36 Care of the vascular surgical patient
Aneurysm: A localized abnormal dilation, distention, or sac in an artery. True aneurysm involves all arterial layers. Dissecting aneurysm allows blood to dissect between the vessel layers. False aneurysm (pseudoaneurysm) develops as a result of disruption of the vessel wall that allows blood to escape from the lumen into a contained sac.4
Angiography (Arteriography): The injection of radiopaque dye into the arteries followed by rapid sequential radiographs of the vascular tree for determination of abnormalities in a specific region.5
Atherectomy: Procedure performed with a special catheter that contains a shaver device at the distal tip. The rotating blade shaves the plaque from the inner lining and removes it from the vessel through a suction device.5
Bypass: Performed to reroute blood flow around an area of stenosis in a blood vessel.5
Cryoplasty: A technique that uses cooling and balloon angioplasty to open stenotic vessels.6
Embolectomy: Extraction of an embolus from an artery to restore blood flow.4
Endarterectomy: Surgical removal of atheromatous plaque from a stenotic vessel.5
Endograft: Device designed to exclude an area of a blood vessel and provide a new conduit through which blood flows. It is primarily used to exclude aneurysmal vessels.7
Fibrinolytic (Thrombolytic) Therapy: Technique that uses clot-dissolving agents to dissolve clot material in a blood vessel.7
Ischemia: Lack of adequate blood flow to an area to meet the needs of the tissues.8
Ligation: Transection and tying off of a blood vessel.8
Stent: Device made of metal or other material used to maintain patency of a blood vessel after angioplasty. The device may be balloon expandable or self expanding.7
Sympathectomy: Interruption of the sympathetic nerve chain performed to produce vasodilation of blood vessels distal to the surgical site.7,9
Thrombectomy: Surgical removal of a thrombus.7
Thrombus: Stationary blood clot or atheromatous plaque that partially or totally occludes a blood vessel.7
Transluminal Angioplasty: Use of a special catheter with a balloon at the distal tip that is passed through the vessel to area of stenosis and inflated to compress stenosis and widen the vessel lumen. The balloon is deflated before removal of the catheter from the vessel. The procedure may be done percutaneously, with only a puncture site, or open through an incision in the vessel, and may be done in conjunction with a stent, atherectomy, or cryoplasty.5
Peripheral arterial disease (PAD) is a “diverse group of disorders that lead to progressive stenosis or occlusion, or aneurysmal dilatation of the aorta and its noncoronary branch arteries.”1,2 PAD is the preferred clinical term, and the definition includes the carotid arteries, upper extremity, visceral and lower extremity arterial branches.1,2 It is estimated that PAD affects 8 million people in the United States.2 Men are more affected than women, and the disease is age-related. As many as 20% of the population older than 70 years may have PAD.2 The consequences of PAD include a decrease in quality of life with reduction in everyday activities and a greater risk of cardiovascular morbidity and mortality.2 Treatment is aimed at prevention or reduction of long-term complications.
Venous insufficiency is estimated to affect as many as 25% of women and 15% of men and is a chronic, debilitating disease that consumes much of the health care budget.3 The treatment of venous disease is aimed at preventing long-term complications such as chronic edema and ulceration.
General considerations
Persons with PAD commonly have other underlying disease processes, some of which may contribute to the development of disease and increase the morbidity and mortality rates associated with surgery. Among the underlying conditions are chronic tobacco use with chronic obstructive pulmonary disease, diabetes, peripheral neuropathy, hypertension, dyslipidemia, obesity, and advancing age.2
The progression of atherosclerosis, which leads to most vascular surgical procedures, is a systemic disease that affects all arterial beds, including those in the extremities, heart, kidneys, and brain.2 A vital part of treatment of arterial disease is risk factor modification to include smoking cessation and control of underlying comorbidities such as diabetes, hypertension, and dyslipidemia.10 Venous surgical procedures have also increased in number as a result of improvements in technology and the use of minimally invasive techniques.3 This chapter is limited to care of the patient undergoing surgery on blood vessels outside the heart.
Diagnostic procedures
Noninvasive diagnostics, such as the ankle-brachial index (ABI), ultrasonography, computed tomography angiogram, magnetic resonance angiography and imaging, have contributed greatly to the early treatment of vascular disease.5,7 These procedures do not usually require sedation.
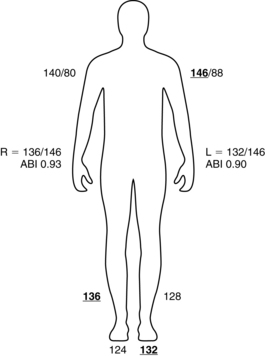
The ABI is a ratio of ankle to brachial blood pressure. PAD is present if the ABI is 0.90 or less.2 Box 36-1 describes the calculation of an ABI. This test may also be used postoperatively to assess patency of vessels following stent placement, percutaneous transluminal angioplasty (PTA), bypass graft placement, or endarterectomy. In patients with diabetes who have developed calcification of the large vessels, an ABI might not be obtainable because of the inability to fully compress the vessels to obtain a pressure. In these patients, toe-brachial index is a useful tool for assessing distal blood flow.8
BOX 36-1 Obtaining an Ankle Brachial Index
1. Obtain the blood pressure in both arms with the patient lying supine.
2. Obtain the blood pressure with a Doppler scan at the dorsalis pedis and posterior tibial pulses on each ankle.
3. Calculate the ABI with the highest ankle pressure divided by the highest arm pressure. (Example: Right BP 140/80 mm Hg; left BP 146/88 mm Hg; right DP 136 mm Hg; right PT 124 mm Hg; left DP 128 mm Hg; left PT 132 mm Hg; right ABI = 136 mm Hg/146 mm Hg [0.93]; left ABI = 132 mm Hg/146 mm Hg [0.90].)
Arteriography continues to be the gold standard for invasive diagnostic testing and usually requires intravenous (IV) sedation. This test requires direct injection of contrast media into the arterial bed and is used to examine the arterial supply of a specific region. Arteriography can also be done in conjunction with various treatment methods, including angioplasty, cryoplasty, atherectomy, and stenting.5 Computed tomographic angiography (CTA) and magnetic resonance angiography (MAR) may also be used in the diagnosis of vascular disease.8
Interventional procedures
Interventional treatments for peripheral vascular disease include PTA, PTA with stent placement, atherectomy, cryoplasty, and fibrinolytic therapy.5 These endovascular procedures are performed by a variety of specialists, including interventional radiologists, vascular surgeons, and cardiologists. These procedures may require only local anesthetic and IV sedation depending on the patient’s condition and physician’s preferences. The procedures can also be performed alone or in conjunction with other vascular surgical procedures in the operating room and may be done with local plus IV sedation, epidural, spinal, or general anesthesia.7
Percutaneous transluminal angioplasty may be performed on carotid, aortic, mesenteric, renal, iliac, femoral, popliteal, and tibial vessel stenosis. This procedure may be used alone or in conjunction with stent placement. Major complications after PTA or stenting include bleeding, hematoma, thrombus formation, and intimal tears (disruption of the inner lining of the vessel).5 Other complications may occur specific to the vascular bed being treated, such as transient ischemic attack or stroke for carotid stenting11 and worsening renal failure in treatment of renal artery stenosis.5 Stents are used to compress and hold the plaque against the vessel wall and are associated with longer patency rates of the vessel.5 They can also be used to treat an intimal tear in the vessel wall. Stents and balloons with drug coating, such as sirolimus and paclitaxel, are being evaluated for their potential to reduce in-stent stenosis as a result of their effects on smooth muscle cell proliferation. The goal is to increase long-term patency rates. Long-term data will help to determine whether these will be an effective tool for PAD.12
Atherectomy is a technique designed for removing plaque from the vessel wall with a special rotating blade and suction apparatus. Angioplasty or stenting may follow atherectomy.13,14 Cryoplasty uses a freezing technique with nitrous oxide inside a balloon for the opening of occluded vessels and theoretically carries the advantage of less risk of intimal hyperplasia, vessel recoil, and dissection.6
After these procedures, patients are monitored for recovery from IV sedation and for bleeding and hematoma formation at the puncture site. Distal pulses are assessed bilaterally to detect any change in blood flow that may be related to formation of an embolism or thrombus for procedures that involve the abdominal vessels or extremities.7 These pulses should be compared with the baseline pulses documented before the procedure. Intake and output should be monitored closely and adequate hydration should be maintained after any procedure with IV contrast. IV contrast can be toxic to the renal system leading to contrast-induced nephropathy; this is characterized by an increase in creatinine of 25% from baseline within 48 hours of contrast administration. This is of greater concern in patients with preexisting diabetic nephropathy.15 Treatments such as additional IV fluids, N-acetylcysteine, diuretics, sodium bicarbonate, and fenoldopam can be used to provide additional protection against contrast-induced nephropathy.15
Bed rest is maintained for 6 to 8 hours after the procedure with the extremity in a straight position to prevent bleeding at the puncture site. If a closure device is used at the puncture site, the patient may be allowed out of bed sooner.5,16 Any patient who undergoes an arteriogram, angioplasty, or stenting that involves the carotid or cranial circulation should undergo frequent neurologic assessment after the procedure. Special protection devices are used during angioplasty and stenting to trap any free-floating particles of plaque or thrombus that may be dislodged during the procedure. These devices serve to minimize postprocedural complications such as stroke.11
Fibrinolytic therapy is used when an embolus or thrombus has occluded a vessel. Special catheters are placed in the area of the thrombus, and agents such as alteplase, tenecteplase, or reteplase are used to lyse the clot.5 This process can be done by initial bolus and then completed via infusion and may take hours for complete lysis of the thrombus. These patients need close observation throughout the infusion for signs of bleeding, bruising, anaphylaxis, hematoma at the puncture site, and hematuria. Blood pressure should be monitored closely to decrease the risk of cerebral hemorrhage.17 Assessing for signs of cerebral bleeding is critical. If treatment is needed, aminocaproic acid (Amicar) can be used to inhibit the fibrinolytic process.5 Frequent assessment of the limb is also needed as reperfusion occurs. As the limb reperfuses, pain may actually worsen initially as microemboli break away from the thrombus and move distally to smaller vessels. As the infusion continues, pain improves as these emboli are dissolved. Frequent laboratory work includes serial monitoring of complete blood count, fibrinogen, prothrombin time and international normalized ratio (PT/INR), and partial thromboplastin time.18 Periodic assessment in radiology is done to follow the progress of the lytic agent. The infusion is discontinued when lysis is complete, fibrinogen levels drop to less than 100, bleeding occurs that necessitates transfusion, or no response to the agent is found.19
Medications used in vascular surgery
Anticoagulants are among the most commonly used medications in the treatment of the patient for vascular surgery. Unfractionated heparin can be administered before, during, and after surgery. Its actions occur at multiple points in the coagulation cascade to ultimately inactivate thrombin and prevent conversion of fibrinogen to fibrin. Heparin has a short, 60- to 90-minute half-life and may be administered IV or subcutaneously. The response to heparin is measured with the activated partial thromboplastin time and is targeted at 1.5- to 2.5-fold greater than normal to obtain a therapeutic response and prevent thromboembolism.20 Complications associated with the use of heparin include increased risk of bleeding and heparin-induced thrombocytopenia (HIT). Platelet counts should be monitored for decrease of 40% to 50% from baseline or any decrease to less than 100,000. If HIT develops, heparin must be discontinued and alternative anticoagulants should be used.20 Protamine is the antidote for heparin; its action occurs within 5 minutes of administration. Care must be taken to avoid overly rapid administration of protamine. When administration is too rapid, side effects can include hypotension, pulmonary hypertension, shortness of breath, and flushing. The usual target dose for reversal is 1 mg of protamine for every 90 units of heparin.20
Low-molecular-weight heparins (LMWHs), such as enoxaparin (Lovenox), dalteparin (Fragmin), and tinzaparin (Innohep), may also be used in the care of the patient for vascular surgery. These drugs are administered subcutaneously and have a significantly lower molecular weight than unfractionated heparin, which gives them improved predictability in the dose response and a longer half-life. This advantage greatly reduces the need for laboratory monitoring. If testing is needed, anti-factor Xa level is the test of choice for monitoring. The LMWHs are administered subcutaneously and have a significantly lower incidence rate of HIT associated with their use; they are primarily used to prevent thromboembolism after surgery, but are also approved in the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE). They may also be used to bridge patients before and after surgery who require long-term anticoagulation with warfarin (Coumadin). The decision to bridge is based on diagnosis and thromboembolic risk. Patients with renal disease may need a dose reduction depending on the severity of their disease. Complications are similar to those of unfractionated heparin. Laboratory testing should be monitored for signs of HIT, although it occurs much less frequently with the use of LMWH.20
Warfarin is an oral anticoagulant that inhibits vitamin K–dependent coagulation factors and the anticoagulant proteins C and S; it has a half-life of 36 to 42 hours. Monitoring of warfarin is done with the PT and the international normalized ratio.20 The PT/INR is laboratory dependent, and specific methods vary among institutions. Caregivers should be familiar with institutional methods. Warfarin is used to treat a variety of thromboembolic disorders, and it can be used to promote long-term patency of infrainguinal bypass grafts, particularly following thrombosis of previously placed grafts. Complications include increased risk of hemorrhage and skin necrosis. Patients receiving warfarin must be counseled to discontinue the drug several days before any invasive procedure to allow time for the PT/INR levels to decrease to normal. Reversal of warfarin is achieved with vitamin K or fresh-frozen plasma.20
Two additional oral anticoagulants are currently available. Dabigatran (Pradaxa), a direct thrombin inhibitor, and rivaroxaban (Xarelto), a direct factor Xa inhibitor, are currently approved for the prevention of stroke in nonvalvular atrial fibrillation. Rivaroxaban is also approved for short-term thrombophyphylaxis after elective hip or knee surgery.20 Dabigatran has a shorter half-life than warfarin and requires twice daily dosing. Patients receiving dabigatran should be counseled to discontinue its use the day before surgery if renal function is normal. Earlier discontinuation may be needed in patients with creatinine clearance less than 30 mL/min.21 Rivaroxaban is administered 10 mg daily. There are no reversal agents for dabigatran and rivaroxaban.20
IV direct thrombin inhibitors act at the active site of thrombin. These drugs provide an alternative to heparin in the patient with HIT. Current drugs available in this category are lepirudin (Refludan), bivalirudin (Angiomax), and argatroban (Acova).5,20 Desirudin (Iprivask) is a direct thrombin inhibitor that is administered subcutaneously. It may be used in patients who cannot use fondaparinux (Aristra) or LMWH.22
Parenteral factor X inhibitors currently include only fondaparinux (Arixtra). Fondaparinux activates antithrombin III, leading to inactivation of factor X. It is administered subcutaneously and requires no laboratory monitoring. Its primary use is preventing DVT and treating acute coronary syndromes. There is no reversal agent for fondaparinux.20
Antiplatelet agents, such as aspirin, clopidogrel (Plavix), prasugrel (Effient), dipyridamole (Persantine), dipyridamole ER/aspirin (Aggrenox), ticlopidine (Ticlid), and cilostazol (Pletal), may also be used in the patient with vascular disease as a preventative measure for myocardial infarction and stroke or as part of medical management for patients following placement of infrainguinal bypass grafts, carotid endarterectomy, and peripheral and carotid stenting.5,20,23 These drugs exhibit an irreversible permanent effect on the platelet for its lifespan and produce a qualitative effect on the platelet that is measured with the bleeding time. Platelet counts are not affected by these agents. Cilostazol also has some vasodilatory effects and is contraindicated in patients with heart failure. Patients should be counseled regarding the discontinuation of these drugs 7 to 10 days before invasive procedures to decrease the risk of bleeding.20 Patients at moderate to high risk for cardiovascular events may continue their antiplatelet agents to the time of surgery.20
Glycoprotein IIb/IIIa inhibitors are parenteral agents that also interfere with platelet aggregation and include abciximab (Reopro), eptifibatide (Integrilin), and tirofiban (Aggrastat). These agents are administered by IV infusion. Patients who have had drug-eluting coronary stent placement within the previous 12 months of a surgical procedure may need to be bridged with a GP IIb/IIIa inhibitor to discontinue their antiplatelet drugs for surgery or may be instructed to continue their aspirin and clopidogrel (Plavix) up to surgery.20
Perioperative use of beta blockers has been associated with decreased morbidity and mortality in the high-risk vascular surgery patient, particularly those undergoing lower extremity bypass or open abdominal procedures.24,25 There is evidence that, although cardiac events are decreased, there may be an increased risk of stroke.25,26 There is also some indication that atenolol may be associated with reduced mortality compared with metoprolol.27 Incidence of preoperative myocardial infarction ranges from 14% to 17.8% for open abdominal procedures and 5% to 15% for lower extremity bypass procedures.26,28 Preoperative stress testing has assisted in determining a plan of care before surgery, including coronary intervention or beta blockade, and may help to lower the risk of cardiac events postoperatively.29 Women are at particular risk for complications after vascular surgery and also have lower survival rates. Their responses to beta blockade may also be less beneficial than in men.29
Arterial surgical procedures
Extremity vessels and extraanatomic procedures
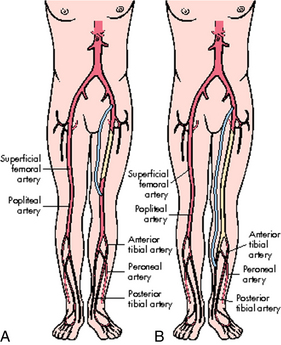
Arterial surgical procedures that involve the extremities include bypass, endarterectomy, embolectomy, and thrombectomy.7 A bypass is performed to reroute blood flow around an area of stenosis and is named for the vessels it arises from and connects into. Examples of these in the lower extremities are femoral-popliteal, femoral-tibial, and femoral-peroneal. The distal anastomosis for femoral-popliteal bypass procedures may be above the knee or below the knee depending on the location of the stenosis (Fig. 36-1). Distal bypasses may also connect into the anterior tibial or posterior tibial artery.7 Bypass procedures may also be performed in the upper extremities, but are much less common and may include bypass to circumvent lesions of the axillary artery, such as carotid-axillary bypass. Extraanatomic bypass procedures route blood in a more unusual fashion and may be done based on a patient’s condition or inability to tolerate a more major procedure. Examples of these types of bypass include axillofemoral, axillary-axillary, and femoral-femoral (Fig. 36-2).7 Endarterectomy can be done alone or in conjunction with a bypass and involves removal of plaque from a stenotic vessel. The most commonly endarterectomized vessels are the carotid, subclavian, iliac, and femoral arteries. Embolectomy and thrombectomy can also be performed to remove a clot from a vessel.7
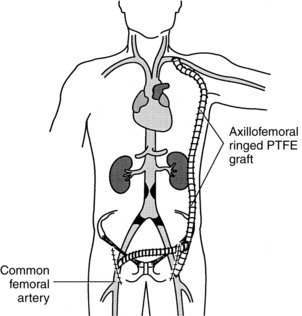
FIG. 36-2 Axillofemoral bypass and femoral-femoral bypass. PTFE, polytetrafluoroethylene.
(From Fahey V: Vascular nursing, ed 4, Philadelphia, 2004, Saunders.)
Materials used for bypass procedures can be autogenous (vein) or synthetic (artificial). The use of the greater saphenous vein can be accomplished in a reversed fashion or in situ. In the reverse method, the vein is reversed, and the small end is attached to the proximal artery and the large end to the smaller artery distally. Arm veins can be used, but this is much less common. Synthetic grafts that can be used include polytetrafluoroethylene (PTFE), which can be with or without supporting rings and knitted or woven Dacron. PTFE is commonly used in the lower extremities and for other low flow states such as extraanatomic bypass. In some cases, a combination of autogenous and synthetic grafts may be used.7
Choice of anesthesia is based on the type of procedure and patient and physician preference. General endotracheal, epidural, and spinal anesthesia have all been used safely in lower extremity vascular surgery procedures.28 Some upper extremity procedures can be performed using regional block. Many procedures that involve smaller incisions or the use of endovascular techniques can be done with local plus IV sedation. Epidural and spinal anesthesia can be used, but in some situations are not the preferred choice because of the effects on the sympathetic nervous system that result in vasodilation of the vasculature in the lower extremities. This vasodilation can lead to hypotension, which places the patient at risk for thrombosis of the graft after surgery. General anesthesia is associated with more postoperative cardiac events.28,30
Positioning
Immediately after surgery, the patient is supine with the head of the bed elevated. Care should be taken to place the limb in a position of comfort. For the patient with lower extremity surgery, care must be taken to prevent compression of the popliteal area and avoid flexion of the extremity. The limb should not be significantly elevated unless edema is present. Turn the patient to the operative side only to avoid external pressure on the graft. Both heels should be protected from pressure, and the toes should be protected from heavy bed linens. Bed rest is usually maintained for the first 24 hours after surgery.7,19
Rich evaluated the effects of positioning on transcutaneous oxygen (TcPO 2) measurements as a means of estimating the underlying circulation and tissue oxygenation in a convenience sample of patients who underwent arterial revascularization of the lower extremity. A repeated-measures experimental design was used, and subjects served as their own controls. Patients were randomly assigned to one of two leg positions, sitting upright with legs extended or positioned with the foot of the bed elevated 20 degrees. Assessments were made preoperatively and on postoperative days 1 and 2. Skin temperatures, TcPO2, limb volumes, and other physiologic data were evaluated. The study found that neither position significantly affected the postoperative TcPO2 measurements in patients with PAD.
Source: Rich K: The effects of leg/body positioning transcutaneous oxygen measurements after lower-extremity arterial revascularization, J Vasc Nurs 26:6–14, 2008.
Cardiopulmonary status
Vital signs are monitored at least hourly initially. Because of the atherosclerosis, patients who undergo vascular procedures have much higher mortality rates. Myocardial ischemia is most common in this patient population and occurs more often in the postoperative period than during surgery. Continuous cardiac monitoring and pulse oximetry are standard monitoring parameters for these patients. Arterial lines and pulmonary artery catheters may be used selectively in this patient population. The perianesthesia nurse should observe for signs of shock and monitor wound sites for hemorrhage or hematoma.19
Neurovascular status
The dorsalis pedis pulses and the posterior tibial pulses should be assessed frequently following lower extremity procedures. For extraanatomic bypass procedures such as the axillofemoral bypass, monitor pulses in the donor arm and the revascularized limb.7 Recommendations are for every 1 to 2 hours in the first 24 hours after surgery. These results should be compared with baseline pulses documented before surgery. Pulses should be assessed based on a scale of 0 to 3 (Box 36-2).7 In some cases of distal bypass procedures, the position of the palpable pulse may be in a different location than before surgery. This information should be provided by the physician. A Doppler scan should be available for assessment of pulses because pulses are commonly present but not palpable. Routine assessment of Doppler-scanned pulses also provides a method of early detection of a change in blood flow. Normal blood flow is heard as a triphasic (three whooshing sounds) or biphasic (two whooshing sounds) flow. If assessment indicates a change from a triphasic or biphasic sound to a monophasic sound (one whooshing sound), blood flow may have changed in the extremity and the physician should be notified. Establishment of a baseline includes knowledge of whether the patient had monophasic flow before surgery.19
Assessment of ABIs is a vital part of assessment for the vascular patient. This measurement is an indicator of blood flow to the lower extremities. The steps for assessing an ABI are listed in Box 36-1. ABIs are not assessed in the patient with a distal bypass graft, because it can cause an occlusion of the graft. Report any ABI that decreases more than 0.15.19
Assess for changes in motor and sensory function, the presence of foot drop, and pain out of proportion for surgical procedure. These symptoms may be indicative of compartment syndrome, which occurs as a result of bleeding into the muscle compartment and leads to a decrease in arterial perfusion.7,19 Color and temperature changes should also be assessed. The extremity should be warm and dry and of normal color. Acute ischemia is recognized quickly with assessment for the six P’s: pain, paralysis, paresthesia, pulselessness, poikilothermia, and pallor (Box 36-3).7,19
Dressings
Dressings should be dry and intact throughout the postanesthesia care unit stay. After distal bypass procedures, dressings may extend the full length of the extremity. Frequent observation for bleeding or hematoma at the area of the incisions is critical. Signs of bleeding should be reported to the physician as soon as possible.7,19
Pain relief
Pain and discomfort are common after lower extremity arterial surgery. This pain must, however, be assessed and differentiated from ischemic pain and that of compartment syndrome. Sudden severe pain may be indicative of graft occlusion, especially when located across the foot at the metatarsal heads. Occlusions usually result in pain out of proportion to the usual postoperative pain. Patients may have pain in the thigh and medial aspect of the leg away from the incision that could characterized as a burning type of pain; this pain is related to neuropathy that can occur as a result of operative injury to the nerve. Most neuropathies resolve after a few months.19
Intake and output
Renal status must be monitored closely after surgery. Intake and output should be monitored every 1 to 2 hours in the first 24 hours. Hydration is important in the patient with vascular disease because of the potential for renal insufficiency; the use of contrast during the procedure increases the risk of contrast-induced nephropathy. Electrolytes, blood urea nitrogen, glomerular filtration rate, and creatinine should be monitored closely to detect changes in renal status.7
Carotid vessels
The most common procedure performed on the carotid arteries is endarterectomy. Other procedures performed on the carotid arteries include bypass, angioplasty, and stenting. Endarterectomy is performed for the removal of plaque from the carotid artery bifurcation and internal carotid artery. This removal is done to improve cerebral circulation and decrease the risk of embolization to the brain, which can result in a stroke. On completion of the endarterectomy, the carotid artery can be closed in a primary fashion by suturing of the edges of the artery together or with a patch graft. This procedure can be performed using general anesthesia or IV sedation with a field block at the site of operation.8,31
For lesions located in the common carotid artery closer to the aortic arch, a bypass to the subclavian artery may be the only means of restoring sufficient blood flow to the brain. A bypass from one carotid to another can also be performed.7
Carotid stenting is usually performed using IV sedation and can be done in the operating room or radiologic suite. A major difference in the procedure for carotid angioplasty and stenting is that the procedure uses distal protection devices designed to trap any debris that may be dislodged during the procedure. These devices trap debris in a basket-type apparatus that removes the debris at the conclusion of the procedure as the catheter is withdrawn. This debris, if not trapped, can lead to cerebral embolism and increase the risk of a stroke. Other complications of stenting include acute stent thrombosis, arterial dissection of the common or internal carotid, contrast-induced encephalopathy, and hyperperfusion syndrome.32 In addition, the patient preparing to undergo carotid stenting begins antiplatelet therapy 4 to 5 days before the procedure or is administered a loading dose of clopridogrel (Plavix; 300 mg) 24 hours before and the day of the procedure.33
Positioning
Immediately after the procedure, the patient is positioned supine with the head of the bed elevated for airway protection and prevention of venous pooling at the incision. This positioning is maintained until vasopressors are discontinued.7,31 Activity is progressed as tolerated. Most patients are ambulatory 6 to 8 hours postoperatively and are discharged the day following surgery.31
Cardiopulmonary status
Vital signs, including oxygen saturation, and neurologic checks are assessed every hour during the first 24-hour period. Intake and output are initially monitored every 4 hours. Uncontrolled hypertension can threaten the anastomosis and is also believed to contribute to the development of hyperperfusion syndrome. Agents that cause vasodilation should be avoided in the treatment of hypertension, because these may worsen hyperperfusion syndrome. Hypotension may predispose the patient to carotid artery thrombosis. Changes in the baroreceptors as a result of surgery can lead to hypotension. Underlying cardiac disease may also be aggravated by extremes in blood pressure. Vasoactive infusions may be necessary to control blood pressure and maintain the levels at less than 140 mm Hg systolic and 90 mm Hg diastolic after endarterectomy. Patients should also be monitored for respiratory distress and hematoma formation.7,31
Neurologic status
Level of consciousness, movement of extremities, and cranial nerve assessment are included in the assessment of vital signs after endarterectomy. Because of the close proximity of cranial nerves to the operative site, risk of injury during the procedure exists. Cranial nerve assessment is summarized in Table 36-1. Cranial nerve injuries are usually temporary and heal over time as the patient recovers from surgery.7 Transient focal neurologic deficits may be indicative of embolization, hyperperfusion syndrome, and contrast encephalopathy after stenting. In the case of hyperperfusion syndrome, if intracerebral hemorrhage occurs, the patient may experience vomiting along with changes in level of consciousness. This complication may prove fatal if not treated immediately.33,34
| CRANIAL NERVE | FUNCTION | ABNORMAL RESPONSE |
|---|---|---|
| Facial (VII) | Facial expression, saliva secretion |
Dressings
Immediately after surgery, the incision can be covered with a dressing. Some physicians use a drain if oozing is seen in the wound bed at the time of surgical closure. Drainage should be measured and recorded during each shift. Bruising, discoloration, and swelling around the incision are common. However, changes in the patient’s respiratory status or increasing hematoma around the incision require immediate attention. Tracheal compression can lead quickly to respiratory compromise. Reoperation may be necessary to locate and correct a suture line bleed and drain the hematoma.7,31
Pain relief
Most patients need little for pain relief after endarterectomy. Opioids are avoided as much as possible because of their effects on level of consciousness and interference with accurate assessment of neurologic function. Acetaminophen is commonly used for postoperative pain. Dosing should not exceed 650 mg per dose or the maximum daily dose of 4000 mg.31 Over-the-counter guidelines have been revised to a limit of 3000 mg daily for acetaminophen products to reduce the risk of acute liver failure.32 Close attention should be paid to symptoms of severe headache, because these may indicate hyperperfusion syndrome or cerebral hemorrhage. This is more likely to occur early in the postoperative period for carotid stenting, within 24 hours of the procedure, and 5 to 7 days after endarterectomy as a result of impaired cerebral autoregulation. Severe cases may need treatment with steroids, anticonvulsants, and vasoconstrictors for prevention of seizures and stroke. Patients should be instructed to report headache that is not controlled by routine analgesia after discharge.34,35
Large vessels
Surgical procedures that involve the aorta and iliac vessels may be performed for atherosclerotic disease, aneurysmal disease, dissection, or trauma. Bypass procedures include aortoiliac, aortofemoral, and femoral-femoral procedures and those that involve the renal arteries and the mesenteric vessels. Endarterectomy can also be performed on these vessels.7,8
The development of endovascular techniques has led to the use of devices designed to reduce extensive surgical procedures, operative time, blood loss, and anesthesia. Endovascular aneurysm repair (EVAR) procedures (Fig. 36-3) are used to repair thoracic and abdominal aortic aneurysms. Patients must meet certain inclusion criteria to qualify for EVAR. These devices consist of a metal stent with graft material, Dacron, or PTFE attached to the stent. The graft material may be on the outside or the inside of the stent depending on the manufacturer. Some devices are a unibody (one part to the device) design; others are modular in design. Physician preference and individual patient anatomy are used to determine the best design for each patient. These procedures can be performed with local plus IV sedation and are performed most often through a cutdown into the femoral arteries in each groin. In some instances, these procedures can be performed percutaneously.7,36–38
Open abdominal procedures that involve the large vessels usually involve a midline incision. Some procedures may be done from a retroperitoneal approach, and laparoscopic techniques have also been used in procedures that involve the aorta.38,39 These procedures usually require general anesthesia. However, supplemental epidural anesthesia can be used to allow a lighter level of general anesthesia.7
Positioning
Immediately after the procedure, the patient is positioned supine with the head of the bed elevated to assist with respiratory function and decrease stress on suture lines.7 Bed rest is maintained for the initial 24 hours after open procedure. After endovascular surgery, bed rest is maintained for at least 2 hours after the sheath is removed for percutaneous procedures. Activity is advanced as tolerated in patients with surgical approach to endovascular repair. Endovascular surgery patients are usually discharged within 24 to 48 hours.8,37
Cardiopulmonary status
Because of the high risk associated with aortic procedures, cardiopulmonary assessment is of the utmost importance. Many patients receive beta blockers in the perioperative period to decrease left ventricular workload and the risk of myocardial ischemia.25–27 Cardiac stress is greatly increased as a result of cross-clamping and declamping. The workload of the heart increases with cross-clamping as a result of the increased resistance the heart must pump against.8 The leading cause of death in postoperative patients after open aneurysm repair is myocardial infarction. At declamping, the patient may experience hypotension and shock.4 Arterial pressure monitoring, electrocardiography, pulse oximetry, arterial blood gas analysis, pulmonary artery pressure, and central venous pressure measurements are all tools that may be used during the immediate postoperative period. Normotension reduces the stress on suture lines and decreases the risk of thrombosis in a graft limb. Vasoactive medications may be used to normalize blood pressure. Dysrhythmias can occur as a result of hypoxemia, myocardial ischemia, and electrolyte imbalances. Some patients may need mechanical ventilation after surgery. Because of underlying pulmonary disease in many vascular patients, pulmonary status must be monitored closely for atelectasis as a result of incisional pain and decreased lung function.8
Neurovascular status
The level of cross clamp of the aorta, if necessary during the procedure, influences greatly the potential for complications after surgery. Neurologic checks should be monitored with hourly vital signs for assessment of any signs of paralysis as a result of spinal cord injury related to cross clamp. Lower extremity pulses, color, temperature, and motor and sensory function should be assessed every 4 hours initially. ABIs are the best tool for determining whether the blood flow to the distal arterial bed of the extremities is adequate after surgery. Ischemia in the lower extremities can occur as a result of embolization, graft occlusion, or hypoperfusion of the extremity. Any absence of a previously palpable pulse indicates an occlusion of the vessel or graft and necessitates immediate intervention.7
Dressings
Dry dressings are applied to the abdominal incision for open procedures and to one or both groins at the site of access for endovascular procedures. Dressings and surgical sites should be assessed for signs of bleeding or hematoma.8
Pain relief
Adequate analgesia is critical to reducing the workload of the heart in the postoperative period. It also aids in the reduction of splinting, tachycardia and hypertension.7 Epidural analgesia is commonly used to assist with pain relief.4 Pain management strategies can include pharmacologic and nonpharmacologic measures and should be reassessed at regular intervals to determine effectiveness.8
Intake and output
Cross-clamping above the renal arteries for open repair and increased use of contrast with EVAR may predispose the renal bed to injury and acute renal failure.8,15 Intake and output should be monitored hourly in the first 24 hours after aortic surgery. The patient should be monitored closely for mobilization of fluids in the initial postoperative period. Diuretics can be used to assist in removing excess fluids and reducing the risk of congestive heart failure.8
Sympathectomy
Sympathectomy is performed to interrupt the sympathetic nerve chain.7 It can be performed with radiofrequency ablation, electrocautery, chemical injection, or dissection and excision of a segment of the sympathetic chain.9,40 Newer techniques used for sympathectomy include the use of laparoscopic and thoracoscopic instruments to allow for minimally invasive surgical options.41 The procedure is performed to increase blood flow by reducing sympathetic tone in the skin and subcutaneous tissue. In arterial occlusive disease, pain relief is one indication for sympathectomy.7
Sympathectomy in the cervical and thoracic regions can be used to treat upper extremity conditions, such as Raynaud phenomenon (hyperactive response to cold exposure or emotional stress) and hyperhidrosis (excessive sweating of the hands, axilla, or face). It involves excision of the lower portion of the stellate ganglion and T2 and T3 ganglion.9,40 Complications include Horner syndrome, postsympathetic neuralgia, hemothorax, pneumothorax, bleeding, and impaired functioning of the hand muscles as a result of injury to the first thoracic nerve.19
Lumbar sympathectomy is used to treat critical lower limb ischemia when vascular reconstruction is not an option and may also aid in wound healing after amputation. Patients with complex regional pain syndrome and neuropathic pain may also benefit from sympathectomy. Complications can include damage to the vena cava or ureter, paralytic ileus, and postsympathectomy neuralgia. A dry dressing is placed over the surgical site. Assess for urine on the dressing, which may indicate ureteral injury, or bleeding, which may indicate lumbar vessel injury. Mild analgesics are usually sufficient to manage pain. Severe pain may be indicative of ureteral injury and requires immediate attention and possible reexploration.7
Amputation
Amputation may be performed as a result of gangrene, infection, trauma, malignancy, or failure of arterial reconstruction. It is more common in men than women. More than half of amputees have diabetes, and 80% have PAD.7,8 Although amputation is often seen as a failure of medical and surgical management of disease, this procedure is the critical beginning for rehabilitation and is essential to maximizing the status of the patient and enhancing quality of life. Amputations can be done at many levels depending on the patient’s level of disease, infectious processes, joint function, and blood supply. Types of amputations are listed in Box 36-4. The more tissue preserved, the higher the functioning of the patient and the lower the morbidity and mortality rate.8
Postoperative care
Immediately after the procedure, the patient is positioned supine with the head of the bed elevated. Elevation of the extremity to assist with edema control and pain management aids wound healing.19 Epidural analgesia may be used to assist with pain relief. Some pain may also be related to phantom limb sensations that may require long-term pain management. Dressings and surgical sites should be assessed for signs of bleeding or hematoma and kept clean and dry. Patients who undergo amputation may also have multiple comorbidities that require close observation of cardiopulmonary and renal status. Stump dressings will aid in reduction of flexion contracture and improve the ability to be fitted with a prosthesis.4,8
Venous surgical procedures
Chronic venous insufficiency affects approximately 15% of men and 25% of women42 and occurs when valves within the veins of the lower extremities become incompetent, leading to an increase in the venous pressure in the lower extremities. This condition may develop as a result of venous obstruction as from DVT, congenitally absent valves or veins, or failure of the calf muscle pump.8 Varicose veins often develop as a result of chronic venous insufficiency and significantly affect quality of life.43 Ulcers may occur in up to 4% of patients older than 65 years and is the most serious consequence of venous insufficiency. Traditional procedures for treating venous disease include high ligation of the greater saphenous vein with stab avulsion, stripping of the vein, and perforator vein ligation.44 These procedures can be performed alone or in combination with each other, typically in the operating room.8 Advances in the treatment of venous disease have added procedures that include liquid and foam sclerotherapy, ambulatory phlebectomy, radiofrequency closure, and endovascular laser. These procedures are usually performed in an outpatient setting and may be done in the physician’s office. Many patients undergo more than one type of procedure because of the complexity of venous disease.40,43
Ligation of the greater saphenous vein is performed to limit pressure on the distal saphenous vein to decrease the risk of further incompetence and distal varices. Ligation is performed at the level of the saphenofemoral junction; the vein can then be removed through stripping of the vein from the groin to the knee or to the ankle if the distal portion of the vein is involved. Individual varicose veins can be treated with stab avulsion, a technique in which individual varicose veins are removed through tiny incisions at the point of valvular reflux. Complications include wound infection, DVT, nerve injury that results in numbness in the lateral foot, and hematoma formation.8,43,44
Perforator (communicating) vein ligation is performed to interrupt incompetent perforator veins and can be performed as an open procedure or with a minimally invasive endoscopic technique.8 Perforator veins serve to channel blood from the superficial to the deep veins and may become incompetent, leading to reflux of blood into the superficial veins. Interruption of these incompetent perforators decreases the pressure in the superficial system and may be performed in conjunction with other procedures.44 Many patients with incompetent perforator veins also have ulcers in the lower extremities. This procedure may enhance wound healing as a result of the decreased venous pressure over the gaiter area, where most venous ulcers are located.8
Sclerotherapy uses a sclerosing agent and is injected directly into the vein, which causes the vessel to swell and seal itself. This action prevents blood from reentering the vessel. Foam sclerotherapy has advantages over traditional sclerotherapy in that it has fewer side effects, requires fewer treatments, and usually yields better results. This technique involves the mixing of oxygen into the sclerosant to produce the foam. The foam forces blood from the vein and as the oxygen bubbles dissolve, the vein deflates. The most commonly used agent is sodium tetradecyl sulfate. Hypertonic saline has also been used, but is associated with a higher risk of cutaneous necrosis. Associated complications include allergic reaction, intraarterial injection, multiple needle punctures, pigment discoloration, hematoma, and ulceration.45
Ambulatory phlebectomy is a technique that is performed with tumescent anesthesia, which is produced by diluting lidocaine. It is performed with a phlebectomy hook, and large varicosities are removed through small incisions that do not require suturing. Complications include hemorrhage, superficial hematoma, blisters, hyperpigmentation, nerve injury, scarring, contact dermatitis, and superficial phlebitis.45
Radiofrequency (RF) closure of varicose veins can be performed with local or tumescent anesthesia and involves the use of RF energy through an endovenous electrode that causes controlled heating of the vessel, producing collapse of the vessel from heat-induced vasospasm and collagen shrinkage as the electrode is slowly withdrawn from the vessel.44 Endovenous laser ablation is the most common method for saphenous vein ablation and uses laser energy with an electrode that is painless, bloodless, and shorter duration of procedure than RF. No scarring and a shorter recovery period are seen with this procedure. Rather than shrinking the vessel wall, this procedure leads to thrombotic occlusion from heating of the blood components and thermal damage to the endothelium. Adverse postoperative events include ecchymosis, skin burns, phlebitis, vessel perforation, DVT, PE, hematoma, infection, and paresthesias.44,45
Postoperative care
After venous procedures, most patients are placed in a compression dressing with elastic bandage. Position the extremity to avoid severe joint flexion and assess dressings for signs of bleeding. Circulation should be assessed to ensure adequate blood flow and to be certain that the elastic bandages are not wrapped too tightly. Elevation of the extremities can be used to assist in the management of edema. Ambulation is encouraged as soon as possible after surgery to reduce the risk of thromboembolic events.7,43,45
Vena caval filters
Inferior vena caval (IVC) filters are placed to prevent PE, which is a life-threatening complication of DVT. According to the 2012 ACCP Evidence-Based Clinical Practice Guidelines,20 indications for placement of an IVC filter include inability to use anticoagulation therapy in a patient with proximal DVT or acute PE and/or documented progression of the thrombus. This procedure can be performed percutaneously in the operating room or in the radiology suite with fluoroscopy, and local anesthesia and can be inserted via the jugular or femoral vein.7 Traditionally, these filters were placed permanently. A new generation of devices now allows some devices to be removed after the high-risk period is over. Retrievable filters are used in situations where patients are found to have a short-term risk of PE. Safe indwelling times are still under evaluation, and there is some evidence to suggest that even when retrievable filters are used they may not be removed.46 Placement of an IVC filter does not eliminate the need for concurrent means of prophylaxis for thromboembolic events.47 Complications of filter placement include perforation of the vena cava with retroperitoneal bleeding, migration of the filter, and improper deployment of the filter. Occlusion of the vena cava as a result of a large embolus is also a possibility.48
Summary
The patient undergoing vascular surgery presents many challenges to the perianesthesia nurse. Most patients have multiple comorbidities that require extensive preparation before surgery and critical thinking to prevent complications after surgery. New medications and technology have enabled these patients to survive longer with a higher quality of life than ever before. The perianesthesia nurse plays a vital role in the care and survival of these patients.
1. Hirsch AT, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the AAVS/SVS, SCAI, SVMB, SIR, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). Circulation.2006;113:e463–654.
2. Olin JW, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral arterial disease: a report of the ACCF/AHA Task Force on Performance Measures, the American College of Radiology, the SCAI, the SIR, the SVM, the SVN, and the SVS (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). J Am Coll Cardiol.2010;56:2147–2181.
3. Frasier K, Latessa V. Minimally invasive vein therapy and treatment options for endovenous heat-induced thrombus. J Vasc Nurs. 2008;26:53–57.
4. Rothrock J. Alexander’s care of the patient in surgery, ed 14. St. Louis: Mosby; 2011.
5. Levine B, Miller K. Cardiac vascular nursing review and resource manual, ed 2. Silver Spring, Md: ANCC; 2006.
6. Gonzalo B, et al. Cryoplasty and endovascular treatment in the femoropopliteal region: hemodynamic results and follow-up at one year. Ann Vasc Surg.2010;24:680–685.
7. Lisberger ME. Peripheral Vascular Care. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 2, St. Louis: Saunders, 2010.
8. Lewis P, et al. Core curriculum for vascular nursing. Beverly, Mass: Society for Vascular Nursing; 2007.
9. Durai R, Hoque H. Chemical sympatholysis: indications, technique and complications. Br J Hosp Med.2008;69:635–638.
10. Muir R. Peripheral arterial disease: pathophysiology, risk factors, diagnosis, treatment and prevention. J Vasc Nurs.2009;27:26–30.
11. Custer N. What nurses should know about carotid stents. MedSurg Nurs. 2009;18:277–282.
12. Ansel GM, Lumsden AB. Evolving modalities for femoropopliteal interventions. J Endovasc Ther.2009;16:1182–1197.
13. Korabathina R, et al. Orbital atherectomy for symptomatic lower extremity disease. Catheter Cardiovasc Interv. 2010;76:326–332.
14. Shrikhande GV, et al. Lesion types and device characteristics that predict distal embolization during percutaneous lower extremity interventions. J Vasc Surg. 2011;53:347–352.
15. Prevention of contrast-induced nephropathy: an update. Prescriber’s Letter. 2008;24. detail document 240577
16. Van den berg J. A close look at closure devices. J Cardiovasc Surg.2006;47:285–295.
17. Agle SC, et al. The association of periprocedural hypertension and adverse outcomes in patients undergoing catheter-directed thrombolysis. Ann Vasc Surg. 2010;24:809–814.
18. Morris J, Neaton M. Continuous improvement process for a high-risk population: catheter-directed thrombolytic infusions. J Vasc Nurs.2009;27:8–12.
19. Fahey V. Vascular nursing, ed 4. Philadelphia: Saunders; 2004.
20. American College of Chest Physicians: Antithrombotic therapy and prevention of thrombosis, ed 9: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl).
21. New Drug: Pradaxa (Dabigatran). Prescriber’s Letter. 2011;27. detail document 270101
22. Comparison of injectable anticoagulants. Prescriber’s Letter. 26, 2010. detail document 260512
23. Antiplatelet agents for stroke prevention. Prescriber’s Letter. 24, 2008. detail document 24010
24. Goodney PP, et al. A regional quality improvement effort to increase beta blocker administration before vascular surgery. J Vasc Surg. 2011;17:1–13.
25. Stephens S. State of the science: beta-blockers and reduction of perioperative cardiac events. Crit Care Nurs Clin N Am.2010;22:209–215.
26. Foex P, Sear JW. Challenges of beta-blockade in surgical patients. Anesthesiology. 2010;113:767–771.
27. Wallace A, et al. Atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology. 2011;114:824–836.
28. Singh N, et al. The effects of the type of anesthesia on outcomes of lower extremity infrainguinal bypass. J Vasc Surg. 2006;44:964–969.
29. Matyal R, et al. Preoperative stress testing in high-risk vascular surgery and its association with gender. Gender Med. 2010;7:584–592.
30. Lumb A. Anaesthesia for vascular surgery on extremities. Anaesth and Int Care Med. 2007;8:255–259.
31. Society for vascular nursing 2009 clinical practice guideline for patients undergoing carotid endarterectomy (CEA). J Vasc Nurs. 2010;28:21–46.
32. Acetominophen. Prescriber’s Letter. 2011;18:52.
33. Nicosia A, et al. Classification for carotid artery stenting complications: manifestation, management, and prevention. J Endovasc Ther.2010;17:275–294.
34. Moulakakis K, et al. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg. 2009;49:1060–1067.
35. Medel R, et al. Hyperperfusion syndrome following endovascular cerebral revascularization. Neurosurg Focus. 2009;26:1–5.
36. Tinkham M. The endovascular approach to abdominal aortic aneurysm repair. AORN. 2009;89:289–306.
37. Thompson J, Bertling G. Endovascular leaks: perioperative nursing implications. AORN.2009;89:830–839.
38. Robbins D. Current modalities for abdominal aortic aneurysm repair: implications for nurses. J Vasc Nurs.2010;28:136–145.
39. Dooner J, et al. Laparoscopic aortic reconstruction: early experience. Am J Surg.2006;191:691–695.
40. Gabrhelik T, et al. Percutaneous upper thoracic radiofrequency sympathectomy in raynaud phenomenon. Reg Anesth and Pain Med. 2009;34:425–429.
41. Murphy M, et al. Upper dorsal endoscopic thoracic sympathectomy: a comparison of one-and two-port ablation techniques. Eur J Cardiothor Surg.2006;30:223–227.
42. Tellings SS, et al. Surgery and endovenous techniques for the treatment of small saphenous varicose veins: a review of the literature. Phlebology; 2011:1–6.
43. Brar R, et al. Surgical management of varicose veins: meta-analysis. Vascular.2010;18:205–220.
44. Word R. Medical and surgical therapy for advanced chronic venous insufficiency. Surg Clin N Am. 2010;90:1195–1214.
45. Mowatt-Larssen E. Management of secondary varicosities. Semin Vasc Surg. 2010;27:107–112.
46. Gaspard S, Gaspard DJ. Retrivable inferior vena cava filters are rarely removed. Am Surg.2009;75:426–428.
47. Comerota A. Retrievable IVC filters: a decision matrix for appropriate utilization. Pers Vasc Surg Endovasc Ther.2006;18:11–18.
48. Spencer F, et al. A population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch Intern Med. 2010;170:1456–1462.