53 Care of the patient with thermal imbalance
Active Warming Measures: Include the application of forced air convective warming, circulating-water mattresses, resistive heating blankets, radiant warmers, negative-pressure warming systems, and warmed humidified oxygen.
Core Thermal Compartment: Consists of the organs of the trunk and head, which compose 50% to 60% of the body mass. Tissues are well perfused and maintain a relatively uniform temperature.
Malignant Hyperthermia: A hereditary abnormality of muscle metabolism caused by certain triggering agents and resulting in a life-threatening pharmacogenetic disorder.
Normothermia: A core temperature range of 36° to 38° C.
Passive Insulation: Warmed cotton blankets, reflective blankets, socks, head covering, and limited skin exposure.
Perioperative Hypothermia: A core body temperature less than 36° C.
Peripheral Thermal Compartment: Consists of the arms and legs. Temperature is not homogenous and varies over time.
Preventative Warming: Initiation of passive insulation or active warming measures to maintain normothermia.
Thermal Comfort: A patient’s subjective description of temperature comfort level.
Patients admitted to the postanesthesia care unit (PACU) usually have some form of thermal imbalance. Thermal imbalance is defined as body core temperature that is outside the normothermic rage of 36° to 38° C.1,2 This chapter reviews the physiology of thermoregulation, the concepts of perioperative thermoregulation and hypothermia, malignant hyperthermia, and the effect of these issues on the care of the patient in the PACU.
Overview of thermoregulation
The body maintains its temperature between the narrow range of 36° to 38° C.1–3 Although the peripheral thermal compartment (consisting of the arms and legs) temperatures can vary with environmental and thermoregulatory responses, temperature in the core body compartment is controlled within a 0.2° C range by a balance of heat production and heat loss typically regulated by thermoregulatory mechanisms in the central nervous system (CNS). These mechanisms receive input from various thermoreceptors located in the skin, nose, oral cavity, thoracic viscera, and spinal cord. These thermoreceptors send sensory information in hierarchical order: spinal cord, reticular formation, and primary control in the preoptic hypothalamic region of the brain.3–6
The central temperature controls maintain body temperature with two primary responses: physiologic and behavioral. The physiologic thermoregulatory response consists of sweating, shivering, and alterations in the peripheral vasomotor tone. These responses control the regulatory process of body temperature; consequently, heat loss is reduced with vasoconstriction and increased with vasodilation and sweating. The responses also work by reducing heat production, lowering the metabolic rate, and increasing muscle tone and shivering to enhance heat production. Behavioral thermoregulation is triggered by subjective feelings of discomfort or comfort. For example, in a hot environment, a person seeks air conditioning; in a cold environment, the person seeks heat. The response mechanism is stronger, but does not exhibit fine motor control as in the physiologic thermoregulatory response system.3,4,6
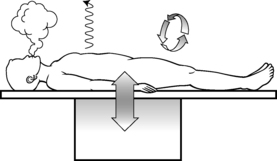
Body heat is produced by metabolism and has a circadian cycle, with the core temperature lower in the morning than in the afternoon.7 Body heat is removed with four methods of heat transfer: radiation, conduction, convection, and evaporation, all of which play a significant role in the development of perioperative hypothermia (Fig. 53-1).
Radiation
Radiation involves the loss of energy, in this case heat, through the radiant electromagnetic waves in the infrared spectrum. It involves no direct contact between the objects involved, but simply occurs as heat radiates from a warmer object to a cooler one. Radiation heat loss accounts for 40% to 60% of all heat loss and occurs in the operating room (OR) as the uncovered skin of the surgical patient radiates energy away from the patient, resulting in a drop in body temperature. Neonates and older adults are particularly prone to heat loss via this mechanism.2–6
Conduction
Conduction involves the transfer of heat energy through direct contact between objects. Conduction loss accounts for as much as 10% of heat loss in the OR and can occur via several mechanisms, including patient contact with a cold OR table, skin preparation solutions, intravenous (IV) fluids, irrigants, and cold sheets and drapes.2–6
Convection
Convection involves the loss of body heat via transfer to the surrounding cooler air and occurs with a temperature gradient between the body and surrounding air. It accounts for 25% to 50% of heat loss in the OR. This transfer can occur in two ways. Passive movement occurs as a loss of body heat from basic skin exposure as warm air rises. Active movement, which can be facilitated by the laminar flow systems in an OR, occurs as a loss of body heat from a fan or wind blowing across the body surface.2–6
Temperature measurement
Patients have rapid core temperature changes during the perioperative period. During such periods of rapid temperature fluctuation, a core temperature measurement provides the most accurate indication of body temperature. Temperature measurement during this period must be accurate and consistent and provide a true reflection of the core temperature. The relationship between temperatures measured at various body sites during this period, however, may differ significantly from a true core reading. Consideration of the best method for obtaining a temperature must also account for accessibility of the measurement site, patient comfort and safety, and the practitioner’s ability to consistently use the device correctly.1,2,4,6,8 The same route of temperature measurement should be used throughout the perianesthesia period to allow for accurate temperature comparisons across the surgical continuum, and any extreme temperature (hypothermic or hyperthermic) detected with a noncore measurement instrument should be interpreted with caution.1
The most accurate core temperature measurement is obtained via use of a pulmonary artery (PA) catheter, because the artery bathes the catheter with blood from the core compartment and its surroundings. Temperature readings at the site can be affected by the rapid infusion of large amounts of warmed or cold IV fluids, by respiratory cycles, and by lower limb pneumatic compression devices. Readings from the distal esophagus and nasopharynx provide accurate alternatives to the PA catheter and are commonly used during surgery; however, like the PA catheter, these methods are invasive in nature and are not appropriate outside the operative setting after the patient has been extubated.2,4,6,8
Oral temperature measurement with electronic digital thermometers is a popular method of temperature measurement that is easily accessible, is less prone to operator error, and quickly reflects changes in core body temperature. Oral temperature readings vary based on placement in the oral cavity (Fig. 53-2).9 Oral temperature measurement taken in the right or left posterior sublingual (buccal) pocket provides an accurate reflection of core temperature, even in the presence of oxygen therapy, warmed and cooled inspired gases, and varied respiratory rates.1,8,10
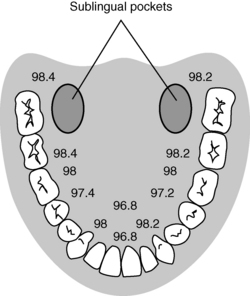
FIG. 53-2 Temperature variations in oral cavity.
(From Nicoll LH: Heat in motion: evaluating and managing temperature, Nursing 32:s12, 2002; Schick L, Windle PE, editors: Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 2, St. Louis, 2010, Saunders.)
Temporal artery thermometry is a noninvasive radiation thermometer used with a scanner probe to scan the forehead and capture the infrared heat from the arterial blood supply and record the highest temperature sensed. Current evidence supports the accuracy of temporal artery readings as a core temperature measurement at normothermic temperatures; however, the accuracy of temporal artery measures at temperature extremes has not been established.1,11–13
Infrared tympanic thermometry, previously considered a preferred method for noninvasive core temperature measurement, can be adversely affected by common sources of instrument error, including poor operator technique, patient anatomy (site), and the calibration, accuracy, and inherent instrument error of the thermometer used.8 Given the issues surrounding temperature measurement with this instrument, it is no longer recommended as an accurate means of temperature measurement during the perianesthesia period.1
Implications for practice
Consistent temperature measurement is critical to the diagnosis and subsequent management of thermoregulatory disturbances in the perianesthesia patient. Invasive core temperature measurement is preferred because it yields the most accurate temperatures; however, such methods are generally not plausible in the preoperative and postoperative periods. Noninvasive core temperature measurement methods vary in their relationship to invasive core temperature measurements. As such, nurses should use the same temperature measurement approach throughout a patient’s perianesthesia experience to establish accurate temperature trends.
Source: Fetzer SJ, Lawrence A: Tympanic membrane versus temporal artery temperatures of adult perianesthesia patients, J Perianesth Nurs 23(4):230–236, 2008.
Perioperative hypothermia
Perioperative hypothermia is defined as a core body temperature less than 36° C.1 As many as 70% of surgical patients have hypothermia in the course of the surgical experience.5 All patients undergoing general or regional anesthesia are at risk for developing unplanned perioperative hypothermia. Additional risk factors include1:
• Systolic blood pressure less than 140 mm Hg
• Length and type of surgical procedure
• Normal or below normal body mass index
• Body surface or wound area uncovered
Adverse effects associated with perioperative hypothermia include1,2:
The typical temperature drop associated with perioperative hypothermia is between 1° and 3° C and depends on the type and dose of anesthesia, amount of surgical exposure, and ambient room temperature. This temperature drop occurs from a loss of normal physiologic thermoregulatory mechanisms that are impaired by anesthetic drugs. As a result, the patient becomes poikilothermic and, without intervention, takes on the cooler temperature of the operative environment.3,14
Intraoperative temperature loss typically occurs in a characteristic pattern as a result of core-to-peripheral redistribution (Fig. 53-3). Redistribution occurs as a result of a reduction in the vasoconstriction threshold from the inhibitory impact of general anesthesia, resulting in a drop in core temperature, and peripheral vasodilation triggered by both general and regional anesthesia, which causes an increase in the blood flow to the skin and a resulting loss in core body heat. An initial heat loss of 1° to 1.5° C occurs during the first hour of surgery, followed by a slower more linear drop over the next 2 to 3 hours. Core temperature loss generally does not stabilize until 2 to 4 hours into the surgical procedure (Fig. 53-4). Postoperative return to normothermia occurs when the brain anesthetic concentration decreases enough to allow a normal thermoregulatory response. This response can take as long as 2 to 5 hours to occur and may be inhibited by residual anesthetics and postoperative opioids.2–4,6,14
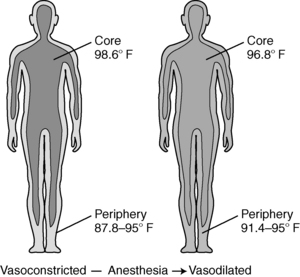
FIG. 53-3 Core-to-peripheral redistribution after administration of anesthesia.
(From Sessler DI: Perioperative heat balance, Anesthesiology 92:583, 2000. In Schick L, Windle PE, editors: Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 2, St. Louis, 2010, Saunders.)
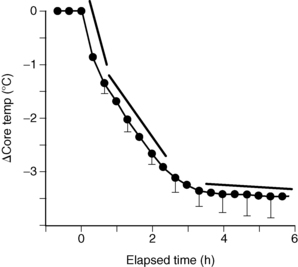
FIG. 53-4 Perioperative heat loss over time.
(From Sessler DI: Perioperative heat balance, Anesthesiology 92:583, 2000. In Schick L, Windle PE, editors: Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 2, St. Louis, 2010, Saunders.)
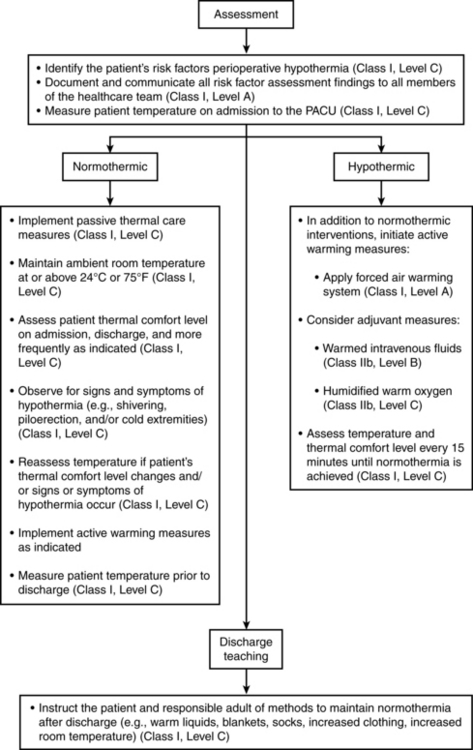
Every patient should be assessed for hypothermia on arrival in the PACU, and postoperative care should be provided per the multidisciplinary American Society of PeriAnesthesia Nurses (ASPAN) Clinical Guideline for the Maintenance of Perioperative Normothermia (Fig. 53-5).1 In the case of normothermia, preventative warming measures and passive insulation should be instituted. The ambient room temperature should be maintained at or greater than 24° C, and the patient’s thermal comfort level should be assessed at least on admission and discharge. In addition to constant observation for the signs and symptoms of hypothermia, the patient’s temperature should be reassessed when the thermal comfort level decreases, with any emerging signs or symptoms of hypothermia, and on discharge from the PACU. In addition to the previous measures, active warming should be initiated for any patient with hypothermia who is admitted. In addition, IV fluids should be warmed; all gases (oxygen) should be humidified and warmed; and temperature should be monitored at least every 15 minutes until normothermia is achieved. The expected outcomes for all patients in PACU Phase I include a return to normothermia (minimum discharge temperature of 36° C), resolution of the signs and symptoms of hypothermia, and patient verbalization of an acceptable level of warmth. Preventative warming measures and continued assessment for hypothermia should continue at whatever location to which the patient is discharged.1
Malignant hyperthermia
Malignant hyperthermia (MH) is a genetic abnormality of muscle metabolism initiated by certain triggering agents resulting in a hypermetabolic state. MH is precipitated by certain general inhalation anesthetics, depolarizing skeletal muscle relaxants, and stress.4,15–17 The incidence of MH ranges from 1 in 5000 to 1 in 100,000 anesthetics. MH averages approximately 600 cases per year in the United States, with hot spots including Wisconsin, Michigan, and West Virginia.17 A study of the clinical manifestations of MH in North America from 1987 to 2006 noted that almost 75% of MH cases occurred in males, with almost 70% of those incidences in whites.18 The study showed that the most commonly presenting clinical symptom was hypercarbia, followed by sinus tachycardia and masseter spasm.18 Additional signs and symptoms include generalized muscle rigidity, unstable blood pressure, tachypnea, mixed respiratory and metabolic acidosis, myoglobinuria, hyperkalemia, and body temperature that may exceed 110° F (43° C).4,16–20 Research shows that time from induction to first sign of MH was less than 30 minutes in the majority of cases.18 After the acute episode is treated in the OR, the patient may be admitted to the PACU. Because successful management of MH depends on early assessment and prompt intervention, the perianesthesia nurse must be knowledgeable of the pathophysiology and treatment of this syndrome.
Identification of patients with malignant hyperthermia susceptibility
Genetics
Humans probably inherit susceptibility to MH with more than one gene or more than one group of possible mutational forms of a gene. The pattern of inheritance can range from recessive to dominant, with graded variations in between. The ease of initiation of an episode of MH seems to depend on the degree of genetic susceptibility and on environmental factors, which explains why some patients who are known to be susceptible show no signs of MH when exposed to confirmed MH-triggering agents. A patient with MH susceptibility (MHS) could be given an anesthetic in the presence of trigger agents, and this patient might not experience an acute MH reaction during surgery, but could have MH develop in the PACU instead.6,15–17,20
Evaluation of susceptibility
Before anesthesia is administered, identification of patients who may be susceptible to MH is of major therapeutic importance. On history and physical examination, patients with MHS usually show some subclinical muscle weakness or abnormality, such as deficient fine motor control. Many patients with MHS have muscle cramps that occur spontaneously, during an infectious illness, or during or after exercise. When these cramps are present, they can be so severe that they are almost incapacitating. The patient may also describe heat prostration during physical exertion that is associated with environmental heat stress. In addition, a positive patient history or a positive genealogy may go back two generations (i.e., the patient or immediate relatives may show MH symptoms during an anesthetic experience). Physical examination of the person with MHS may reveal myopathies such as wasting of the distal ends of the vastus muscles and hypertrophy of the proximal femoral muscles of the thigh. Other myopathies that are associated with MH susceptibility are cryptorchidism, pectus carinatum, kyphosis, lordosis, ptosis, and hypoplastic mandible. Electromyographic changes are seen in fewer than half of patients with MHS. Electrocardiographic results of patients with MHS may reveal ventricular or atrial hypertrophy (or both), bundle branch block, myocardial ischemia, and ventricular dysrhythmias. Measurements of blood creatine phosphokinase are usually approximately 70% reliable in estimating to MHS. The most definitive test for detection of MH susceptibility is the biopsy of skeletal muscle. Samples are obtained from the quadriceps muscle and are subjected to isometric contracture testing. The skeletal muscle of the patient with MHS has an increased isometric tension when exposed to caffeine or halothane.16,17,20
A new molecular genetic screening test has recently been developed that detects anywhere from 30% to 50% of those patients at risk for development of MH. A genetic test and DNA analysis is performed with a simply obtained blood sample. Although this method does not detect all people susceptible to development of MH and thus cannot be used to replace the more definitive muscle biopsy testing, the procedure is less costly, does not require the patient to travel to one of the few muscle biopsy testing centers, and shows great promise for future development.16,17,20
Patients at high risk for development of an acute MH crisis have been classified as follows: (1) patients who have an MH-positive muscle biopsy or who have survived an acute MH crisis; (2) patients who have a first-degree relative known to have MHS or to have had a positive muscle biopsy; (3) patients whose family members have a clinically demonstrated muscle abnormality; and (4) patients who are members of a family whose plasma creatine phosphokinase measurements have been found to be elevated in one or more samples (taken on at least three occasions).17,20
Normal skeletal muscle physiology
Although a complete discussion of skeletal muscle contraction can be found in Chapter 23, a brief synopsis is presented here. The events that lead to the contraction of a skeletal muscle begin with an electric impulse that is transmitted down the axon to the motor nerve terminal, where vesicles that contain acetylcholine are located. On stimulation, the contents of the vesicles are released. This quantum of acetylcholine crosses the myoneural junction and interacts with its receptor on the postsynaptic membrane. This receptor activation causes a transient increase in the permeability for sodium and potassium ions, which ultimately creates an electric action potential (nerve impulse) that is propagated along the muscle membrane. This action potential electrically excites the sarcolemma and releases into the myoplasm calcium ions that are stored in the sarcoplasmic reticulum. These calcium ions then attach to troponin C, an inhibitory muscle protein that, when stimulated by the calcium, permits the actin and myosin protein filaments to interact and cause muscle contraction. The calcium ions in the myoplasm are then taken up via a reuptake mechanism into the sarcoplasmic reticulum. The process by which the electrically excited sarcolemma is coupled to the calcium released from the sarcoplasmic reticulum is known as excitation-contraction (E-C) coupling.20,21
Pathophysiology of malignant hyperthermia
When a susceptible patient is exposed to a trigger agent, such as halothane, that causes MH to occur, the clinical features are produced by an excess of calcium ions in the myoplasm. Although the exact pathophysiology of MH is not known, in MH the reuptake of calcium from the myoplasm by the sarcoplasmic reticulum appears to be decreased. It has also been suggested that the E-C coupling mechanism is defective. With an elevated calcium ion concentration in the myoplasm, the skeletal muscle contraction is intense and prolonged, finally leading to a hypermetabolic state of acid and heat production. More specifically, heat is produced by the accelerated and continued synthesis and use of adenosine triphosphate during glycolysis. The metabolic byproduct of glycolysis, lactic acid, is transported to the liver, where part of it is oxidized to provide the adenosine triphosphate necessary to help make glucose. This glucose, along with glycogen, is released from the liver and transported back to the metabolically active muscle, where the entire cycle repeats. This revolving process liberates much heat and produces a significant amount of metabolic acid. Respiratory and metabolic acidosis develop because of this hypermetabolic state, and symptoms such as tachycardia, tachypnea, ventricular dysrhythmias, and unstable blood pressure appear. Because of intense vasoconstriction, the skin is mottled and cyanotic (Box 53-1). Elevated body temperature can actually be a late sign of MH; for this reason, the nurse should not prolong the assessment of the patient on the assumption that the patient’s body temperature must be significantly elevated before intervention is attempted. When the patient’s body temperature begins to rise, it may increase at a rate of 0.5° C every 15 minutes and may approach levels as high as 46° C.15,20–22
BOX 53-1 Signs and Symptoms of Malignant Hyperthermia
• The most consistent indicator of potential MH in the OR is an unanticipated increase (e.g., doubling or tripling) of end-tidal CO2 when minute ventilation is kept constant.
• Unexpected tachycardia, tachypnea, and jaw muscle rigidity (masseter spasm) are often common signs of MH that follow the significant CO2 increase.
• Respiratory and metabolic acidosis usually indicate fulminant MH. However, metabolic acidosis is not always present before severe temperature increase.
• A specific sign of the MH syndrome is body rigidity (i.e., limbs, abdomen, and chest).
• Temperature elevation is often a late sign of MH. Temperature change during MH is best detected with core temperature measurement (nasopharyngeal or oropharyngeal, esophageal, or pulmonary artery). Forehead skin temperature is less acceptable; it is slower in reflecting changes in core temperature and could be influenced by peripheral vasoconstriction.
• Although most commonly presenting within the first 30 minutes of anesthesia induction, malignant hyperthermia can occur at any time during anesthesia or on emergence from anesthesia, including in the immediate postoperative period.
Muscle rigidity occurs in approximately 75% of patients with MH, especially after the administration of succinylcholine. In fact, the spasm of the masseter muscles after the injection of succinylcholine may be so severe that the anesthesia provider cannot open the patient’s mouth to insert an airway. The onset of skeletal muscle rigidity after the administration of succinylcholine could be a sign of the impending development of MH.15,17,18,20–22
Triggering of malignant hyperthermia
Various environmental stimuli and pharmacologic agents can stimulate an acute episode of MH (Box 53-2). The symptoms of heat exertion and heat stroke are similar to MH. However, most patients with heat-related illness are not MH susceptible. In a few cases, MH susceptibility has been diagnosed with muscle biopsy in patients who had heat stroke (nonfatal), and some experts believe that heat stroke may occur more often in individuals with MH susceptibility. This area is an area of intense interest and investigation.15,17,18,20–22
The anesthetic agents that trigger MH seem to affect the sarcoplasmic reticulum or the E-C coupling mechanism, or both. Because of their wide use, halothane and succinylcholine are the most common trigger agents, although all inhalation agents are triggering agents. In patients with MHS or in patients who have had an episode of acute MH in the OR, all possible trigger agents should be stringently avoided.15,17,18,20–22
Dantrolene sodium (dantrium)
Dantrolene, the primary pharmacologic agent used in the treatment of MH, is a muscle relaxant that is chemically and pharmacologically unrelated to other muscle relaxants. It is the only known pharmacologic agent that is effective in the treatment of MH. The site of action of this drug is distal to the end plate within the muscle fiber. The main pharmacologic action of dantrolene reduces the release of calcium by the sarcoplasmic reticulum without affecting reuptake. Consequently, the concentration of calcium in the myoplasm is reduced, thus inhibiting the E-C coupling mechanism and causing muscle contraction to cease. When administered orally, dantrolene has a half-life of 8 hours; with IV administration, the half-life is 5 hours. When it is used in the treatment of acute MH, the intravenous dosage is 1 to 2 mg/kg, which can be repeated every 5 to 10 minutes, with a maximal dose of 10 mg/kg. If the acute episode of MH occurs in the OR and the patient is treated successfully, dantrolene therapy is continued into the recovery period in the PACU to prevent recurrence of MH. After the acute period in the PACU has passed, the patient is given oral dantrolene in four divided doses. Because dantrolene is poorly soluble, it is supplied in vials in the form of a lyophilized powder. To reconstitute a vial of lyophilized powder, 60 mL of sterile water for injection is added to the vial and is shaken until the solution is clear; many compatibility problems arise when dantrolene is mixed with solutions other than sterile water for injection. In addition, the sterile water for injection, used to reconstitute the dantrolene, should not contain any bacteriostatic agents because it is not unusual to use more than 2000 mL of diluent during the treatment of acute MH in an adult who weighs 70 kg.4,16,17,19–21
Perioperative management of the patient with malignant hyperthermia susceptibility
Although pretreatment with dantrolene was previously recommended for the patient with MHS, current evidence indicates that pretreatment is ineffective and not indicated.16,23 Premedication with anticholinergics, such as atropine, should be avoided because they interfere with the normal heat loss mechanisms and, in the case of atropine, can cause tachycardia that may cause confusion in the diagnosis of acute MH. Phenothiazine should also be avoided in the perioperative period because it can cause a release of calcium from the sarcoplasmic reticulum. Intraoperative anesthesia requires the use of agents that do not trigger an episode of MH (Box 53-3). Although regional anesthesia avoids the use of the general inhalation anesthetic agents and skeletal muscle relaxants, elevated temperatures in patients with MHS have been reported with its use.16,17,21,23
Intraoperative monitoring of the patient with MHS includes electrocardiogram, temperature, arterial blood gas (including acid-base), and precordial stethoscope determinations. These monitoring parameters should be continued into the PACU period (Box 53-4). Because some patients with MHS have had MH triggered in the postoperative period, patients should be followed for a minimum of 24 hours after surgery and should not be subjected to anxiety or stress. These patients should be reassured that physicians and nurses have reliable instruments to monitor for MH and that prompt and effective treatment will be provided if it develops.16,17,20–23
BOX 53-4 Suggested Components of Monitoring of Patient with Acute Malignant Hyperthermia
Treatment of acute malignant hyperthermia in the postanesthesia care unit
The cornerstone of the successful treatment of MH is early detection (see Box 53-1). Box 53-5 lists the suggested equipment and drugs that should be kept in the PACU to be used in the treatment of acute MH. Chapter 1 also provides information on equipment and drugs that should be available for the treatment of acute MH. If assessment indicates that the patient is developing acute MH, the following steps should be taken4,6,19,20,22:
1. Discontinue the use of any trigger agent (see Box 53-3) and send for help.
2. Rapidly ventilate the patient’s lungs with large tidal volumes with a bag-mask system and oxygen (total oxygen flow should exceed 15 L/min). Oral endotracheal intubation should be performed if the patient’s airway is compromised.
3. Insert arterial and central venous lines and send venous and arterial blood samples to the laboratory for immediate results on electrolyte and arterial blood gas analysis. Also obtain creatine kinase (CK), myoglobin, comprehensive metabolic panel, prothrombin time/partial thromboplastin time (PT/PTT), fibrinogen, fibrin split products, complete blood count (CBC), and platelets.
4. Start reconstituting the dantrolene as soon as possible.
5. Administer the intravenous dantrolene, 1 to 2 mg/kg over 1 to 2 minutes, up to 10 mg/kg or until the patient’s temperature starts to decrease.
6. Cool the patient. Cover all exposed surfaces with towels soaked in water. Cover the wet towels with ice. Use cooling blankets and fans if possible. Use cold gastric lavage and hydrate with iced intravenous fluids. To avoid hypothermia, discontinue all the cooling interventions when body temperature decreases to 38° C.
7. Administer sodium bicarbonate IV at a dose of 1 to 2 mEq/kg. When results of the arterial blood gas analysis are available, correct the base deficit with sodium bicarbonate according to the following formula:
BOX 53-5 Suggested Equipment and Drugs to Be Used in Treatment of Acute Malignant Hyperthermia
Equipment needed
• Intravenous lines with assorted cannula gauges
• Central venous pressure sets (2)
• Transducer kits for arterial and central venous cannulation
• Esophageal or other core temperature probes
• Laboratory test tubes for blood chemistry analysis
• Syringes (60 mL × 5) to dilute dantrolene
• Crystalloid solution (ten 1000-mL bottles), labeled for hyperthermia only and stored in PACU refrigerator
• Bucket of cracked ice, labeled for hyperthermia only and stored in freezer of PACU refrigerator
Drugs needed
• Sodium bicarbonate (8.4%): 50 mL × 5
• Furosemide: 40 mg/ampule × 4 ampules
• Calcium chloride (10%): 10-mL vial (2)
• Glucose (two bottles of 50% strength)
• Iced intravenous saline solution (ten 1000-mL bottles in refrigerator)
• Lidocaine for injection: 100 mg per 5 mL or 100 mg per 10 mL in preloaded syringes (3)
• Amiodarone is also acceptable (ACLS protocol for treatment of cardiac dysrhythmias)
• Regular insulin (1 ampule of 100 units; refrigerated)
• Dantrolene (Dantrium) intravenous: 36 vials of lyophilized powder with at least 2200 mL of sterile water for injection, USP (without a bacteriostatic agent), to reconstitute dantrolene
PACU, Postanesthesia care unit; ACLS, advanced cardiac life support.
If the PaCO2 is elevated, increase the tidal ventilation of the patient. Do not administer sodium bicarbonate to correct respiratory acidosis, because the PaCO2 only increases, which may lead to ventricular fibrillation.
8. Constantly monitor the patient’s core temperature, blood pressure, pulse, cardiac rhythm, and pupil size and reactivity and watch for cyanosis (see Box 53-4).
9. Hyperkalemia should be treated with intravenous insulin and glucose (0.25 to 0.5 U/kg of insulin to 0.25 to 0.5 g/kg of glucose) or calcium.
10. If possible, catheterize the bladder and monitor urinary output and appearance. To secure a high urinary output, furosemide (1 mg/kg) or mannitol (1 g/kg) can be given.
11. Avoid calcium channel blockers. Dysrhythmias usually subside with resolution of the hypermetabolic phase of MH. They can be treated with amiodarone, lidocaine, procainamide, adenosine, or other drugs according to the advanced cardiac life support protocol.
12. Hypotension can be treated with the infusion of cold crystalloid solution.
13. Continue to send arterial and venous blood samples to the laboratory for prompt determination of arterial blood gases and electrolytes.
14. Look for such hopeful prognostic signs as lessening coma, hyperactive tendon reflexes, and stabilization of the temperature. When the temperature returns to normal, continue constant observation of the patient.
Complications after acute malignant hyperthermia
Renal failure can occur because of myoglobinuria or hypotension. Consumption coagulopathies, such as disseminated intravascular coagulation, have been reported along with acute heart failure and pulmonary edema. Because the brain is the organ most sensitive to hyperthermia (permanent brain damage at ≥41° C), brain deterioration can occur in patients who are not promptly diagnosed and treated for MH.4,6,18
Summary
Thermal imbalance issues encompass one of the most common, and one of the rarest, complications experienced by the patient in the PACU. Perioperative hypothermia, a commonly occurring yet easily preventable complication, can precipitate many adverse patient events, resulting in increases in both morbidity and mortality. Malignant hyperthermia, although rare, requires rapid diagnosis and treatment to avoid certain death. This chapter has provided an overview of the physiology of thermoregulation, the basic concepts of perioperative thermoregulation and hypothermia, malignant hyperthermia, and the effects of these issues on the care of the patient in the PACU. Astute assessment, appropriate prophylaxis, and aggressive patient management by the PACU nurse are critical to assuring safe quality patient outcomes in the patient with thermal alterations.
1. Hooper VD, et al. ASPAN’s evidence-based clinical practice guideline for the promotion of perioperative normothermia: second edition. J Perianesth Nurs. 2010;25(6):346–365.
2. Kurz A. When is forced-air warming cost-effective. Fleisher LA, ed. Evidence-based practice of anesthesiology, ed 2, Philadelphia: Saunders, 2009.
3. Sessler DI. Perioperative heat balance. Anesthesiology. 2000;92:578–596.
4. Hooper VD. Thermoregulation issues. In: Stannard D, Krenzischek DA. Perianesthesia nursing care: a bedside guide for safe recovery. Sudbury, Mass: Jones & Bartlett Learning, 2012.
5. Welch TC. AANA journal course. AANA Journal. 2002;70(3):227.
6. Hooper VD. Thermoregulation. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing. ed 2. St. Louis: Saunders; 2010.
7. Holtzclaw BJ. Circadian rhythmicity and homeostatic stability in thermoregulation. Biol Res Nurs. 2001;2:221–235.
8. Hooper VD, Andrews JO. Accuracy of noninvasive core temperature measurement in acutely ill adults: the state of the science. Biol Res Nurs.2006;8(1):24–34.
9. Nicoll LH. Heat in motion: evaluating and managing temperature. Nursing.2002;32(5):s1–s12.
10. Lawson L, et al. Accuracy and precision of noninvasive core temperature measurement in adult intensive care patients. American Journal of Critical Care. 2007;16:485–496.
11. Calonder EM, et al. Temperature measurement in patients undergoing colorectal surgery and gynecology surgery: a comparison of esophageal core, temporal artery, and oral methods. J Perianesth Nurs.2010;25(2):71–78.
12. Fetzer SJ, Lawrence A. tympanic membrane versus temporal artery temperatures of adult perianesthesia patients. J Perianesth Nurs. 2008;23(4):230–236.
13. Washington GT, Matney JL. Comparison of temperature measurement devices in post anesthesia patients. J Perianesth Nurs. 2008;23(1):36–48.
14. Sessler DI, Akca O. Nonpharmacological prevention of surgical wound infections. Helathcare Epidemiology. 2002;35:1397–1404.
15. Hernandez JF, et al. Scientific advances in the genetic understanding and diagnosis of malignant hyperthermia. J Perianesth Nurs. 2009;24(1):19–34.
16. Watson CB. Is there an ideal approach to the malignant hyperthermia-susceptible patient. Fleisher LA, ed. Evidence-based practice of anesthesiology. ed 2. Philadelphia: Saunders; 2009.
17. Rosenburg H. Malignant hyperthermia syndrome. available at www.mhaus.org/NonFB/Slideshow_eng/SlideShow_ENG_files/frame.htm, May 29, 2011. Accessed
18. Larach MG, et al. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Analgesia & Anesthesia. 2010;110(2):498–507.
19. Glahn KPE, et al. Recognizing and managing a malignant hyperthermia crisis: guidelines from the european malignant hyperthermia group. British Journal of Anaesthesia. 2010;105(4):417–420.
20. Hommertzheim R, Steinke EE. Malignant hyperthermia-the perioperative nurse’s role. AORN J. 2006;83(1):149–167. [erratum appears in AORN J, 83(3):601, 2006]
21. Gurunluoglu R, et al. Evidence-based patient safety advisory: malignant hyperthermia. Plastic and Reconstructive Surgery.2009;124(4S):68S–81S.
22. Naecsu A. Malignant hyperthermia. Nurs Stand. 2006;20(28):51–57.
23. Wappler F. Anesthesia for patients with a history of malignant hyperthermia. Current Opinion in Anesthesiology. 2010;23:417–422.