48 Care of the patient with chronic disorders
Acetylcholine (ACh) Receptors: Cholinergic neurotransmitter receptors.
Anticholinesterases: Drugs that act to block the cholinesterase enzyme.
Asthma: A lung disease characterized by constriction and spasms of the muscles of the small airways; a component of COPD.
Atelectasis: Collapse of a lung or portion of the lung.
Bronchospasm: Muscle spasm in the bronchi that causes constriction and a reduction in airflow; a component of COPD.
Cardiomegaly: Enlargement of the heart.
Chronic Bronchitis: Lung disease usually caused by chronic infections in the lungs characterized by increased pulmonary secretions.
Cor Pulmonale: Right heart failure as a result of primary lung disease.
Dynamic Compliance: Elasticity of the lungs over the tidal volume range.
Emphysema: A disease of the lungs characterized by a physical breakdown of the pulmonary tissue and a disease component of COPD.
FEV1: Forced expiratory volume in the first second.
Glycosuria: Glucose in the urine.
Hemolyze: Breakdown of red blood cells that causes a release of hemoglobin.
Hypercarbia: Abnormally high levels of carbon dioxide in the blood.
Hypervolemia: An increase in the volume of circulating blood.
Hypoglycemic Agent: A synthetic drug that lowers the blood glucose level for treatment of type 2 diabetes.
Immunosuppressants: Agents that significantly interfere with the ability of the immune system to respond to antigenic stimulation with inhibiting cellular and humoral immunity.
Microangiopathy: A disease of the small blood vessels.
Miosis: Contraction of the sphincter muscle of the iris that causes the pupil to become smaller.
Myasthenic Syndrome: Called the Eaton-Lambert syndrome; chronic fatigability and muscle weakness, especially in the face and throat.
Plasmapheresis: Removal of plasma from previously withdrawn blood via centrifugation, reconstitution of the cellular elements in an isotonic solution, and reinfusion of this solution into the donor or another person who needs red blood cells rather than whole blood.
Polycythemia: Increased number of red blood cells.
Ptosis: Abnormal condition in which the upper eyelids droop because of muscle weakness.
Rales: Crackling sound made inside the lungs during auscultation.
Rhabdomyolysis: A disease of the skeletal muscle characterized by the presence of myoglobin in the urine.
Rhonchi: Sound made inside the lungs during auscultation.
Thalassemia: Microcytic, hypochromic, and short-lived red blood cells caused by deficient synthesis of hemoglobin.
In this chapter, selected chronic disorders will be presented to enhance the use of appropriate and informed perianesthesia care. For example, patients with chronic obstructive pulmonary disease (COPD) can have significant preoperative respiratory dysfunction, of which some improvement can be accomplished with intense and knowledgeable nursing care. COPD is a serious condition that starts to develop up to 30 years before significant symptoms; it affects more than 25 million Americans and is responsible for about 80,000 deaths per year.1 These patients have significant risks for anesthesia and surgery. The COPD disease process can be generalized to many pulmonary dysfunctions, as are the other chronic disorders described in this chapter. Therefore patients suffering from some of the other chronic disorders described in this chapter present a significant challenge to the perianesthesia nurse. In an effort to reduce the incidence of postoperative complications in patients suffering from chronic disorders, a complete understanding of the pathophysiology of the disease process will facilitate the appropriate evidence-based nursing interventions will lead to a positive outcome for the perianesthesia patient.
Chronic obstructive pulmonary disease
Description of COPD
The hallmark of COPD is the evidence of a productive cough and a progressive decrease in the patient’s exercise tolerance. Three major diseases are part of COPD: asthma, emphysema, and chronic bronchitis.2 All are characterized by airway obstruction. These diseases may have medically reversible components, such as bronchospasm, or they may have irreversible components, such as alveolar septal destruction. Some of the reversible components of asthma, such as retained secretions, bronchospasms, and infections, can be corrected with the interaction of the physician, nurse, physical therapist, and respiratory therapist. The treatment of asthma can include oxygen therapy, bronchodilators, chest physiotherapy, and proper hydration.
Chronic bronchitis is associated with chronic cigarette smoking. The nurse can contribute greatly to the patient’s future health with strong influences to refrain from smoking.3 Other therapy for the reversible components can include the use of bronchodilators, chest physiotherapy, and oxygen.
Cigarette smoking as A precursor to the development of COPD
Cigarette smoking has been well established as one of the major precursors to chronic bronchitis and emphysema—two of the three disease components of COPD. Cigarette smoking affects the manner in which a patient recovers from an anesthetic.4,5 The perianesthesia nurse should be aware of the diverse reactions that smoking can have on the patient who is emerging from an inhalation anesthetic. Studies on the relationship between smoking and its effects on anesthesia indicate an increase in the risk factor in the patient who smokes. Although the incidence rate of smoking is decreasing slowly, it continues to rise in the teenage population.
Respiratory effects of smoking
A growing body of convincing scientific literature suggests that almost all pulmonary disease is related in some way to the inhalation of infectious or irritant particulate material. Cigarette smoke in its gaseous phase contains nitrogen, oxygen, carbon dioxide, carbon monoxide, hydrogen, argon, methane, hydrogen cyanide, ammonia, nitrogen dioxide, and acetone. In the particulate phase, cigarette smoke contains nicotine, tar, acids, alcohol, phenols, and hydrocarbons. The bottom line is that cigarette smoke contains oxidants, and the oxidants can damage cells and the extracellular matrix components of the lung, leading to significant damage to the tissue in the lungs. Smokers who inhale nicotine from a cigarette into the lungs actually receive 25% to 30% of the nicotine contained in the cigarette. Thirty percent is destroyed with combustion, and 40% is lost in the side stream. Therefore, if a person inhales the smoke from a cigarette that contains 2.5 mg of nicotine, 1 mg of nicotine is actually absorbed by the lungs. In addition, filters are known to make little difference in this absorption. Contrary to some opinions, the smoking of cigars and pipes also presents a risk for pulmonary disease.6 Carbon monoxide combines with the hemoglobin molecule at the same point as oxygen does. It has an affinity for this receptor point that is 210-fold greater than that of oxygen7; therefore the oxygen-carrying capacity of hemoglobin is reduced, and the end result is that less oxygen is relinquished to the tissues by the hemoglobin. When carbon monoxide combines with hemoglobin, a compound called carboxyhemoglobin is formed. The amount of carboxyhemoglobin in the blood is especially important in the patient who has a diseased myocardium, because myocardial oxygenation is limited by the flow of the blood through the coronary arteries. During stress, such as in surgery and anesthesia, the amount of carboxyhemoglobin saturation could lead to severe myocardial hypoxia in patients who smoke heavily and have coronary artery disease because the diseased coronary arteries cannot increase the flow significantly. The only means of prevention of hypoxia is an increase in the extraction of oxygen from the hemoglobin. Small amounts of carboxyhemoglobin can hinder the uncoupling of the oxygen and thus result in yet more oxygen retention at any given tension. This effect clearly is greater when the oxygen tension is further reduced by local ischemia and any additional vasoconstriction associated with smoking.
Smoking is an important causative factor in chronic pulmonary disease, especially the obstructive type.8 The characteristic pulmonary function alterations in smokers usually include a reduction in vital capacity, an increase in residual volume to total lung capacity, an uneven distribution of inspired gas, a decrease in dynamic compliance, and an increase in nonelastic resistance. Most critically, chronic cigarette smoking ultimately causes the forced expiratory volume in the first second (FEV1) to be less that 80% of normal, a critical sign of COPD.
Chronic bronchitis is the disease most often associated with smoking and is seen often by the perianesthesia nurse. Hypertrophy of bronchial mucous glands with production of excessive mucus is the hallmark of this disease. A vicious cycle develops as this failure to remove the mucus leads to retention of pathogenic organisms and irritants. The resulting distorted alveolar septa and the increased pressure on the alveoli from chronic bronchitis can lead to emphysema.
Surgical considerations
The incidence rate of pulmonary complications in patients who have undergone abdominal or thoracic surgery is high. Changes occur in the pulmonary status of the patient who undergoes anesthesia and surgery. In the postoperative phase, these changes are characterized by gradual or abrupt alveolar collapse. The patient with COPD, when subjected to surgery, then represents an even higher risk for postoperative complications. These patients must be given meticulous preoperative care so that they are in the best possible health when they enter surgery. This preoperative medical treatment usually includes hydration, nutrition, chest physiotherapy, bronchodilators, and prophylactic antibiotics if an infection is present. Serial pulmonary function tests and arterial blood gas determinations are used to monitor the progression of the preoperative treatment.7
Care of the COPD patient
Perianesthesia care focuses on prevention of complications. The modified stir-up regimen should include frequent cascade coughing, sustained maximal inspirations (SMIs), and repositioning of the patient (see Chapters 12 and 28). An appropriately implemented modified stir-up regimen is of great importance, especially in patients who are recovering from upper abdominal or thoracic operations. Surgery at these sites can cause decreased ventilatory effort and a complete absence of sighs by the patient. Given that the patient already has compromised respiratory function, the possibility of retained secretions and atelectasis is magnified. As a result, these patients represent a significant challenge to the perianesthesia nurse (Box 48-1).
Postoperative
• Continue tracheal intubation and mechanical ventilation (likely after abdominal or intrathoracic surgery and a preoperative PaCO2 > 50 mm Hg and FEV1/FVC < 0.5; maintain PaO2 at 60 to 100 mm Hg and PaCO2 in a range that maintains the pH at 7.35 to 7.45).
• Institute lung-volume expansion maneuvers (voluntary deep breathing, incentive spirometry, continuous positive airway pressure).
• Maximize analgesia (neuraxial opioids, intercostals nerve blocks, patient-controlled analgesia).
FEV1, Forced expiratory volume in the first second; FVC, forced vital capacity.
From Stoelting RK, Dierdoff SF: Handbook for anesthesia and co-existing disease, ed 2, New York, 2002, Churchill Livingstone; Data from Smetana GW: Preoperative pulmonary evalution, N Engl J Med 340:93−944, 1999.
Patients with COPD have some component of reactive airways disease. Consequently, the airway becomes compliant and can become compressed during a forced expiratory maneuver. This dynamic compression of the airways is a function of the equal pressure point theory, as discussed in Chapter 12. To reduce the amount of dynamic compression of the airway during exhalation, the patient should be encouraged to use pursed-lip breathing. Breathing through pursed lips during exhalation can be the same as adding 5 to 10 cm H2O of positive end-expiratory pressure. Increasing the pressure inside the airway during exhalation reduces the amount of dynamic compression of the airways and decreases the amount of air trapping that commonly occurs in patients with COPD.
Audible wheezing will require the use of a fast-acting bronchodilator such as albuterol. Arterial blood gases may be ordered, and capnography may be used to monitor end-tidal CO2.9
The cardiac status should be monitored meticulously because of the frequent involvement of the heart in the pathologic disorders of these patients. Kidney function should also be monitored because it may be altered, especially in patients with fluid retention and edema of the extremities.
Patients who are cigarette smokers have significant postanesthesia risks.6 Cigarette smokers who have smoked for a long period of time and have an FEV1 less than 80% usually have an increased risk of pulmonary complications in comparison with nonsmokers. Patients who smoke more than two packs of cigarettes per day are especially prone to perianesthetic complications. In addition, patients who have had a long history of smoking (>20 pack years) and are presently in a nonsmoking situation still can have pulmonary complications. Many of these complications develop when cigarette smokers have a preexisting chronic respiratory disease, usually bronchitis. The major postoperative complications associated with smoking are infection, atelectasis, pleural effusion, pulmonary infarction, and bronchitis.
Complications associated with chronic cigarette smoking revolve around the inability of the patient to clear secretions. The goal of nursing care in the PACU centers on clearing the tracheobronchial tree, which necessitates frequent suctioning, cascade coughing, and the SMI maneuver. If rales and rhonchi are heard on auscultation, percussion and postural drainage should be initiated.
Respiratory depressant drugs, such as opioids, should be given in low doses, or they should be avoided completely if the COPD is severe. Repositioning of the patient and splinting of the incision site, in addition to reducing the anxiety usually seen in these patients, reduces the need for opioids. Some form of regional analgesia may be beneficial for these patients.8
Myasthenia gravis
The patient with MG deserves special consideration in the PACU because of the respiratory dysfunction and possible pharmacologic ramifications of the disease. The incidence rate of MG has been estimated to be between 1 in 7500 and 1 in 10,000.1 MG occurs twice as often in females than in males and at earlier ages, and it occurs most often between the ages of 30 and 40 years.7 The main symptoms are weakness in one or more of the muscle groups, fatigability on effort, and at least some partial restoration of muscle function after rest.7
Treatment for this disease consists of various pharmacologic interventions designed to enhance neuromuscular transmission and slow the progression of the disease. Therefore the treatment may include cholinesterase inhibitors, corticosteroids, specific immunosuppressants, plasmapheresis, intravenous immunoglobulin, and thymectomy.1
Because thymectomy has been used as a therapeutic intervention in the treatment of MG, the perianesthesia nurse will probably render nursing care to many patients with MG. Because of the location of the incision, the myasthenic patient does not usually receive any intraoperative skeletal muscle relaxants. Patients with myasthenia can have an exacerbation of symptoms in the PACU; therefore critical monitoring of the patient’s ventilatory status should be the primary focus of the perianesthesia nursing care. Patients with MG who are recovering from any type of surgical procedure and who have been administered any form of anesthesia (general, inhalation, or regional) can have exacerbated symptoms and myasthenic crisis develop in the PACU. Consequently, respiratory support should always be available for these patients.
Care of the patient with myasthenia gravis
If a muscle relaxant is given during surgery, the patient has the neuromuscular blockade reversed. After reversal in the operating room, the patient must have a complete sustained return of skeletal muscle strength before extubation. If the patient does not meet the criteria for extubation, the endotracheal tube remains in place and the patient is taken to the PACU for ventilatory support. In patients with MG, the skeletal muscle strength may appear to be appropriate immediately after surgery, but may deteriorate a few hours thereafter.7
The PACU nurse should monitor for a myasthenic crisis, which is a severe exacerbation of the symptoms associated with MG. It can occur when an anticholinesterase is underdosed and does not reduce the amount of muscle weakness sufficiently. Alternatively, a cholinergic crisis can occur when too much anticholinesterase is administered, resulting in a surplus of acetylcholine at the myoneural junction and causing a depolarizing type block that leads to skeletal muscle weakness, which could be severe. For all the previous reasons, during PACU care of the patient with MG in the immediate postoperative phase, airway equipment must be kept at the patient’s bedside. Along with the skeletal muscle weakness, muscarinic side effects occur, such as abdominal cramping, miosis, bradycardia, salivation, and diarrhea. See Chapters 10, 11, and 23, which provide the reader an in-depth discussion of the myoneural junction and nicotinic and muscarinic effects.
Diabetes mellitus
Diabetes mellitus (DM) affects approximately 29 million people in the United States and is the seventh leading cause of death. It is also believed that DM as a comorbidity is underreported.10 However, it is what transpires between the diagnosis and death that determines the level of health, the health care needed, and the number of surgical interventions of the patient with DM.
Diabetes is a chronic, progressive disease characterized by the body’s inability to metabolize carbohydrates, fats, and proteins leading to hyperglycemia.11 The degree of hyperglycemia and the incidence of acute or long-term diabetes complications depend on the type of diabetes and the level of DM control over the lifetime of the person with diabetes.
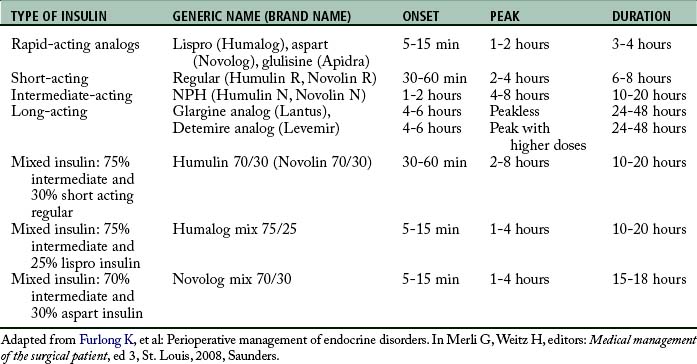
The two forms of diabetes are insulin dependent and non–insulin dependent. Insulin-dependent diabetes, now called type 1, was formerly called juvenile onset diabetes. Type 1 diabetes may be genetic in predisposition, but the majority of those diagnosed with type 1 DM have no first-degree relatives with DM.11 It is characterized by complete dependence on exogenous insulin therapy. Type 1 DM is caused by a vigorous autoimmune destruction of the beta cells in the islets of Langerhans in the pancreas. The onset of DM is usually before the age of 40 years; however, it can develop at any age. By the time of diagnosis, approximately 80% to 90% of the beta cells have been destroyed. Approximately 10% of the people with DM have type 1.11 Treatment centers on meal planning, exercise, and insulin administration (Table 48-1).
The other main form of DM is non-insulin dependent diabetes, or type 2 diabetes. Formerly called adult onset diabetes, type 2 affects approximately 90% of people with DM. This type of DM is characterized by impaired insulin secretion or peripheral resistance to one’s own insulin, resulting in a degree of endogenous insulin production, but not at a level sufficient to produce normal carbohydrate homeostasis. Most people with type 2 DM are older than 40 years and have a family history of diabetes, and 85% are obese.11 As the U.S. population ages, becomes more obese, and is less active, a continued rise in the number of persons with DM is to be expected. Treatment focuses on meal planning, exercise, and weight loss with the possible inclusion of oral hypoglycemics, injectable noninsulin products, and treatment with insulin (Table 48-2).
Table 48-2 Oral Medication or Non-Insulin Injectable Medications for Persons with Diabetes
| CHEMICAL CLASS | AGENTS | ACTION |
|---|---|---|
| Biguanides | Metformin | Decreases endogenous hepatic glucose production and increases peripheral glucose uptake |
| Sulfonylureas | Glipizide, glyburide, glimepiride | Increases the secretion of insulin from B cells |
| Meglitinides | Repaglinide, nateglinide | Increases the secretion of insulin from B cells |
| Thiazolidinediones | Pioglitazone, rosiglitazone | Improves insulin action in the periphery and the liver |
| α-Gglycosidase inhibitors | Acarbose, miglitol | Inhibits intestinal α-glucosidase, delaying glucose absorption |
| GLP-1 receptor agonist | Exenatide (injectable) | Stimulates insulin secretion and facilitates homeostasis follow food ingestion |
| Amylinomimetic | Pramlintide (injectable) | Regulates glucose concentration after a meal, increases satiety, slows gastric emptying, suppresses glucagon secretion |
Adapted from Fain J: Management of clients with diabetes mellitus. In Black J, Hawks J, editors: Medical surgical nursing; clinical management for positive outcomes, ed 8, St. Louis, Saunders.
The Centers for Disease Control and Prevention estimates that 25.8 million persons in the United States have DM, comprising 8.3% of the total population.10 Of these, 18.8 million have been diagnosed, whereas 7 million remain undiagnosed. As the U.S. youth become more sedentary and obese, the incidence of type 2 DM in children is skyrocketing. In 2010, approximately 215,000 people younger than 20 years old had a diagnosis of type 1 or type 2 DM (Table 48-3).10
Table 48-3 Diagnosed Persons with Diabetes Aged 20 Years or Older – U.S. 2010
| GROUP | NUMBER WITH DIABETES (%) |
|---|---|
| Age ≥20 years | 25.6 million (11.3) |
| Age ≥65 years | 10.9 million (26.9) |
| Men | 13.0 million (11.8) |
| Women | 12.6 million (10.8) |
| Non-Hispanic whites | 15.7 million (10.2) |
| Non-Hispanic blacks | 4.9 million (18.7) |
From Centers for Disease Control, U.S. Department of Health and Human Services. National Diabetes Fact Sheet 2011, available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed May 5, 2012.
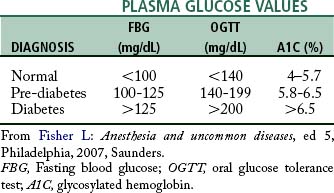
The diagnosis of DM has traditionally been determined by the fasting blood glucose (FBG) level. FBG greater than 126 mg/dL (7.0 mmol/L) indicates DM. An oral glucose tolerance test (OGTT) with a 75-g sugar load may also be used, with a resultant blood glucose (BG) of greater than 200 mg/dL (11.1 mmol/L) indicating DM. The glycosylated hemoglobin (HbA1c), now referred to as the A1C level, is a newer determinant of a diagnosis of DM. The A1C level was used for two decades to determine diabetes control, and in 2010 the American Diabetes Association allowed a laboratory-based standardized A1C to be used for diagnosis.12 As the red blood cell travels throughout the body on its approximately 6-week life cycle, glucose molecules attach to the hemoglobin molecule in the red blood cell. The A1C test reports a percentage, correlating to the average BG level over the last 2 to 3 months (Table 48-4).
Although hyperglycemia is most often cited as the primary problem with diabetes, it is usually the resultant long-term physiologic changes that cause the person with DM to require surgery. It is estimated that people with diabetes have a 50% chance of requiring surgery in their lifetime and that approximately 20% of all surgical patients will have DM.13 DM is the leading cause of nontraumatic lower limb amputations, increasing the likelihood of intervention in any surgical setting.
Determination of any long-term diabetes complications should be made in the preoperative period, hopefully weeks before the scheduled surgery (Box 48-2). Input from the primary care physician or diabetes specialist should be sought. Patient information from the medical record can also provide a thorough assessment for the emergent surgical patient with diabetes. Results of a current A1C provide an overview of the glucose control over the last 2 to 3 months; A1C values under 7% help to ensure fewer postoperative infections.13 None of these complications are unique to people with diabetes, but the incidence of these complications is higher in those with diabetes. Macrovascular disease reflects an increase in atherosclerosis with deposits of lipids within the inner layer of vessel walls. Increased levels of BG over time cause a thickening of the cell basement membrane and intracellular edema and altered cell function. The resulting thickness causes a greater distance of travel for nutrients and cellular waste decreasing the oxygen and nutrition entering the cell and causing cellular waste buildup.11
Anesthesia and diabetes
Preoperative guidelines for medications depend on the person’s home regimen and should be discussed with the person’s primary care physician and diabetes specialist before the surgical intervention. Varied combinations of medications make it unlikely to find a standardized preoperative plan that works for all patients with type 1 or type 2 DM. If possible, the person with diabetes should be scheduled for an early morning surgery to reduce the time without oral intake.11 Ongoing preoperative monitoring of BG levels helps to prevent acute complications during the perioperative and postoperative periods.
In general, people with DM who take oral medications or non-insulin injectables should be advised to hold those medications the evening before or the day of surgery, primarily for prevention of hypoglycemia. Sulfonylureas can interfere with ischemic myocardial preconditioning and theoretically increase the risk of perioperative myocardial ischemia and infarction.14 Metformin is withheld to prevent the possibility of lactic acidosis.
Persons with type 1 DM may be advised to reduce the bedtime insulin to prevent hypoglycemia while NPO before surgery; however, some form of maintenance insulin must be continued to prevent hyperglycemia from the lack of basal insulin. Basal insulin is the amount of exogenous insulin per unit of time necessary to prevent hepatic gluconeogenesis and ketogenesis. People with type 1 DM are usually maintained on a basal dose of one or two injections of a long-acting insulin or multiple injections of an intermediate insulin or continuous subcutaneous insulin infusion (i.e., insulin pump with a rapid acting analog).15 Nutritional insulin is the amount of exogenous insulin necessary to prevent hyperglycemia associated with any type of nutritional supplement, such as discrete meals, total parenteral nutrition, and enteral feedings. Correctional or supplemental insulin is used to correct unexpected hyperglycemia that occurs before or between meals.15 Nutritional and correctional insulin is usually a rapid-acting insulin or a short-acting insulin may be chosen. Each hospital tends to adopt different procedures for the person with diabetes using an insulin pump. Refer to individual hospital policies for details. In general, the pump may be set to the “suspend” mode for minor or short-duration surgeries. Longer procedures from which the person will have a longer period of sedation necessitate the removal of the pump and implementation of an IV insulin drip. The bagged and labeled pump and equipment should be sent home with the family and that action documented.
Care of the patient with diabetes
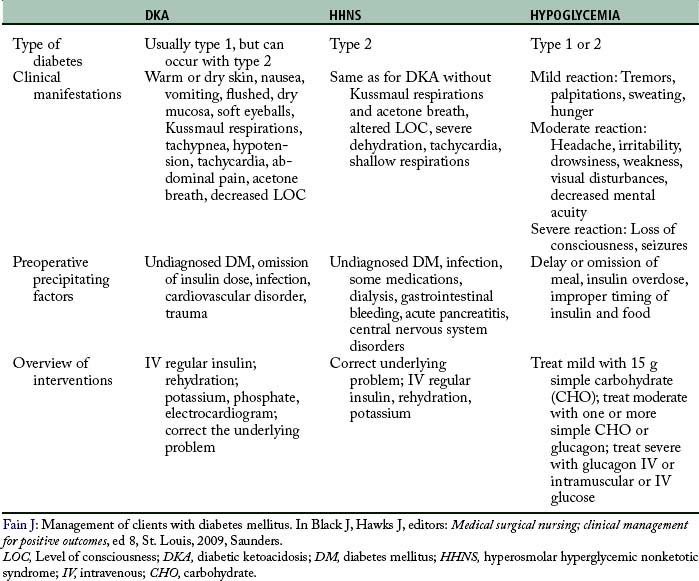
Perioperative goals for glycemic control include: the maintenance of fluid and electrolyte balance, prevention of ketoacidosis, avoidance of marked hyperglycemia, and avoidance of hypoglycemia.13 If any of these problems occur, immediate treatment is imperative and diligent investigation is required to determine the underlying cause. Surgery induces a stress response mediated by the neuroendocrine system through the release of catecholamines, glucagon, and cortisol. Surgery and possibly anesthesia cause an elevation of sympathetic tone with a release of cortisol and catecholamines. The relative insulin deficiency (type 2) and the absolute insulin deficiency (type 1) limit the body’s ability to compensate for this deficiency, requiring supplemental insulin during the perioperative period. This stress response of hyperglycemia may also occur with surgical patients without DM, demonstrating a need for glucose control for all surgical patients. Multiple studies have shown that control of the perioperative serum glucose level has a positive effect on the outcomes of all patients (Table 48-5).14
Source: Smith DK, et al: A study of perioperative hyperglycemia in patients with diabetes having colon, spine, and joint surgery, J Perianesth Nurs 24:362−369, 2009.
The nature and duration of the surgical procedure affects the glucose levels and the insulin interventions needed. In patients who require minor surgical procedures of short duration or limited general, local, epidural, or spinal anesthesia might need only minimal changes in their diabetes regimen. Procedures of longer than 2 hours under general anesthesia will have greater glucose variations and require more frequent monitoring and treatment.15 Intravenous regular insulin is used for glucose control during surgery. Doses and frequency may be determined by hospital or surgical center protocols. Subcutaneous insulin is not used because the absorption is affected by the person’s body temperature, circulating blood volumes, and certain anesthetics.11
Postoperative goals for the patient with DM include the universal outcomes of stabilizing vital signs, correcting fluid and electrolyte imbalances, preventing wound infection and promoting wound healing. Prevention of hypoglycemia depends on BG monitoring and physical indicators such as a decrease in blood pressure or an increased heart rate in the still unresponsive patient. Patients with peripheral vascular disease, neuropathy, or both require frequent skin inspection for signs of breakdown, pressure spots, or shearing injuries to the skin.11 However, for the person with diabetes, reestablishing control of their DM is imperative.
What is considered to be the optimal BG levels for the postoperative period has been one of debate for several years. However, statistics demonstrate that infection accounts for 66% of postoperative complications for those with DM. Mortality rates in people in diabetes have been estimated at fivefold greater than for those without DM. Both infection and mortality are thought to be caused by impaired leukocyte function, altered chemotaxis, and phagocytic activity.14
Furnary and colleagues16 and Van den Berghe et al17 determined that tight glycemic control decreased the incidence of deep sternal wound infections and decreased mortality, respectively. A postoperative BG level between 80 mg/dl (4.4 mmol/L) and 110 mg/dl (6.1 mmol/L) was designated as the standard for cardiac surgical or critical care patients. The basic goal of balancing the BG levels while still preventing hypoglycemia remains paramount in the medical and surgical efforts to decrease mortality and morbidity. Current studies have shown that although tight glycemic control may still be beneficial for the postcardiac surgical patient, others benefit from less stringent goals of between 80 (4.4 mmol/L) and 150 mg/dL (8.3 mmol/L). Other guidelines propose “reasonable” normoglycemia with the majority of BG levels less than 180 to 200 mg/dL (<10 to 11 mmol/L).13 The American Diabetes Association Standards of Medical Care has endorsed an FBG level of less than 140 mg/dL (7.8 mmol/L) for general hospitalized patients with random BG readings less than 180 mg/dL (10 mmol/L).12,18
Diabetes and BG control throughout the perianesthesia period determines the success of the procedure, the adverse or positive outcomes, and the overall health of the person with diabetes. DM remains the leading cause of kidney failure and is a major cause of heart disease and stroke; all of these are risks that may have an impact on the outcome of any surgical case. Minimizing BG variability during the perianesthesia period should be part of any glycemic control strategy.18 Nursing consideration of the preoperative DM status, the perioperative BG levels, and the postoperative outcomes influence the person with DM for months beyond the surgical intervention portion of their health care.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a relatively common disease that affects the connective tissue of the body. The clinical course varies, but it tends to be progressive and lead to characteristic deformities. Many patients become incapacitated over time. The disease affects more women than men, and its incidence rate in temperate climates is approximately 3%. The cause is not completely understood, but the disease is thought to be an autoimmune phenomenon. The outstanding clinical feature of this disease is proliferative inflammation. The patient often appears chronically ill, undernourished, and anemic.19
These patients often undergo surgery to correct restrictive deformities caused by the disease process (Table 48-6). On arrival in the PACU, they require comprehensive nursing management. Some of the hazards to be aware of in patients with rheumatoid arthritis are listed in Table 48-7.
| OPERATIVE SITE | COMMON OPERATIVE PROCEDURE |
|---|---|
| Neck | Atlantoaxial arthrodesis |
| Shoulder | Synovectomy and partial excision of acromion |
| Elbow | Synovectomy and radial head excision, resection arthroplasty |
| Wrist | Synovectomy and excision of distal ulna |
| Hand | Metacarpal phalangeal arthroplasty and flexor and extensor tenosynovectomy |
| Hip | Cup or total replacement, arthroplasty |
| Knee | Synovectomy (often bilateral), arthroplasty |
| Foot | Resection arthroplasty (often bilateral) |
From Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.
Table 48-7 Perianesthesia Hazards in Patients with Rheumatoid Arthritis
| AREA OF CONCERN | COMPLICATION |
|---|---|
| Respiratory System | |
| Airway | Hypoplastic mandible restriction, cervical spine motion, atlantoaxial subluxation, laryngeal tissue damage |
| Ventilation | Rheumatoid nodules in lung, chronic diffuse interstitial fibrosis, costovertebral joint disorder that inhibits ventilation, thoracic vertebrae flexion deformity that inhibits ventilation, tuberculous lung |
| Cardiovascular System | |
| Pericardial, myocardial, coronary artery disorders, aortic valve regurgitation, arrhythmias | |
| Hemopoietic, Hepatic, and Renal Systems | |
| Anemia, leukopenia, bleeding tendency (decreased platelets), renal amyloidosis | |
| Miscellaneous | |
| Skin fragility; postoperative chest complications, such as atelectasis, hypercarbia, and hypoxia; multiple joint disease | |
Modified from Bready L, et al: Decision making in anesthesiology, ed 4, St. Louis, 2007, Mosby.
Care of the patient with rheumatoid arthritis
Lungs
The patient with RA usually has pulmonary dysfunction, such as diffuse interstitial fibrosis, granulomatous lesions, or large silicotic nodules. These pulmonary dysfunctions lead to stiff lungs, and these patients are prone to atelectasis, hypoxemia, and hypercarbia in the PACU (see Chapter 12). Postoperative blood gas analysis and good pulmonary support are therefore important. Respiratory depressant opioids should be given with caution, if at all. Deaths in patients with RA have resulted from drug-induced respiratory failure during this period.20
Blood
The patient with RA usually has anemia, most commonly of the hypochromic microcytic variety. In most instances, this type of anemia can be treated with blood transfusion. Postoperative hematocrit and hemoglobin levels should be determined when the patient arrives in the PACU. Blood loss should be extensively monitored, including observation of the stools for blood. The contents recovered from the nasogastric tube (if present) should be checked for blood, because these patients may have a bleeding peptic ulcer from long-term aspirin and steroid therapy.
Sickle cell disease
More than 100 abnormal hemoglobins have been described in humans. When these hemoglobins are exposed to low oxygen tensions, this particular form of hemoglobin causes the red blood cell to distort its shape (sickle) and cause infarction and other complications. Normal hemoglobin is labeled hemoglobin A, whereas this sickling hemoglobin is labeled hemoglobin S.7
Sickle cell trait is found in about 8% to 12% of the African-American population21, which is heterozygous for sickling, and represents a combination of sickle hemoglobin (SA) and normal hemoglobin (AA). The red blood cells of such persons contain 20% to 40% hemoglobin S, but are not misshapen in normal living conditions. The person may have sickling with exposure to any conditions that cause hypoxia, such as depressed respiratory function from anesthetics in the PACU.
The most common form of sickle cell disease is the homozygous sickle cell disease. It occurs in 1 in 400 to 500 African Americans. These persons have inherited sickling genes from both parents, and they usually have 80% to 100% hemoglobin S. Sickling is present all the time, and minor reductions in oxygen tension can cause a sickle cell crisis. The onset of symptoms occurs around the age of 2 years; rarely do these persons live past the age of 40 years.21
Care of the patient with sickle cell disease
Prevention of sickle cell crisis is the main objective in the PACU phase. A general guideline for preoperative preparation is a hemoglobin A level of at least 50% and a hematocrit value of 35%. A recent study indicated that a conservative transfusion regimen is effective as an aggressive regimen in preventing postoperative complications. The tendencies to administer preoperative intravenous fluid and to transfuse blood to patients with sickle cell increased with disease severity and extensiveness of the surgical procedure. Although 89% of respondents to a recent survey felt comfortable managing patients with sickle cell disease, 73% thought an advisory statement on optimal perioperative management was needed. Ultimately, there is a wide variation in the management of children with sickle cell disease, and clinicians determine management based on disease severity and procedure type.22
Sickle cell crisis
The types of crisis seen in sickle cell anemia are vasoocclusive, aplastic, sequestration, and hemolytic. The vasoocclusive crisis is the most common type and is characterized by tissue ischemia, infarction, and necrosis. The bones, tendons, synovia, spleen, liver, and intestine are common sites of occlusion. Infections, dehydration, high altitudes, extreme physical exertion, and emotional upsets can trigger this type of crisis.23
The aplastic crisis is most grave and constitutes a medical emergency. It is characterized by a sudden drastic decrease in red blood cell production. The patient initially appears weak and has signs of cardiac decompensation.24
If crisis occurs, the following modes of treatment are recommended: keep the patient warm; treat infections; and maintain oxygenation, hydration, and alkalinization. Heparin may be administered to reduce the risks of embolus formation, and magnesium sulfate may also be indicated for its vasodilator and anticoagulant properties.
Summary
In many cases, the chronic disorders presented in this chapter have a pathophysiologic process that can be generalized to other patients. It is certainly important to acquire specific information on the disease process for a patient who is suffering from chronic diseases not specifically discussed in this chapter.
1. Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
2. Fisher L. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
3. Warner D. Helping surgical patients quit smoking: who, when, and how. Anesth Analg.2005;101:481–487.
4. O’Rourke J, et al. The effects of exposure to environmental tobacco smoke on pulmonary function in children undergoing anesthesia for minor surgery. Ped Anesth. 2006;16:560–567.
5. Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review. Tob Control.2006;15:352–358.
6. Moores L. Smoking and postoperative pulmonary complications: an evidence-based review of the recent literature. Clin Chest Med.2000;21:139–146.
7. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
8. Marley R, Hoyle BL. Respiratory care. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 2, St. Louis: Saunders, 2010.
9. Daley K. Patients with chronic diseases. In: Stannard D, Krenzischek DA. Perianesthesia nursing care. Sudbury, Mass: Jones & Bartlett, 2012.
10. Centers for Disease Control, US Department of Health and Human Services: National diabetes fact sheet, 2011. available at: www.cdc.gov/diabetes/pubs/factsheet11.htm, November 27, 2011. Accessed
11. Fain J. Management of clients with diabetes mellitus. Black J, Hawks J. Medical surgical nursing; clinical management for positive outcomes, ed 8, St. Louis: Saunders, 2009.
12. American Diabetes Association: Diagnosis and classification of diabetes. Diabetes Care.2011;34(suppl1):S62–S69. S1–2
13. Khan N, et al. Perioperative management of diabetes mellitus, UpToDate. available at www.uptodate.com/contents/perioperative-management-of-diabetes-mellitus?source=search_result&search=perioperative+management+of+diabetes+mellitus&selectedTitle=1%7E150, December 30, 2011. Accessed
14. Loh-Trivedi M. Perioperative management of the diabetic patient. available at http://emedicine.medscape.com/article/284451-overview, May 5, 2012. Accessed
15. Furlong K, et al. Perioperative management of endocrine disorders. Merli G, Weitz H. Medical management of the surgical patient, ed 3, St. Louis: Saunders, 2008.
16. Furnary A, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Annals of Thoracic Surgery. 1999;67(2):352–360.
17. Van den Berghe G, et al. Intensive insulin therapy in the critically ill patients. New England Journal of Medicine. 2001;345:1359.
18. Raju TA, et al. Perioperative blood glucose monitoring in the general surgical population. Journal of Diabetes Science and Technology. 2009;3(6):1282–1287.
19. MacKenzie C, Sharrock N. Perioperative medical considerations in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 1998;24:1–17.
20. Longnecker D, et al. Anesthesiology. New York: McGraw Hill Medical; 2007.
21. Davis P, et al. Smith’s anesthesia for infants and children. ed 8. St. Louis: Mosby; 2011.
22. Firth PG, et al. A survey of perioperative management of sickle cell disease in North America. Pediatric Anesthesia. 2011;21:43–49.
23. Miller R, Pardo M. Basics of anesthesia, ed 6. Philadelphia: Saunders; 2011.
24. Stoelting R. Pharmacology and physiology in anesthetic practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
AACN. Core curriculum for progressive care nursing. Philadelphia: Saunders; 2010.
Aguilar D. Glycated hemoglobin as a prognostic marker in nondiabetic patients after acute myocardial infarction: what now. Circulation.2011;124:666–668.
Barash P, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
Barrett K, et al. Ganong’s review of medical physiology, ed 23. New York: McGraw-Hill Medical; 2009.
Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill Professional; 2010.
Drake R, et al. Gray’s anatomy for students, ed 2. Philadelphia: Churchill Livingstone; 2009.
Deutschman C, Netigan P. Evidence-based practice of critical care. Philadelphia: Saunders; 2010.
Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
Hemmerling T, et al. Comparison of a continuous glucose-insulin-potassium infusion versus intermittent bolus application of insulin on perioperative glucose control and hormone status in insulin-treated type 2 diabetics. J Clin Anesth. 2001;13:293–300.
Hines R, Marschall K. Handbook for Stoelting’s anesthesia and co-existing disease, ed 3. Philadelphia: Saunders; 2009.
Mason R. Murray and Nadel’s textbook of respiratory medicine, ed 5. Philadelphia: Saunders; 2011.
Michaelian N, et al. Perioperative glycemic control: use of a hospital-wide protocol to safely improve hyperglycemia. J Perianesth Nurs.2011;26:242–251.
Miller R, et al. Miller’s anesthesia, ed 7. Philadelphia: Churchill Livingstone; 2009.
Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis: Mosby; 2011.
Pasero C, McCaffery M. Orthopaedic postoperative pain management. J Perianesth Nurs. 2007;22(3):160–174.
Townsend C, et al. Sabiston’s textbook of surgery, ed 18. Philadelphia: Saunders; 2008.
Vincent J, et al. Textbook of critical care, ed 6. Philadelphia: Saunders; 2011.