Chapter 101
Carotid Artery
Stenting
Piergiorgio Cao, Paola De Rango
In 1977 and 1980, Mathias et al1,2 and Kerber et al3 reported successful results with percutaneous angioplasty for carotid stenosis using technology derived from peripheral arterial angioplasty. Balloon-expandable stents were first deployed in the carotid artery in 1989 but were prone to extrinsic compression and had a high rate of major adverse events.4–6 These issues were resolved with self-expanding mesh wire Elgiloy carotid stents and, later, nitinol stents.7 Nevertheless, the high likelihood of embolic stroke during carotid stent deployment was a major concern that limited the early enthusiasm for endovascular treatment of carotid stenosis. It was with the introduction of embolic protection devices (EPDs) in 19908 that carotid artery stenting (CAS) developed rapidly and reached widespread expansion in the subsequent years. The feasibility of CAS to treat carotid stenosis outside surgical settings has accelerated the use of the procedure as an alternative to surgery for treatment of patients with carotid stenosis. Proponents of CAS have stressed the apparent simplicity, quickness, and comfort of the new, less invasive procedure compared with carotid endarterectomy (CEA). However, the procedure has undergone large scrutiny in the last 15 years,9–41 and today there are still concerns about the consideration of CAS as an “equivalent” therapeutic option to CEA in most patients with carotid stenosis because of increased periprocedural stroke risks and costs.9,27 After the publication of a number of randomized clinical trials (RCTs),10–22,28–37 multiple meta-analyses,42–49 and several large registries and worldwide series,50–109 there are data to support a twofold higher perioperative stroke risk with CAS compared with CEA when it is performed in unselected patients with symptomatic carotid stenosis. Currently, with better patient and operator selection and improved technology, CAS may be considered an alternative to CEA only in certain subgroups of patients.
Patient Selection
The incidence of complications of CAS strongly depends on how patients are selected and on the presence of factors that are associated with increased periprocedural risks. Better patient subgroup selection is associated with notably decreased CAS complications. However, most data for subgroup analyses are provided by smaller samples not powered to assess treatment differences and should be interpreted with caution. The detailed decision-making process to select CAS, CEA, or medical management in patients with carotid stenosis is developed in Chapter 99.
Clinical Considerations
Age
Advanced age is one of the most commonly used factors in surgical risk stratification. Given the less invasiveness and avoidance of general anesthesia, CAS was initially considered a promising alternative to CEA, especially in elderly patients. Today, there is compelling information supporting CEA rather than CAS in the treatment of old patients, particularly octogenarians.20,31,110,111
In a post hoc analysis of the SPACE trial, it was found that age (≥68 years) was significantly associated with the risk of stroke and death in the CAS group (P = .001) but not in the CEA group (P = .534).20 Similarly, CREST showed that older age increases the risk for the combined endpoint of stroke/death or myocardial infarction (MI) for CAS (P = .02), with the efficacy of CAS and CEA approximately equal at the age of 70 years.31 CAS risk for the primary endpoint increased with age (P < .0001) by 1.77 times (95% confidence interval [CI], 1.38-2.28) per 10-year increment, whereas there was no evidence of increased risk for CEA (P = .27). The treatment-by-age interaction for CAS and CEA was not altered by symptomatic status (P = .96).31
Pooled analysis of the three European RCTs (EVA-3S, SPACE, and ICSS) from the Carotid Stenting Trialists’ Collaboration found that in patients 70 years or older, there was a twofold increase in the 120-day stroke or death risk with CAS over CEA: 12.0% versus 5.9% (risk ratio [RR], 2.04; 95% CI, 1.48-2.82; P = .0053). For patients younger than 70 years, the risk was comparable: 5.8% in CAS and 5.7% in CEA (RR, 1.00; 95% CI, 0.68 to 1.47).110
In the Cochrane meta-analysis of pooled RCTs, the odds ratio (OR) of CAS versus CEA for 30-day death or any stroke risk was 1.16 (95% CI, 0.80-1.67) in patients younger than 70 years and 2.20 (95% CI, 1.47-3.29) in patients 70 years or older.42
The Society for Vascular Surgery Vascular Registry stratified outcomes of CAS and CEA by age and compared the composite outcome of death, stroke, or MI at 30 days among 1347 CEA and 861 CAS patients younger than 65 years and 4169 CEA and 2536 CAS patients 65 years and older. In patients 65 years and older, CEA had lower death (0.91% vs. 1.97%; P < .01), stroke (2.52% vs. 4.89%; P < .01), and composite death/stroke/MI (4.27% vs. 7.14%; P < .01) rates.80 Significant difference in composite outcome for old patients persisted when the analysis was performed separately in symptomatic (5.27% vs. 9.52%; P < .01) and asymptomatic (3.31% vs. 5.27%; P < .01) subgroups.80
Last, in-hospital data derived from a large number of carotid procedures (495,331) retrieved through the Nationwide Inpatient Sample (NIS) showed age 70 years and older to be an important predictor of postoperative stroke (OR, 1.7; 95% CI, 1.2-2.5; P < .0025) and cardiac complications (OR, 1.3; 95% CI, 1.0-1.6; P < .045) after CAS.111
Although CAS is a less invasive approach, older patients frequently have extensive atherosclerotic diseases that translate into more frequent occurrence of multiple embolic sources, adverse conditions for endovascular approach (such as diffuse calcification or “shaggy” aortic arches), and impaired cerebrovascular reserve with higher sensitivity to minor cerebral emboli damage. Manipulations with wires, catheters, and sheaths in tortuous or diseased arteries proximal to the target lesion can contribute to the increased hazards of CAS in older patients.
Sex
Sex-specific differences in cardiovascular disease and CEA have been well described. However, whether such disparities apply to CAS remains uncertain as randomized and nonrandomized studies report conflicting results.33,48,112–115
A secondary analysis of the CREST trial suggested that periprocedural risk of stroke/death appeared to be higher in women who had CAS than in those who had CEA: 5.5% versus 2.2% (P = .013).33 The difference in rates was more evident in symptomatic women (7.5% vs. 2.7% in CAS vs. CEA, respectively; P = .030), whereas it was not confirmed in asymptomatic women (P = .28).33
In the meta-analysis of the three European RCTs (Carotid Stenting Trialists’ Collaboration), an opposite trend was shown; a lower CAS to CEA risk ratio in women than in men (1.22 vs. 1.68) was assessed at 120 days.48 A further meta-analysis including CREST and CAVATAS was consistent with the not real effect of sex on the risks of CAS or CEA.112
Outside RCTs, there is conflicting information in reporting the effect of sex on CAS outcome.113–117 The potential and uncertain higher stroke risk in symptomatic women after CAS may be related to greater embolic potential in female carotid plaque.117 This hypothesis needs to be substantiated by more data.
Statewide risk-adjusted analysis of 18,320 women undergoing CEA and 2293 undergoing CAS found that the risk of in-hospital stroke/death was 1.7-fold higher in symptomatic and 3.4-fold higher in asymptomatic women receiving CAS compared with CEA.115
The ALKK-CAS registry showed that for the group of 1443 women, the total composite in-hospital endpoint of nonfatal stroke and death was comparable to that of men: 3.0% versus 3.4%. Despite the older age of women, all in-hospital outcomes of CAS in women were comparable to those of men in both symptomatic and asymptomatic patients.116
Currently, sex cannot be considered among high-risk criteria for CAS.
Timing in Symptomatic Patients
In recent years, there is evidence recommending treatment with “early” CEA in symptomatic patients to prevent the risk of recurrent stroke, which is four to five times higher in the early period after the onset of symptoms.118 Whereas for patients undergoing CEA there is increased benefit from early surgery performed within the first 7 days of the symptomatic event, the same data are conflicting when they are applied to CAS.119–122 The Carotid Stenting Trialists’ Collaboration meta-analysis of the three European RCTs observed that patients undergoing CAS within 14 days of their most recent symptom were almost three times more likely to have a procedural stroke or death (8.6% vs. 3.2%; OR, 2.7; 95% CI, 1.36-5.45; P = .30) than patients undergoing CEA.48 Updated data from the same group recently showed that the stroke risk in CAS compared with CEA appeared to be greatest in patients treated within 7 days of symptoms. Patients treated with CAS in this period had 9.4% risk of periprocedural stroke/death compared with 2.8% for CEA, leading to more than threefold increased risk of CAS versus CEA adjusted for age, sex, and type of event (hazard ratio [HR], 3.4; 95% CI, 1.01-11.8).119
Analysis of 482 symptomatic patients included in the CAPTURE registry showed that an interval of 0 to 13 days from symptoms (stroke or transient ischemic attack) to CAS was an independent predictor of adverse 30-day outcome (OR, 2.52; 95% CI, 1.33-4.78; P = .0047).63 Topakian et al120 analyzed the effect of timing in 77 CAS patients and identified old age and treatment within 2 weeks as the only predictors of increased risk for 30-day complications; stroke and death rate was 26% versus 1.9% in those treated later.
Conversely, in a cohort of 320 patients from two centers described by Gröschel et al,121 the 30-day complication rate after CAS was 8.45%, but early stenting (<2 weeks) was not associated with an increased risk of stroke.
Lin et al,122 using a single-center prospective registry with 224 CAS procedures in symptomatic patients, found that the risk of periprocedural stroke was 3.45% in the early (<4 weeks) and 5.95% in the late (>4 weeks) intervention groups (P = .5). The 30-day stroke/death and MI rates were also similar (6.03% vs. 8.33%).
It is intuitive that endovascular manipulation of a carotid lesion recently symptomatic would have increased embolic risk based on knowledge of carotid plaque pathology as reviewed in Chapter 97. Nevertheless, management of patients by CAS in the early period after the onset of a transient ischemic attack or minor stroke is a challenge to be solved by larger prospective studies focused on timing.
Asymptomatic Carotid Stenosis
Data from CREST and SAPPHIRE suggest that in properly selected asymptomatic patients, CAS, performed by experienced interventionalists, may be equivalent to CEA. Nevertheless, these findings must be interpreted in light of recent studies and guidelines suggesting that for many asymptomatic patients, especially those with short life expectancy or with recurrent stenosis more than true carotid plaque (like many of those included in SAPPHIRE), best medical therapy may be the optimal treatment modality.123–128 Currently, there are insufficient data for CAS to be recommended as a primary therapy for asymptomatic patients with severe carotid stenosis. RCTs are ongoing.38–41
Combined Carotid and Coronary Artery Disease
Because the incidence of concomitant carotid and coronary atherosclerosis is significant and presence of carotid disease has been associated with an increase in the incidence of stroke in the perioperative period for cardiac surgery, management of concomitant carotid and coronary disease has been the subject of considerable debate. The use of CAS would be a logical alternative to CEA in this setting because of its less invasiveness. However, data are not sufficiently robust to support definitive recommendations.123–129 A review of the NIS found that the risk of perioperative stroke was 62% greater in patients undergoing CEA plus coronary artery bypass grafting (CABG) than in those undergoing staged CAS before CABG (OR, 1.62; 95% CI, 1.1-2.5; P = .02). Nevertheless, rates of in-hospital mortality were similar with the two approaches (5.2% vs. 5.4%).130
A meta-analysis of published studies of CAS plus CABG by Naylor et al131 showed a stroke/death rate at 30 days of 9.1%. Although CAS was suggested as a valuable alternative in combined carotid and coronary operations, the stroke/death risks remained questionable because most carotid stenoses treated were asymptomatic.131 Whether the low rate of complications with CAS plus CABG shown in other small studies132–134 reflects case selection bias or an intrinsic safety advantage remains uncertain and should be clarified in properly designed prospective studies.
One of the most challenging issues to be solved is that catheter-based carotid interventions require treatment with a potent platelet inhibitor such as clopidogrel and dual antiplatelet therapy, which can increase the risk of major bleeding associated with cardiac surgery. Conversely, delay of antiplatelet therapy raises the risk of stent thrombosis and stroke.
Anatomic Features
Several anatomic factors may influence the outcome of CAS. Recent analysis from the EVA-3S trial found that the risk of stroke or death in CAS was higher in patients with internal carotid artery (ICA)–common carotid artery (CCA) angulation of 60 degrees or more (RR, 4.96; 95% CI, 2.29-10.74).135 A systematic review of literature by the same EVA-3S authors including 56 studies and 34,398 patients with CAS confirmed that the risk of 30-day stroke/death was higher in patients with increased carotid vessel angulation (RR, 3.41; 95% CI, 1.52-7.63) and when the target ICA lesion was more than 10 mm in length (RR, 2.36; 95% CI, 1.28-3.38). There was no significant increase in risk of stroke/death in patients with type III aortic arch, arch calcification, ostial involvement, calcification, ulceration, degree of stenosis, or contralateral carotid occlusion as also suggested by Sayeed.135,136
A similar analysis of CREST on morphology predictors of outcomes showed that lesion length, eccentric lesions, ulcerated lesions, percentage stenosis, and procedural time were all potential factors not contributing to the age-related risk differences in the CAS treatment group.31
Preprocedural assessment of carotid stenosis is generally based on duplex ultrasound, which provides morphologic and hemodynamic information at the extracranial carotid level. However, in patients with diffuse vascular disease, it is advisable to obtain full supra-aortic vessel imaging with magnetic resonance angiography or computed tomographic angiography before scheduling intervention with CAS. This allows careful evaluation of the aortic arch, the origin and possible tortuosity of all supra-aortic trunks, and the severity of stenosis. In addition, computed tomographic angiography can provide information on the presence and extent of calcification and the morphology of the plaque, and it can make accurate measurements of the diameter of the CCA and ICA for selection of the devices to be used.
Aortic Arch
Factors involving the aortic arch that may increase technical complexity during CAS include the presence of extensive aortic wall irregularities with multiple atheromas (shaggy aorta) and severe aortic calcification (eggshell aorta). A patient with a shaggy aorta may have an absolute contraindication to CAS from the high risk of catastrophic distal embolism not only to the brain but, rarely, to the viscera and lower extremities. An eggshell aorta is associated too with the risk for intimal disruption and embolism because of lack of compliance while the guide wires, catheters, and sheaths are being directed. Last, the stiffness of the vessels may decrease the torqueability of wires and catheters and cause resistance to progression of the sheath or stent delivery system to the target lesion.
Arch morphology can be variable and becomes more elongated and tortuous with advancing age. The upper inner aspect of the arch becomes a fulcrum; with increasing tortuosity, the ascending aorta and the transverse arch can elongate and push the aortic valve and the origins of the innominate artery and left CCA downward. As the arch becomes more tortuous, the origins of the major branches become more difficult to negotiate by remote endoluminal access.
The shape and curvature of the aortic arch can be categorized into three types, depending on the position of the innominate artery as defined by two horizontal lines drawn across the highest point of the outer and inner curvatures of the arch. In a type I aortic arch, the great vessels arise above or in the same horizontal plane as the outer curvature of the arch. In a type II aortic arch, the origin of the innominate artery lies between the horizontal planes of the outer and inner curvatures of the aortic arch. In a type III arch, the innominate artery lies below the horizontal plane of the inner curvature of the arch (Fig. 101-1). The more inferior the origin of the supra-aortic vessels (type II or III arch), the greater the difficulty in gaining access to the carotid arteries and catheter guidance and exchange. A type III aortic arch may lead to prolonged catheter manipulation with a possible risk for aortic plaque embolization.
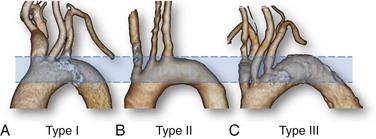
Figure 101-1 Aortic arch classification. A, Type I: the great vessels arise above or in the same horizontal plane of the outer curvature of the arch. B, Type II: the origin of the innominate artery lies between the horizontal planes of the outer and inner curvatures of the aortic arch. C, Type III: the innominate artery lies below the horizontal plane of the inner curvature of the arch.
In addition to these arch configurations, some congenital variations in the origin of the great vessels are relatively common. The so-called bovine arch, present in up to 27% of patients,137 may take two different anatomic patterns. In the more frequent type 2 (8% to 10% of cases), the innominate artery and left CCA share a common origin (Fig. 101-2); in the other type, the common carotid branch takes off from the innominate artery. A “pure” bovine arch, extremely rare, occurs when there is a common brachiocephalic arterial trunk originating from the arch that gives rise to both subclavian arteries (right and left) and a single bicarotid trunk. The bicarotid trunk then branches into two separate trunks, left and right CCA.137
Carotid Tortuosity and Calcification
Heavy circumferential calcification, especially if it is associated with tortuosity along the carotid vessels, increases the difficulty of accessing the lesion. Severe distal ICA kinking or coiling may prevent positioning of the distal EPD with a landing zone sufficient for stent deployment and may predispose to vascular spasm at the end of the procedure. When the angulation is located at the distal end of carotid plaque, stent deployment may change the conformation of the vessel and cause flow-limiting angulation. On occasion, extreme tortuosity may preclude a patient from undergoing intervention.
Finally, extensive calcification in the area of the stenosis may make stent delivery more difficult and may lead to insufficient expansion after deployment and recoiling of the dilated stenosis after deflation of the balloon.
Plaque Morphology
Quantitative and qualitative carotid plaque analysis, primarily achieved with duplex ultrasound, has been suggested as an important parameter for assessing the risk of stroke during CAS.138–142 Details of carotid duplex ultrasound are provided in Chapter 98. There appears to be a higher potential for embolism during CAS in the presence of hypoechoic or echolucent soft plaque.139–142 However, the definition of plaque echogenicity is operator dependent and difficult to classify in terms of different grades of severity. Novel computerized ultrasound technology, such as the gray scale median score, has been proposed141; however, its validation and reproducibility are not universally accepted for routine use in clinical practice.143 In general, the reliability of ultrasound evaluation and correlation with pathologic specimens remain under study, and the clinical applicability of these observations awaits solid confirmation.
Contralateral Carotid Occlusion
There are conflicting data in the literature supporting the increased risks of stroke for patients with contralateral carotid occlusion.144–147 The ALKK-CAS Registry showed overall major stroke and death rates in patients undergoing CAS comparable to those of patients without contralateral carotid occlusion.144 Similar information was provided by the Illinois experience, which found only 2.6% periprocedural stroke in CAS patients with contralateral carotid occlusion.145 Brewster et al,147 in a small prospective study, found two transient ischemic attacks and no perioperative stroke in the group of CAS patients with contralateral carotid occlusion, and 30-day and midterm outcomes were comparable to those of patients without contralateral carotid occlusion.
Currently, the observed outcomes do not support use of contralateral carotid occlusion as a selection criterion for CAS over CEA in the absence of other indications.
Adverse Neck Conditions
CAS is preferred to CEA in patients with local factors that increase complexity of CEA, such as tracheostomy, prior contralateral nerve palsy, lesions that extend proximally near to the clavicle or distally to the C2 vertebral body, and situations in which local tissues are scarred and fibrotic from prior ipsilateral surgery or external beam radiotherapy.123–127 A meta-analysis of 27 articles including 361 CAS and 172 CEA procedures performed for carotid stenosis after radiation therapy showed perioperative risks for any cerebrovascular events of 3.9% with CAS and 3.5% with CEA. However, risk of cranial nerve injury was 0% after CAS and 9.2% after CEA, whereas CAS was followed by higher risk of restenosis over time.148 Another systematic review including 211 surgical procedures and 510 CAS procedures for post-radiotherapy carotid stenosis showed 10.3% cranial nerve palsy occurrence (0.6% permanent) in the surgical group. There were no statistically significant differences in 30-day rates of stroke/death between surgery and CAS in both symptomatic (OR, 0.52; 95% CI, 0.14-1.98; P = .38) and asymptomatic (OR, 0.55; 95% CI, 0.06-5.42; P = .99) patients.149
A complete outline for decision making based on clinical and anatomic criteria to select CAS, CEA, or medical management in patients with carotid stenosis is described in Chapter 99.
Periprocedural Medical Management
Adequate medical management involves intraprocedural therapy and treatment in preparation for or after CAS.
Intraprocedural Therapy
Adequate Fluid Administration
Adequate administration of fluids is the first fundamental step in reducing the risk for contrast-induced nephropathy and intraprocedural hypotension in preparing patients for CAS.
Anticoagulation
As in most vascular procedures, intraprocedural anticoagulation is necessary with CAS. Adequate anticoagulation is usually achieved with unfractionated heparin (70 to 100 units/kg) administered after arterial access is gained and before manipulation of catheters in the aortic arch. An activated clotting time not longer than 250 to 300 seconds is recommended to avoid the risk for intracerebral hemorrhage from reperfusion. Heparin is rarely reversed at the end of the procedure. Given that the half-life of heparin is about 90 minutes, the procedure is usually finished before the anticoagulant effect of the initial dose has completely worn off. Bivalirudin may be used as an alternative.124
Atropine
Hypotension associated with marked bradycardia is a common feature after balloon dilatation, especially in elderly patients with heavily calcified stenoses.150–152 Intravenous atropine (0.4 to 1 mg given intravenously) may be administrated before stent deployment and balloon inflation to suppress the hemodynamic response to stretching of carotid baroreceptors. In the case of a severe hemodynamic response, aggressive volume expansion and an additional dose of atropine or use of vasopressors, including intravenous phenylephrine (1 to 10 µg/kg/min) and dopamine (5 to 15 µg/kg/min) infusion, may be necessary.124 Moderate hypotension may last 24 to 48 hours before the carotid sinus adapts to the radial force of the self-expanding stents, and pharmacologic support is occasionally required. The hemodynamic response may be exaggerated in patients with baseline bradycardia or those taking beta blockers or digoxin, whereas patients with a denervated carotid sinus because of previous CEA or with pacemakers are at lower risk. Intraprocedural transcutaneous temporary cardiac pacing is rarely indicated for CAS.153,154
Vasodilators and Hypotensive Drugs
Accurate pressure control during and after the procedure is essential in decreasing the risk of complications. Hypertension occasionally develops immediately before, during, or rarely after CAS, and maintenance of systolic blood pressure below 180 mm Hg is advised to minimize the risk of intracranial hemorrhage.124 More commonly, hypotension may develop after stent deployment and persist a few days as described before.
Spasm may appear after EPD retrieval in the distal ICA. Most cases of spasm resolve spontaneously in a few minutes. However, when spasm is severe and persistent, administration of vasodilators may be required. Commonly, nitroglycerin (100 µg) is administered directly into the ICA through the introducer sheath or guiding catheter (500 µg diluted in 10 mL and 2 mL). Additional doses may be administered every 3 to 5 minutes. The patient must be observed for possible exaggerated hypotension.
Periprocedural Therapy
Several developments in medical treatment in preparation for or after CAS have evolved since the 1990s, including newer antiplatelet agents, statins, and the recognized benefits of lowering blood pressure.123–125,127–129
Antiplatelet Drugs
Rapid thrombus formation inside the stent and consequent potential embolization immediately after CAS provide the rationale for double antiplatelet therapy.123–125,127–129,155 Aspirin, the most studied antiplatelet agent, causes irreversible inhibition of platelet cyclooxygenase by decreasing production of thromboxane A2.155–158 However, there is interpatient variability in the response of platelets to aspirin as well as occasional resistance to the drug.159 Thienopyridine derivatives, such as ticlopidine and clopidogrel, or the newer ticagrelor and prasugrel, which act as adenosine diphosphate receptor antagonists through a separate pathway from inhibition of cyclooxygenase, have been considered a valid alternative or adjunctive treatment to aspirin.160–162
Clinical evidence supporting the use of double antiplatelet treatment to prevent vascular events is mainly derived from studies involving coronary interventions; there are few data in CAS patients.163–170 The benefit is less clear when dual treatment is applied to patients who had ischemic stroke or transient ischemic attack.171,172 The MATCH trial (Management of Athero Thrombosis with Clopidogrel in High-risk patients with recent transient ischemic attack or ischemic stroke) found only a nonsignificant 6.4% reduction in the relative risk for primary endpoints (stroke, MI, vascular death, rehospitalization) with a dual antiplatelet regimen.172 Similar results were obtained in the CHARISMA trial (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance).173,174 The benefit of combination therapy became marginally significant in high-risk subgroups, such as patients with symptomatic vascular disease; conversely, the related risk of bleeding obviated the benefits of treatment in low-risk subgroups.173,174
Despite uncertain information about the effectiveness of dual antiplatelet treatment in preventing cerebrovascular ischemic events during CAS, there is consensus that patients undergoing CAS should receive a regimen similar to that of those undergoing coronary stenting, as recommended by guidelines from the Society for Vascular Surgery, American College of Cardiology, American Heart Association, European Society of Cardiology, and European Society for Vascular Surgery.123–125,127 The standard regimen includes aspirin, 75 to 325 mg/daily, and clopidogrel, 75 mg/daily, both starting at least 4 days before the procedure. Alternatively, a loading single dose of clopidogrel (300 to 600 mg) at least 4 to 6 hours before the procedure is advocated. For patients intolerant to clopidogrel, ticlopidine (250 mg twice daily) may be used.123
There is limited evidence to support the benefit of dual antiplatelet therapy with aspirin and dipyridamole after CAS.128,175,176
Today, most centers administer dual antiplatelet therapy for 4 weeks after carotid stenting as suggested by studies documenting the occurrence of adverse events during the first 24 days and up to 30 days after the procedure and taking into account that stent endothelialization lasts 28 to 96 days.124,127,177 Longer dual antiplatelet treatment is suggested in patients at high risk for restenosis or major cardiovascular events.127,163,165,168 A low dose of aspirin (75-100 mg/daily) may be considered effective for long-term treatment in standard-risk patients.
Regarding the patients scheduled for combined carotid and coronary surgery, the American College of Cardiology and American Heart Association guidelines recommend stopping clopidogrel therapy at least 5 days before CABG.123–125,127,178 Recent data suggest that clopidogrel may be safely continued through the perioperative period without excessive bleeding risk, and the last Society for Vascular Surgery carotid guidelines suggest that it is reasonable to individualize perioperative management of clopidogrel therapy.123
Antithrombotic Drugs
Few studies have compared antiplatelet with anticoagulant therapy after CAS. In a small RCT that included 47 patients undergoing CAS, the 30-day neurologic complications rate was 0% with clopidogrel and aspirin versus 25% (P = .02) with aspirin plus heparin. The unacceptable level of complications in the anticoagulant group resulted in early termination of the study.179
Warfarin is recommended for primary and secondary prevention of stroke in patients with atrial fibrillation.180,181 On the basis of these trials in patients with carotid stenosis and a concurrent risk for cardioembolic stroke, it is reasonable to maintain the antithrombotic therapy without adjunctive antiplatelet drugs to avoid the increased hemorrhagic risk. Warfarin may be converted to intravenous heparin before the procedure.
Statins
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) have been found to be highly effective in preventing stroke in both male and female patients with cardiovascular disease.182 Review and large multicenter studies analyzing the effect of statins in patients undergoing vascular surgery showed significant improvement in postoperative outcomes.183–185 A number of studies have specifically focused on outcomes of carotid surgery, raising the question of whether similar effects would affect the outcomes of CAS, but few studies have addressed this issue.185–189 The supposed benefit in patients undergoing carotid revascularization appears to be largely independent of the cholesterol-lowering effect of the drug; it is due to so-called pleiotropic effects because statins have been shown to stabilize atherosclerotic plaque and show anti-inflammatory, antithrombogenic, antiproliferative, and antileukocyte adhesion properties.
In a single-center experience, the incidence of cardiovascular events after CAS was 4% in statin users versus 15% in nonusers (P < .05).188 Another large series showed 1.5% versus 4% 30-day stroke/death (P < .009) for users and nonusers.189 On the basis of available data, it is reasonable to prescribe statins early before the procedure but also to monitor liver function and creatine kinase levels thereafter to detect potential side effects of the drug.124,127
Procedure Technique
The CAS procedure widens the carotid stenosis by plaque dilatation and attempts to prevent future embolization by scaffolding the ruptured plaque against the vessel wall. The specific technique of CAS may vary according to operator experience. However, some steps are widely accepted in standardized protocols.
Technologic Requirements
In the optimal setting, carotid intervention should be performed in angiography suites specifically equipped with a sterile environment and all facilities necessary for an operating room, as in the so-called hybrid operating rooms.23,152,178,190 In general, specific minimum requirements are the following: appropriate equipment and sufficient space to allow positioning of patient monitoring and anesthesia equipment while preserving the sterile field; high-resolution image intensifier with the ability to acquire and to store high-quality images with the lowest radiation exposure; and adequate physiologic monitoring system and prompt access to surgical and medical emergency support.
It has been suggested that a bank of three monitors be used to show simultaneous working and reference images as well as hemodynamic data. The monitors should be positioned in front of the operator to allow thorough understanding of the patient’s condition and progress of the procedure. Prompt availability of a large inventory of endovascular supplies as well as any emergency equipment and medication is critical for a successful carotid stent program.
Periprocedural Monitoring
One important aspect of performing CAS safely is ensuring that the patient is adequately monitored before, during, and after the procedure. Intraprocedural management includes continuous assessment of neurologic and hemodynamic parameters and anticoagulation. Continuous electrocardiographic, pulse oximetry, and intra-arterial pressure monitoring through the side arm of the introducer sheath should be routinely used during the procedure to detect early and to treat hemodynamic alterations.
The level of alertness, speech, and motor function must be continuously evaluated by asking the patient to answer simple questions and to squeeze a plastic toy in the contralateral hand. However, because subtle ischemic neurologic changes may be overlooked, more accurate assessment is advisable after completion of the procedure. Transcranial Doppler (TCD) may be used as an adjunctive technique to monitor the patient during and immediately after the procedure. Indeed, it is known that cerebral embolic events may occur during any phase of CAS. High-intensity transient signals can be detected as warning signs.191 Because reflection of ultrasound power depends on both the size and composition of the embolus, the use of multifrequency TCD has been suggested for automatic recognition of high-intensity transient signals and differentiation between particulate emboli and air bubbles or artifacts.192,193 Nevertheless, sources of error are frequent, and the ability of TCD to differentiate emboli with relevant clinical consequences from artifacts is questionable.141,142,194–197
Mild sedation may be offered to CAS patients, but an adequate level of consciousness under local anesthesia should be maintained to monitor the neurologic condition. Noninvasive hemodynamic monitoring should be maintained until the following morning. Postprocedural management for 24 hours should include ultrasound evaluation to assess the morphology of the treated vessel and the arterial access site. Laboratory chemistry including cardiac enzymes to rule out renal and cardiac damage should be performed before discharge and after 1 week.
At discharge, the patient should be instructed to comply with the follow-up schedule and to inform the practitioner in the event of any new neurologic symptoms. Serial follow-up assessment by trial convention should be performed at 1 month, 6 months, and annually thereafter and should include duplex ultrasound imaging to detect restenosis.124
Procedure
Access
Transfemoral.
Retrograde right femoral access followed by a 6F to 9F introducer access sheath is the most commonly used technique for catheter manipulation by a right-handed operator. The left common femoral is the second choice if the right femoral route is not available. Insertion of guide wires and catheters should follow the method for any endovascular procedure. Full-dose heparin is administered intravenously at this step.
Transcervical.
A direct CCA access at the base of the neck has recently been introduced and can be used in selected cases.198–202 The rationale of the transcervical CCA access approach is to avoid catheter manipulation in a diseased aortic arch, which can increase the risk of plaque embolization into the brain.198–202 A percutaneous approach with puncture of the CCA at the base of the neck with ultrasound guidance can be used to obtain a stable platform to next advance the EPD and stent to the proper carotid site. A direct percutaneous CCA approach may be complicated with dissection or embolization in vessels with diffuse atherosclerotic lesions. In such situations, a surgical cutdown with a 2- to 3-cm neck incision and surgical exposure of the CCA, even if it is more invasive, might be safer. In all cases of transcervical approach, a careful evaluation of the level of carotid bifurcation is needed because a short CCA may not allow a stable placement of a short introducer sheath.
Transbrachial and Transradial.
A transbrachial access may be employed with a 6F guiding catheter in case of aortoiliac occlusion or extremely difficult access to the CCA, as in a type III arch with diffuse calcification. A transradial approach has recently been suggested as an alternative with the same indications.203
Remote Access to the Target Lesion
Stable sheath access in the proximal CCA is a critical step. Vessels that originate below the apex of the aortic arch are more difficult to cannulate because wires and catheter insertion and exchange become increasingly difficult with a type II or III aortic arch.
In procedures with remote access, two main techniques to advance the catheter into the CCA are commonly used. Once it is in place, the guiding catheter or long sheath side port is intermittently irrigated or attached to a slow continuous infusion of saline to avoid stagnation of blood in the sheath and then connected to a blood pressure monitoring system.
Sheath Based.
In the sheath-based platform, preshaped 5F catheters, such as the Judkins 4, Headhunter, Simmons 1, or other models, may be used for CCA cannulation according to the preference of the operator. Through these catheters, an exchange-length stiff 0.035-inch guide wire is placed in the terminal branches of the external carotid artery (ECA), and a 6F 90-cm sheath (e.g., Shuttle, Cook, Inc.; Destination, Terumo Interventional Systems) with its dilator is then advanced over the wire and positioned with a pull-and-push maneuver in the distal CCA a few centimeters below the bifurcation. Care must be taken to identify the tip of the dilator because it should be kept away from the carotid bulb. Otherwise, a 125-cm curved catheter premounted into the sheath is used for direct cannulation and advancement of the guide wire and sheath (telescopic technique). The telescopic method is preferable in patients with a calcified and tortuous aortic arch because the catheter and guide wire assembly provides additional support with fewer maneuvers. One possible disadvantage is the mismatch between the size of the catheter (5F) and the sheath, which may cause the tip of the sheath to scrape the arterial wall (“snowplowing”) and result in distal embolization.
Guiding Catheter Based.
As an increasingly used alternative, CCA access is achieved directly with a preshaped guiding catheter (e.g., multipurpose curve, vertebral or reversed-angle Vitek catheter) with a 6F to 7F diameter that is left at the CCA level for the entire procedure. The advantage of this technique is that it does not require exchange wires and catheter, with consequent reduced manipulation approaching the target lesion. A shortcoming of the approach is the need to reach a stable position well above the CCA origin to avoid displacement of the guiding catheter into the aortic arch during stent progression. When the guiding catheter is judged not to be sufficiently stable in the CCA, a high-support 0.014-inch coronary “buddy wire” placed into the ECA may be used to better stabilize the working platform.
Arteriography
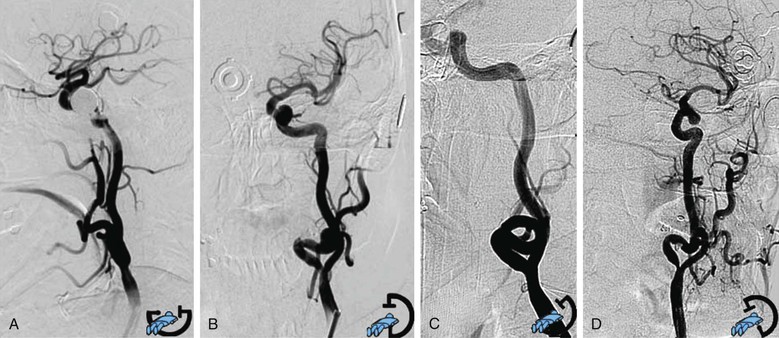
When a stable working platform has been reached in the CCA, an arteriogram through the guiding catheter or sheath should be obtained. Anteroposterior or lateral projection is needed to obtain minimal overlap of the ICA and ECA, optimal visualization of the target lesion, and complete intracranial carotid vessel distribution. When differentiation between the two carotid branches is not easily obtained with two projections, an adjunctive oblique projection is performed following the best view obtained from the preprocedural three-dimensional computed tomographic angiography reconstruction. Rotational angiography (the image intensifier rotates around the patient and acquires a series of images that are then reconstructed through software algorithms into a three-dimensional image) is occasionally used, when it is available, in selected cases of difficult visualization of the carotid lesion (Fig. 101-3).
Stent System Delivery
Embolic Protection Device Deployment.
After full angiographic evaluation, a decision must be made about use of an EPD. Several EPDs are available, and selection depends on lesion characteristics and anatomic considerations. With the most commonly used distal filter, the stenosis is crossed with a 0.014-inch guide wire that is a fixed or mobile component of the EPD in use, usually with a road mapping technique. Distal filters are deployed into the ICA, before its petrous segment; the position must be checked throughout the entire procedure. It is important not to advance the tip of the device any farther because the intracranial portion of the carotid artery is highly prone to dissection and spasm. Different types of EPDs are presented later in this chapter.
Predilatation.
When the stenosis is extremely severe, after placement of the EPD, predilatation with a 2.5- or 4.0-mm coronary balloon at relatively low inflation pressure (4 to 6 atm) may prevent difficulty in stent crossing and release. Rarely, careful predilatation may be required to allow EPD deployment through a preocclusive stenosis. In these settings, use of a flow reversal EPD is an alternative (see later section on selection of EPD type for details).
Stent Delivery.
The stent is deployed under road mapping control or by use of the vertebral bones as landmarks. The stent is usually placed across the bifurcation because most of the time the plaque extends to the bulb area. A self-expanding stent is used; a variety of diameters (7 to 10 mm), lengths (3 to 4 cm), and shapes (cylindrical or tapered) are available. The diameter of the stent must be sized to the largest portion of the vessel, typically the distal CCA. Different models of stents can be used to better adapt to the vessel morphology and plaque characteristics. This issue is discussed later in the chapter.
Postdilatation.
After stent deployment, short (2 cm), usually 5- to 5.5-mm balloons are used to dilate the narrowest portion of the stent. Balloon diameter should never exceed the diameter of the distal ICA. The balloon is always maintained within the stent. Higher pressure might be needed for heavily calcified plaque, which has a tendency to recoil.
Retrieval of the Embolic Protection Device and Completion Angiography.
A completion arteriogram of the carotid bifurcation at highest magnification is obtained before removal of the protection device to ensure the accuracy of deployment and to evaluate possible residual stenosis, spasm at the stent endpoint, or filling defects inside the stent due to plaque protrusion. After retrieval of the guide wire and EPD, a final completion angiogram of the cervical carotid and intracranial circulation is obtained, usually in double projection. ICA spasm may occur distally from filter movements during guide wire manipulations. Typically, watchful waiting and occasionally the administration of small doses of nitroglycerin (100-200 µg) through the guiding sheath allow resolution of the problem.
Care should be taken in the total amount of contrast agent used because of potential toxicity for the brain parenchyma. The total amount injected is optimally maintained as low as possible and never exceeding 60 mL.
Access Hemostasis.
Heparin’s action is not usually reversed. Access site hemostasis may be achieved with a percutaneous closure device or groin manual compression.
Embolic Protection Devices
EPD use is suggested to reduce the occurrence of distal cerebral embolization and, potentially, the risk of stroke. However, debate concerning the routine use of EPDs during CAS is still open because of different results reported. The EVA-3S trial provided strong support for the use of EPDs: a 30-day risk of stroke of 7.9% with protected CAS versus 25% with unprotected CAS.17 On the other hand, the SPACE trial found the same rate of events in both groups; the risk of 30-day death or any ipsilateral stroke was 6.2% in the protected group and 8.3% in the unprotected group (P = .40).204 In the CREST and in the postmarketing CAPTURE 2 registry, the use of an EPD was mandatory when feasible, and it was used in 96% of CREST patients. The systematic use of EPDs in CREST has been proposed as a main factor in the relatively low rate of adverse events in this RCT compared with others.28,62–64 In a meta-analysis by Garg et al205 that included a total of 12,263 protected and 11,198 unprotected CAS procedures, the relative risk for stroke was 0.62 (95% CI, 0.54-0.72) in favor of protected CAS. The pooled stroke rate at 30 days was 2.6% with use of an EPD versus 4.2% in patients without protection (P < .001).
Use of an EPD reduced the risk of stroke during CAS by 38% in all patients (33% in the symptomatic and 39% in the asymptomatic patients; P = .05).205 However, conflicting comments have been raised about the specific risk for stroke from improper placement of the EPD, and some investigators argue for selective use.88,94,206–210
Currently, use of an EPD is required by the Centers for Medicare and Medicaid Services (CMS) to qualify for reimbursement. The updated Society for Vascular Surgery guidelines for management of carotid disease of 2011 released a grade I, level B recommendation for the use of an EPD during CAS to reduce the risk of cerebral embolization.123
Categories of Embolic Protection Devices
Today there are a large variety of EPD models with different mechanisms that extend its applicability to disparate anatomic conditions. Three conceptually different methods, each with advantages and drawbacks, have been developed.
Distal Filter.
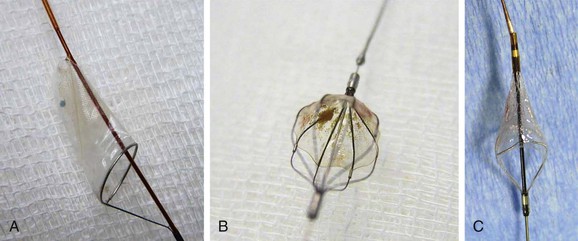
Filter-type EPDs, the most widely used devices,77,104,208–211 allow antegrade cerebral flow during the entire procedure. Filter design varies, with no demonstrated superiority of one model over another212; some are advanced over a 0.014-inch wire, and others are attached to the guide wire with a steerable wire tip. Crossing profiles usually range from 1.7F to 3.9F. Distal filter systems function like an umbrella or basket that is opened in the ICA and placed in the distal ICA after the lesion is crossed to capture any debris during the procedure (Fig. 101-4). After successful angioplasty and stent placement, the distal filter containing the debris is removed with a dedicated retrieval system. The main advantages of these EPDs are the maintenance of blood flow to the brain, the availability of angiographic control throughout the procedure, and the reduced need of device manipulation inside the carotid vessels in proximity of the target lesion. The main drawbacks are the need to cross the ICA lesion before delivery of the EPD, the difficulty in crossing very tight and tortuous lesions, and the uncertainty of capturing all debris for incomplete arterial wall apposition.
Distal Occlusion Balloon.
Distal occlusion balloons are available but have generally been abandoned.8,93 Protection is obtained by occluding the distal ICA above the lesion with an inflated balloon and flushing and suctioning after stent deployment. Their main disadvantages include the need to cross the lesion without protection, as with filters; the potential inability to remove all of the embolic material; and the risk for distal spasm or wall damage as a result of balloon inflation in the distal ICA.
Proximal Protection Devices with Flow Stasis or Reversal.
Proximal protection systems allow embolic protection before the lesion is crossed by use of flow stasis or flow reversal. The Mo.Ma system uses two occlusion balloons, one each in the CCA and the ECA, in a single delivery catheter. Debris is recruited with intermittent syringe aspiration through the long introducer sheath, after stent deployment and balloon dilatation but before deflation of the occlusion balloon.52
The Gore Flow Reversal System is based on the same principle of ECA and CCA balloon occlusion, but an external shunt, filtering all the debris, is placed between the long introducer sheath in the carotid artery and a previously placed short introducer in the femoral vein.57 Similarly, the most recent MICHI Neuroprotection System allows a reverse-flow arteriovenous circuit to be obtained by connecting an 8F transcervical arterial access sheath and an 8F venous return sheath with a large blood flow line with flow controller.201
The main advantage of these new EPDs is that no interaction with the plaque occurs until the stasis or reversal of flow is initiated. The main disadvantages are the increased need of device manipulation in areas close to the target lesion; the relatively large size of the access sheath (9F-8F for femoral approaches, 8F for transcervical approach); and the need to occlude both CCA and ECA, causing flow stasis or reversal, with increased possibility of neurologic intolerance. This condition is generally encountered in about 5% to 9% of patients and can be managed by short intermittent occlusion times.123,201,211
Selection of Embolic Protection Device Type
The selection of filter device versus proximal or flow reversal systems is guided by the patient’s anatomy but often related to operator preference. It is hoped that technologic improvements including lower profile of the device, better efficacy in recruiting debris, and reduced need of manipulation for the proximal occlusion balloon systems can improve the safety of the procedure.*
A number of studies, mostly industry sponsored, have been conducted to show the safety of different EPDs.203,213,214 Randomized (e.g., PROFI trial)214 and mainly not randomized studies, including small samples of patients, on direct comparison between proximal occlusion and distal filter, seem to show lower rates of periprocedural stroke213,214 or reduced rates of microembolic lesions with the proximal occlusion systems,198,201,202,215 but this awaits confirmation in larger population studies.
Despite considerable improvements, no EPD model has been shown to be completely effective, and the problem of cerebral emboli during CAS in different steps of the procedure (cannulation, placement of the EPD, balloon dilatation, and stenting) cannot be considered completely solved. The operator’s skill is crucial, and each interventionalist should be familiar with at least two different classes of EPD so that the technique can be tailored to the individual case.178
Stent Selection
Different self-expandable carotid stent models composed of either stainless steel (a cobalt alloy) or nitinol (a nickel-titanium alloy) can be applied to cover the entire carotid lesion. Flexibility (the ability to conform to vessel tortuosity) and scaffolding (the amount of support given to cover the plaque) are the two main characteristics that drive stent choice. In the case of tortuous anatomy, a rigid stent may create a kink at the distal end of deployment. Insufficient scaffolding may cause protrusion of plaque material through the stent strut and produce distal embolization. In general, nitinol stents have higher radial force, which is useful in counteracting recoiling of severely calcified plaque, but no one type of self-expanding stent has been shown to be superior to another.
Closed Cell versus Open Cell Stents
An important technologic feature of stents for the carotid artery has been the development of two main configurations: closed cell, in which all stent struts are interconnected; and open cell, in which not all struts are interrelated. Open cell stents are more conformable than closed cell stents and preferred in tortuous anatomy (Figs. 101-5 and 101-6).
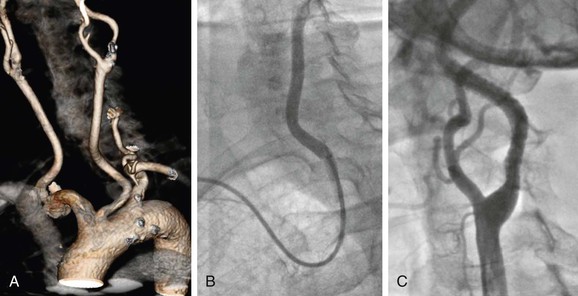
Figure 101-5 Difficult access procedure. A, Bovine aortic arch with left carotid stenosis. B, Right brachial access with 6F guiding catheter for difficult target vessel approach. C, Open cell stent has been placed for better conformability.
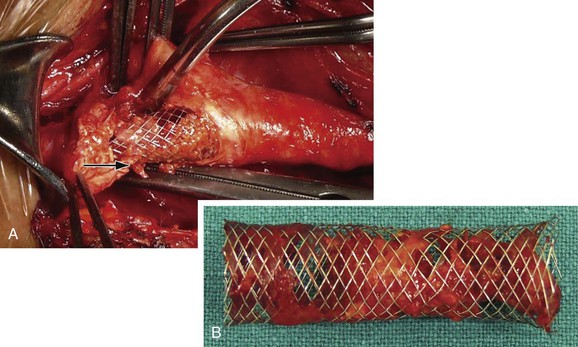
Figure 101-6 Postprocedural conversion to open surgery because of a transient ischemic attack. A, Plaque can be seen prolapsing through the stent strut (arrow). B, Carotid stent after removal with trapped plaque.
Stent selection is often based on the physician’s preference. Although it has been suggested that closed cell stents with a smaller free cell area and a greater percentage of wall coverage offer better scaffolding, reducing embolization risk compared with the open cell structure, there is no consensus on that point.72
In a multicenter experience of 3179 CAS procedures, Bosiers et al72 reported a significantly lower 1.3% adverse event rate in patients with closed cell stents and 3.4% in those with open cell stents (P < .0001), mainly for symptomatic patients. At the opposite, in another multicentric study of 1648 patients, Schillinger et al73 reported a similar 30-day stroke and death rate for closed cell and open cell stents (3.1% vs. 2.4%; P = 0.38). A small randomized study of open versus closed cell stents by Timaran et al216 failed to show increased stroke/death rate or new cerebral lesion by magnetic resonance angiography (53% vs. 47%) or cerebral embolic Doppler signals (264 vs. 339) in procedures performed with the two different types of stents. Histologic analysis of material retrieved within the distal filter suggested that the use of open cell stents was associated with a larger mean particle size but had no impact on procedural outcomes or total number of embolic particles compared with closed cell.217 More data are needed for final conclusions to be drawn about open versus closed cell stent selection or other characteristics that may ultimately determine optimal stent performance.
Complications
Neurologic Complications
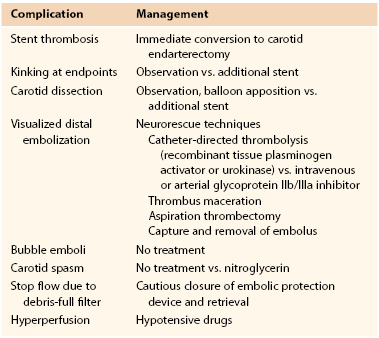
Neurologic complications are the most common and serious risks reported during CAS. Details on types of procedural neurologic complications and options for their management are summarized in Table 101-1.
Spasm and Thrombosis
Some ischemic complications may be related to the use of an EPD. Spasm at the site of filter deployment is common but usually minor and does not require intervention or antispasmodic drug use (e.g., nitrate 200 µg). On occasion, severe spasm, dissection, or debris-full filter may be severe enough to cause flow interruption. Arrest of the flow should be managed by slow closure and withdrawal of the filter and eventually spasmolytic drug use (Fig. 101-7). Flow-limiting dissection would best be managed with further stenting.127
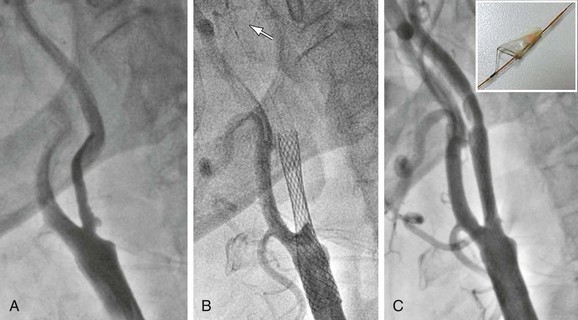
Figure 101-7 Ulcerated severe carotid stenosis in a symptomatic patient. A, Preoperative angiogram. B, Post-stent deployment angiogram with carotid flow interruption due to debris-full embolic protection device filter. The arrow indicates inferior marker of FilterWire. C, Completion angiogram after filter removal showing normal flow to the internal carotid artery (patient asymptomatic throughout the procedure and thereafter). Upper right inset shows FilterWire containing debris.
Acute stent thrombosis is a rare but potentially disastrous complication after CAS. It can be caused by stent misplacement, severe recoiling due to annular calcification, or massive plaque protrusion and requires immediate stent removal with endarterectomy.218
Embolization
Distal brain embolization from atherosclerotic plaque or catheter-generated thrombus is the most common complication reported after CAS. The incidence varies and in some cases may be related to operator experience and patient selection. Advanced age, difficult arch, and long or multiple lesions have been implicated as predictors of embolic stroke.219 When neurologic symptoms occur and intracranial arterial occlusion is visible on the angiogram, a number of neurorescue maneuvers have been suggested for retrieval of distal emboli, including catheter-directed chemical thrombolysis with urokinase or recombinant tissue plasminogen activator, thrombus maceration, aspiration thrombectomy, snare removal, and glycoprotein IIb/IIIa receptor inhibitor (e.g., ReoPro) administration, all with variable results. Presently, prevention remains the best option to avoid disastrous consequences of distal cerebral embolization.23,24,73
In general, consequences of cerebral embolization can vary from silent cerebral infarction to transient ischemic attack and stroke of varying severity. Studies with TCD or diffusion-weighted magnetic resonance imaging (MRI) showed increased incidence of cerebral infarcts in patients undergoing CAS than in CEA.220 In the ICSS trial, about three times more patients in the CAS group than in the CEA group had new ischemic lesions on the diffusion-weighted MRI postprocedural scan (50% vs. 17%; adjusted OR, 5.21; 95% CI, 2.78-9.79; P < .0001).221 Echolucent lipid-rich carotid plaques are more likely to be associated with increased embolic risks,222,223 whereas recurrent carotid stenosis from intimal hyperplasia and fibrous lesions are associated with a decreased occurrence. Postprocedural ischemic brain lesions detected by diffusion-weighted MRI can similarly occur in patients with symptomatic or asymptomatic carotid stenosis and are found in approximately half of asymptomatic patients after CAS.220,224 The proportion of patients with new ipsilateral diffusion-weighted MRI lesions might be significantly lower after protected versus nonprotected CAS, but data are not robust to confirm this hypothesis.202,215,224
Although most studies with TCD and diffusion-weighted MRI showed varying and at times high rates of intraprocedural embolization with different plaque compositions and size, most of these signals were not associated with acute clinical consequences. However, in general, when symptoms of focal neurologic injury develop during CAS and no immediate cause is found, it is best to complete the procedure, to retrieve the catheters, and to reassess the patient.
Neurocognitive Complications
Although most of the identified new cerebral infarcts after CAS are subclinical, there are concerns that these lesions might be associated with subtle long-term neurologic changes and deterioration of cognition.225 The ICSS trial in a subgroup of patients undergoing neuropsychological examination showed that differences between CAS and CEA on cognition at 6 months after revascularization were small and not statistically relevant, despite new ischemic lesions found twice as often after CAS than after CEA (RR, 2.1; 95% CI, 0.0 to 4.4; P = .04).226 Wasser et al227 prospectively analyzed neuropsychological outcomes with a multiple domains test battery and diffusion-weighted MRI lesions after 24 CAS and 31 CEA procedures. New diffusion-weighted lesions were detected in 71% of CAS patients and 4% of CEA patients; however, none of the cognitive domains decreased significantly at 72 hours, and the overall cognitive performance was not significantly different between patients with and without new diffusion-weighted MRI lesions.227 A number of studies and literature reviews seem to support similar negative findings, whereas some studies reported an overall improvement in cognitive function after CAS. Neurocognitive effects after silent cerebral embolization from CAS remain largely uncertain.227–230 The most common finding in the literature is that new brain subclinical lesions after CAS seem not to affect cognitive performance of the patient.
Intracranial Hemorrhage
Intracranial hemorrhage is a rare (<1%) and devastating complication after carotid revascularization that has been particularly detrimental after CAS.231,232 Large NIS data including 215,012 CEA and 13,884 CAS procedures showed that patients undergoing CAS had sixfold increased risk of intracranial hemorrhage (OR, 6.07; 95% CI, 4.7-7.8; P < .0001).231 Reasons for these CAS versus CEA disparities are unclear and may be related to the dual antiplatelet regimen used for CAS or baroreceptor deregulation after stent deployment in the carotid body, leading to cerebral hyperperfusion.233 However, similar higher risks of intracranial hemorrhage for CAS are not supported by findings from RCTs comparing CAS and CEA. In a subgroup analysis, the SPACE trial investigators found ipsilateral intracerebral bleeding after one CAS procedure (0.17%) and five CEA procedures (0.86%), resulting in a nonsignificant lower trend in favor of CAS (OR, 0.19; 95% CI, 0.004 to 1.74).19 The CREST trial found that intracranial hemorrhages were severe and devastating (two thirds fatal) but not more common in the CAS arm (4 in 1262 CAS vs. 3 in 1240 CEA).234 Similarly, in the EVA-3S trial, three intracranial hemorrhages occurred in the CAS (n = 261) and three in the CEA (n = 259) arm.17 In these RCTs, intracranial hemorrhage occurred days after intervention, raising doubts about the benefit of the underlying dual antiplatelet therapy and suggesting the imperative need of blood pressure control days after carotid revascularization.
Patients with symptomatic carotid stenosis, hypertension, and preocclusive internal carotid stenosis, especially in association with contralateral severe carotid stenosis or occlusion, are more likely to develop intracranial hemorrhage after CAS.235,236
Cardiovascular Complications
The risk of periprocedural MI after CAS is generally reported with rates about half those of CEA: 0.4% versus 0.8% in the EVA-3S trial; 0.4% versus 0.6% in the ICSS trial; and 1.1% versus 2.3% in the CREST trial.17,19,28 This may lead to decreased cardiac mortality from CAS in high-risk patients for cardiac diseases. Nevertheless, different definitions of MI (silent with enzymatic or biomarker rise, electrocardiographic changes) were used in studies comparing CAS and CEA. Concerns remain about congestive heart failure that can deteriorate in patients with reduced cardiac output provoked by the osmotic load associated with administration of contrast agents during CAS.
Systemic cardiovascular complications, such as severe bradycardia and hypotension from stretching of the carotid bulb, are frequent in patients undergoing CAS but rarely led to serious cardiac rhythm or ischemic disorders.124,237,238 In contrast, hypertension is a less commonly encountered systemic complication after CAS than after CEA. A substudy of ICSS found a greater decrease in blood pressure at discharge after CAS (N = 587) than after CEA (N = 637): mean difference in systolic blood pressure between groups, 10.3 mm Hg; in diastolic blood pressure, 4.1 mm Hg; P < .0001. However, during follow-up, blood pressure changes were not different between groups. Adjustment for differences in baseline characteristics did not change the results.238
Hypotension
Hypotension can occur in 19% to 51% of CAS patients immediately after stent deployment and after dilatation; it is usually transient and rarely symptomatic.150,151,239–241 However, it may last longer than 24 hours in 3% to 4% of the cases. There is currently no consensus as to which patients require vasopressor agents.127 Bradycardia is also common after CAS, with a variable incidence (2.3%-37%) in cases of prophylactic atropine administration and more frequent occurrence (23%-62%) without the use of atropine before stent deployment.*
In CREST, 53 cases of severe hypotension (systolic blood pressure ≤80 mm Hg or pressors administrated ≥24 hours) and 41 cases of bradycardia occurred in the CAS arm. Rates were lower after CEA.28 The EVA-3S similarly showed 4.2% hypotension or bradycardia requiring prolonged monitoring or treatment in the CAS group versus 0% in the CEA group.17
Increased age, symptomatic lesions, diabetes, and presence of calcification have been found to be significant predictors of bradycardia.150,151,239 Nitinol stents might be associated more with hypotension and bradycardia, but data are limited.242,243
The clinical relevance of such hemodynamic changes on cardiovascular mortality after CAS remains debatable.150,151,153,239–244 Preventive measures include fluid infusion in the preoperative period, intraprocedural atropine administration, and infusion of pressor amines. Prophylactic placement of a temporary pacemaker is indicated only in the presence of preexisting relevant rhythm disorders. Atropine is routinely administered prophylactically in many centers, but its selective use has been debated because of its potential side effects, including tachycardia, which increases cardiac oxygen demand.127
Local and Other Complications
Major access site complications can occur in around 3% to 5% of patients after CAS and include hemorrhage and arterial occlusion. Like any endovascular procedure, CAS can be followed by local bleeding or hematoma, retroperitoneal hemorrhage, infection, pseudoaneurysm or arteriovenous fistula at the puncture site, and vessel thrombosis or dissection with limb ischemia. Detailed description of local complications after endovascular surgery is provided in Chapter 46.
Most commonly, local complications are self-limited, but blood transfusion can be required in 2% to 3% of cases. In the CREST trial, 24 instances of major bleeding requiring transfusion and 12 cases of retroperitoneal major bleeding or femoral hematoma requiring revision were recorded in the 1262 CAS cohort.28
Other complications (e.g., deterioration in renal function) are generic to endovascular procedures. However, CAS requires injection of a small volume of contrast material, which is usually avoided in patients with severe renal dysfunction.
Results of Carotid Artery Stenting
Percutaneous treatment of carotid stenosis has been extensively investigated and outcomes compared with those of CEA by a large number of RCTs, registries, and clinical studies. The results of CAS can be divided into two main periods because of substantially changed approaches and techniques beginning in the last years: period 1, early carotid endovascular period (1979-1999); and period 2, modern carotid endovascular period (after 2000).
The transition from the early to the modern period was slow because of substantial reluctance to accept evolving techniques with uncertain outcomes. Early studies performed in period 1 were affected by poor technology and operator skills; thereby, patients’ outcomes with endovascular carotid procedures were unpredictable. The results achieved in studies and in a number of RCTs10–14 performed in period 1 are therefore unreliable or no longer relevant to the current treatment method. Most of the studies in this period were applied to small numbers of patients defined at “high risk” for surgery with nonstandardized criteria and showed prohibitive complication rates. It was only after 2000, with major technology advancements, that CAS experienced large dissemination, allowing the shift from period 1 to period 2.
Four main innovations contributed to the substantial shift of CAS technique over time: routine use of stenting, routine use of EPDs, introduction of dedicated materials for carotid endovascular procedures (long introducers and guiding catheters, low-profile guide wires with monorail technique), and implementation of new antiplatelet drugs.
Randomized Clinical Trials
A number of RCTs have been created in period 2 in an effort to establish the noninferiority or comparability of CAS to CEA and thereby expanded its approved use. Five large RCTs have been completed (Table 101-2).15–22,28–37 Three were focused only on patients with symptomatic carotid stenosis. A number of RCTs with asymptomatic patients are ongoing, and results for CAS in these populations remain largely uncertain.38–41 Results are eagerly awaited (see Table 101-2).
Table 101-2
Randomized Controlled Trials of Carotid Artery Stenting Since 1999
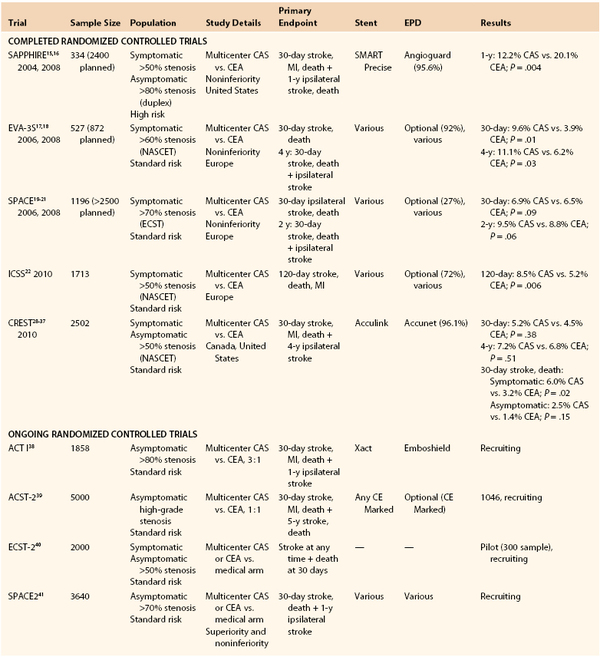
CAS, Carotid artery stenting; CEA, carotid endarterectomy; EPD, embolic protection device.
Sapphire
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE, sponsored by Cordis, Johnson & Johnson Company, Warren, NJ) was designed to test the noninferiority of CAS versus CEA.15,16 The primary endpoint of the trial was the cumulative incidence of death, stroke, or MI within 30 days after the procedure or death or ipsilateral stroke between 31 days and 1 year. The study enrolled patients with 50% or greater symptomatic carotid stenosis or 80% or greater asymptomatic stenosis with conditions that potentially increased the risk of surgery. An ancillary registry included patients ineligible for randomization. All patients undergoing CAS were treated with a self-expandable nitinol stent (Smart or Precise, Cordis) with EPD (Angioguard or Angioguard XP, Cordis). The randomized trial was ended earlier than planned because of slow enrollment; 334 patients were randomly assigned to CEA (n = 167) or CAS (n = 167), and an additional 406 patients had been included in an ancillary registry of the study. The 1-year cumulative primary endpoint rate was lower in the CAS than in the CEA group (12.2% vs. 20.1%; P = .004). The difference was more evident in the asymptomatic (9.9% in CAS vs. 21.5% in CEA) than in the symptomatic group of patients (16.8% in CAS vs. 16.5% in CEA) and became less evident over time (at 3 years, 24.6% for CAS vs. 26.9% for CEA; P = .71).16
Critique.
A major criticism for this trial relates to the concern that 67% of the enrolled patients had asymptomatic carotid stenosis and were at high surgical risk, suggesting that they should have been managed medically. Poor outcome in patients treated by CEA versus CAS was mainly related to cardiac morbidity (MI rates of 2.5% in CAS vs. 8.1% in CEA; P = .03); MI was generally not an endpoint in most published CEA trials. Differences in medical therapy (CAS patients received clopidogrel, whereas CEA patients did not) may have had a role in decreasing the incidence of MI in the CAS group. Furthermore, no subsequent study or RCT has recorded cardiac events for CEA approaching that recorded in SAPPHIRE. However, the trial included a population with high cardiac morbidity that compares worse with more recent studies of CEA in average-risk patients.
Three other trials were subsequently developed to investigate the safety and comparability of CAS versus CEA in average-risk patients with symptomatic carotid stenosis.17–22
EVA-3S
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial is a multicenter noninferiority European RCT that compared CAS with CEA in 527 patients with greater than 60% symptomatic carotid stenosis.17,18 Various models of carotid stents and EPDs were used, but EPD use was not routine in the first phase of the trial. The primary endpoint was any stroke or death within 30 days after the procedure.17 The trial was stopped prematurely because of a significant 2.5-fold higher risk for 30-day stroke and death in the CAS group (9.6% vs. 3.9% in CAS vs. CEA). However, it was noted that in the CAS group, the 30-day incidence of stroke or death was lower among patients who received cerebral protection (7.9%) than among those treated without (25%; P = .03). At 4 years, the combined rate of 30-day stroke or death was higher with CAS than with CEA (11.1% vs. 6.2%; HR, 1.97; 95% CI, 1.06-3.67; P = .03).18 The 4-year difference was largely accounted for by the higher periprocedural risk of CAS. The conclusion of this trial was that the safety of carotid stenting needed to be improved before it could be used as an alternative to CEA in patients with symptomatic carotid stenosis.
SPACE
The Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial randomized 1196 European patients with symptomatic carotid stenosis (≥70% according to European Carotid Surgery Trial criteria) to CAS (n = 607) or CEA (n = 589) to test the noninferiority of CAS.19–21 The primary endpoint was ipsilateral stroke or death between randomization and 30 days after treatment. Different models of carotid stents and EPDs were used, but an EPD was used in only 27% of the procedures. The trial was stopped before reaching the planned sample for reasons of futility and costs.19,20 The rate of death or ipsilateral stroke was 6.9% with CAS and 6.5% with CEA (90% CI, −1.89 to 2.91; P = .09), but the difference in upper CI limits was greater than the predefined threshold to establish noninferiority of CAS versus CEA. No significant differences were detected in procedures with and without the use of an EPD. At 2 years, the cumulative rate of any periprocedural stroke or death plus ipsilateral strokes did not differ between CAS and CEA (9.5% vs. 8.8%; HR, 1.10; 95% CI, 0.75-1.61; P = .6).21 The authors concluded that their trial failed to prove noninferiority of CAS compared with CEA for the higher periprocedural complication rate. Consequently, the widespread use of CAS for treatment of carotid artery stenosis had yet to be considered justified.
Critique.
Criticism of European trials, specifically the EVA3-S and the SPACE trials, focused on the technical inexperience of most interventionalists allowed to join the trials, which might have greatly contributed to the high periprocedural stroke/death rates in the CAS arm. In the EVA-3S trial, the participating centers were required to have performed 12 CAS procedures or 35 supra-aortic stenting procedures, with at least 5 in the carotid artery, under the supervision of a tutor.17 In the SPACE trial, the minimum requirement to allow participation for CAS was generically defined as 25 consecutive successful angioplasty or stent procedures.19 There is consensus today that requirements for training in CAS are higher than those for endovascular procedures to be safely performed in other anatomic regions.23–27
ICSS
The International Carotid Stenting Study is a multicenter international trial that enrolled 1713 patients with symptomatic carotid stenosis of greater than 50% (North American Symptomatic Carotid Endarterectomy Trial criteria).22 The trial established a minimum of 50 stenting procedures with at least 10 in the carotid artery (or with proctoring) as credentialing requirements for operators to join the study. Use of an EPD, with different device models, was not mandatory (used in 72%). The primary outcome measure, the 120-day rate of stroke, death, or MI, was double in the CAS group (8.5% vs. 5.2%; HR, 1.69; 95% CI, 0.16-2.45; P = .006). MI rates were comparable. The majority of adverse events recorded at 120 days occurred during the initial 30-day postprocedural period; cumulative incidence of stroke/death and MI at 30 days was 7.4% versus 4.0% (P = .003) in CAS and CEA, respectively. Thus, while waiting for completion of long-term data, the authors concluded that CEA should remain the treatment of choice for patients with symptomatic carotid stenosis suitable for surgery.22
CREST
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) is a U.S. trial cosponsored by the National Institutes of Health and an industry partner.28–37 To date, it is the most recent and largest RCT designed to compare the efficacy of CAS and CEA in standard-risk patients with statistical power to identify significant differences between the two treatments.28 The trial included 2502 patients with symptomatic or asymptomatic carotid stenosis of 70% or greater (based on ultrasound criteria) or 50% or greater (based on angiographic North American Symptomatic Carotid Endarterectomy Trial criteria). CAS was performed with the RX Acculink stent (Carotid Stent System, Abbott Vascular, Abbott Park, Ill), and a distal EPD (RX Accunet Embolic Protection System, Abbott Vascular) was used in 96.1%. The primary endpoint was the composite of stroke, death, or MI during the periprocedural period or any ipsilateral stroke within 4 years after randomization. The 477 surgeons and 224 interventionalists who were allowed to perform procedures within the trial each met a set of standards for training and experience. In CREST, there was no significant difference in the estimated 4-year rate of the primary endpoint between the CAS (n = 1262) and the CEA (n = 1240) groups (7.2% vs. 6.8%, respectively; HR, 1.11; P = .51; 95% CI, 0.81-1.51). There was also no differential treatment effect at 4 years according to symptomatic status (8.0% vs. 6.4% in symptomatic patients and 4.5% vs. 2.7% in asymptomatic patients after CAS and CEA, respectively). Nevertheless, there were important differences detected among treatment groups at 30 days; risk of periprocedural stroke/death was significantly higher in CAS than in CEA (4.4% vs. 2.3%; P = .005), and that of MI was significantly higher in CEA (1.1% vs. 2.3%; P = .003). Periprocedural risk of stroke/death was higher after CAS for symptomatic patients (6.0% vs. 3.2%; P = .02), whereas it did not achieve statistical significance in the asymptomatic group (2.5% vs. 1.4%; P = .15). Thus, CREST confirmed higher periprocedural stroke hazards from CAS in symptomatic patients but raised concerns about the cardiac risks after CEA.
Although it has been argued that MI in the setting of carotid revascularization is not as important as stroke in terms of overall health, there are also data to support that MI as well as cranial nerve lesions may indeed be relevant complications in carotid procedures. A subanalysis of CREST showed that perioperative MI complications were independently associated with a nearly threefold increased future mortality. Mortality at 4 years was significantly higher for both patients who developed MI (HR, 3.40; 95% CI, 1.67-6.92) and those with cardiac biomarker increase only (HR, 3.57; 95% CI, 1.46 to 8.68) after the carotid procedure. The increased risk remained unchanged after adjustment for baseline risk factors.30 However, perioperative strokes have an important short- and long-term impact on carotid revascularization. In CREST, quality of life analyses among survivors at 1 year indicated that periprocedural stroke had a greater adverse effect on health status domains (physical and mental) than did MI.28 Furthermore, CREST supported that stroke complications implied a poorer long-term survival, with almost three times more risk of death at 4 years; the estimated 4-year mortality after periprocedural stroke (including minor) was 21.1% versus 11.6% for patients without stroke (HR, 2.78; 95% CI, 1.63-4.76).37 The CREST authors suggested that the relative increase in long-term mortality risk in patients with perioperative stroke was similar in magnitude to that of patients who had a perioperative MI.37
Critique.
Criticism of CREST includes the diagnosis of MI based on elevated enzymes and usually not used as an endpoint to assess the safety and efficacy of carotid revascularization. Another criticism was due to the enrollment of both symptomatic and asymptomatic patients, leaving a decreased statistical power to detect differences between treatments (CAS and CEA) in each group.
Meta-Analyses
In the past few years, several meta-analyses combining results from RCTs were published with the aim to increase the strength of the evidence on CAS.42–49 Nevertheless, results are biased by the frequent inclusion of RCTs performed in period 1 together with those in period 2 and the large heterogeneity among single RCTs. These meta-analyses confirmed that CAS is associated with increased periprocedural stroke risks in symptomatic patients (Table 101-3).
Table 101-3
Meta-Analyses of Trials Comparing Carotid Artery Stenting and Carotid Endarterectomy
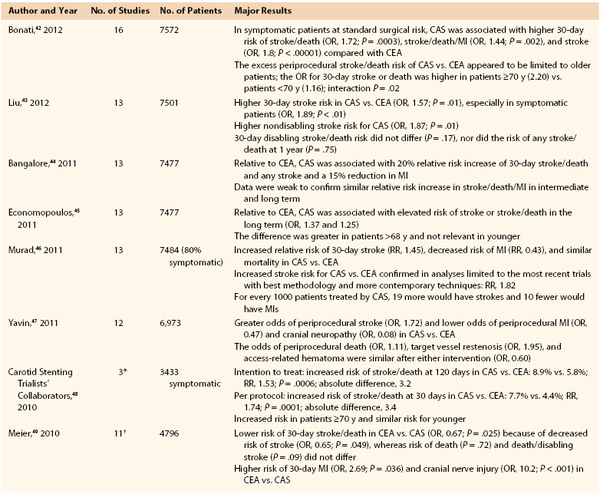
* Three European symptomatic trials: EVA-3S, SPACE, ICSS.
† CREST not included.
CAS, Carotid artery stenting; CEA, carotid endarterectomy; MI, myocardial infarction.
The Cochrane group provided one of the most comprehensive and updated meta-analyses that included 16 trials (7572 patients).42 In patients with symptomatic carotid stenosis at standard surgical risk, CAS was associated with higher risk than CEA of 30-day death or any stroke (OR, 1.72; 95% CI, 1.29-2.31; P = .0003) and any stroke alone (OR, 1.81; 95% CI, 1.40-2.34; P < .00001). The rate of death or disabling stroke did not differ significantly between treatments (OR, 1.28; 95% CI, 0.93-1.77; P = .13). CAS was associated with lower risks of MI (OR, 0.44; 95% CI, 0.23-0.87; P = .02), cranial nerve palsy (OR, 0.08; 95% CI, 0.05-0.14; P < .00001), and access site hematomas (OR, 0.37; 95% CI, 0.18-0.77; P = .008). From these pooled data, it was concluded that CAS was associated with an increased risk of periprocedural stroke or death compared with CEA. However, this excess risk appeared to be limited to older symptomatic patients.
As of today, all RCTs have failed to provide consistent data to support noninferiority of CAS compared with CEA. Nevertheless, study designs and sample size are vastly heterogeneous, and the overall evidence of recommendations is of limited strength.50
Registries
A large number of independent and industry-sponsored registries51–67 and multicenter data sets29,68–82 were established across North America and Europe to record the outcomes of CAS in period 2 (Tables 101-4 and 101-5). Several industry-sponsored studies were conducted for the purpose of device approval by the U.S. Food and Drug Administration (FDA) in patients deemed at high risk for CEA (see Table 101-4). Stroke rates at 30 days varied from 1.8% to 6.9%, and combined stroke, death, and MI rates varied from 2.7% to 7.5%.51–61 Most data are not published in peer-reviewed journals (see also http://www.fda.gov/cdrh/mda/docs/p040038.html). Other CAS registries, published after FDA device approval,62–67 were larger than the previous ones, enrolling more than 1000 patients each (see Table 101-4).
Table 101-4
Registry and Industry-Sponsored Studies of High-Risk Patients in Period 2
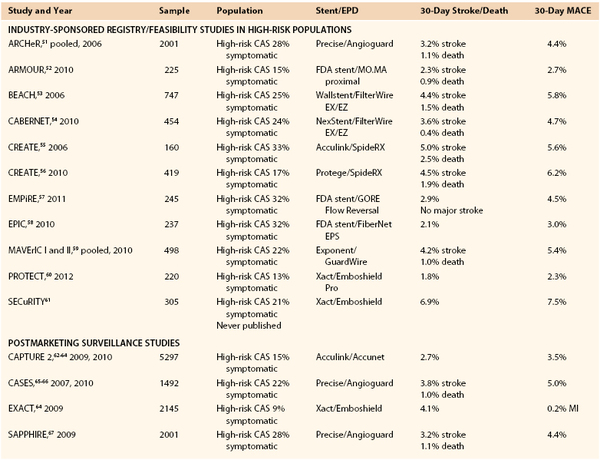
CAS, Carotid artery stenting; EPD, embolic protection device; MACE, major adverse clinical events (stroke, death, myocardial infarction); MI, myocardial infarction.
Table 101-5
Multicenter Registries and Studies of Carotid Artery Stenting in Period 2
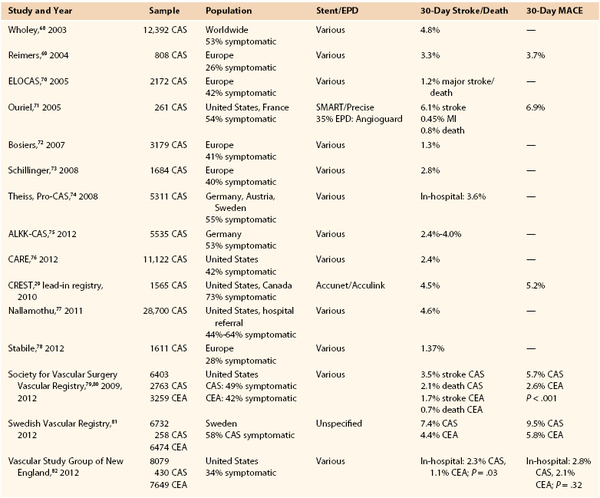
CAS, Carotid artery stenting; CEA, carotid endarterectomy; EPD, embolic protection device; MACE, major adverse clinical events (stroke, death, or myocardial infarction); MI, myocardial infarction.
EXACT (Emboshield and Xact post Approval Carotid stent Trial)64 enrolled 2145 high-risk patients (213 symptomatic), with overall 30-day major adverse clinical events (death/stroke or MI) of 4.1%. Stroke/death rates were 7% in symptomatic and 3.7% in asymptomatic patients.64 CASES (Carotid Artery Stenting with Emboli Protection Surveillance–Post-Marketing Study with the use of the Precise stent and Angioguard cerebral protection devices) included 1493 high-risk patients and showed a 30-day stroke rate of 3.8% and major adverse clinical events rate of 5.0% (6.2% in symptomatic and 4.7% in asymptomatic patients).65,66
CAPTURE (Carotid Acculink/Accunet Post-Approval Trial to Uncover Unanticipated or Rare Events) is one of the largest registries that represented the postmarket analysis of the devices used in the ARCHER registry (RX Acculink and RX Accunet) applied to high-risk patients. The second phase of CAPTURE (CAPTURE 2) was a prospective multicenter study to assess CAS outcomes in the general practice settings for patients at high risk for surgery. Data from the 5297 (721 symptomatic) patients showed a 30-day stroke rate of 2.7% (4.6% in symptomatic and 2.3% in asymptomatic patients).62,63
Other data from large non-FDA registries and multicenter data sets are shown in Table 101-5.29,68–82 In the European Registry of Carotid Artery Stenting that enrolled 1611 patients receiving CAS with EPD in eight centers in Europe, the 30-day stroke/death, death, and stroke rates were 1.36%, 0.24%, and 1.12%, respectively.78
Critique.
Registries have many limitations, such as various definitions or incomplete follow-up of enrolled patients. Furthermore, carotid registries were mainly focused on patients defined at high risk for surgery by variable inclusion criteria based on mixed anatomic and systemic factors that have questionable negative impact on the outcome. Many patients were commonly labeled at high risk for carotid surgery merely because of the presence of recurrent carotid stenosis or age older than 80 years. There are no consistent data for use of these factors to define high risk for CEA,245 whereas old age has indeed been shown to be a risk factor for stroke in CAS more than in CEA.31 Despite several drawbacks, registries may improve the understanding of how the technique may be incorporated and safely practiced at the community level.50
Case Series and Population-Based Studies
In a review of 20 CAS series including more than 24,000 procedures, most performed after 2002, Goodney et al83 found an average 30-day stroke rate of 3% and combined major adverse clinical events rate of 4%. Burton et al84 performed a review of CAS studies, all using EPDs, published after 2002. Data from 3091 procedures showed estimated average 30-day stroke and death rates of 2.4% ± 0.3% and minor stroke rates of 1.1% ± 0.2%.
Several large case series and population-based studies published results of CAS after 2002.81,85–108 However, these outcomes from nonrandomized series should be interpreted with caution because of the high variability among different studies and wide complication ranges.
Controversy currently exists about the results in the so-called real-world practice with CAS compared with CEA. There are data to support that results achieved today with CEA across a wide spectrum of surgeons and hospitals may equate with those seen in RCTs (e.g., CREST). The National Surgical Quality Improvement Program database showed that outcomes of 13,622 CEA procedures performed in Veterans Affairs and private hospitals compared favorably with other contemporary studies with CEA procedures performed in more experienced centers.245 Fokkema et al246 found an overall 2.2% 30-day stroke/death rate among 35,916 CEA procedures within the National Surgical Quality Improvement Program. On the contrary, the outcomes from CAS are more heterogeneous, with large discrepancies among registries and administrative databases. Low complication rates in CAS patients are supported mainly by registries (and case series) performed by experienced operators in both symptomatic and asymptomatic patients. Worldwide results are worse when CAS is applied in hospitals without high-level expertise because of different patient selection and technical performance, as shown by administrative data. The NIS database derived from a stratified sample of U.S. community hospitals and including 81,638 patients treated by CAS showed rates of in-hospital mortality and major adverse clinical events significantly higher among patients treated outside clinical U.S. studies, suggestive of less experienced operators (mortality: 1.12% vs. 0.53%, P = .0005; major adverse clinical events: 4.91% vs. 3.85%, P = .023).109 A retrospective analysis of 7649 CEA and 430 CAS procedures from the Vascular Study Group of New England involving 14 academic and 14 community centers showed that the rate of periprocedural stroke/death was 2.3% with CAS versus 1.1% with CEA (P = .028). The periprocedural risk of CAS particularly increased in symptomatic patients, with stroke/death rates of 5.1% versus 1.6% (P = .001) and major adverse clinical events rates of 5.8% versus 2.7% (P = .022) for CAS and CEA, respectively.82
Critique.
All the studies based on administrative data should be interpreted with caution because most rely on early outcomes recorded during in-hospital stay. True rates of stroke and death after a carotid procedure may be superior when they are recorded at 30 days. In the study of Fokkema, in more than 35,916 CEA procedures performed within the National Surgical Quality Improvement Program, 38% of combined adverse events (stroke, death, or cardiac) took place after hospital discharge.246
Role of Interventionalist Specialty
CAS outcomes may be comparable to those of CEA regardless of procedures performed by different specialists (cardiologists, interventionalists, surgeons, neurosurgeons), provided similar high-expertise level is achieved. The overall center experience may also have an important effect on new trainees practice.247 An observational analysis of Medicare beneficiaries undergoing CAS in 306 hospital referral regions identified 28,700 CAS procedures performed in 2005-2007 in the United States by different specialty operators, mainly cardiologists (52% of procedures). Whereas significant differences were noted in the characteristics of patients treated by cardiologists and other specialists, 30-day standardized risk of mortality or stroke was comparably low (4.6%, 4.7%, and 4.8%) in centers with a prevalence of cardiologists, surgeons, and radiologists, respectively.77 Data by CREST showed periprocedural stroke/death/MI of 4.8% with CAS and 3.8% with CEA (HR, 1.26; 95% CI, 0.61-2.60; P = .54) in CAS procedures performed exclusively by trained vascular surgeons, suggesting slightly lower stroke/death risks for these operators.248
Conclusion: Literature Results of Carotid Artery Stenting
On the basis of published evidence, two important observations should be considered:
Long-Term Results of Carotid Artery Stenting
A main concern of CAS, as for all endovascular approaches, is the durability of the procedure, which is defined as effectiveness in preventing stroke and restenosis.
Stroke Prevention
Although there is a tendency in the literature to support a limited risk of stroke after the periprocedural phase, the overall clinical durability of CAS in preventing stroke in the long term is not yet well established. CREST and EVA-3S reported 4-year higher rates of stroke/death after CAS than after CEA, 6.4% versus 4.7% (HR, 1.5; P = .03) for CREST28 and 11.1% versus 6.2% (HR, 1.97; P = .03) for EVA-3S,18 but the higher rates were mainly accounted for by the higher periprocedural risks of CAS. Considering only ipsilateral stroke events occurring after 30 days, the risks were similar at 4 years in CREST (2.0% CAS vs. 2.4% CEA) and in EVA-3S (4.5% CAS vs. 4.9% CEA).18,28 Similar information is provided at 2 years by SPACE (2.2% after CAS vs. 1.9% after CEA).21
However, currently, the clinical durability of CAS beyond 5 years cannot be determined from RCTs, but it is supported by case series and registries reporting low risk of stroke death in the long term.71,102,103,107 Gröschel et al249 performed a systematic review of midterm results in 3814 patients from different studies and found an extremely low ipsilateral stroke rate. However, according to the same authors, the disparate case mix, accuracy of follow-up, and potential selection bias made the results questionable.
Restenosis
Another limiting factor in the wider application of CAS is the perceived problem of carotid restenosis. An analysis of long-term data at 10 years or more from the randomized trial CAVATAS showed an alarming restenosis rate of 30.7% in the endovascular arm and 10.5% in the endarterectomy arm. However, in this trial, the majority of the procedures (78%) were performed only with balloon angioplasty without stenting. Patients in the endovascular arm who were treated with a stent (n = 50) had a significantly lower risk for development of restenosis compared with those treated with balloon angioplasty alone (HR, 0.43; 95% CI, 0.19-0.97; P = .04).250
In more recent studies performed in period 2, the incidence of restenosis after CAS appears to be low. A secondary analysis of CREST found that carotid restenosis or occlusions occurred in similar rates after CAS (6.0%) and CEA (6.3%) at 2 years after randomization (HR, 0.90; 95% CI, 0.63-1.29; P = .58) and were affected by diabetes, female sex, and dyslipidemia more than by the type of procedure.234 Nevertheless, 2-year data from the SPACE trial showed still higher carotid restenosis after CAS than after CEA, 10.7% versus 4.6% (P = .0009).21
From most clinical series, restenosis rates after CAS appear comparable to those after CEA. Unfortunately, not all the authors used life-table estimates and similar stenosis severity cutoff to give reliable information on the issue. In the review article by Gröschel et al249 that included 34 studies reporting on restenosis rates after CAS during a median of 13 months (6-31 months), the cumulative restenosis rate was 6% at 1 year and 7.5% at 2 years for the studies using a threshold of 50% and 4% for the studies using a threshold of greater than 70%.
The risk for in-stent restenosis may be increased in patients treated for recurrent stenosis after CEA, contralateral occlusion, diabetes, hyperlipidemia, or previous neck irradiation or cancer.237,250–254 There are also data suggesting that patients in whom restenosis develops after CEA are also more prone to the development of restenosis after CAS.252–255
Of relevance, the incidence of restenosis after carotid treatment depends on the method used for detection, and the rates of in-stent restenosis may be overestimated by conventional ultrasound evaluation. There is increasing evidence that the ultrasound criteria are different from those used in native, nonstented arteries.256–262 Stent placement can alter the biomechanical properties of the carotid territory by decreasing vessel compliance. The enhanced stiffness of the stent–arterial wall complex renders the flow-pressure relationship of the carotid artery closer to that observed in a rigid tube, and as a consequence, the degree of stenosis may be overvalued. Although standardized flow velocity patterns are not well established for carotid stents, new modified criteria have been proposed. Peak systolic velocity as high as 300 cm/s or greater and an ICA/CCA peak systolic velocity ratio of 4.3 have been defined as thresholds for detection of greater than 70% restenosis.256–262 Further studies should clarify this issue.
After CAS, restenosis is frequently asymptomatic, but larger experiences to assess its clinical relevance are needed. The decision of retreatment should involve rapid progression or the onset of symptoms as in restenosis after CEA. The majority of in-stent restenoses are manageable by repeated balloon dilatation or occasionally cutting balloons. In some cases, additional stenting may need to be performed.263
Stent Fracture
Stent fracture has been reported after CAS. However, despite the concerns of a carotid ruptured stent, these complications appear of limited clinical relevance.264–267 A systematic review from Sfyroeras et al265 identified 55 published stent fractures occurring from 0 to 37 months after CAS. Only 6 patients developed symptomatic fractures, whereas 55% of fractures were associated with carotid restenosis. The incidence of stent fracture seems to be more commonly reported after nitinol stent placement and in the presence of calcified plaque.264–267 Treatment currently applied for carotid stent fracture is subjective, varying for de novo stent, CEA, bypass graft, and follow-up alone. Larger and longer studies would clarify the actual incidence, clinical relevance, and optimal treatment.
On the basis of these data, accurate and prolonged surveillance after CAS is advisable. Duplex ultrasonography should be performed during the same admission and baseline velocities recorded to serve as a basis for comparison with future assessments. The examination should be repeated every 6 months for the first 2 years, when the incidence of restenosis secondary to intimal hyperplasia is higher, and then annually thereafter. Investigation with imaging (computed tomographic angiography) should be integrated, especially in the case of unclear duplex findings.
Quality of Life
There are data to support that in the long term, quality of life perception after CAS is not inferior to that after CEA. CREST specifically analyzed health-related quality of life in randomized patients and found that CAS was associated with better health-related quality of life during the early recovery period compared with CEA, particularly with regard to physical limitations and pain domains. These differences were not evident after 1 year. Event-specific analyses showed that stroke had a great and more sustained impact on deterioration of health-related quality of life than MI did.32
Training Issues
Results of CAS are strictly related to training (including technical skills), appropriate patient selection, and availability of adequate equipment (Fig. 101-8).
A number of studies have demonstrated that increased operator experience with CAS decreases the incidence of stroke.25,26,247,268,269 CREST, differently from the other European RCTs, required a significant lead-in phase for interventionalists and documentation of low procedural morbidity.28,29 In the multicenter Pro-CAS registry of 5341 CAS procedures, the risk of stroke decreased with center experience from 5.9% for the first 50 to 3.0% for the last procedures (P < .0010) and with the total center volume from 4.6% in low-volume versus 2.9% in high-volume (>50 CAS/year) hospitals (P < .0014).74 CMS patients treated by very-low-volume (<6/year) operators had a higher risk of 30-day mortality compared with patients treated by high-volume (>24/year) operators (adjusted OR, 1.9; 95% CI, 1.4-2.7; P < .001).268
Unfortunately, there are no universally accepted standards in formal training requirements for CAS, and there is a high variability in the number of cases judged to be adequate before systematic use of this procedure is undertaken.23,25–27,270 The Society for Cardiovascular Angiography and Interventions, the Society for Vascular Medicine and Biology, and the Society for Vascular Surgery multidisciplinary consensus document of 2005 supported a minimum of 30 diagnostic cervicocerebral angiograms and 25 carotid interventions before performance of CAS independently.271–273 The multidisciplinary document of the American Academy of Neurology, American Association of Neurological Surgeons, American Society of Interventional and Therapeutic Neuroradiology, American Society of Neuroradiology, Congress of Neurological Surgeons, and Society of Interventional Radiology defined 100 supervised cerebral angiograms and either 10 supervised CAS procedures or 25 noncarotid stent placements, 4 supervised carotid stent placements, and 16 hours of continued medical education with acceptable results as a minimum requirement.274,275 An interdisciplinary consensus document in Europe, ICCS-SPREAD (Italian Consensus Carotid Stenting/Stroke PRevention and Educational Awareness Diffusion), set a minimum standard of at least 150 supra-aortic vessel angiography procedures (>100 as the primary operator), at least 75 CAS procedures (>50 as the primary operator), and an additional 50 CAS procedures per year for acquisition and maintenance of competence with CAS.23
The use of virtual training simulators for reproducing the procedure and interacting with the operator may represent first-line training but is not intended as a substitute for live experience.276 As a second step, a number of tutoring or training programs or device-specific programs offered by industry are widely available and recommended to improve skills and to shorten the training phase while preserving patient safety. Each institution must have a clearly designed program for carotid stent procedures and for assessing outcomes with independent postprocedural neurologic assessment.
Cost and Reimbursement
Costs related to CAS, especially because of the more advanced and expensive technology required and higher stroke rates, seem to be higher than those of CEA.27,277–279 However, there is no overall agreement. Data from the NIS from 2005 to 2007 showed that in the United States, resource utilization was significantly higher for CAS than for CEA despite some improved outcomes with CAS in the last years; in 2007, median total hospital charges were estimated at $21,169 for CEA and $33,485 for CAS.280
A retrospective analysis based on costs (including supply, labor, facility, and miscellaneous categories) of the index hospitalization in a single tertiary center found mean hospital costs of $9426 for CAS and $6734 for CEA (P < .0001). However, there were significantly fewer costs for patients undergoing CAS enrolled in a trial or registry ($7779) compared with those treated outside ($11,279; P = .0001).281
Opposite findings by others supported similar costs of the two carotid procedures. Cost-effectiveness analysis from CREST indeed showed that despite initial procedural costs of $1025/patient higher with CAS than with CEA, postprocedure and physician costs were lower, such that the total costs for the index hospitalization were similar: $15,055 versus $14,818 (mean difference of $239/patient). Projected 10-year outcomes from this controlled randomized trial demonstrated only minor differences in overall healthcare costs between the two strategies.36
The poorer outcomes and potentially higher costs of CAS compared with CEA raised concerns about reimbursement policies and national coverage determination actions. Under the current U.S. national coverage determination policy, there is CMS payment for CAS only in patients who are at high risk for CEA with use of FDA-approved CAS systems in CMS-approved centers or in patients enrolled in post-approval registries or category B Investigational Device Exemption trials. National coverage determination policy does not cover CAS in any standard-risk patients, whereas there are no restrictions for CEA reimbursement.
Improvements in technology and patient selection in the future would also clarify the reimbursement policies and costs related to CAS.
Acknowledgment
The authors thank Dr. Ciro Ferrer for assistance with imaging and writing.
Selected Key References
Bonati LH, Lyrer P, Ederle J, Featherstone R, Brown MM. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2012;(9).
Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Neuro Interventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. 2011;57:1002–1044.
The clinical expert consensus documents on carotid stenting of the American College of Cardiology, updated 2011..
Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23.
International Carotid Stenting Study investigators, Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, Macdonald S, Lyrer PA, Hendriks JM, McCollum C, Nederkoorn PJ, Brown MM. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997.
Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lièvre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touzé E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X, EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671.
Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK, Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–e31.
SPACE Collaborative Group, Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247.
Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Mathias K. A new catheter system for percutaneous transluminal angioplasty (PTA) of carotid artery stenoses. Fortschr Med. 1977;95:1007–1011.
2. Mathias K, et al. Percutaneous catheter dilatation of carotid stenoses. Rofo. 1980;133:258–261.
3. Kerber CW, et al. Catheter dilatation of proximal carotid stenosis during distal bifurcation endarterectomy. AJNR Am J Neuroradiol. 1980;1:348–349.
4. Diethrich EB, et al. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3:42–62.
5. Marks MP, et al. Stent placement for arterial and venous cerebrovascular disease: preliminary experience. Radiology. 1994;191:441–446.
6. Roubin GS, et al. Carotid stent-supported angioplasty: a neurovascular intervention to prevent stroke. Am J Cardiol. 1996;78:8–12.
7. Roubin GS, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation. 2001;103:532–537.
8. Theron J, et al. New triple coaxial catheter system for carotid angioplasty with cerebral protection. AJNR Am J Neuroradiol. 1990;11:869–874.
9. Paraskevas KI, et al. Comparison of the five 2011 guidelines for the treatment of carotid stenosis. J Vasc Surg. 2012;55:1504–1508.
10. Naylor AR, et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1988;28:326–334.
11. Alberts MJ. Results of a multicentre prospective randomized trial of carotid artery stenting vs carotid endarterectomy. Stroke. 2001;32:325.
12. CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–1737.
13. Brooks WH, et al. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589–1595.
14. Brooks WH, et al. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery. 2004;54:318–324.
15. Yadav JS, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. [Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators] N Engl J Med. 2004;351:1493–1501.
16. Gurm HS, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. [SAPPHIRE Investigators] N Engl J Med. 2008;358:1572–1579.
17. Mas JL, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. [EVA-3S Investigators] N Engl J Med. 2006;355:1660–1671.
18. Mas JL, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. [EVA-3S Investigators] Lancet Neurol. 2008;7:885–892.
19. SPACE Collaborative Group, Ringleb PA, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247.
20. Stingele R, et al. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol. 2008;7:216–222.
21. Eckstein HH, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902.
22. International Carotid Stenting Study investigators, Ederle J, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997.
23. Cremonesi A, et al. Carotid artery stenting: first consensus document of the ICCS-SPREAD Joint Committee. Stroke. 2006;37:2400–2409.
24. Schneider PA. Management of the complications of carotid interventions. Schneider PA, Bohannon WT, Silva MB Jr. Carotid interventions. Marcel Dekker: New York; 2004:275–283.
25. Verzini F, et al. Appropriateness of learning curve for carotid artery stenting: an analysis of periprocedural complications. J Vasc Surg. 2006;44:1205–1212.
26. Ahmadi R, et al. Carotid artery stenting: effect of learning curve and intermediate-term morphological outcome. J Endovasc Ther. 2001;8:539–546.
27. Paraskevas KI, et al. Cost implications of more widespread carotid artery stenting consistent with the American College of Cardiology/American Heart Association guideline. J Vasc Surg. 2012;55:585–587.
28. Brott TG, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. [CREST Investigators] N Engl J Med. 2010;363:11–23.
29. Hopkins LN, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial: credentialing of interventionalists and final results of lead-in phase. J Stroke Cerebrovasc Dis. 2010;19:153–162.
30. Blackshear JL, et al. Myocardial infarction after carotid stenting and endarterectomy: results from the carotid revascularization endarterectomy versus stenting trial. [CREST Investigators] Circulation. 2011;123:2571–2578.
31. Voeks JH, et al. Age and outcomes after carotid stenting and endarterectomy: the carotid revascularization endarterectomy versus stenting trial. [CREST Investigators] Stroke. 2011;42:3484–3490.
32. Cohen DJ, et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: results from CREST (Carotid Revascularization Endarterectomy versus Stenting Trial). [CREST Investigators] J Am Coll Cardiol. 2011;58:1557–1565.
33. Howard VJ, et al. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). [CREST Investigators] Lancet Neurol. 2011;10:530–537.
34. Silver FL, et al. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). [CREST Investigators] Stroke. 2011;42:675–680.
35. Voeks JH, et al. Age and outcomes after carotid stenting and endarterectomy: the carotid revascularization endarterectomy versus stenting trial. [CREST Investigators] Stroke. 2011;42:3484–3490.
36. Vilain KR, et al. Costs and cost-effectiveness of carotid stenting versus endarterectomy for patients at standard surgical risk: results from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). [CREST Investigators] Stroke. 2012;43:2408–2416.
37. Hill MD, et al. Stroke after Carotid Stenting and Endarterectomy in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Circulation. 2012;126:3054–3056.
38. Carotid stenting vs. surgery of severe carotid artery disease and stroke prevention in asymptomatic patients (ACT I). [Available at] www.clinicaltrials.gov/show/NCT00106938.
39. Rudarakanchana N, et al. Asymptomatic Carotid Surgery Trial-2 (ACST-2): rationale for a randomised clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. Eur J Vasc Endovasc Surg. 2009;38:239–242.
40. European Carotid Surgery Trial 2 (ECST-2). [Available at] www.controlled-trials.com/ISRCTN97744893.
41. Reiff T, et al. Stent-protected angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy: SPACE2—a three-arm randomised-controlled clinical trial. [SPACE2 Study Group] Int J Stroke. 2009;4:294–299.
42. Bonati LH, et al. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2012;(9).
44. Bangalore S, et al. Carotid artery stenting vs carotid endarterectomy: meta-analysis and diversity-adjusted trial sequential analysis of randomized trials. Arch Neurol. 2011;68:172–184.
45. Economopoulos KP, et al. Carotid artery stenting versus carotid endarterectomy: a comprehensive meta-analysis of short-term and long-term outcomes. Stroke. 2011;42:687–692.
46. Murad MH, et al. A systematic review and meta-analysis of randomized trials of carotid endarterectomy vs stenting. J Vasc Surg. 2011;53:792–797.
47. Yavin D, et al. Carotid endarterectomy versus stenting: a meta-analysis of randomized trials. Can J Neurol Sci. 2011;38:230–235.
48. [Carotid Stenting Trialists’ Collaboration] Bonati LH, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–1073.
49. Meier P, et al. Short term and intermediate term comparison of endarterectomy versus stenting for carotid artery stenosis: systematic review and meta-analysis of randomised controlled clinical trials. BMJ. 2010;340:c467.
50. Bowens NM, et al. Carotid artery stenting: clinical trials and registry data. Semin Vasc Surg. 2010;23:148–155.
51. Gray WA, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. [ARCHeR Trial Collaborators] J Vasc Surg. 2006;44:258–268.
52. Ansel GM, et al. Safety and effectiveness of the INVATEC MO.MA proximal cerebral protection device during carotid artery stenting: results from the ARMOUR pivotal trial. [Investigators for the ARMOUR Pivotal Trial] Catheter Cardiovasc Interv. 2010;76:1–8.
53. White CJ, et al. Carotid stenting with distal protection in high surgical risk patients: the BEACH trial 30 day resuls. [BEACH Trial Investigators] Catheter Cardiovasc Interv. 2006;67:503–512.
54. Hopkins LN, et al. Carotid artery revascularisation in high-surgical-risk patients with the NexStent and the FilterWire EX/EZ: 3-year results from the CABERNET trial. Euro Intervention. 2010;5:917–924.
55. Safian RD, et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. [CREATE Pivotal Trial Investigators] J Am Coll Cardiol. 2006;47:2384–2389.
56. Safian RD, et al. Protected carotid stenting in high risk patients: results of the SpideRX arm of the carotid revascularization with ev3 arterial technology evolution trial. [CREATE SpideRX Trial Investigators] J Interv Cardiol. 2010;23:491–498.
57. Clair DG, et al. Neuroprotection during carotid artery stenting using the GORE flow reversal system: 30-day outcomes in the EMPiRE Clinical Study. [EMPiRE Clinical Study Investigators] Catheter Cardiovasc Interv. 2011;77:420–429.
58. Myla S, et al. Carotid artery stenting in high surgical risk patients using the FiberNet embolic protection system: the EPIC trial results. Catheter Cardiovasc Interv. 2010;75:817–822.
59. Higashida RT, et al. Evaluation of the Medtronic exponent self-expanding carotid stent system with the Medtronic guardwire temporary occlusion and aspiration system in the treatment of carotid stenosis: combined from the MAVErIC (Medtronic AVE Self-expanding CaRotid Stent System with distal protection In the treatment of Carotid stenosis) I and MAVErIC II trials. [MAVErIC I and II Investigators] Stroke. 2010;41:102–109.
60. Matsumura JS, et al. Results of carotid artery stenting with distal embolic protection with improved systems: Protected Carotid Artery Stenting in Patients at High Risk for Carotid Endarterectomy (PROTECT) trial. J Vasc Surg. 2012;55:968–976.
61. Xact Carotid Stent System. Summary of safety and effectiveness data. P040038. [Available at] http://www.accessdata.fda.gov/cdrh_docs/pdf4/p040038a.pdf.
62. Matsumura JS, et al. CAPTURE 2 risk-adjusted stroke outcome benchmarks for carotid artery stenting with distal embolic protection. [CAPTURE 2 Investigators and Executive Committee] J Vasc Surg. 2010;52:576–583.
63. Gray WA, et al. The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. [CAPTURE Trial Collaborators] Catheter Cardiovasc Interv. 2007;70:1025–1033.
64. Gray WA, et al. Thirty-day outcomes for carotid artery stenting in 6320 patients from 2 prospective, multicenter, high-surgical-risk registries. [Investigators and the Executive Committees] Circ Cardiovasc Interv. 2009;2:159–166.
65. Katzen BT, et al. Carotid artery stenting with emboli protection surveillance study: thirty-day results of the CASES-PMS study. [CASES-PMS Investigators] Catheter Cardiovasc Interv. 2007;70:316–323.
66. Schreiber TL, et al. Carotid artery stenting with emboli protection surveillance study: outcomes at 1 year. [CASES-PMS Investigators] J Am Coll Cardiol. 2010;56:49–57.
67. Massop D, et al. Stenting and angioplasty with protection in patients at high-risk for endarterectomy: SAPPHIRE Worldwide Registry first 2,001 patients. [SAPPHIRE Worldwide Investigators] Catheter Cardiovasc Interv. 2009;73:129–136.
68. Wholey MH, et al. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003;60:259–266.
69. Reimers B, et al. Routine use of cerebral protection during carotid artery stenting: results of a multicenter registry of 753 patients. Am J Med. 2004;116:217–222.
70. Bosiers M, et al. Does carotid artery stenting work on the long run: 5-year results in high-volume centers (ELOCAS Registry). J Cardiovasc Surg (Torino). 2005;46:241–247.
71. Ouriel K, et al. Feasibility trial of carotid stenting with and without an embolus protection device. J Endovasc Ther. 2005;12:525–537.
72. Bosiers M, et al. Does free cell area influence the outcome in carotid artery stenting? Eur J Vasc Endovasc Surg. 2007;33:135–141.
73. Schillinger M, et al. Does carotid stent cell design matter? Stroke. 2008;39:905–909.
74. Theiss W, et al. Predictors of death and stroke after carotid angioplasty and stenting: a subgroup analysis of the Pro-CAS data. [German Society of Angiology/Vascular Medicine; German Society of Radiology] Stroke. 2008;39:2325–2330.
75. Staubach S, et al. The role of endovascular expertise in carotid artery stenting: results from the ALKK-CAS-Registry in 5,535 patients. Clin Res Cardiol. 2012;101:929–937.
76. Hawkins BM, et al. Pre-procedural risk quantification for carotid stenting using the CAS score: a report from the NCDR CARE Registry. J Am Coll Cardiol. 2012;60:1617–1622.
77. Nallamothu BK, et al. Physician specialty and carotid stenting among elderly Medicare beneficiaries in the United States. Arch Intern Med. 2011;171:1804–1810.
78. Stabile E, et al. European Registry of Carotid Artery Stenting: results from a prospective registry of eight high volume European institutions. Catheter Cardiovasc Interv. 2012;80:329–334.
79. Sidawy AN, et al. Risk-adjusted 30-day outcomes of carotid stenting and endarterectomy: results from the SVS Vascular Registry. [Outcomes Committee for the Society for Vascular Surgery] J Vasc Surg. 2009;49:71–79.
80. Jim J, et al. Society for Vascular Surgery (SVS) Vascular Registry evaluation of comparative effectiveness of carotid revascularization procedures stratified by Medicare age. [SVS Outcomes Committee] J Vasc Surg. 2012;55:1313–1320.
81. Lindström D, et al. Outcome after 7 years of carotid artery stenting and endarterectomy in Sweden—single centre and national results. Eur J Vasc Endovasc Surg. 2012;43:499–503.
82. Nolan BW, et al. Comparison of carotid endarterectomy and stenting in real world practice using a regional quality improvement registry. [Vascular Study Group of New England] J Vasc Surg. 2012;56:990–996.
83. Goodney PP, et al. Current status of carotid artery stenting. J Vasc Surg. 2006;43:406–411.
85. Becquemin JP, et al. Carotid stenting versus carotid surgery: a prospective cohort study. J Endovasc Ther. 2003;10:687–694.
86. Bosiers M, et al. Belgian experience with FilterWire EX in the prevention of embolic events during carotid stenting. J Endovasc Ther. 2003;10:695–701.
87. Cernetti C, et al. Carotid artery stenting with cerebral protection in 100 consecutive patients: immediate and two-year follow-up results. Ital Heart J. 2003;4:695–700.
88. Cremonesi A, et al. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003;34:1936–1941.
89. Hobson RW 2nd, et al. Carotid artery stenting: analysis of data for 105 patients at high risk. J Vasc Surg. 2003;37:1234–1239.
90. Kastrup A, et al. Early outcome of carotid angioplasty and stenting versus carotid endarterectomy in a single academic center. Cerebrovasc Dis. 2003;15:84–89.
91. Lal BK, et al. In-stent recurrent stenosis after carotid artery stenting: life table analysis and clinical relevance. J Vasc Surg. 2003;38:1162–1168.
92. Tan KT, et al. Timing and frequency of complications after carotid artery stenting: what is the optimal period of observation? J Vasc Surg. 2003;38:236–243.
93. Henry M, et al. Carotid angioplasty under cerebral protection with the Percu Surge Guard Wire System. Catheter Cardiovasc Interv. 2004;61:293–305.
94. Sztriha LK, et al. Favorable early outcome of carotid artery stenting without protection devices. Stroke. 2004;35:2862–2866.
95. Sganzerla P, et al. The treatment of carotid artery bifurcation stenoses with systematic stenting: experience of first 100 consecutive cardiological procedures. J Invasive Cardiol. 2004;16:592–595.
96. Bush RL, et al. A comparison of carotid artery stenting with neuroprotection versus carotid endarterectomy under local anesthesia. Am J Surg. 2005;190:696–700.
97. Vos JA, et al. Carotid angioplasty and stent placement: comparison of transcranial Doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patients. Radiology. 2005;234:493–499.
98. Yen MH, et al. Symptomatic patients have similar outcomes compared with asymptomatic patients after carotid artery stenting with emboli protection. Am J Cardiol. 2005;95:297–300.
99. Powell RJ, et al. Comparison of embolization protection device–specific technical difficulties during carotid artery stenting. J Vasc Surg. 2006;44:56–61.
100. Zhou W, et al. Management of in-sent restenosis after carotid artery stenting in high-risk patients. J Vasc Surg. 2006;43:305–312.
101. Eskandari MK, et al. Technical limitations of carotid filter embolic protection devices. Ann Vasc Surg. 2007;21:403–407.
102. Younis GA, et al. Predictors of carotid stent restenosis. Catheter Cardiovasc Interv. 2007;69:673–682.
103. Setacci C, et al. Carotid artery stenting in a single center: are six years of experience enough to achieve the standard of care? Eur J Vasc Endovasc Surg. 2007;34:655–662.
104. Rhee-Moore SJ, et al. Periprocedural complication rates are equivalent between symptomatic and asymptomatic patients undergoing carotid angioplasty and stenting. Ann Vasc Surg. 2008;22:233–237.
105. Meyer SA, et al. Outcomes of carotid artery stenting in high-risk patients with carotid artery stenosis: a single neurovascular center retrospective review of 101 consecutive patients. Neurosurgery. 2010;66:448–453.
106. Stabile E, et al. Proximal endovascular occlusion for carotid artery stenting: results from a prospective registry of 1,300 patients. J Am Coll Cardiol. 2010;55:1661–1667.
107. De Rango P, et al. Long-term prevention of stroke: a modern comparison of current carotid stenting and carotid endarterectomy. J Am Coll Cardiol. 2011;57:664–671.
108. Krasniqi N, et al. Carotid artery stenting: a single center “real world” experience. PLoS One. 2012;7.
109. Qureshi AI, et al. A comparison of outcomes associated with carotid artery stent placement performed within and outside clinical trials in the United States. J Vasc Surg. 2012;56:317–323.
110. Bonati LH, et al. Age modifies the relative risk of stenting versus endarterectomy for symptomatic carotid stenosis—a pooled analysis of EVA-3S, SPACE and ICSS. [Carotid Stenting Trialists’ Collaboration] Eur J Vasc Endovasc Surg. 2011;41:153–158.
111. Khatri R, et al. Age differential between outcomes of carotid angioplasty and stent placement and carotid endarterectomy in general practice. J Vasc Surg. 2012;55:72–78.
112. Brown MM, et al. Should sex influence the choice between carotid stenting and carotid endarterectomy? Lancet Neurol. 2011;10:494–497.
113. De Rango P, et al. A comparative analysis of the outcomes of carotid stenting and carotid endarterectomy in women. J Vasc Surg. 2010;51:337–344.
114. Bisdas T, et al. The impact of gender on in-hospital outcomes after carotid endarterectomy or stenting. Eur J Vasc Endovasc Surg. 2012;44:244–250.
115. Vouyouka AG, et al. Analysis of Florida and New York state hospital discharges suggests that carotid stenting in symptomatic women is associated with significant increase in mortality and perioperative morbidity compared with carotid endarterectomy. J Vasc Surg. 2012;56:334–342.
116. Werner N, et al. Carotid artery stenting in clinical practice: does sex matter? Results from the carotid artery stenting registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK). [Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK)] Clin Cardiol. 2012;35:111–118.
117. Spyris CT, et al. Sex-related differences in embolic potential during carotid angioplasty and stenting. Ann Vasc Surg. 2012;26:93–101.
118. Rerkasem K, et al. Systematic review of the operative risks of carotid endarterectomy for recently symptomatic stenosis in relation to the timing of surgery. Stroke. 2009;40:e564–e572.
119. Rantner B, et al. The risk of carotid artery stenting compared with carotid endarterectomy is greatest in patients treated within 7 days of symptoms. [Carotid Stenting Trialists’ Collaboration] J Vasc Surg. 2013;57:619–626.
120. Topakian R, et al. Timing of stenting of symptomatic carotid stenosis is predictive of 30-day outcome. Eur J Neurol. 2007;14:672–678.
121. Gröschel K, et al. Early treatment after a symptomatic event is not associated with an increased risk of stroke in patients undergoing carotid stenting. Eur J Neurol. 2008;15:2–5.
122. Lin R, et al. The impact of timing on outcomes of carotid artery stenting in recently symptomatic patients. J Neurointerv Surg. 2010;2:55–58.
123. Ricotta JJ, et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. [Society for Vascular Surgery] J Vasc Surg. 2011;54:e1–e31.
124. Brott TG, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Neuro Interventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. 2011;57:1002–1044.
125. European Stroke Organisation, Tendera M, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). [ESC Committee for Practice Guidelines] Eur Heart J. 2011;32:2851–2906.
127. Liapis CD, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. [ESVS Guidelines Collaborators] Eur J Vasc Endovasc Surg. 2009;37(Suppl):1–19.
128. Liapis CD, et al. ESVS Guidelines: Section A—prevention in patients with carotid stenosis. [ESVS Guidelines Collaborators; European Society for Vascular Surgery] Curr Vasc Pharmacol. 2010;8:673–681.
129. Kakisis JD, et al. The European Society for Vascular Surgery guidelines for carotid intervention: an updated independent assessment and literature review. Eur J Vasc Endovasc Surg. 2012;44:238–243.
130. Timaran CH, et al. Trends and outcomes of concurrent carotid revascularization and coronary bypass. J Vasc Surg. 2008;48:355–360.
131. Naylor AR, et al. A systematic review and meta-analysis of 30-day outcomes following staged carotid artery stenting and coronary bypass. Eur J Vasc Endovasc Surg. 2009;37:379–387.
132. Velissaris I, et al. Synchronous carotid artery stenting and open heart surgery. J Vasc Surg. 2011;53:1237–1241.
133. Van der Heyden J, et al. Carotid artery stenting and cardiac surgery in symptomatic patients. JACC Cardiovasc Interv. 2011;4:1190–1196.
134. Versaci F, et al. Simultaneous hybrid revascularization by carotid stenting and coronary artery bypass grafting: the SHARP study. JACC Cardiovasc Interv. 2009;2:393–401.
135. Naggara O, et al. Anatomical and technical factors associated with stroke or death during carotid angioplasty and stenting: results from the endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial and systematic review. [EVA-3S Investigators] Stroke. 2011;42:380–388.
136. Sayeed S, et al. Angiographic lesion characteristics can predict adverse outcomes after carotid artery stenting. J Vasc Surg. 2008;47:81–87.
137. Layton KF, et al. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27:1541–1542.
138. Redgrave JN, et al. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113:2320–2328.
139. Tedesco MM, et al. Postprocedural microembolic events following carotid surgery and carotid angioplasty and stenting. J Vasc Surg. 2007;46:244–250.
140. Hellings WE, et al. The carotid atherosclerotic plaque and microembolisation during carotid stenting. J Cardiovasc Surg (Torino). 2006;47:115–126.
141. Biasi GM, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756–762.
142. Nicolaides AN, et al. Effect of image normalization on carotid plaque classification and the risk of ipsilateral hemispheric ischemic events: results from the asymptomatic carotid stenosis and risk of stroke study. [Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group] Vascular. 2005;13:211–221.
143. Lovett JK, et al. A critical appraisal of the performance, reporting, and interpretation of studies comparing carotid plaque imaging with histology. Stroke. 2005;36:1091–1097.
144. Mehta RH, et al. Effectiveness and safety of carotid artery stenting for significant carotid stenosis in patients with contralateral occlusion (from the German ALKK-CAS Registry experience). [German Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte Carotid Artery Stent (ALKK-CAS) Registry] Am J Cardiol. 2009;104:725–731.
145. Keldahl ML, et al. Does a contralateral carotid occlusion adversely impact carotid artery stenting outcomes? Ann Vasc Surg. 2012;26:40–45.
146. Goodney PP, et al. Impact of practice patterns in shunt use during carotid endarterectomy with contralateral carotid occlusion. [Vascular Study Group of New England] J Vasc Surg. 2012;55:61–71.
147. Brewster LP, et al. Contralateral occlusion is not a clinically important reason for choosing carotid artery stenting for patients with significant carotid artery stenosis. J Vasc Surg. 2012;56:1291–1295.
148. Fokkema M, et al. Stenting versus surgery in patients with carotid stenosis after previous cervical radiation therapy: systematic review and meta-analysis. Stroke. 2012;43:793–801.
149. Kasivisvanathan V, et al. Periprocedural outcomes after surgical revascularization and stenting for postradiotherapy carotid stenosis. J Vasc Surg. 2012;56:1143–1152.
150. Gupta R, et al. Rate, predictors, and consequences of hemodynamic depression after carotid artery stenting. J Am Coll Cardiol. 2006;47:1538–1543.
151. Cieri E, et al. Is haemodynamic depression during carotid stenting a predictor of peri-procedural complications? Eur J Vasc Endovasc Surg. 2008;35:399–404.
152. Nonaka T, et al. Prediction of prolonged postprocedural hypotension after carotid artery stenting. Neurosurgery. 2005;57:472–477.
153. Mlekusch W, et al. Hypotension and bradycardia after elective carotid stenting: frequency and risk factors. J Endovasc Ther. 2003;10:851–859.
154. Im SH, et al. Transcutaneous temporary cardiac pacing in carotid stenting: noninvasive prevention of angioplasty-induced bradycardia and hypotension. J Endovasc Ther. 2008;15:110–116.
155. Taylor DW, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet. 1999;353:2179–2184.
156. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
157. SALT Collaborative Group. Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338:1345–1349.
158. Farrell B, et al. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054.
159. Assadian A, et al. Aspirin resistance among long-term aspirin users after carotid endarterectomy and controls: flow cytometric measurement of aspirin-induced platelet inhibition. J Vasc Surg. 2007;45:1142–1147.
160. Eshaghian S, et al. Role of clopidogrel in managing atherothrombotic cardiovascular disease. Ann Intern Med. 2007;146:434–441.
161. Oh EY, et al. A comprehensive comparative review of adenosine diphosphate receptor antagonists. Expert Opin Pharmacother. 2012;13:175–191.
162. Floyd CN, et al. Comparative pharmacokinetics and pharmacodynamics of platelet adenosine diphosphate receptor antagonists and their clinical implications. Clin Pharmacokinet. 2012;51:429–442.
163. Brener SJ, et al. Prolonged dual antiplatelet therapy after percutaneous coronary intervention reduces ischemic events without affecting the need for repeat revascularization: insights from the CREDO trial. [CREDO Investigators] J Invasive Cardiol. 2007;19:287–290.
164. Steinhubl SR, et al. Optimal timing for the initiation of pre-treatment with 300 mg clopidogrel before percutaneous coronary intervention. [CREDO Investigators] J Am Coll Cardiol. 2006;47:939–943.
165. Mukherjee D, et al. Extracardiac vascular disease and effectiveness of sustained clopidogrel treatment. [CREDO Investigators] Heart. 2006;92:49–51.
166. Weintraub WS, et al. Long-term cost-effectiveness of clopidogrel given for up to one year in patients with acute coronary syndromes without ST-segment elevation. [CURE Study Investigators] J Am Coll Cardiol. 2005;45:838–845.
167. Squizzato A, et al. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev. 2011;(1).
168. Hirsh J, et al. Comparative benefits of clopidogrel and aspirin in high-risk patient populations: lessons from the CAPRIE and CURE studies. Arch Intern Med. 2004;164:2106–2110.
170. Chen ZM, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. [COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group] Lancet. 2005;366:1607–1621.
171. Liao JK. Secondary prevention of stroke and transient ischemic attack: is more platelet inhibition the answer? Circulation. 2007;115:1615–1621.
172. Diener HC, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. [MATCH investigators] Lancet. 2004;364:331–337.
173. Bhatt DL, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. [CHARISMA Investigators] N Engl J Med. 2006;354:1706–1717.
174. Wang TH, et al. An analysis of mortality rates with dual-antiplatelet therapy in the primary prevention population of the CHARISMA trial. [CHARISMA Investigators] Eur Heart J. 2007;28:2200–2207.
175. ESPRIT Study Group, Halkes PH, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673.
176. Chaturvedi S. Acetylsalicylic acid + extended-release dipyridamole combination therapy for secondary stroke prevention. Clin Ther. 2008;30:1196–1205.
177. Grewe PH, et al. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol. 2000;35:157–163.
178. American College of Cardiology Foundation; American Society of Interventional & Therapeutic Neuroradiology, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, Bates ER, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting). J Am Coll Cardiol. 2007;49:126–170.
179. McKevitt FM, et al. The benefits of combined anti-platelet treatment in carotid artery stenting. Eur J Vasc Endovasc Surg. 2005;29:522–527.
180. Sacco RL, et al. Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin-Aspirin Recurrent Stroke Study. [WARSS Investigators] Cerebrovasc Dis. 2006;22:4–12.
181. Kasner SE, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. [Warfarin Aspirin Symptomatic Intracranial Disease Trial Investigators] Circulation. 2006;113:555–563.
182. Gutierrez J, et al. Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med. 2012;172:909–919.
183. Hindler K, et al. Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology. 2006;105:1260–1272.
184. Paraskevas KI, et al. Can statins reduce perioperative morbidity and mortality in patients undergoing non-cardiac vascular surgery? Eur J Vasc Endovasc Surg. 2006;32:286–293.
185. Perler BA. The effect of statin medications on perioperative and long-term outcomes following carotid endarterectomy or stenting. Semin Vasc Surg. 2007;20:252–258.
186. Brooke BS, et al. Preoperative statin and diuretic use influence the presentation of patients undergoing carotid endarterectomy: results of a large single-institution case-control study. J Vasc Surg. 2007;45:298–303.
187. Kennedy J, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke. 2005;36:2072–2076.
188. Gröschel K, et al. Statin therapy at carotid angioplasty and stent placement: effect on procedure-related stroke, myocardial infarction, and death. Radiology. 2006;240:145–151.
189. Verzini F, et al. Effects of statins on early and late results of carotid stenting. J Vasc Surg. 2011;53:71–79.
190. Lin PH, et al. Carotid artery stenting with neuroprotection: assessing the learning curve and treatment outcome. Am J Surg. 2005;190:850–857.
191. Basic identification criteria of Doppler microembolic signals. Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium. Stroke. 1995;26:1123.
192. Ringelstein EB, et al. Consensus on microembolus detection by TCD. International Consensus Group on Microembolus Detection. Stroke. 1998;29:725–729.
193. Moehring MA, et al. Pulse Doppler ultrasound detection, characterization and size estimation of emboli in flowing blood. IEEE Trans Biomed Eng. 1994;41:35–44.
194. Dietrich R, et al. The use of embolic signal detection in multicenter trials to evaluate antiplatelet efficacy: signal analysis and quality control mechanisms in the CARESS (Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic carotid Stenosis) trial. Stroke. 2006;37:1065–1069.
195. Naylor AR, et al. Reducing the risk of carotid surgery: a 7-year audit of the role of monitoring and quality control assessment. J Vasc Surg. 2000;32:750–759.
196. Ackerstaff RG, et al. TCD-detected cerebral embolism in carotid endarterectomy versus angioplasty and stenting of the carotid bifurcation. [Antonius Carotid Endarterectomy, Angioplasty, and Stenting Study Group] Acta Chir Belg. 2004;104:55–59.
197. Markus HS, et al. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke. 2005;36:971–975.
198. Alvarez B, et al. Transcervical carotid stenting with flow reversal is a safe technique for high-risk patients older than 70 years. J Vasc Surg. 2012;55:978–984.
199. Christopoulos D, et al. The results of a simplified technique for safe carotid stenting in the elderly. J Vasc Surg. 2011;54:1637–1642.
200. Dorfer C, et al. Direct percutaneous puncture approach versus surgical cutdown technique for intracranial neuroendovascular procedures: technical aspects. World Neurosurg. 2012;77:192–200.
201. Pinter L, et al. Safety and feasibility of a novel transcervical access neuroprotection system for carotid artery stenting in the PROOF Study. J Vasc Surg. 2011;54:1317–1323.
202. Leal I, et al. A diffusion-weighted magnetic resonance imaging–based study of transcervical carotid stenting with flow reversal versus transfemoral filter protection. J Vasc Surg. 2012;56:1585–1590.
203. Dahm JB, et al. The concept of an anatomy related individual arterial access: lowering technical and clinical complications with transradial access in bovine- and type-III aortic arch carotid artery stenting. Vasa. 2011;40:468–473.
204. Jansen O, et al. Protection or nonprotection in carotid stent angioplasty: the influence of interventional techniques on outcome data from the SPACE Trial. Stroke. 2009;40:841–846.
205. Garg N, et al. Cerebral protection devices reduce periprocedural strokes during carotid angioplasty and stenting: a systematic review of the current literature. J Endovasc Ther. 2009;16:412–427.
206. Iyer V, et al. The type of embolic protection does not influence the outcome in carotid artery stenting. J Vasc Surg. 2007;46:251–256.
207. Atkins MD, et al. Embolic protection devices for carotid artery stenting: have they made a significant difference in outcomes? Semin Vasc Surg. 2007;20:244–251.
208. Schonholz CJ, et al. Is there evidence that cerebral protection is beneficial? Clinical data. J Cardiovasc Surg (Torino). 2006;47:137–141.
209. Macdonald S. The evidence for cerebral protection: an analysis and summary of the literature. Eur J Radiol. 2006;60:20–25.
210. Barbato JE, et al. A randomized trial of carotid artery stenting with and without cerebral protection. J Vasc Surg. 2008;47:760–765.
212. Siewiorek GM, et al. The association of clinical variables and filter design with carotid artery stenting thirty-day outcome. Eur J Vasc Endovasc Surg. 2011;42:282–291.
213. Brewster LP, et al. Carotid revascularization outcomes comparing distal filters, flow reversal, and endarterectomy. J Vasc Surg. 2011;54:1000–1004.
214. Bijuklic K, et al. The PROFI study (Prevention of Cerebral Embolization by Proximal Balloon Occlusion Compared to Filter Protection During Carotid Artery Stenting): a prospective randomized trial. J Am Coll Cardiol. 2012;59:1383–1389.
215. Faraglia V, et al. Cerebral embolization during transcervical carotid stenting with flow reversal: a diffusion-weighted magnetic resonance study. Ann Vasc Surg. 2009;23:429–435.
216. Timaran CH, et al. Randomized clinical trial of open-cell vs closed-cell stents for carotid stenting and effects of stent design on cerebral embolization. J Vasc Surg. 2011;54:1310–1316.
217. Tadros RO, et al. Comparing the embolic potential of open and closed cell stents during carotid angioplasty and stenting. J Vasc Surg. 2012;56:89–95.
218. Iancu A, et al. Acute carotid stent thrombosis: review of the literature and long-term follow-up. Cardiovasc Revasc Med. 2010;11:110–113.
219. Narins CR, et al. Patient selection for carotid stenting versus endarterectomy: a systematic review. J Vasc Surg. 2006;44:661–672.
220. Tulip HH, et al. Cerebral embolization in asymptomatic versus symptomatic patients after carotid stenting. J Vasc Surg. 2012;56:1579–1584.
221. Bonati LH, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). [ICSS-MRI study group] Lancet Neurol. 2010;9:353–362.
222. Montorsi P, et al. Microembolization during carotid artery stenting in patients with high-risk, lipid-rich plaque. A randomized trial of proximal versus distal cerebral protection. J Am Coll Cardiol. 2011;58:1656–1663.
223. Giannakopoulos TG, et al. Association between plaque echogenicity and embolic material captured in filter during protected carotid angioplasty and stenting. Eur J Vasc Endovasc Surg. 2012;43:627–631.
224. Kastrup A, et al. Effects of age and symptom status on silent ischemic lesions after carotid stenting with and without the use of distal filter devices. AJNR Am J Neuroradiol. 2008;29:608–612.
225. Gress DR. The problem with asymptomatic cerebral embolic complications in vascular procedures: what if they are not asymptomatic? J Am Coll Cardiol. 2012;60:1614–1616.
226. Altinbas A, et al. Cognition after carotid endarterectomy or stenting: a randomized comparison. Neurology. 2011;77:1084–1090.
227. Wasser K, et al. New brain lesions after carotid revascularization are not associated with cognitive performance. J Vasc Surg. 2011;53:61–70.
228. Lal BK, et al. Cognitive changes after surgery vs stenting for carotid artery stenosis. J Vasc Surg. 2011;54:691–698.
229. De Rango P, et al. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke. 2008;39:3116–3127.
230. Zhou W, et al. Prospective neurocognitive evaluation of patients undergoing carotid interventions. J Vasc Surg. 2012;56:1571–1578.
231. McDonald RJ, et al. Intracranial hemorrhage is much more common after carotid stenting than after endarterectomy: evidence from the National Inpatient Sample. Stroke. 2011;42:2782–2787.
232. Timaran CH, et al. Intracranial hemorrhage after carotid endarterectomy and carotid stenting in the United States in 2005. J Vasc Surg. 2009;49:623–628.
233. Park ST, et al. Atherosclerotic carotid stenoses of apical versus body lesions in high-risk carotid stenting patients. AJNR Am J Neuroradiol. 2010;31:1106–1112.
234. Lal BK, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. [CREST Investigators] Lancet Neurol. 2012;11:755–763.
235. Abou-Chebl A, et al. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol. 2004;43:1596–1601.
236. Goldberg JB, et al. Brain injury after carotid revascularization: outcomes, mechanisms, and opportunities for improvement. Ann Vasc Surg. 2011;25:270–286.
237. Cayne NS, et al. Carotid angioplasty and stent-induced bradycardia and hypotension: impact of prophylactic atropine administration and prior carotid endarterectomy. J Vasc Surg. 2005;41:956–961.
238. Altinbas A, et al. Effects of carotid endarterectomy or stenting on blood pressure in the International Carotid Stenting Study (ICSS). Stroke. 2011;42:3491–3496.
239. Lin PH, et al. Factors associated with hypotension and bradycardia after carotid angioplasty and stenting. J Vasc Surg. 2007;46:846–853.
240. Trocciola SM, et al. Analysis of parameters associated with hypotension requiring vasopressor support after carotid angioplasty and stenting. J Vasc Surg. 2006;43:714–720.
241. Taha MM, et al. Periprocedural hemodynamic instability with carotid angioplasty and stenting. Surg Neurol. 2008;70:279–285.
242. Diehm N, et al. Influence of stent type on hemodynamic depression after carotid artery stent placement. J Vasc Interv Radiol. 2008;19:23–30.
243. Nii K, et al. Incidence of hemodynamic depression after carotid artery stenting using different self-expandable stent types. Neurol Med Chir (Tokyo). 2011;51:556–560.
244. Widecka-Ostrowska K, et al. Haemodynamic depression during carotid angioplasty and stenting. Pol J Radiol. 2010;75:34–37.
245. Stoner MC, et al. Defining the high-risk patient for carotid endarterectomy: an analysis of the prospective National Surgical Quality Improvement Program database. J Vasc Surg. 2006;43:285–295.
246. Fokkema M, et al. In-hospital versus postdischarge adverse events following carotid endarterectomy. J Vasc Surg. 2013;57:1568–1575.
247. Parlani G, et al. Safety of carotid stenting (CAS) is based on institutional training more than individual experience in large-volume centres. Eur J Vasc Endovasc Surg. 2013;45:424–430.
248. Timaran CH, et al. Differential outcomes of carotid stenting and endarterectomy performed exclusively by vascular surgeons in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). [CREST Investigators] J Vasc Surg. 2013;57:303–308.
249. Gröschel K, et al. Systematic review of early recurrent stenosis after carotid angioplasty and stenting. Stroke. 2005;36:367–373.
250. Bonati LH, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. [CAVATAS Investigators] Lancet Neurol. 2009;8:908–917.
251. Wasser K, et al. Clinical impact and predictors of carotid artery in-stent restenosis. J Neurol. 2012;259:1896–1902.
252. Lal BK. Recurrent carotid stenosis after CEA and CAS: diagnosis and management. Semin Vasc Surg. 2007;20:259–266.
253. Setacci C, et al. In-stent restenosis after carotid angioplasty and stenting: a challenge for the vascular surgeon. Eur J Vasc Endovasc Surg. 2005;29:601–607.
254. Skelly CL, et al. Risk factors for restenosis after carotid artery angioplasty and stenting. J Vasc Surg. 2006;44:1010–1015.
255. de Borst GJ, et al. Carotid angioplasty and stenting for postendarterectomy stenosis: long-term follow-up. J Vasc Surg. 2007;45:118–123.
256. Nederkoorn PJ, et al. Optimal cut-off criteria for duplex ultrasound for the diagnosis of restenosis in stented carotid arteries: review and protocol for a diagnostic study. BMC Neurol. 2009;9:36.
257. Kim ES, et al. Characteristics of duplex sonographic parameters over time after successful carotid artery stenting. J Ultrasound Med. 2012;31:1169–1174.
258. Zhou W, et al. Ultrasound criteria for severe in-stent restenosis following carotid artery stenting. J Vasc Surg. 2008;47:74–80.
259. Lal BK, et al. Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg. 2008;47:63–73.
260. AbuRahma AF, et al. Carotid duplex velocity criteria revisited for the diagnosis of carotid in-stent restenosis. Vascular. 2007;15:119–125.
262. Telman G, et al. Duplex ultrasound verified by angiography in patients with severe primary and restenosis of internal carotid artery. Ann Vasc Surg. 2006;20:478–481.
263. Reimers B, et al. Endovascular treatment of in-stent restenosis after carotid artery stenting: immediate and midterm results. J Endovasc Ther. 2006;13:429–435.
264. Coppi G, et al. Carotid artery stent fracture identification and clinical relevance. J Vasc Surg. 2010;51:1397–1405.
265. Sfyroeras GS, et al. Clinical relevance and treatment of carotid stent fractures. J Vasc Surg. 2010;51:1280–1285.
266. Chang CK, et al. Prevalence and clinical significance of stent fracture and deformation following carotid artery stenting. J Vasc Surg. 2011;54:685–690.
267. Ling AJ, et al. Stenting for carotid artery stenosis: fractures, proposed etiology and the need for surveillance. J Vasc Surg. 2008;47:1220–1226.
268. Nallamothu BK, et al. Operator experience and carotid stenting outcomes in Medicare beneficiaries. JAMA. 2011;306:1338–1343.
269. Vogel TR, et al. Carotid artery stenting: impact of practitioner specialty and volume on outcomes and resource utilization. J Vasc Surg. 2009;49:1166–1171.
270. Cronenwett JL, et al. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537.
271. Rosenfield KM. Clinical competence statement on carotid stenting: training and credentialing for carotid stenting—multispecialty consensus recommendations. [SCAI/SVMB/SVS Writing Committee] J Vasc Surg. 2005;41:160–168.
272. Beller GA, et al. ACC revised recommendations for training in adult cardiovascular medicine. Core Cardiology Training II (COCATS 2). (Revision of the 1995 COCATS training statement.). J Am Coll Cardiol. 2002;39:1242–1246.
273. Creager MA, et al. ACC/ACP/SCAI/SVMB/SVS clinical competence statement on vascular medicine and catheter-based peripheral vascular interventions: a report of the American College of Cardiology/American Heart Association/American College of Physician Task Force on Clinical Competence (ACC/ACP/SCAI/SVMB/SVS Writing Committee to develop a clinical competence statement on peripheral vascular disease). J Am Coll Cardiol. 2004;44:941–957.
274. Connors JJ 3rd, et al. Training, competency, and credentialing standards for diagnostic cervicocerebral angiography, carotid stenting, and cerebrovascular intervention: a joint statement from the American Academy of Neurology, the American Association of Neurological Surgeons, the American Society of Interventional and Therapeutic Neuroradiology, the American Society of Neuroradiology, the Congress of Neurological Surgeons, the AANS/CNS Cerebrovascular Section, and the Society of Interventional Radiology. J Vasc Interv Radiol. 2009;20(Suppl):S292–S301.
275. Connors JJ 3rd, et al. Training, competency, and credentialing standards for diagnostic cervicocerebral angiography, carotid stenting, and cerebrovascular intervention: a joint statement from the American Academy of Neurology, American Association of Neurological Surgeons, American Society of Interventional and Therapeutic Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, AANS/CNS Cerebrovascular Section, and Society of Interventional Radiology. [Neuro Vascular Coalition Writing Group; American Academy of Neurology; American Association of Neurological Surgeons; American Society of Interventional and Therapeutic Radiology; American Society of Neuroradiology; Congress of Neurological Surgeons; AANS/CNS Cerebrovascular Section; Society of Interventional Radiology] Radiology. 2005;234:26–34.
276. Van Herzeele I, et al. Validation of video-based skill assessment in carotid artery stenting. [European Virtual Reality Endovascular Research Team EVEResT] Eur J Vasc Endovasc Surg. 2009;38:1–9.
277. Abbott AL, et al. Why the United States Center for Medicare and Medicaid Services (CMS) should not extend reimbursement indications for carotid artery angioplasty/stenting. Eur J Vasc Endovasc Surg. 2012;43:247–251.
278. Safian RD. Carotid artery stenting: payment, politics, and equipose. J Am Coll Cardiol. 2012;59:1390–1391.
279. Pawaskar M, et al. Economic evaluation of carotid artery stenting versus carotid endarterectomy for the treatment of carotid artery stenosis. J Am Coll Surg. 2007;205:413–419.
280. Eslami MH, et al. National trends in utilization and postprocedure outcomes for carotid artery revascularization 2005 to 2007. J Vasc Surg. 2011;53:307–315.
281. Sternbergh WC 3rd, et al. Carotid endarterectomy is more cost-effective than carotid artery stenting. J Vasc Surg. 2012;55:1623–1628.