34 Care of the thoracic surgical patient
Atelectasis: Collapse of the alveoli, caused primarily by obstruction of lower airways. Most commonly, this obstruction is caused by accumulation of respiratory secretions, but it may also be caused by diminished lung volumes, tumors, prolonged bronchospasm, and foreign bodies.
Bronchoscopy: Direct visualization of the tracheobronchial tree with use of a lighted scope. It is used for diagnostic and therapeutic interventions for visualization of structures of the tracheobronchial tree; removal of secretions, washings, mucous plugs, or foreign bodies; and performance of a tissue biopsy or application of medication. Bronchoscopy can be combined with yttrium aluminium garnet (YAG) laser therapy for ablation of tracheal and bronchial obstructions. It can be performed in the operating room, special procedures unit, or at the patient’s bedside, depending on the degree of urgency and the patient’s status.
Chest Tube: A drainage tube into the intrapleural space to remove air, fluid, or blood with the goal of restoring normal negative pressure and to allow reexpansion of the lung. The tube is placed on the operative side after open chest procedures.
Chest Wall Reconstruction: Repair of chest wall defects caused by trauma, tumor, or chest wall deformities, with the use of muscle or omentum (underlying abdominal tissue). It provides for protection of underlying structures and organs and provides support for respiration.
Decortication of the Lung: Removal of fibrous deposits or restrictive membranes on the visceral or parietal pleura that interfere with ventilatory action. The goal is restoration of normal lung function.
Endobrachial Ultrasound: A procedure that may be performed during a bronchoscopy, to provide further information to diagnose or determine the stage of a lung cancer. This allows visualization of the lungs and surrounding chest area, which have traditionally required more invasive surgical procedures to evaluate.
Hemothorax: Accumulation of blood or serosanguineous fluid or both within the pleural cavity, compromising lung expansion.
Lobectomy: Removal of one or more lobes of the lung. Lobectomy is the preferred procedure when a cancerous lesion involves a single lobe of the lung. It is used primarily in the treatment of bronchial cancer and is also used in the treatment of bronchiectasis, emphysematous blebs, large benign tumors, fungal infections, and congenital anomalies.
Mediastinoscopy: Direct visualization of lymph nodes or tumors at the tracheobronchial junction, subcarina, or upper lobe bronchi via a lighted scope. This procedure is done by passing the mediastinoscope through a small incision at the suprasternal area and then down along the anterior course of the trachea. It is a diagnostic procedure for patients with identified changes on chest radiograph results.
Needle Biopsy: Insertion of a needle with subsequent aspiration of lung tissue or fluid for diagnostic purposes. It is generally performed with local anesthesia via a percutaneous approach.
Pneumonectomy: Removal of an entire lung, most commonly for lung cancer when lobectomy cannot be performed for total removal of bronchial cancer. It is occasionally indicated for removal of a lung destroyed by chronic infections.
Pneumothorax: Accumulation of air or gas within the pleural cavity, thus compromising lung expansion. Pneumothorax can occur as a direct result of a thoracotomy incision or after chest wall trauma, such as a stab wound.
Segmentectomy (Segmental Resection): Excision of individual bronchovascular segments of the lobe of the lung with ligation of segmental branches of the pulmonary artery and vein and division of the segmental bronchus. Segmentectomy conserves healthy tissue while allowing for removal of localized lesion.
Sleeve Resection: Surgical removal of part of the bronchi, with healthy tissue left for reanastomosis, thus preserving some tissue and lung function. Sleeve resection is used primarily for metastatic disease in either the right or left upper bronchus.
Sternotomy: Incision through the sternum.
Thoracentesis: Insertion of a needle through the chest wall into the pleural space to remove either air or fluid to relieve lung compression or for diagnostic purposes. Removed fluid is evaluated for chemical, bacteriologic, and cellular composition. This procedure can be performed at the bedside, generally with local anesthesia.
Thoracoplasty: Removal of ribs or portions of the ribs to reduce the size of the thoracic space and to collapse a diseased lung.
Thoracoscopy: The insertion of an endoscope, a narrow-diameter tube with a viewing mirror or camera attachment, through a small incision in the chest wall for examination of the lungs or other structures in the chest cavity, without a large incision. The procedure may be diagnostic or therapeutic.
Thoracostomy: An incision of the chest wall for the purpose of drainage. Closed thoracostomy is used to place chest tubes or catheters for drainage of air or fluid to restore normal negative pressure within pleural space. It also can be used to create a surgical access port for video-assisted lobectomy and other endoscopic procedures. Open thoracostomy (partial rib resection) allows healing and reinflation of an infected lung.
Thoracotomy: Incision into the chest cavity that can be used as a diagnostic tool to diagnose or stage cancer. It allows the surgeon access to the thoracic organs including the heart, esophagus, great vessels, or the lungs. Surgery can result from benign or malignant conditions.
Transplantation: Removal of a diseased recipient lung with an immediate replacement of a cadaveric donor lung.
Volume Reduction Surgery: Incision and removal of the parts of the lung that are the most destroyed, most commonly from emphysema, to allow for full function of remaining lung structures.
Wedge Resection: Excision of a small wedge-shaped section from the peripheral portion of the lobe of a lung. It is commonly used to remove cancerous growths in the outer section of the lung to spare lung tissue and function.
Thoracic surgery involves procedures in the structures within the chest cavity, including the lungs, heart, great vessels, and esophagus. In this chapter, discussion focuses on procedures of the lungs and respiratory system. Postanesthesia care after cardiac surgery is discussed in Chapter 35, care after surgery of the great vessels is discussed in Chapter 36, and care after surgery of the esophagus is discussed in Chapter 40.
Lung surgery may be recommended for the diagnosis and treatment of:
• Abnormal chest radiograph results
• Tumors (solitary pulmonary nodules)
• Small areas of long-term infection (highly localized tuberculosis or mycobacterium)
• Pockets of infection (abscess)
• Permanently enlarged (dilated) bronchus (bronchiectasis)
• Permanently enlarged (dilated) section of lung (lobar emphysema)
• Permanently collapsed lung tissue (atelectasis)
• Injuries with collapsed lung tissue (atelectasis, pneumothorax, hemothorax)
• Correction of congenital or acquired chest wall deformities
Anesthesia
Invasive surgery that involves the chest cavity is generally performed with general anesthesia, although diagnostic procedures such as bronchoscopy, needle biopsy, and thoracentesis are commonly performed with local (topical) anesthesia, often with small titrated amounts of intravenous sedation.1 Epidural catheters can also be placed before surgery for use during surgery and for extended postoperative pain control after pneumonectomy or lobectomy. Because these procedures all involve the airway in addition to anesthesia, patients are given nothing by mouth before any procedure.
Epidural anesthesia involves placement of a catheter into the epidural space of the thoracic vertebrae with subsequent instillation of an infusion combination of an opioid and local anesthetic to achieve sensory blockade of pain without compromising motor function needed for coughing, deep breathing, and ambulating. Thoracic epidural anesthesia can be used in combination with either sedation or general anesthesia.1 The catheter can be left in place for up to 3 days after surgery for pain control and may be regulated solely by medical personnel or controlled by the patient. Epidural anesthesia is commonly used as an adjunct to general anesthesia.
Paravertebral or intercostal blockade is a regional technique used for the thoracic surgery patient. The advantage of these blocks is neural blockade; the disadvantage is that they last only until the local anesthetic is metabolized.1 The most commonly used anesthetics for these blocks are lidocaine, bupivacaine, and ropivacaine. Intrapleural local anesthetic instillation can be used for postoperative analgesia, but has the potential for systemic absorption and toxicity.1 See Chapters 24 and 25 for information on local anesthetics and regional anesthesia.
The patient and family should receive detailed information preoperatively. When possible, taking time to improve the patient’s pulmonary, physical, and nutritional status is desirable.2 Smoking cessation is an important preoperative aspect of surgery; however, the effects of smoking linger after cessation with benefits noted after a year. Smokers who have recently quit have no difference in pulmonary complications than current smokers.3 Preoperative medications should be continued with the exception of anticoagulant medications.2 The perianesthesia nurse should review the diagnostic and laboratory tests preoperatively. Preoperative evaluation of the patient who will undergo thoracic surgery may include laboratory tests and pulmonary function tests listed in Table 34-1.
Table 34-1 Laboratory Studies for Assessment of Patients Undergoing Thoracic Procedures
| LABORATORY STUDY | NORMAL RESULTS | SIGNIFICANCE OF ABNORMAL FINDINGS |
| Perfusion Studies—Arterial Blood Gases | ||
| pH | 7.35-7.45 | Changes indicate metabolic or respiratory acidosis. |
| PaCO2 | 35-45 mm Hg | Elevations indicate possible COPD, asthma, pneumonia, anesthetic effects, or use of opioids (respiratory acidosis). Decreased levels indicate hyperventilation/respiratory alkalosis. |
| HCO3− | 21-28 mEq/L | Elevations indicate possible respiratory acidosis as compensation for primary metabolic alkalosis. Decreased levels indicate possible respiratory alkalosis as compensation for primary metabolic acidosis. |
| PaO2 | 80-100 mm Hg | Elevations may indicate possible excessive oxygen administration. Decreased levels indicate possible COPD, asthma, chronic bronchitis, cancer of bronchi and lungs, respiratory distress syndrome, or any other cause of hypoxia. |
| O2 saturation | 95%-100% | Decreased levels indicate possible impaired ability of hemoglobin to release oxygen to tissues. |
| Complete Blood Count | ||
| RBCs | ||
COPD, Chronic obstructive pulmonary disease; HCO3, bicarbonate ion; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; RBC, red blood cell; WBC, white blood cell.
From Pagana KD, Pagana TJ: Mosby’s diagnostic and laboratory test references, ed 9, St. Louis, 2009, Mosby; Rees HC: Assessment of the respiratory system. In Ignatavicius DD, Workman ML, editors: Medical-surgical nursing: patient-centered collaborative care, ed 6, Philadelphia, 2010, Saunders.
Surgical procedures
Surgical procedures can be diagnostic or therapeutic in nature. Diagnostic procedures can include bronchoscopy, mediastinoscopy, laryngoscopy, and thoracoscopy. Bronchoscopies are performed to visualize the airway or remove abnormal tissue, mucous plugs, or foreign bodies. They also aid in evaluating lung lesions and staging of lung cancer. Complications can include airway obstruction, hypoxemia, pneumothorax, hemorrhage, or cardiovascular problems such as dysrhythmias or hypotension (Fig. 34-1).
Mediastinoscopy is performed for direct visualization of lymph nodes or tumors at the tracheobronchial junction, subcarina, or upper lobe bronchi via a lighted scope. The potential for hemorrhage is present because of the close proximity of the innominate vessels and aortic arch to the mediastinoscope. Other complications can include venous air embolism; vagally mediated reflex bradycardia from compression of the trachea or great vessels; airway or esophageal injury, including subcutaneous emphysema; chest pain; or pneumothorax. Recurrent laryngeal nerve injury can occur and manifest symptoms such as hoarseness or vocal cord paralysis.4 A laryngoscopy is performed to visualize or biopsy the oropharynx, laryngopharynx, larynx, or proximal trachea. Complications include trauma to the lips, mucous membranes, teeth, or eyes; rupture of the esophagus; hypoxemia; or laryngospasm. Endobronchial ultrasound (EBUS) is a new minimally invasive technique that allows the proceduralist to see beyond the lumen of the airway. There are two EBUS systems currently available—the radial probe EBUS allows for evaluation of central airways, accurate definition of airway invasion, and facilitates the diagnosis of peripheral lung lesions; and the linear EBUS guides transbronchial needle aspiration of hilar and mediastinal lymph nodes.5
Thoracoscopy is the insertion of an endoscope, a narrow-diameter tube with a camera attachment, through a small incision in the chest wall for examination of the lungs or other structures in the chest cavity, without a large incision. It is performed for basic diagnostic (undiagnosed pleural fluid or pleural thickening) and therapeutic procedures (pleurodesis). Complications can include bleeding, infection of the pleural space, and injury to intrathoracic organs, atelectasis, and respiratory failure.6 This procedure is different from video-assisted thoracoscopic surgery (VATS), an invasive procedure that uses a high-level access platform and multiple ports for separate viewing and working instruments to access pleural space.6 VATS can be diagnostic or therapeutic and is used often for biopsy of mediastinal masses, to perform wedge resections, to obtain hemostasis, or to evacuate blood clots. A variety of procedures can be performed via thoracoscopy, from lung volume reduction to a biopsy and excision of mediastinal lesions. Robotic-assisted thoracic procedures can enhance the speed and safety of VATS. Smaller incisions are used for robotic surgery which may contribute to less postoperative pain and morbidity.7
A significant advantage of thoracoscopy is that it is minimally invasive and results in less incisional pain. It can also decrease recovery time and length of hospital stay. In some facilities, patients come to the PACU with a small chest tube that is pulled if chest radiograph results are clear; the patient then is allowed to go home in a few hours. VATS may be converted to an open surgery if there is an inability to achieve one-lung ventilation, extensive pleural adhesions, uncontrolled or significant intraoperative bleeding, an inability to identify target lesion for biopsy, or technical difficulties with or rarely, primary failure of video equipment and/or endoscopic instruments.8
Perianesthesia nursing care after thoracic procedures
Admission assessment in the PACU is the same as for any other surgical patient (see Chapters 27 and 28). Common problems that lead to delayed discharge from the hospital for the patient who has undergone a thoracic procedure include inadequate pain control, prolonged air leak, severe nausea, fever, debility, and arrhythmias.2 Postoperative care should target prevention or speedy treatment of these complications. Some specific issues for the patient after thoracic surgery are discussed.
Positioning
Positioning after thoracic procedures varies; therefore, medical orders must be checked. The patient may be kept in a side-lying position until awake, and then the head of the bed is elevated 30 to 45 degrees to facilitate ventilation. This position allows the diaphragm to drop into normal position, thus enhancing lung expansion and, if present, facilitating chest tube drainage. After lobectomy, segmentectomy, and wedge resection, the patient can be turned freely from side to side to allow full expansion of lung tissue on both the operative and nonoperative side. After pneumonectomy, the patient may be placed on the back or on the operative side.9 The patient is not positioned side lying on the nonoperative side because the mediastinum is no longer confined by lung tissue and may move freely, thereby compressing the remaining lung or creating traction or torsion of the vena cava. In addition, if the bronchial stump ruptures and bleeds profusely, the unaffected lung is compressed by secretions from the pneumonectomy site.
Respiratory assessment and care
On arrival, the patient is placed on oxygen via the delivery system required per the extent of the patient’s surgery, preexisting medical conditions, and need for continued assistance. Continued assistance may include a nasal cannula after bronchoscopy, face mask or face tent, or mechanical ventilation. Delivered oxygen should be given with humidification to help thin tracheobronchial secretions and thus permit the ciliary mechanism and coughing to clear the airway.
Pain management
Although pain after bronchoscopy is usually limited to a sore throat, the patient undergoing thoracic surgery should be told before surgery to expect a fair amount of postoperative incisional pain. The patient should also be told that pain relief measures are available and may include epidural analgesia, patient-controlled analgesia, and nurse-administered opioids. The thoracotomy incision is an extremely painful incision because of irritation from respiratory effort and any upper body movement (Fig. 34-2).1 Because acute pain after thoracic surgery has been linked to chronic thoracic pain months later, appropriate pain relief must occur. Severe pain during the first couple of days after surgery is predictive of chronic postthoracotomy pain with as many as 67% of patients who underwent a thoracotomy developed persistent postsurgical pain.10
Implications for practice
The use of ketamine postoperatively in conjunction with an opioid provides safe and effective pain management for patients after thoracic surgery. The incidence of hallucination requiring intervention was 2.9%. Other randomized controlled trials found no hallucination or psychological side effects. The perianesthesia nurse should know the indications and side effects for the use of ketamine for acute postoperative pain in thoracic surgery patients.
Source: Mathews TJ, et al: Does adding ketamine to morphine patient-controlled analgesia safely improve post-thoracotomy pain? Interact Cardiovasc Thorac Surg, 2011 [Epub ahead of print].
An effective modality for postsurgical thoracotomy pain is nerve conduction blockade with local anesthetics.1 Pain management via epidural catheter has been shown to provide more effective pain relief after thoracotomy. Of best benefit is epidural analgesia with an opioid and local anesthetic that has begun at least 30 minutes before induction of anesthesia. Pain medications should be given in adequate doses and in a timely manner because pain interferes with needed activities after surgery, including deep breathing, coughing, and progressive mobilization. Because opioids can diminish respiratory function, care must be taken in their administration, especially after general anesthesia. However, a patient whose pain is not adequately controlled is unable to deep breath effectively to maintain oxygenation and prevent atelectasis (Box 34-1).
BOX 34-1 Postoperative Analgesia Modalities
Systemic analgesia
Local anesthetics and nerve blocks
Other techniques
Epidural analgesia
• Spinal injection of opioids can have a duration of analgesia that approaches 24 hours after thoracotomy.
• Epidural techniques reduce the incidence of respiratory complications.
• The majority of thoracotomies received a thoracic epidural between T3 and T8, with infusions of bupivacaine plus either fentanyl or hydromorphone.
• Risks of respiratory depression and hemodynamic instability are due to the use of bolus injection techniques.
• Use of epidural infusions has an excellent record for patient safety when used on routine postoperative surgical wards.
• The majority of thoracotomies receive a thoracic epidural between T3 and T8, with infusions of bupivacaine plus either fentanyl or hydromorphone.
Paravertebral block
• Paravertebral local anesthetics provide a reliable multilevel intercostal blockade. Clinically, the analgesia is comparable to that from epidural local anesthetics.
• Advantages over epidural include comparable analgesia, fewer failed blocks, decreased risk of neuraxial hematoma, and less hypotension, nausea, or urinary retention.
From Slinger PD, Campos JH: Anesthesia for thoracic surgery. In Miller RD, et al: Miller’s anesthesia, ed 7, Philadelphia, 2010, Churchill Livingstone.
In addition to analgesics, pain relief measures can include use of a pillow to splint the incision while the patient coughs. Because coughing is the most effective way to clear secretions, pain medication should be offered and given regularly. See Chapter 31 more detailed information about pain management in the PACU.
Chest tube management
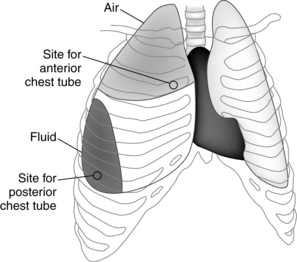
Surgery that involves entry into the thoracic cavity results in air entry and the development of a pneumothorax (atmospheric pressure admitted into the pleural cavity and collapse of the lung). Placement of a pleural chest tube after open-chest procedures allows for drainage of air and blood, restoration of normal negative pressure, and reexpansion of the collapsed lung. Because blood is heavier than air, blood pools in the lower portion of the pleural space, whereas air accumulates in the upper portion. Therefore one or two chest tubes are placed through the chest wall via a stab wound or incision. Most surgeons still place one tube anteriorly and one posteriorly.2 The upper or anterior chest tube is placed in the second intercostal space to allow for air removal. The lower or posterior chest tube is placed in the sixth to eighth intercostal space to allow for drainage from the pleural space (Fig. 34-3). The chest tubes are sutured in place with pursestring sutures and covered with a dressing. The chest tube insertion site should be palpated for the presence of crepitus (also known as subcutaneous emphysema) caused by air trapping in subcutaneous tissue. Crepitus feels like crunchy cereal under the skin. If noted, the surgeon should be notified for probable repeated securing of the chest tube.
The goal of chest tube drainage is to use positive pressure, gravity, and suction to facilitate evacuation of air and fluid that surrounds the lung for reexpansion of the collapsed lung. The air trapped in the chest creates the positive pressure. Gravity assists primarily in fluid evacuation, and suction, when applied, facilitates removal of both air and fluid. Suction is generally established at 20 cm negative pressure, unless specifically ordered differently. Wall units have a manometer in place to allow for correct setting of suction pressure. Some surgeons now prefer to use no suction if the lung is fully expanded.2 When the air and fluid are removed, the visceral and parietal pleura are brought back together again, and the pressure in the interpleural space becomes negative again, thus reexpanding the lung. Disposable prefabricated chest drainage units such as the Pleur-Evac or Atrium systems are used for chest drainage (Fig. 34-4). The chest tubes can be connected to chest drainage with a wet seal or a dry seal. Either dry or wet seal chest drainage has its positive attributes and is capable of evacuating air or fluid. The air flow and negative pressure depends on the type of chest drainage system. The dry seal system is optimal when the transporting the patient.11


FIG. 34-4 Pleur-Evac chest drainage systems. A, Dry and wet seal. B, Dry suction and dry seal.
(Courtesy Teleflex Medical, Research Triangle Park, NC.)
Because the goal of chest tube placement is evaluation of air and fluid, the system must remain patent. The perianesthesia nurse must ensure patency of the chest tubes, drainage tubing, and the system through periodic regular assessments. A chest radiograph is often performed on admission to the PACU to ensure placement and lung function. Proper functioning of the system is evidenced by fluctuation or bubbling of the fluid in the water seal tubing in response to the patient’s respiration.2 If no fluctuation is noted, the system should be evaluated for proper functioning. The tubing must not kink and should form a straight line from patient to collection unit to allow for unobstructed gravitational flow. Milking or stripping of the chest tube may dislodge clots of blood that block the tubing.2 This procedure should be done in the direction away from the patient, toward the drainage system, to prevent forcing clots back into the pleural space. If the system shows no fluctuation or bubbling with respiration and the tubing has been deemed clear, the physician should be notified, especially in the immediate postoperative period. In the latter days of recovery, the absence of fluctuation or bubbling signals reexpansion of the lung, with further evidence provided by full return of breath sounds and chest radiograph results. However, in the PACU, failure of the system prevents lung reexpansion. Any acute respiratory difficulty or pain should be referred immediately to the surgeon.
New modalities exist for use at home when a patient requires palliation of symptoms associated with recurrent pleural effusions (Fig. 34-5). The PleurX catheter system can be used for patients who require minimal intervention. After placement, usually outpatient surgery, the tunneled catheter eliminates the need for thoracentesis.12
Complications
Bleeding
After bronchoscopy, bleeding should be limited to no more than lightly pink-tinged or slightly blood-streaked sputum. Grossly bloody sputum and coughing of frank blood should be reported to the surgeon and anesthesia provider immediately. After a more invasive procedure such as lobectomy or pneumonectomy, the surgical site must be inspected for bleeding and the chest tube for the presence of bloody drainage. Postoperative hemorrhage occurs in approximately 3% of thoracotomies and may be associated with up to 20% mortality. Signs of hemorrhage include increased chest tube drainage (>200 mL/h), hypotension, tachycardia, and a low hematocrit.5 If chest tube drainage seems excessive (>100 mL/h) or does not decrease in volume over time or if fresh bleeding is noted, the physician should be notified to evaluate for hemorrhage. Even if drainage is excessive, the chest tube should never be clamped unless specifically ordered. Clamping of the chest tube may result in the development of a tension pneumothorax, which is considerably more dangerous than an open pneumothorax.
Summary
Care of patients undergoing thoracic surgery or procedures of the lungs and respiratory system was discussed in this chapter. These procedures range from minimally invasive procedures to open thoracotomies. The procedures are performed on patients with underlying conditions such as emphysema or malignant disease that must be surgically treated. Care of these patients in the PACU involves respiratory function, observation for complications, and promotion of oxygenation and ventilation.
1. Barrick BP, Kyle RW. Conduct of anesthesia. Shields TW, et al. General thoracic surgery, ed 7, Philadephia: Lippincott Williams & Wilkins, 2009.
2. Shaw JP, LoCicero JIII. General principles of postoperative care. Shields TW, et al. General thoracic surgery, ed 7, Philadephia: Lippincott Williams & Wilkins, 2009.
3. Barrera R, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest.2005;127:1977–1983.
4. Morgan GEJr, et al. Anesthesia for thoracic surgery. Morgan G, et al. Clinical anesthesiology, ed 4, New York City: McGraw-Hill, 2006.
5. Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc. 2009;6(2):180–186.
6. Ernst A, et al. Interventional pulmonary procedures: Medical thoracoscopy/pleuroscopy. available at: www.medscape.com/viewarticle/455720_16, December 30, 2011. Accessed
7. Blanchard B. Thoracic surgery. Rothrock JC, ed. Alexander’s care of the patient in surgery, ed 14, St. Louis: Mosby, 2001.
8. Yim APC, et al. Video-assisted thoracic surgery as a diagnostic tool. Shields TW, et al. General thoracic surgery, ed 7, Philadephia: Lippincott, Williams & Wilkins, 2009.
9. Marley RA, Hoyle BL. Respiratory care. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing. ed 2. St. Louis: Saunders; 2010.
10. Pasero C. Persistent postsurgical and posttrauma pain. J Perianesth Nurs. 2011;26:38–42.
11. Manzanet G, et al. A hydrodynamic study of pleural drainage systems: some practical consequences. Chest.2005;127:2211–2221.
12. Warren K, et al. Identification of clinical factors predicting PleurX catheter removal in patients treated for malignant pleural effusion. Eur J of Cardio-Thoracic Surg. 2008;33:89–94.
Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
Barash PG, et al. Clinical anesthesia, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2005.
Coughlin AM, Parchinsky C. Go with the flow of chest tube therapy. Nursing. 2006;36(3):L36–42.
Fibla JJ, et al. Early removal of chest drainage and outpatient program after videothoracoscopic lung biopsy. Eur J Cardiothor Surg. 2005;28:604–606.
Fleisher LA. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
Gotoda Y, et al. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5:89–96.
Mason RJ, et al. Murray and Nadel’s textbook of respiratory medicine, ed 5. Philadelphia: Saunders; 2010.
Miller R, et al. Miller’s anesthesia, ed 7. Philadelphia: Churchill Livingstone; 2009.
Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis: Mosby; 2011.
Rieker M. Anesthesia for thoracic surgery. Nagelhout J, Plaus K. Nurse anesthesia, ed 4, Philadelphia: Saunders, 2010.
Senturk M, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002;94:11–15.
Slinger P. Principles and practice of anesthesia for thoracic surgery. New York: Springer; 2011.
Stoelting R, Miller R. Basics of anesthesia, ed 6. Philadelphia: Churchill Livingstone; 2011.