapy, but patients are typically treated with parenteral culture-specific antibiotics for 4 to 6 weeks followed by oral antibiotics for 3 to 6 months or for life (see Chapter 42).
Special Considerations
Pediatric Patients
Pourhassan et al26 reviewed their experience and the available reports of ECAAs in children. The review cited 27 case reports of ECAAs occurring in the pediatric population within the past 25 years. The etiology of these aneurysms included infectious (such as peritonsillar abscess), traumatic (penetrating, blunt, and post-tonsillectomy injury), and congenital causes. Fourteen of the 27 cases were categorized as mycotic pseudoaneurysms with an associated antecedent serious oropharyngeal infection. Six were thought to be congenital or a manifestation of a systemic disease process (i.e., Behçet’s disease, type IIb hyperlipoproteinemia). Five infected pseudoaneurysms were thought to be secondary to surgical trauma after recent tonsillectomy; this conclusion emphasizes the anatomic proximity of the carotid artery to the tonsillar fossa. As seen in adults, the majority of aneurysms involved the common carotid artery or the ICA. If the external carotid artery is involved, it usually represents a pseudoaneurysm secondary to trauma or infection.
The usual initial symptom of carotid artery aneurysm in children is a pulsatile mass in the neck. Rupture of a carotid artery aneurysm is seen more frequently in children than in adults. In the review by Pourhassan et al,26, 42% of the patients were initially evaluated for either hematemesis or epistaxis. The greater frequency of aneurysm rupture seen in children is probably a manifestation of the etiology. Traumatic and mycotic aneurysms seem to have the highest risk for rupture, and these causes represent the majority of cases occurring in childhood.
Operative intervention is clearly recommended for all symptomatic carotid aneurysms with manifestations of cerebral ischemic events and local discomfort. Given the higher rates of rupture seen in children, aggressive surgical intervention is warranted. Multiple carotid artery reconstructions have been proposed, depending on the size, location, and etiology of the aneurysm. As in adults, resection plus interposition grafting is the treatment of choice. In the pediatric population, resection of the aneurysm should be followed by interposition grafting with saphenous vein. An autologous conduit is recommended because of the infectious etiology in many cases. It also allows longitudinal growth of the vessels as the child ages.
Selected Key References
Bush RL, Lin PH, Dodson TF, Dion JE, Lumsden AB. Endoluminal stent placement and coil embolization for the management of carotid artery pseudoaneurysms. J Endovasc Ther. 2001;8:53–61.
Coldwell DM, Novak Z, Ryu RK, Brega KE, Biffl WL, Offner PJ, Francoise RJ, Burch JM, Moore EE. Treatment of posttraumatic internal carotid arterial pseudoaneurysms with endovascular stents. J Trauma. 2000;48:470–472.
El-Sabrout R, Cooley DA. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000;31:702–712.
The largest single-center experience with extracranial carotid artery aneurysms..
Saatci I, Cekirge HS, Ozturk MH, Arat A, Ergungor F, Sekerci Z, Er U, Turkoglu S, Ozcan OE, Ozgen T. Treatment of ICA aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol. 2004;25:1742–1749.
The largest series to date of the use of covered stents for ICA aneurysms..
Zhou W, Lin PH, Bush RL, Peden E, Guerrero MA, Terramani T, Lubbe DF, Nguyen L, Lumsden AB. Carotid artery aneurysm: evolution of management over two decades. J Vasc Surg. 2006;43:493–496.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. McCormick WF, et al. The size of intracranial saccular aneurysms: an autopsy study. J Neurosurg. 1970;33:422–427.
2. Dell S. Asymptomatic cerebral aneurysm: assessment of its risk of rupture. Neurosurgery. 1982;10:162–166.
3. El-Sabrout R, et al. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000;31:702–712.
4. Schechter DC. Cervical carotid aneurysms: part I. N Y State J Med. 1979;79:892–901.
5. Schechter DC. Cervical carotid aneurysms. part II. N Y State J Med. 1979;79:1042–1048.
6. Johnston KW, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13:452–458.
7. de Jong KP, et al. Extracranial carotid artery aneurysms. Eur J Vasc Surg. 1989;3:557–562.
8. Cooper A. Account of the first successful operation performed on the common carotid artery for aneurysm, in the year 1808: with the post-mortem examination, in 1821. Guys Hosp Rep. 1836;1:53–59.
9. Winslow N. Extracranial aneurysm of the internal carotid artery: history and analysis of the cases registered up to Aug 1, 1925. Arch Surg. 1926;689–729.
10. Padayachy V, et al. Carotid artery aneurysms in patients with human immunodeficiency virus. J Vasc Surg. 2012;55:331–337.
11. Bower TC, et al. Brachiocephalic aneurysm: the case for early recognition and repair. Ann Vasc Surg. 1991;5:125–132.
12. Welling RE, et al. Extracranial carotid artery aneurysms. Surgery. 1983;93:319–323.
13. McCollum CH, et al. Aneurysms of the extracranial carotid artery: twenty-one years’ experience. Am J Surg. 1979;137:196–200.
14. Zwolak RM, et al. Atherosclerotic extracranial carotid artery aneurysms. J Vasc Surg. 1984;1:415–422.
15. Rhodes EL, et al. Aneurysms of extracranial carotid arteries. Arch Surg. 1976;111:339–343.
16. Pratschke E, et al. Extracranial aneurysms of the carotid artery. Thorac Cardiovasc Surg. 1980;28:354–358.
17. Krupski WC, et al. Aneurysms of the carotid arteries. Aust N Z J Surg. 1983;53:521–525.
18. Moreau P, et al. Surgical treatment of extracranial internal carotid artery aneurysm. Ann Vasc Surg. 1994;8:409–416.
19. Faggioli GL, et al. Extracranial internal carotid artery aneurysms: results of a surgical series with long-term follow-up. J Vasc Surg. 1996;23:587–594.
20. Sundt TM Jr, et al. Surgical management of aneurysms of the distal extracranial internal carotid artery. J Neurosurg. 1986;64:169–182.
21. Schievink WI, et al. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery. 1994;35:809–815.
22. Pulli R, et al. Extracranial carotid artery aneurysms. J Cardiovasc Surg (Torino). 1997;38:339–346.
23. Zhou W, et al. Carotid artery aneurysm: evolution of management over two decades. J Vasc Surg. 2006;43:493–496.
24. Lesley WS, et al. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. AJNR Am J Neuroradiol. 2003;24:975–981.
25. Painter TA, et al. Extracranial carotid aneurysms: report of six cases and review of the literature. J Vasc Surg. 1985;2:312–318.
26. Pourhassan S, et al. Extracranial carotid arteries aneurysms in children: single-center experiences in 4 patients and review of the literature. J Pediatr Surg. 2007;42:1961–1968.
27. Rosset E, et al. Surgical treatment of extracranial internal carotid artery aneurysms. J Vasc Surg. 2000;31:713–723.
28. Berne JD, et al. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma. 2006;60:1204–1209.
29. Rich NM, et al. Acute arterial injuries in Vietnam: 1,000 cases. J Trauma. 1970;10:359–369.
30. Stonebridge PA, et al. Traumatic aneurysm of the extracranial internal carotid artery due to hyperextension of the neck. Eur J Vasc Surg. 1990;4:423–425.
31. Biffl WL, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845–853.
32. Fabian TC, et al. Blunt carotid injury: importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223:513–522.
33. Coldwell DM, et al. Treatment of posttraumatic internal carotid arterial pseudoaneurysms with endovascular stents. J Trauma. 2000;48:470–472.
34. Ohta H, et al. Endovascular stent therapy for extracranial and intracranial carotid artery dissection: single center experience. J Neurosurg. 2011;115:91–100.
35. Pham MH, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856–866.
36. Coffin O, et al. Results of surgical management of internal carotid artery aneurysm by the cervical approach. Ann Vasc Surg. 1997;11:482–490.
37. Xu C, et al. Aneurysmal and occlusive atherosclerosis of the human abdominal aorta. J Vasc Surg. 2001;33:91–96.
38. Zarins CK, et al. Atherosclerotic enlargement of the human abdominal aorta. Atherosclerosis. 2001;155:157–164.
39. Shipley AM, et al. Aneurysm in the cervical portion of the internal carotid artery: an analytical study of the cases recorded in the literature between August 1, 1925, and July 31, 1936. Report of two new cases. Ann Surg. 1937;105:673–699.
40. Mokri B, et al. Extracranial internal carotid artery aneurysms. Mayo Clin Proc. 1982;57:310–321.
41. Goldstone J. Aneurysms of the extracranial carotid artery. Rutherford RB. Vascular surgery. ed 6. Elsevier Saunders: Philadelphia, PA; 2005.
42. Kato K, et al. Balloon occlusion test of the internal carotid artery: correlation with stump pressure and 99mTc-HMPAO SPECT. Acta Radiol. 2006;47:1073–1078.
43. Dare AO, et al. Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayed hemodynamic ischemia after therapeutic carotid occlusion. Surg Neurol. 1998;50:147–155.
44. Linskey ME, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15:829–843.
45. Watson WL, et al. Ligature of the common carotid artery in cancer of the head and neck. Ann Surg. 1939;109:1–27.
46. Brackett CE Jr. The complications of carotid artery ligation in the neck. J Neurosurg. 1953;10:91–106.
47. Kirby CK, et al. Aneurysm of the common carotid artery. Ann Surg. 1949;130:913–920.
48. Pemberton JD, et al. Surgical treatment of carotid body tumors: value of anticoagulants in carotid ligation. Ann Surg. 1951;133:837–852.
49. Ehrnefeld WK, et al. Relation of carotid stump pressure to safety of carotid artery ligation. Surgery. 1983;93:299–305.
50. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191–1200.
51. Candon E, et al. Cervical-to-petrous internal carotid artery saphenous vein in situ bypass for the treatment of a high cervical dissecting aneurysm: technical case report. Neurosurgery. 1996;39:863–866.
52. Skau T, et al. Surgical treatment of distal extracranial internal carotid artery aneurysms involving the base of the skull: a multidisciplinary approach. Eur J Vasc Endovasc Surg. 2000;20:308–311.
52a. Shea PC Jr, et al. Anastomosis of common and internal carotid arteries following excision of mycotic aneurysm. Surgery. 1955;37:829–832.
53. Dimitza A. Aneurysms of the carotid arteries: report of 2 cases. Angiology. 1956;7:218–227.
54. Beall AC Jr, et al. Extracranial aneurysms of the carotid artery: report of seven cases. Postgrad Med. 1962;32:93–102.
56. Hertzer NR. Extracranial carotid aneurysms: a new look at an old problem. J Vasc Surg. 2000;31:823–825.
57. Bush RL, et al. Endoluminal stent placement and coil embolization for the management of carotid artery pseudoaneurysms. J Endovasc Ther. 2001;8:53–61.
58. Hori Y, et al. Double stent technique for the treatment of an internal carotid artery pseudoaneurysm caused by zone III stab injury. J Vasc Interv Radiol. 2007;18:1300–1304.
59. Marotta TR, et al. Autologous vein-covered stent repair of a cervical internal carotid artery pseudoaneurysm: technical case report. Neurosurgery. 1998;42:408–412.
60. Zimmerman MC, et al. Treatment of impending carotid rupture with detachable balloon embolization. Arch Otolaryngol Head Neck Surg. 1987;113:1169–1175.
61. Kubaska SM 3rd, et al. Internal carotid artery pseudoaneurysms: treatment with the Wallgraft endoprosthesis. J Endovasc Ther. 2003;10:182–189.
62. Holder R, et al. Percutaneous thrombin injection of carotid artery pseudoaneurysm. J Endovasc Ther. 2002;9:25–28.
63. Baril DT, et al. Endovascular repair of an infected carotid artery pseudoaneurysm. J Vasc Surg. 2004;40:1024–1027.
64. Saatci I, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol. 2004;25:1742–1749.
65. Li Z, et al. Endovascular stenting of extracranial carotid artery aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2011;42:419–426.
66. Comerota AJ, et al. Antiplatelet therapy for vascular interventions. Perspect Vasc Surg Endovasc Ther. 2008;20:28–35.
67. McGirt MJ, et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg. 2005;42:829–836.
68. Kennedy J, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke. 2005;36:2072–2076.
69. Groschel K, et al. Statin therapy at carotid angioplasty and stent placement: effect on procedure-related stroke, myocardial infarction, and death. Radiology. 2006;240:145–151.