51 Care of the pregnant patient
Aortocaval Compression (Scott Syndrome): Obstruction of the inferior vena cava and the pelvic veins by the enlarging uterus.
Aspiration Pneumonitis: An inflammatory condition of the lungs and bronchi caused by material in the stomach regurgitated into the pharynx and inhaled through the epiglottis into the lungs and bronchi.
Cesarean Section: A surgical procedure in which the abdomen and pregnant uterus are incised and the baby is delivered transabdominally.
Cricoid Pressure (Sellick Maneuver): Used in rapid sequence intubation of the trachea in which the cricoid cartilage is pushed against the body of the sixth cervical vertebra in an effort to compress the esophagus to prevent passive regurgitation.
Defasciculation: Administration of a nondepolarizing muscle relaxant 1 to 3 minutes before the administration of succinylcholine to prevent the muscle twitches that usually occur after the administration of a depolarizing muscle relaxant with the intended outcome of reducing the amount of gastric pressure created by the muscle twitches or fasciculations.
Esophagitis: The inflammation of the mucosal lining of the esophagus.
Gastric Motility: The spontaneous peristaltic movements of the stomach that move the stomach contents through the pyloric sphincter into the duodenum.
Gastroesophageal Reflux: Often referred to as heartburn; a result of a backflow of stomach contents as a result of an incompetent lower esophageal sphincter muscle.
Hypovolemia: A reduction from normal in blood volume.
Physiologic Anemia of Pregnancy: A normal reduction in hemoglobin in the blood as a result of the normal physiologic process of pregnancy.
Plasma Cholinesterase: An enzyme in the plasma that is responsible for the metabolic breakdown of acetylcholine to choline and acetate.
Pruritus: The symptom of itching of the skin.
Retained Placenta: After the birth of the infant, the placenta is usually delivered within 30 minutes; if it is expelled after 30 minutes, it is considered to be a retained placenta.
Sepsis: Infection or contamination.
Thromboembolism: A situation in which a blood vessel becomes obstructed by a clot or thrombus that had been carried by the bloodstream from its site of formation.
The incidence rate of surgery performed on pregnant women for reasons unrelated to the pregnancy itself has been reported to be as high as 50,000 to 75,000 cases per year, with a frequency range of 0.75% to 2%.1 The most common conditions that require surgical intervention are acute appendicitis, ovarian cysts, and breast tumors, with laparoscopy being the most common first-trimester procedure. However, more complicated procedures have been reported and include craniotomy, open-heart surgery, and aneurysm repair that have been performed successfully in pregnant patients.1,2
In care of the pregnant patient after surgery, one must remember that two patients require nursing care and assessment: the mother and the fetus. Perianesthesia nursing care should be directed toward emotional support for the mother and avoidance of uterine stimulation that could produce preterm labor. Also of prime importance are prevention of respiratory depression in the mother and maintenance of normal uterine placental blood flow to ensure adequate fetal supply of oxygen and nutrients.1–3
Physiologic changes of pregnancy
Almost every system in the body is affected in some way during pregnancy, either from hormonal changes or from the increasing size of the uterus. The changes that affect perianesthesia nursing care are outlined in Box 51-1 and are discussed in the following sections.
BOX 51-1 Physiologic Changes in Pregnancy
Cardiovascular system
Cardiovascular changes
Hemodynamic alterations
The cardiovascular system undergoes significant change as pregnancy advances. Cardiac output and heart rate increase progressively during pregnancy until, at 30 to 34 weeks’ gestation, the cardiac output is 30% to 50% higher than normal and the heart rate is approximately 15% greater than the nonpregnant normal level, with electrocardiographic changes and heart sounds possibly developing (Box 51-2). The systolic blood pressure is minimally affected by pregnancy, with a maximum decline of approximately 8% during early to middle gestation and a return to the pregnant level at term. Diastolic blood pressure falls to a greater degree than does systolic pressure, with early to middle gestational decreases of approximately 20%. It also returns to a prepregnancy level at term.4
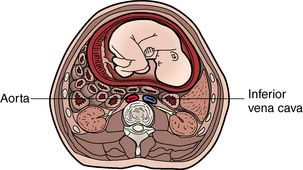
Perhaps the most significant effect on the cardiovascular system for the nurse to consider in routine postanesthesia management is obstruction of the inferior vena cava and the pelvic veins by the enlarging uterus (Figs. 51-1 and 51-2). This condition, known as aortocaval compression or Scott syndrome, can develop by the second trimester and cause supine hypotension. Avoidance of the supine position becomes mandatory after surgery, because it can significantly aggravate the obstruction. Treatment includes the administration of additional intravenous crystalloid, the placement of the mother in the side-lying position, and the administration of supplemental oxygen (Fig. 51-3).5,6
FIG. 51-1 Cross section of lower abdomen (nonpregnant).
(From Bontrager KL, Lampignano JP: Textbook of radiographic positioning and related anatomy, ed 7, St. Louis, 2010, Mosby.)
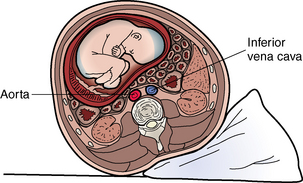
FIG. 51-3 Uterine displacement with wedge under hip to relieve aortocaval compression.
(From Lowdermilk DL, et al: Maternity and women’s health care, ed 10, St. Louis, 2012, Mosby.)
Collateral circulation for venous return develops through the intervertebral venous plexus and the azygos vein. This condition reduces the volume of the epidural and subarachnoid spaces; therefore the amount of drug during regional anesthesia should be decreased. With this in mind, the perianesthesia nurse should assess the patient on admission for a high block and monitor dermatome levels frequently thereafter (see Chapter 25).5,6
In the nonpregnant patient, the sympathetic nervous system plays a role in promoting venous return to the heart from the lower extremities. This sympathetic stimulation of vasomotor tone is enhanced during pregnancy in an effort to counteract the negative effects of uterine compression of the vena cava. Clinically, this protective mechanism is abolished with spinal or epidural anesthesia because it acts as a pharmacologic sympathectomy. Without an appropriate preload of fluids (500 to 1000 mL intravenous lactated Ringer solution), the pregnant patient could have a 30% to 50% decrease in blood pressure during the anesthesia. The pregnant patient must receive an appropriate preload of fluids before epidural or spinal anesthesia. Hemodynamic stability can be secured with the infusion of 15 mL/kg of colloid solution or 30 mL/kg of crystalloid solution. If the patient is to receive an inhalation anesthetic agent such as isoflurane or sevoflurane, similar fluid preloading is given because the inhalation anesthetic agents produce peripheral vasodilation.7
This increased fluid requirement has significant implications for the perianesthesia care of the pregnant patient. Consequently, the patient’s cardiac and hydration status must be monitored closely throughout the emergence phase of regional anesthesia (see Chapter 25).7,8
Hematologic alterations
Blood volume along with the number of platelets, fibrinogen levels, and the level of activity of several clotting factors (VII, VIII, IX, X, and XII) increases by 15% during the first trimester, rises rapidly during the second trimester to 50% above the pregnant levels, and changes little during the remainder of the pregnancy.7,8 However, a smaller rise in the number of circulating red blood cells occurs. This difference results in lower hematocrit and hemoglobin levels (see Box 51-1), although red blood cell mass actually increases. This condition is known as physiologic anemia of pregnancy.7,8
The plasma concentration of the enzyme cholinesterase is decreased during pregnancy. Because plasma cholinesterase is involved in the mechanisms of clotting, the perianesthesia nurse should monitor the pregnant patient for thromboembolism. Plasma cholinesterase is also involved in the destruction of the depolarizing muscle relaxant succinylcholine. The recovery time from succinylcholine is unaltered and in fact may be somewhat faster in pregnant women, which is explained by the fact that the volume distribution of succinylcholine increases during pregnancy because of an elevation in the plasma volume. In the immediate postpartum period, the plasma cholinesterase concentration and the plasma volume distribution are further reduced.7–10
Respiratory changes
Upper airway anatomy
During pregnancy, capillary engorgement of the upper respiratory tract includes the nasal and oropharyngeal mucosa and larynx, and pregnant women may have nasal stuffiness. In addition, nose breathing is difficult, and nosebleeds can occur. This capillary engorgement of the respiratory mucosa during pregnancy predisposes the upper airways to trauma, bleeding, and obstruction. Gentle laryngoscopy and the use of small endotracheal tubes (6 to 6.5 mm) should be used during general anesthesia.7–10
Lung mechanics and ventilation
The diaphragm elevates, and the rib cage flares; therefore at term, 85% of respiratory effort is intercostal and 15% diaphragmatic (normally, approximately 70% is intercostal, and 30% is diaphragmatic). Because of the mechanical changes in the lungs and chest wall, the lung volumes and capacities change during pregnancy. Overall, the inspiratory lung volumes and capacities moderately increase, and the expiratory lung volumes and capacities decrease. The inspiratory reserve volume and the inspiratory capacity increase by 5% to 15%. The functional residual capacity (FRC) decreases by approximately 20% to 60%. The residual volume and expiratory reserve volume, which compose the FRC, are decreased. The combination of decreased FRC and increased oxygen consumption promotes rapid oxygen desaturation during periods of apnea.7–10
The tidal volume also increases 20% to 45%, and the respiratory rate does not change, which leads to a 30% to 45% increase in the alveolar ventilation and the minute ventilation. Therefore, during pregnancy, the arterial oxygen level ranges from 95 to 105 mm Hg and the arterial carbon dioxide level is approximately 30 mm Hg, with an arterial pH of 7.44. Consequently, the pregnant patient has some respiratory alkalosis for which the renal excretion of bicarbonate compensates. As a result, the normal bicarbonate level during pregnancy is approximately 19 mEq/L, and the base excess is reduced by 2 mEq/L.7–10
Limited information exists regarding the effects of pregnancy on characteristics of the central chemoreflex control of breathing. Some studies suggest that the increased circulation of hormones, particularly progesterone and estradiol, significantly lowers the threshold and increases the sensitivity of the central ventilatory chemoreflex response to CO2. In regard to flow rate changes, the forced expiratory volume in 1 second and the flow-volume loop remain unchanged. In addition, the closing capacity (see Chapter 12) does not change during gestation.7–10 Consequently, the conductance and resistance of the small and large airways do not change during pregnancy.
Gastrointestinal changes
Motility and secretions
Gastric emptying slows during pregnancy because the stomach is displaced as the uterus enlarges, which leads to gastroesophageal reflux and esophagitis during pregnancy. All parturients have a gastric pH less than 2.5, and more than 60% have gastric volumes greater than 25 mL. The gastric volume also increases during hours 1 to 8 in the postpartum period; therefore the perianesthesia nurse must be cognizant of the potential for vomiting and aspiration, particularly in patients who have had general anesthesia. Muscle relaxants may have been used and can result in the patient’s normal protective mechanisms being obtunded. Again, the side-lying position becomes of significant importance.7–10
Renal changes
Early in pregnancy, the kidneys receive an increased blood flow because of renal vasodilation, and glomerular filtration and urine formation rates increase. This increase is necessary to handle the increased amount of waste products produced. Monitoring of output should reflect this expected increase in volume. Intervention may be necessary for hypovolemia even though the urine output is within ranges acceptable in a nonpregnant patient.7–10
Increased water retention is a basic chemical alteration of pregnancy. This alteration leads to a decrease in plasma sodium concentration from 140 to 136 mmol/L and a decrease in plasma osmolality from 290 to 280 mOsmol/kg. Serum potassium levels decrease by an average of 0.2 to 0.3 mEq/L.7–10
Care of the obstetric patient
Positioning
The supine position causes a reduction in uterine blood flow in the pregnant patient; therefore the semi-Fowler position is used when possible. To prevent aortocaval compression, the patient is placed in the lateral decubitus position, and the right hip is elevated with a pillow, or the uterus is displaced to the left with devices on the operating table.11,12
Gastrointestinal considerations
The pregnant patient has a reduced gastric emptying time and a reduced gastric pH. Research has shown that gastric volume and acidity in the pregnant patient do not differ significantly from those in the nonpregnant patient. However, many anesthesia clinicians believe strongly that the pregnant patient, especially the patient with pyrosis (heartburn), is at risk of developing aspiration pneumonitis. Consequently, preoperative pharmacologic interventions are usually taken. Drugs that can be administered include a nonparticulate antacid such as 30 mL of 0.3 mmol/L sodium citrate (Bicitra) to increase the gastric pH; cimetidine (Tagamet) or 50 mg ranitidine (Zantac) or granisetron (Kytril), which are histamine-2 receptor blockers that reduce gastric acid secretion; and 10 mg metoclopramide (Reglan), which accelerates gastric emptying time and elevates lower esophageal tone.13–15
General anesthesia
Induction
Because of the strong full-stomach considerations, the pregnant patient is intubated with a rapid-sequence endotracheal intubation technique (see Chapter 30) that includes intravenous (IV) propofol, etomidate, or ketamine followed by succinylcholine. A defasciculation dose of a nondepolarizing muscle relaxant can be given before the administration of the succinylcholine to avoid the increase in intragastric pressure. Some clinicians do not administer a defasciculating dose of a nondepolarizing muscle relaxant, because most pregnant patients do not have fasciculation after succinylcholine. Cricoid pressure (Sellick maneuver) is applied with an assistant’s thumb and index fingers exerting downward pressure on the cricoid cartilage to displace the cartilaginous cricothyroid ring posteriorly and thus compress the underlying esophagus against the cervical vertebrae.16–19
The endotracheal tube should be a small tube, usually 6.0 to 6.5 mm in diameter or smaller, because of increased mucosal engorgement in the nasal and oropharyngeal areas. Nasotracheal intubation is not used because of the high risk of tissue trauma.16–19
Maintenance
Nitrous oxide in 50% concentration with oxygen is usually administered. Inhalation agents such as sevoflurane, isoflurane, and desflurane can be used. Analgesic concentrations of 0.5 minimum alveolar concentration (see Chapter 20) or less to avoid significant uterine relaxation can be used safely for these inhalation drugs. However, these inhalation agents, particularly sevoflurane, may be used in high concentrations for a short period to produce uterine relaxation for intrauterine manipulation of the fetus or removal of a retained placenta. The clinical implication in the postanesthesia care unit (PACU) for the patient who received a high concentration of an inhalation agent, even for a short period (less than 2 minutes) is monitoring for postpartum hemorrhage and maternal hypotension. The uterine response to oxytocic drugs is reduced when high concentrations of these inhalation agents are used.16–19
Source: American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies: Practice guidelines for perioperative blood transfusion and adjuvant therapies, Anesthesiology 105:198–208, 2006; Borgman MA, et al: The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital, J Trauma 63:805–813, 2007.
In regard to skeletal muscle relaxation, succinylcholine infusion or short-acting nondepolarizing muscle relaxants such as rocuronium and vecuronium can be used safely, because they lack autonomic side effects and have a low degree of placental transfer. In the immediate postpartum period, the neuromuscular-blocking effects of vecuronium are prolonged.16–19
Regional anesthesia
Regional anesthesia, primarily spinal and epidural, is used extensively for anesthesia in the pregnant patient because it produces analgesia without causing neonatal depression. This technique also reduces the risk of maternal hypoventilation and the need for opioids and sedatives. Regional anesthesia does not require airway management, preserves airway reflexes, and allows the mother to remain awake during birth. It is contraindicated in patients with severe coagulation problems, severe hypovolemia, sepsis, and infection at the needle insertion site; in situations in which immediate delivery is crucial, such as in fetal distress; and when the patient refuses the procedure.16,20,21
The level of sensory blockade for either spinal or epidural anesthesia for cesarean section is from T4 to S4. The commonly used local anesthetic drugs for spinal anesthesia are lidocaine and bupivacaine (see Chapter 24). For epidural anesthesia, the commonly used local anesthetic agents are 2-chloroprocaine (Nesacaine), lidocaine with epinephrine, bupivacaine, and ropivacaine, which may be preferable because of possibly less motor blockade and reduced potential for cardiotoxicity.16,20,21
In comparison with the spinal approach, the epidural approach is the preferred technique because drugs can be administered throughout the surgical procedure via a continuous epidural catheter. The anesthesia clinician then has the ability to control the onset, distribution, and duration of anesthesia. In addition, the incidence rate of postdural puncture is much lower in comparison with the spinal technique.16,20–21
Perianesthesia care of the mother and the fetus
The care of the pregnant patient after surgery should be the same as for any patient who undergoes that procedure or for one who recovers from that particular anesthetic. However, additions to the routine nursing care must be instituted for all pregnant patients.22–24
Psychologic and emotional support
The mother’s concern for her unborn child is paramount. Constant reassurance is mandatory. If possible, allow the mother to listen to the fetal heartbeat frequently during the recovery phase. Explain all procedures and why they are being done before they are followed. If the PACU allows visitors, involvement of the father should also be considered.25–27
Fetal monitoring
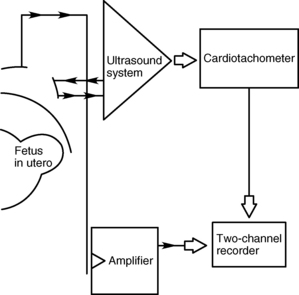
The fetal heart rate must be monitored every 15 minutes if the fetus has reached viability (Table 51-1). If available, an indirect fetal monitoring system should be used for constant assessment of fetal stability (Fig. 51-4). If the fetus is considered previable, it is generally sufficient to ascertain the fetal heart rate by Doppler before and after the procedure.25,28
| DESCRIPTION | RATE (BEATS/MIN) |
|---|---|
| Normal fetal heart rate | 120-160 |
| Moderate tachycardia | 160-180 |
| Marked tachycardia | >180 |
| Moderate bradycardia | 100-120 |
| Marked bradycardia | <100 |
The second type of monitoring required is close observation of the patient for signs of premature labor. These signs include spontaneous rupture of membranes, increased fetal heart rate, presenting of vaginal mucus plug, uterine palpitations, uterine contractions, and restlessness of the mother.25,28
Initially, the patient might not feel the contractions or be aware of membrane rupture; therefore palpation of the abdomen and assessment of vaginal discharge must be performed by the nurse. If premature labor begins, transfer of the patient to the labor and delivery area as soon as possible is recommended. A drug may be necessary to stop labor. These drugs should be administered by personnel familiar with proper protocols for administration and side effects.25,28
Pain management
The patient after cesarean section
The patient after cesarean section presents unique challenges in regard to pain management. More specifically, more women desire to care for their newborns within the first 24 hours. Heavy sedation with opioids and IV or epidural catheters inhibits the mother’s ability to care for the infant. In addition, the infant can be affected through the transfer of the drug in breast milk.20,23,29
Patient-controlled analgesia (PCA) is becoming popular in pain management of the patient after cesarean section (see Chapters 22, 27, and 31). PCA interrupts the pain cycle, gives the patient a feeling of control, hastens the time to ambulation, and reduces the length of stay in the hospital. Morphine is the preferred drug to be administered via PCA. The usual dose is 1 to 1.5 mg, with a lockout of 10 minutes and an hourly limit of 10 to 12 mg.16,29
Epidural administration of opioids is another option that can be used for the control of pain in the PACU. Morphine is the preferred drug because of its demonstrated safety and prolonged duration of action after a single dose of 5 mg. The primary side effects of epidural morphine are pruritus and nausea. Some clinicians have advocated the use of the opioid antagonist naloxone (5 to 10 mcg/kg/h) to treat these side effects; however, problems arise with some reversal of the analgesia. Most clinicians treat the nausea with 2 to 4 mg of ondansetron (Zofran) IV and the pruritus with 12.5 mg of IV diphenhydramine (Benadryl).16,29
Fentanyl is an alternative to morphine for epidural analgesia. The difficulty with fentanyl is that its duration of action is less than 5 hours and that it must be administered via continuous infusion or intermittent boluses. The epidural fentanyl technique provides excellent analgesia; however, most women desire to be ambulatory as soon as possible and do not like to be encumbered with the catheter, tape, and pump.16,29,30
When administered epidurally with opioids, 2-chloroprocaine inhibits the analgesic effects of the opioids. This inhibitory action of 2-chloroprocaine is probably caused by the ethylenediaminetetraacetic acid (EDTA) that is used in the solution of the drug. EDTA is an antioxidant that has analgesic antagonism properties because it is a strong chelator of calcium. Clinically epidural opioids should be avoided for at least 6 to 8 hours after 2-chloroprocaine has been administered.16,29,30
A variety of receptor-specific drugs to include opioids, alpha2-adrenergic agonists, and local anesthetics are being evaluated for use via the intraspinal approach. With the refining of the spinal technique to include the use of small needles, this technique is gaining in popularity for post–cesarean section pain relief. Morphine, 0.3 mg administered intrathecally, has a longer duration of action than 4 mg of morphine administered via the epidural route. Some anesthesia clinicians are now adding morphine to bupivacaine during surgery, with the outcome of excellent analgesia that lasts well into the postanesthesia period.16,29,30
Along with the various opioid analgesics, the patient after cesarean section should be administered supplemental oxygen during anesthesia and for the first 2 hours after surgery, not only to enhance pulmonary function but also to potentially help in the prevention of postoperative nausea and vomiting. More research is needed to evaluate the effectiveness during the immediate postanesthesia period.16,29,30
The pregnant patient
Because of the growth in consumer awareness, the administration of medication during pregnancy has become a controversial issue that must be addressed on an individual basis. For pain management in the PACU, when the pregnant patient is in a hypersuggestive state, distraction techniques such as guided imagery and breathing exercises have had favorable results in place of drugs.31,32
If a mild analgesic is needed, the drug of choice is acetaminophen (Tylenol). For moderate pain management, oral or sublingual opioids (morphine, methadone, codeine, and in some countries oxycodone, buprenorphine, and fentanyl) can be used safely for short periods during pregnancy. If prolonged administration is expected, drugs without active metabolites are preferable, such as methadone rather than morphine for short-term pain management. Opioid analgesia may be warranted for severe pain but must be used judiciously, with the respiratory depressive effects kept in mind.31–33 Ketorolac (Toradol), a prostaglandin synthetase inhibitor, is becoming popular in postoperative pain therapy. However, nonsteroidal antiinflammatory agents, such as ketorolac, are not recommended in the parturient, because they suppress uterine contractions and promote closure of the fetal ductus arteriosus.31–34
The gravid patient in the PACU is a rare occurrence that requires the nurse to have a great deal of knowledge and the ability to provide continual support during this stressful situation. The nurse must be able to provide a quiet calm reassuring atmosphere for the patient. The objective of the care delivered is an optimal environment for both the mother and the fetus.31–34
Summary
The perianesthesia nursing care of the obstetric patient who is undergoing a nonobstetric surgical procedure is both challenging and rewarding. Statistics indicate that approximately 1 in 500 pregnancies are complicated by nonobstetric surgical conditions. The intent of this chapter is to provide information on the pregnant patient and fetus undergoing nonobstetric surgery. The physiologic changes, types of surgery, and perianesthesia nursing care were highlighted and should provide the perianesthesia nurse with valuable knowledge to facilitate the appropriate outcomes for the mother and fetus.35
1. Abboud T. Nonobstetric surgery during pregnancy. Semin Anesth. 1992;11(1):51–54.
2. ACOG Committee Opinion Number 474: Nonobstetric surgery during pregnancy. Obstetrics and Gynecology.2011;117(2):420–421.
3. Alspach J. Core curriculum for critical care nursing. ed 6. Philadelphia: Saunders; 2005.
4. Barash P, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
5. Benumof J, Saidman L. Anesthesia and perioperative complications, ed 2. St. Louis: Mosby; 1999.
6. Belfort M, et al. Critical care obstetrics, ed 5. Oxford: Wiley-Blackwell; 2010.
7. Chestnut D. Obstetrical anesthesia: principles and practice. ed 4. Philadelphia: Mosby; 2009.
8. Chichester M. When your patient is from the obstetric department: postpartum hemorrhage and massive transfusion. J Perianesth Nurs.2005;20(3):167–176.
9. Conklin K. Maternal physiological adaptations during gestation, labor, and the puerperium. Semin Anesth. 2005;10(4):221–234.
10. Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
11. Bowdle T, et al. The pharmacologic basis of anesthesiology. New York: Churchill Livingstone; 1994.
12. Martin J. Positioning in anesthesia and surgery, ed 3. St. Louis: Mosby; 1997.
13. Miller R, et al. Miller’s anesthesia, ed 7. Philadelphia: Churchill Livingstone; 2009.
14. Moran D, Dewan D. Anesthesia for cesarean delivery. Semin Anesth. 1991;10(4):286–294.
15. Morgan E, et al. Clinical anesthesiology, ed 4. New York: McGraw-Hill; 2006.
16. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. Philadelphia: Saunders; 2010.
17. Shnider S, Levinson G. Anesthesia for obstetrics. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2002.
18. Stoelting R. Pharmacology and physiology in anesthetic practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
19. Miller R, Pardo MCJr. Basics of anesthesia, ed 6. New York: Saunders; 2011.
20. Eisenach J. Pain management in the parturient: theoretical and practical aspects. Semin Anesth.1992;11(1):55–65.
21. Estafanous F, et al. Cardiac anesthesia, ed 2. Philadelphia: Lippincott Williams & Wilkins; 2001.
22. Coté C, et al. A practice of anesthesia for infants and children. ed 4. Philadelphia: Saunders; 2008.
23. Gbods A, Set al. Effect of postoperative supplemental oxygen on nausea and vomiting after cesarean birth. J Perianesth Nurs. 2005;20(3):200–205.
24. Torgersen K. Communication to facilitate care of the obstetric surgical patient in a postanesthesia care setting. J Perianesth Nurs. 2005;20(3):177–183.
25. Lake C, et al. Clinical monitoring: practical applications for anesthesia and critical care. St. Louis: Mosby; 2001.
26. Davidson M, et al. Maternal-newborn nursing and women’s health across the lifespan, ed 9. Upper Saddle River, NJ: Pearson Education; 2011.
27. Curran C. Perianesthesia care following obstetric emergencies at risk for multisystem organ dysfunction. J Perianesth Nurs. 2005;20(3):185–199.
28. Motoyama E. Smith’s anesthesia for infants and children, ed 8. St. Louis: Mosby; 2011.
29. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing. St. Louis: Saunders; 2010.
30. Hardman J, Limbird L. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill; 2010.
31. Chestnut DH, et al. Chestnut’s obstetric anesthesia. ed 4. Philadelphia: Mosby; 2009.
32. Shaver S, Shaver D. Preoperative assessment of the obstetric patient undergoing abdominal surgery. J Perianesth Nurs. 2005;20:160–166.
33. Fleisher LA. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
34. Katzung B. Basic and clinical pharmacology, ed 11. Los Altos, CA: Appleton & Lange; 2009.
35. Ziadlourad F, Conklin K. Anesthesia for obstetric emergencies. Semin Anesth. 1989;8(3):222–231.