47 Care of the laser/laparoscopic surgical patient
Absorption: The action of the tissue taking up the laser energy, which causes a reaction within the tissue. Thermal damage caused by laser absorption depends on the wavelength and fluence of the laser beam and the tissue consistency, color, and water content.
Coherence: A state in which all the waves travel in the same phase and direction and all the peaks and troughs of the waves are synchronized.
Collimation: A state in which light waves travel parallel to each other and do not diverge or spread, which reduces the loss of power and allows for better focus and precision.
Laparoscopic Surgery: A form of endoscopic surgery with a fiberoptic laparoscope inserted into the peritoneum for surgical assessment or treatment of a wide and continually expanding range of conditions.
Laser (Light Amplification by Stimulated Emission of Radiation): A process by which energy is converted into a light form or light energy.
Monochromatic: Light composed of one color or wavelength.
Pneumoperitoneum: Created when gas is delivered into the abdominal cavity through a Veress needle that is advanced through the abdominal wall. A mechanical insufflator with a pressure-limiting function is connected with tubing to the Veress needle to inflate the peritoneum. When a pneumoperitoneum is achieved, the surgical team is able to visualize the abdomen and perform the indicated procedure.
Reflection: Occurs when the direction of the laser beam is changed after it comes in contact with a surface.
Scattering: Process in which the laser beam is distributed in many different paths after entering the tissue.
Transmission: Occurs when the laser beam passes through or is transmitted through a medium, such as fluids or tissue, with little or no thermal effect.
Laser surgery
The term laser is actually an acronym for light amplification by stimulated emission of radiation. It describes a process by which energy is converted into a light form or light energy. The theory on which laser technology is based was developed by Albert Einstein in 1917. Schawlow and Townes further explored this theory while Gordon Gould also researched light technology. The principle of LASER was introduced in 1958 by these researchers, and the first true laser device was built by Theodore H. Maiman in 1960. Laser devices, although initially controversial, revolutionized surgical procedures; technology and use continue to expand.1,2 The benefits of laser-assisted surgery are many (Box 47-1).2,3
BOX 47-1 Benefits of Laser Surgery
• Seals small blood vessels, possibly reducing intraoperative and postoperative blood loss
• Often decreases postoperative edema and the chance of the spread of malignant cells with sealing effects of lymphatic and vascular vessels
• Sometimes seals nerve endings, thus reducing postoperative pain in certain procedures
• Decreases scarring through the possible reduction of postoperative stenosis and decrease in collagen reformation
• Laser beam precision usually minimizes adjacent tissue damage
• Usually decreases operative and anesthesia time
• Allows for increased use of local anesthetic techniques as opposed to general anesthesia
• More procedures can be done on an ambulatory basis
• Often quickens recovery time and return to activities of daily living
Modified from Houck PM: Comparison of operating room lasers: uses, hazards, guidelines, Nurs Clin North Am 41(2):193-218, 2006; Ball KA: Surgical modalities. In Rothrock JC: Alexander’s care of the patient in surgery, ed 14, St. Louis, 2011, Mosby; Babin-Ebell J: Transmyocardial laser revascularization combined with intramyocardial endothelial progenitor cell transplantation in patients with intractable ischemic heart disease ineligible for conventional revascularization: preliminary results, Thorac Cardiovasc Surg 58(1):11-16, 2010.
Laser light
Laser energy is measured by the wavelength of the light, which is the distance between two successive peaks of a wave. Laser wavelengths are often measured in nanometers. One nanometer is equal to 10–9 m.3 Some of the lasers used in surgery today are longer (e.g., the CO2 laser at 10,600 nm or the Nd:YAG laser at 1064 nm) and are within the infrared section of the electromagnetic spectrum. Other laser wavelengths are in the visible area of the electromagnetic spectrum (400 to 750 nm), whereas shorter wavelengths are found in the ultraviolet region of the electromagnetic spectrum (100 to 400 nm; Fig. 47-1).1,3
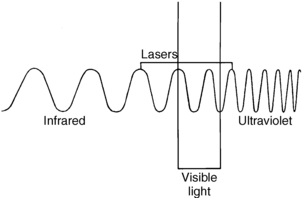
FIG. 47-1 Electromagnetic spectrum.
(From Rothrock JC: Alexander’s care of the patient in surgery, St. Louis, 2011, Mosby.)
Laser light differs from ordinary light in three distinct ways that make it both unique and effective in the surgical setting.1,3
1. Laser light is monochromatic, which means one color or wavelength. This pure color can determine how it reacts with certain tissues. Ordinary light, in comparison, is polychromatic, which means that it comprises a multiple array of colors or wavelengths.
2. Laser light is collimated. The light waves travel parallel to each other and do not diverge or spread. Collimation reduces the loss of power and allows for better focus and precision. Ordinary light spreads out in space as it travels away from its source.
3. Laser light is coherent. All the waves travel in an orderly manner in the same phase and direction, and all the peaks and troughs of the waves are synchronized. This coherence gives the laser beam its power. Ordinary light, in comparison, is incoherent as its waves travel out in random directions.
Tissue interaction
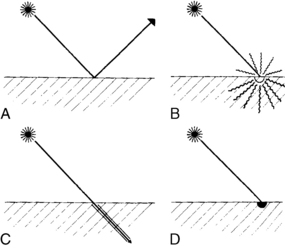
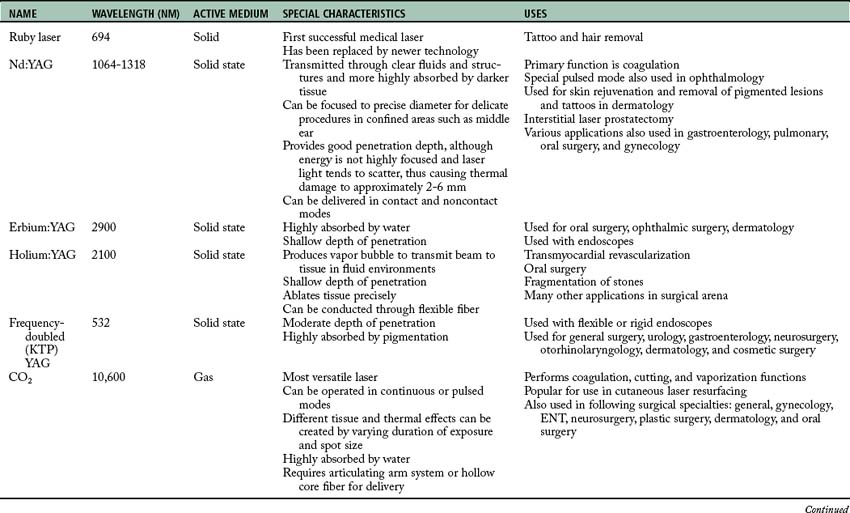
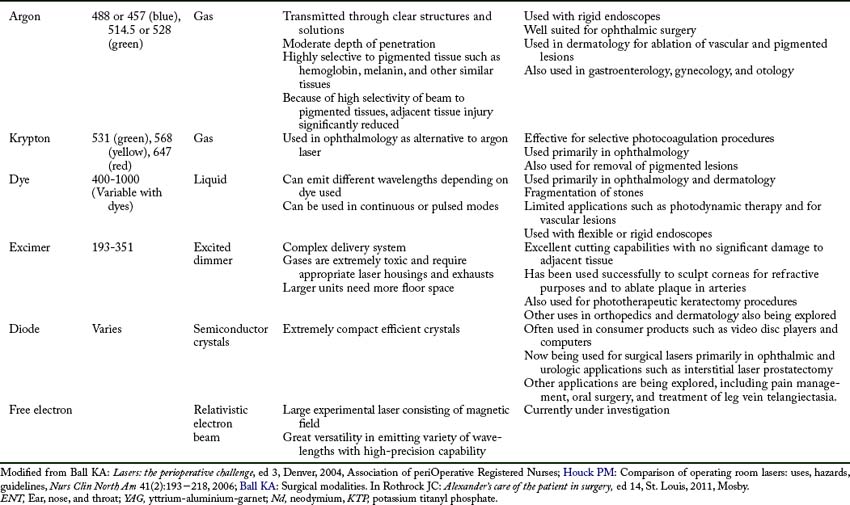
Four different interactions can occur when laser energy comes into contact with human tissue (Fig. 47-2). These interactions include reflection, scattering, transmission, and absorption.3 The extent of this interaction is dependent on the wavelength of the laser, power settings, spot size, duration of exposure of the laser beam with the tissue, and the characteristics of the tissue. These interactions can have both positive and negative effects.1–3
• Reflection. Reflection occurs when the direction of the laser beam is changed after it comes into contact with a surface. This direction change can be intentional or accidental and thus can have both positive and negative effects. Mirrors can be used to intentionally reflect the laser beam to direct the beam to a hard-to-reach area. This action must be done carefully, however, to prevent an inadvertent strike and possible damage to a nontargeted area. Reflection can also occur if the laser beam hits an obstacle (e.g., a surgical instrument) and then is inadvertently reflected to another area, thus causing a tissue burn. Reflection can be either specular (direct reflection) or diffuse (scattered reflection).
• Scattering. The laser beam can also scatter as it enters certain tissues. This scattering causes the beam to disperse over a large area and weakens its strength. Backscattering can also occur as the beam scatters backward up the endoscope, thus causing damage to the operator’s eye, the endoscope optics, or the distal end of the scope.
• Transmission. Transmission occurs when the laser beam passes or is transmitted through fluids or tissue with little or no thermal effect. Transmission depends on the wavelength of the laser beam and the tissue with which it comes in contact. For example, an argon laser beam can be transmitted through the clear structures and solutions of the eye to coagulate a bleeding vessel on the retina. This action occurs because the argon energy is not absorbed by clear structures and solutions; therefore no thermal effect is noted on these tissues.
• Absorption. Thermal effects and tissue response occur only when tissue absorbs the energy of the laser that contacts it. The amount of absorption and penetration depends on the beam’s wavelength and power, the characteristics of the contact tissue (color, consistency, and water content), the duration of the beam exposure, and the beam spot size. A thermal response can occur when tissue absorbs laser energy, thus heating the target cells. The degree of tissue change or thermal damage depends on the temperature to which the cells are heated. This temperature change is purposely regulated to affect the desired tissue response (Table 47-1).
| TEMPERATURE (°C) | VISUAL CHANGE | BIOLOGIC CHANGE |
|---|---|---|
| 37-60 | No visual change | Warming, welding |
| 60-65 | Blanching | Coagulation |
| 65-90 | White/gray | Protein denaturization |
| 90-100 | Puckering | Drying |
| >100 | Smoke plume | Vaporization, carbonization |
From Ball KA: Lasers: the perioperative challenge, ed 3, Denver, 2004, Association of periOperative Registered Nurses.
Laser surgery can be categorized into three different types of tissue response, including thermal, mechanical, and chemical effects.1 The thermal effect, as discussed previously, is the most common laser effect as tissue is vaporized, coagulated, ablated, cut, and welded depending upon the degree of thermal interaction. The mechanical (acoustical) effect from laser energy results when sound energy is created by the laser beam which disrupts tissue. The chemical effect is produced as the laser energy is used to activate a light-sensitive dye to disrupt and change tissue.
Types of lasers
Lasers are classified by the four active mediums used to generate the laser energy: gas, solid, liquid, and semiconductor crystals. In a gas medium, electric energy is pumped through a gas, such as argon, to produce the laser energy. A solid medium uses a special rod doped with an element that is activated with exposure to flash lamps to create the laser energy. Liquid mediums are organic dyes that produce a wide range of wavelengths when activated with another laser beam. Semiconductor crystals are used in the medical field and in consumer products and fiberoptic communication systems. Experimental mediums that are currently being explored include free electron lasers. The actual laser name is usually derived from the active medium substance that is used to generate the laser energy.1–3 A summary of the various lasers currently in use can be found in Table 47-2.
Preoperative care
For example, transmyocardial revascularization with the laser is generally limited to patients with advanced cardiovascular disease who have hemodynamically stable conditions and are not candidates for traditional bypass surgery. This new laser treatment may offer a method to revascularize the heart muscle for patients with intractable ischemic heart disease.1,4 Dermatologic procedures may require extensive skin preparation at home, preoperative administration of prophylactic antibiotics or antivirals, multiple treatments, and extensive postoperative skin care regimens that may last up to 1 month or more.1,2,5Preoperative care must include education concerning these issues and must be used to determine whether the patient will be able to comply with the treatment regimen.
The patient must also be prepared for expectations both during and after surgery. Many of these procedures are conducted without any anesthesia or with moderate sedation. The patient must be prepared for the sights, smells, and other sensations that will be experienced. Eye protection must be provided. Odors can include the smell of tissue burning or being vaporized. The patient may also have burning or stinging types of painful sensations with certain procedures.1,6
Intraoperative issues
Intraoperative issues with laser procedures primarily concern safety. Lasers are considered a class III medical device and, as such, are subject to U.S. Food and Drug Administration jurisdiction.3 Many other regulatory, industry, and professional bodies also address the safe use of lasers. Regulations addressed include the registration of laser devices, training requirements, laser safety officer responsibilities, and safety rules.1,3
Lasers must be further classified by the manufacturer according to their potential to cause biologic harm and their inherent level of hazard. The classification system is based on the laser output power, wavelength, exposure duration, and emergent beam exposure. The classification system ranges from I to IV; the higher the class, the greater the potential hazard. Most lasers used in surgery are classified as a class IV and can damage eyes and skin and present a fire hazard.1,3 Because of the many provider and patient risks associated with laser use, a laser safety program should be in place in any facility in which laser procedures are conducted. This includes freestanding ambulatory facilities and physician’s offices. A laser safety committee complete with a laser safety officer should be established and responsible for guiding and overseeing all laser use in the facility. Issues that should be addressed include staff education, physician credentialing, and the monitoring of quality and safety issues. All staff involved in laser use must receive appropriate education before using or being involved in laser procedures. Topics included in these special training classes include laser biophysics, laser equipment, laser-tissue interaction, safety procedures, and clinical applications. Knowledge and skills should be verified through a competency-based credentialing program, and the skills should be reassessed and updated on a regular basis.1–3 The three most important areas of safe laser use include eye protection, smoke evacuation, and fire safety.
The eyes are susceptible to damage from laser radiation. The damage may occur acutely or may go unnoticed and develop gradually over time. The type of damage also varies with the type of laser being used. Anyone who enters an operating room or treatment area where a laser is in use (including the patient) is at risk for eye damage and therefore should wear protective eyewear specific to the laser in use. Filtering devices should also be placed on operative microscopes and endoscopes. The patient’s eyes should be protected with either the appropriate eyewear or moist gauze pads.1–3 The protective eyewear should have inscribed on the side of the frame the laser wavelength that the lenses are protecting against along with the optical density (i.e., the lenses’ filtering capabilities).1–3
Another major safety concern with the use of laser technology is the control of the smoke that the laser energy produces as it impacts tissue. This surgical smoke is also called laser plume or surgical plume. Surgical smoke contains extremely small particles of vaporized tissues, toxins, and steam. If inhaled, this particulate can end up in the alveoli of surgical team members or can coat the inside lumen of unprotected suction lines if used for smoke evacuation. Even short exposure to smoke particulate and odor from toxic gases may be related to headaches, nausea, myalgia, rhinitis, conjunctivitis, and respiratory conditions and complications.1–3 In one study, perioperative nurses were shown to have twice the incidence of targeted respiratory problems, probably because of the repeated inhalation of surgical smoke.7–9 In addition, there is a high chance that surgical smoke can transmit viable pathogenic material within the plume.1–3,7
Patients are also exposed to hazards when surgical smoke is not evacuated appropriately. One study demonstrated that laparoscopic surgery patients can absorb the byproducts of laser tissue interaction (surgical smoke), thus increasing the level of the patient’s methemoglobin and carboxyhemoglobin. This in turn, will decrease the oxygen-carrying capabilities of the red blood cells. The patient is absorbing the toxins produced within surgical smoke and then exhibits symptoms of headache, double vision, or nausea in the postanesthesia care area.10 When surgical smoke is properly evacuated during laparoscopy, vision of the surgical site is maintained, smoke is not absorbed by the patient, and these untoward symptoms are not routinely present in the recovering patient.
Implications for practice
For smoke evacuation recommendations to be followed, a comprehensive educational program on smoke hazards along with smoke evacuation equipment training needs to be implemented. Leadership support and active communication among health care providers need to be fostered. Barriers to compliance must be addressed to minimize any obstacles preventing the achievement of a 100% smoke-free surgical environment.
Source: Ball K: Surgical smoke evacuation guidelines: compliance among perioperative nurses, AORN J 92(8):e1−e23, 2010.
Postoperative care
Only one type of laser procedure requires special nursing care in the postanesthesia care unit. If the patient has undergone photodynamic therapy for selective destruction of a malignancy, the laser is used to activate a special light-sensitive dye that has been injected into the patient approximately 2 days before the procedure. The laser is introduced intraoperatively, which may be during a laparoscopic procedure, to activate the dye that in turn causes singlet oxygen to be formed, thus destroying a malignancy. Because some of the light-sensitive dye is retained for approximately 6 weeks in the skin cells, the patient cannot be exposed to bright lights or sunlight. Precautions to avoid this exposure must be followed in the postanesthesia phase and especially if the patient is discharged. Sometimes these procedures are performed later in the day so that the patient can be discharged during the evening hours when it becomes dark outside.1
Laparoscopic surgery
Laparoscopic surgery is a form of endoscopic surgery with a fiberoptic laparoscope inserted into the peritoneum for surgical assessment or treatment of a wide and continually expanding range of conditions.11 This surgical approach affords many benefits to the patient and surgeon, including smaller incisions, decreased hospital stays and recovery time, and better visualization and magnification of surgical anatomy and pathology.12 To understand the history of laparoscopy, one must first examine the origins of endoscopy, which began in ancient times and were driven by the innate human curiosity to peer inside body cavities. Speculums were first developed and used to examine various areas of the body, such as the rectum and vagina, as early as 400 bc. An Arabian physician first used a mirror to reflect light and examine the cervix in ad 1012. The first crude endoscope was developed in 1585 and used the sun as a light source for examination of the nasal cavity.3,13,14
The 1800s saw the addition of more reliable, but crude, light sources to these endoscopic examinations. The Italian physician Phillip Bozinni developed a device that used a candle for illumination to examine the urethra of a living patient. Later devices used alcohol lamps and a wick. Thomas Edison’s development of the incandescent light bulb in 1880 truly spurred the evolution of modern endoscopy and laparoscopy as we know it today.3,13,14
True laparoscopy was first accomplished by George Kelling in 1901 when he viewed the abdominal viscera of a living dog with a cystoscope. Kelling is also credited with creating the first pneumoperitoneum with this procedure. Equipment and techniques continued to evolve, and the first laparoscopic tubal ligation was performed in 1941. By 1973, more than 500,000 gynecologic laparoscopic procedures had been performed. Laparoscopic cholecystectomy procedures all but replaced open procedures within 3 years of its introduction in 1987. The technology continues to expand today into multiple therapeutic and diagnostic procedures across most surgical specialties.3,14
Preoperative issues
Preoperative care should be focused on the adequate assessment and preparation of the patient. Routine diagnostics and assessments that are conducted for all general anesthesia or surgical patients should be completed. Special attention should be paid to establishing the appropriateness of a laparoscopic procedure for this patient because laparoscopy, and the creation of a pneumoperitoneum, brings its own inherent risks and problems. A recommended preoperative checklist should include the following14:
• History and physical examination
• Evaluation of medical problems
• Thorough evaluation of the cardiac and respiratory systems
• Normalization of fluids and electrolytes
• Deep vein thrombosis prophylaxis
• Genitourinary system evaluation
Numerous relative and absolute contraindications to laparoscopic procedures are well established (Box 47-2), and the patient should be closely evaluated in regard to these issues. Previous abdominal surgery should be thoroughly evaluated, because possible scarring or adhesions can affect the performance of the laparoscope or limit the surgeon’s view of the surgical field. A comprehensive evaluation of the cardiovascular and pulmonary systems is mandated before any laparoscopic procedure, because a pneumoperitoneum can greatly stress these systems. Patients with significant pulmonary disease are at particular risk for developing respiratory acidosis from the accumulation of insufflation carbon dioxide in the abdomen. Large abdominal wall hernias, diaphragmatic defects, and previous scars can affect trocar placement. Pregnancy was once considered an absolute contraindication to laparoscopic surgery; however, these procedures have now been shown to be safe and effective well into the second trimester. Obese patients should also be closely evaluated with specific attention to cardiac and pulmonary status.12,15,16
Modified from Wadlund DL: Laparoscopy: risks, benefits and complications, Nurs Clin North Am 41(2):219−229, 2006; Gerges FJ, et al: Anesthesia or laparoscopy: a review, J Clin Anesthesia 18(1):67−78, 2006; Jobe BA, Hunter JG: Minimally invasive surgery. In Brunicardi FC, et al, editors: Schwartz’s principles of surgery, ed 8, New York, 2005, McGraw Hill; Soper NJ, et al, editors: Mastery of endoscopic and laparoscopic surgery, ed 2, Philadelphia, 2004, Lippincott Williams & Wilkins.
Intraoperative issues
The primary difference between laparoscopic surgeries and their open counterparts are the creation of a pneumoperitoneum and patient positioning, both of which can create patient management challenges during the operative and recovery phases.
Pneumoperitoneum
The creation of a pneumoperitoneum involves the insufflation of the abdomen with a gas. The most commonly used gas for insufflation is CO2 because of its relatively low risk of venous gas embolism (rapidly dissolves) and noncombustibility. Other gases that have been evaluated in clinical and experimental settings include nitrous oxide, helium, and argon, but CO2 gas has been chosen as the preferred insufflation gas for laparoscopic procedures.12,17
A pneumoperitoneum is created during laparoscopic surgery to allow the surgical team to visualize the abdomen and perform the indicated procedure. Unfortunately, the creation and maintenance of this pneumoperitoneum can have varying effects on the patient and is associated with many of the complications generally associated with laparoscopic surgery. The patient’s position during surgery can exacerbate these adverse affects.3,12,15
The pneumoperitoneum is created when gas is insufflated into the abdominal cavity after the Veress needle is inserted through the abdominal wall. A mechanical insufflator with a pressure-limiting function is connected to the Veress needle with tubing to deliver the insufflations gas into the peritoneum. Normal insufflation pressures are maintained at 14 to 16 mm Hg.3 Insufficient pressure produces an inadequate pneumoperitoneum and impairs surgical visualization of the target site. Excessive pressure creates even greater cardiovascular and respiratory compromise than that commonly associated with the procedure.3,12 Insufflation control panels should monitor and display the rate of flow of the insufflations gas, volume of gas delivered, and the intraabdominal pressure.3 High-flow insufflators that deliver 15 to 20 L/min are more effective than those delivering gas at slower rates.3 When higher pressures are achieved in the abdomen, the insufflator must immediately stop the insufflation and release some of the gas if overpressurization is sensed.3
Cardiovascular changes
A wide variety of hemodynamic effects have been reported with the insufflation of a CO2 pneumoperitoneum. The increased abdominal pressure compresses veins within the abdominal cavity and results in an initial increase in cardiac preload; however, true preload is ultimately decreased because of impaired venous return. Cardiac afterload is also increased as a result of the increased abdominal pressure and the resultant neurohumoral reflexes. The most common net effects from these changes include increases in heart rate, systemic vascular resistance, and central venous pressure. Cardiac output drops, and mean arterial pressure may increase, decrease, or remain unchanged, depending on the relative changes in cardiac output and systemic vascular resistance. Hemodynamic monitoring can be used to monitor for pressure changes and myocardial compromise in patients at extremely high risk. Pneumoperitoneum can cause dysrhythmias to include sinus tachycardia, bigeminy, and premature ventricular contractions. When pneumoperitoneum has been established, a resultant increase in the abdominal pressure causes vagal stimulation that can lead to severe bradycardia and possible asystole.12,15 As stated previously, automatic sensors are available within insufflators that provide continual monitoring and adjustment of the abdominal pressure so that cardiac problems are minimized.
Respiratory changes
The creation of a CO2 pneumoperitoneum also has several adverse effects on the respiratory system. Oxygenation may be impaired because of reductions in lung volume and the associated atelectasis that results from an elevated diaphragm. Ventilation may also be impaired and result in CO2 retention and hypercarbia. Other untoward effects include reduced pulmonary compliance, increased airway resistance, and reduced vital capacity. All these effects are exacerbated by the commonly used Trendelenburg position.3 These respiratory changes are also further aggravated by the following conditions: surgery that lasts more than 4 hours, a history of chronic obstructive pulmonary disease, age, obesity, and an American Society of Anesthesiologists physical status of III or greater.3,12,15 A summary of these effects can be found in Table 47-3.
| ELEVATED | REDUCED | |
|---|---|---|
| Respiratory | Respiratory rate | pH |
| PaCO2, mixed venous CO2 tension, alveolar volume | Forced expiratory | |
| CO2 tension | Forced vital capacity | |
| Arterial-venous CO2 difference | Functional residual capacity | |
| Peak airway pressure | Total lung capacity | |
| Plateau airway pressure | Compliance | |
| Intrathoracic pressure | ||
| Airway resistance | ||
| Atelectasis | ||
| Cardiovascular | Heart rate with initial insufflation | Stroke volume |
| Systemic blood pressure | Cardiac output | |
| Mean arterial pressure | Venous return unchanged or reduced | |
| Central venous pressure | Bradycardia with maintenance of pneumoperitoneum | |
| Pulmonary artery pressure | ||
| Systemic vascular resistance | ||
| Myocardial oxygen demand |
Modified from Wadlund DL: Laparoscopy: risks, benefits and complications, Nurs Clin North Am 41(2):219−229, 2006; Gerges FJ, et al: Anesthesia or laparoscopy: a review, J Clin Anesthesia 18(1):67−78, 2006; Soper NJ, et al, editors: Mastery of endoscopic and laparoscopic surgery, ed 2, Philadelphia, 2004, Lippincott Williams & Wilkins.
Other system effects
In addition to the extensive cardiopulmonary changes affected by the creation of a pneumoperitoneum, various other body systems may be affected as well. The patient should be closely monitored for the development of hypothermia, and preventive measures should be taken to avoid this complication.3 Hypercarbia can lead to increased cerebral blood flow with a net result of increased intracranial blood pressure, possible cerebral edema, and potential brain stem herniation. Renal failure can result from the impaired renal blood flow caused by the increased abdominal pressure18 or hypercarbia. Increased abdominal pressure also compromises venous return and puts the patient at risk for development of deep vein thrombosis. Stress hormones are also elevated because of peritoneal distention, which can increase anesthetic time, pain, and acidosis, and decrease venous return.
Concerns about the effect of a pneumoperitoneum on the implantation and spread of tumor cells also arise. The role of laparoscopic surgery for the treatment of cancer remains controversial with questions still being posed about the possibility of the CO2 gas used to establish the pneumoperitoneum being the vehicle for dissemination of malignant cells causing port site metastasis.19,20 More research needs to be conducted before a conclusive answer can be determined.
Gasless laparoscopy
Several systems are currently being evaluated for use in gasless laparoscopy. These systems work with various slings and retractors to lift the abdominal wall away from the intraabdominal contents and create a surgical space in which the procedure can be performed. The primary advantage of this technique, of course, is the elimination of the need for a pneumoperitoneum. Disadvantages center around the inability to establish adequate surgical field exposure. Patient indications for gasless laparoscopy essentially parallel those for similar open and laparoscopic (with insufflation) cases. The gasless approach is better suited to lower abdominal procedures, because greater abdominal distension can be accomplished in this area, particularly with women.21
Patient positioning
Exaggerated surgical positions are often necessary with laparoscopic surgery to affect adequate organ exposure. The two most commonly used positions are the Trendelenburg, or head-down, position for bowel surgery and the reverse Trendelenburg, or head-up tilt, position for upper abdominal procedures. Both positions result in changes in cardiac filling pressures and lung volumes that affect ventilation, oxygenation, and lower extremity venous stasis. These changes are exacerbated with the addition of a pneumoperitoneum (Table 47-4).3,12,21
Table 47-4 Physiologic Effects of Patient Position During Laparoscopic Surgery
| SYSTEM | TRENDELENBURG | REVERSE TRENDELENBURG |
|---|---|---|
| Cardiovascular | Increased central filling pressures | Decreased central filling pressures |
| Increased MAP | Decreased MAP | |
| No change in CO | Decreased CO | |
| Pulmonary | No change in oxygenation | No change in oxygenation |
| No change in ventilation | No change in ventilation | |
| Venous stasis | No change in lower extremity venous blood flow | No change in lower extremity venous blood flow |
MAP, Mean arterial pressure; CO, cardiac output.
Modified from Wadlund DL: Laparoscopy: risks, benefits and complications, Nurs Clin North Am 41(2):219−229, 2006; Gerges FJ, et al: Anesthesia or laparoscopy: a review, J Clin Anesthesia 18(1):67−78, 2006; Soper NJ, et al, editors: Mastery of endoscopic and laparoscopic surgery, ed 2, Philadelphia, 2004, Lippincott Williams & Wilkins; Henny CP, et al: Laparoscopic surgery: pitfalls due to anesthesia, positioning, and pneumoperitoneum, Surg Endosc 19(9):1163−1171, 2005.
Postoperative issues
Care of the patient immediately after any laparoscopic procedure should include basic PACU care and monitoring specific to the procedure and type of anesthesia administered. Postoperative pain management is typically easier after laparoscopic procedures than after open procedures and can often be accomplished with a small amount of opioids in combination with nonsteroidal agents and local anesthetics. Visceral discomfort is often more difficult to treat and more unpredictable. This pain is triggered by the retained gas in the peritoneal cavity and the resulting irritation of peritoneal surfaces; it commonly manifests as shoulder pain and may persist for several days after surgery. The patient should be prepared for this discomfort as a part of the preoperative education. The pain can generally be managed with oral analgesics.15,21
Postoperative nausea and vomiting can pose a significant problem with any intraabdominal surgery. Routine drainage of the stomach at the end of the case before removal of the nasogastric tube helps to reduce the incidence but does not completely eliminate it. Prophylactic treatment with an antiemetic is not indicated for all laparoscopic cases, although it may be appropriate when multiple risk factors for postoperative nausea and vomiting are present.15
If a recovering laser laparoscopic surgery patient complains of headaches, nausea, or double vision, these symptoms may be from the lack of smoke evacuation when the plume is created. A study by Ott10 demonstrates that the patient may absorb surgical smoke toxic gases, such as carbon monoxide, when plume is not removed.10 Untoward symptoms, such as methemoglobin and carboxyhemoglobin, can be avoided through appropriate smoke evacuation during laparoscopic procedures.10
In addition to basic PACU care, careful attention should be paid to monitoring the patient for any complications associated with laparoscopic intervention. Laparoscopic procedures are remarkably safe when correctly performed; their major complication rate is less than 1%, and the overall mortality rate is 4 to 8 deaths per 1000 procedures.21 Complications can occur, however, and are divided into two categories: those associated with the pneumoperitoneum and those associated with the procedure.
Pneumoperitoneum complications
The complications associated with the creation of the surgical pneumoperitoneum are directly related to the physiologic changes associated with this procedure. Most complications occur during the initiation and maintenance of the pneumoperitoneum; however, the perianesthesia nurse may be the one who identifies the complication or is responsible for the continued care and management of the patient. Table 47-5 provides a summary of pneumoperitoneum complications and their causes. Nursing care should be based on the complication presented.
| SYSTEM | COMPLICATION | POSSIBLE MECHANISM |
|---|---|---|
| Cardiovascular | Tension pneumothorax |
IAP, Increased abdominal pressure.
Modified from Wadlund DL: Laparoscopy: risks, benefits and complications, Nurs Clin North Am 41(2):219−229, 2006; Gerges FJ, et al: Anesthesia or laparoscopy: a review, J Clin Anesthesia 18(1):67−78, 2006; Soper NJ, et al, editors: Mastery of endoscopic and laparoscopic surgery, ed 2, Philadelphia, 2004, Lippincott Williams & Wilkins.
Laparoscopy complications
Complications associated with the laparoscopic technique are usually trocar-related injuries and involve the bowel, vasculature, or bladder.3,12,15,21 Early recognition and treatment in the operating room, of course, results in the best outcome; however, many injuries may go unrecognized at the time of surgery. As a result, vigilant PACU assessment, care, and thorough discharge teaching are essential to a positive patient outcome.
Bowel injuries
Bowel injuries are most troubling because they tend to go unrecognized at the time of surgery. The most common bowel injury involves perforation of the small intestine. Injury to the colon, duodenum, and stomach can also occur. Almost 50% of these injuries go unrecognized for at least 24 hours.12 Perforations that go unrecognized in the operating room can develop into peritonitis sometime after discharge. Delayed onset of sepsis is also common with these injuries. The mortality rate associated with unrecognized bowel injuries can be as high as 5%.12,21 These injuries may go unrecognized in the PACU because the patient may be asymptomatic at that time. Discharge teaching that emphasizes reporting of unrelieved pain, nausea and vomiting, and unresolved fever is particularly important in the recognition and resolution of this complication.
Vascular injuries
Although rare (occurring 0.02% to 0.03% of the time), vascular injuries carry a significant mortality rate of 15%.12 Vascular injuries are most commonly associated with pelvic procedures and tend to occur near the distal aorta and its branches, the inferior vena cava, or iliac veins. Abdominal wall hemorrhage can also occur from inadvertent trocar insertion. Major vascular injury during laparoscopic procedures is rare. Most injuries are usually recognized quickly and repaired in the operating room with direct suture ligation, although a patch or synthetic graft may be necessary for more extensive damage.12,21 Injuries that go unrecognized in the operating room again pose the greatest challenge to the perianesthesia nurse. Unresolved tachycardia and hypotension must be closely evaluated as possible signs and symptoms of hemorrhage. Unresolved or extremely severe postoperative pain and abdominal distension are also possible signs and symptoms of unexpected bleeding. Recognition of surgical hemorrhage and immediate evaluation is critical to a positive patient outcome.
Bladder injuries
The risk of bladder injury can be decreased during laparoscopic procedures with the routine insertion of a urinary catheter to decompress the bladder; however, occasional bladder perforation still occurs. The risk of perforation is greatest in patients with previous abdominal or bladder surgery; risks are also elevated in patients with congenital anomalies. The most common signs and symptoms of bladder perforation are the appearance of air in the urinary drainage bag or unexplained urinary tract bleeding during the procedure. Diagnosis can be confirmed with a retrograde cystogram, and surgical repair can be performed.12,21
Other complications
Other complications of interest include postoperative infection and laparoscopic electrosurgery complications. Antibiotic medications may be used for laparoscopic procedures and other types of surgical procedures as prophylaxis. Studies show that wound infection rates range from 0.1% for diagnostic laparoscopy to as high as 1% for laparoscopic cholecystectomies.12 Electrosurgery has replaced laser energy as the preferred power supply during laparoscopic surgery because it is less expensive and more convenient. This energy, however, has been associated with secondary thermal injuries that may go unrecognized (direct coupling, insulation failure, or capacitive coupling) because they occur outside the surgeon’s view through the laparoscope.3 As with bowel perforations, these injuries often are seen days to weeks after surgery as peritonitis or sepsis, which again highlights the importance of thorough discharge instructions regarding the signs, symptoms, and management of postoperative infections.1,3
Summary
Technologic advances in surgical techniques have had a significant effect on surgical procedures and perianesthesia care as more complex procedures can be conducted with less trauma to the patient. Laser and laparoscopic techniques form the foundation for many of these surgeries. Laser devices, which create a collimated beam of light energy, have revolutionized surgical procedures as technology and acceptance continue to expand. Laparoscopic surgery, a form of endoscopic surgery with a fiberoptic laparoscope inserted into the peritoneum for surgical assessment or treatment of a wide range of conditions, also continues to expand into multiple therapeutic and diagnostic procedures across most surgical specialties. This chapter provided an overview of laser and laparoscopic technologies and how the use of these surgical tools affects perianesthesia patient care. Details of the care of patients who undergo specific procedures may be found in the appropriate systems chapters throughout this book.
1. Ball KA. Lasers: the perioperative challenge. ed 3. Denver: AORN; 2004.
2. Houck PM. Comparison of operating room lasers: uses, hazards, guidelines. Nurs Clin North Am.2006;41(2):193–218.
3. Ball KA. Surgical modalities. Rothrock JC, ed. Alexander’s care of the patient in surgery. ed 14. St. Louis: Mosby; 2011.
4. Babin-Ebell J. Transmyocardial laser revascularization combined with intramyocardial endothelial progenitor cell transplantation in patients with intractable ischemic heart disease ineligible for conventional revascularization: preliminary results. Thoracic & Cardiovascular Surgeon.2010;58(1):11–16.
5. Tiffany J, et al. Patient compliance as a major determinant of laser tattoo removal success rates: A 10-year retrospective study. Journal of Cosmetic & Laser Therapy. 2010;12(4):166–169.
7. Ball K. Surgical smoke evacuation guidelines: compliance among perioperative nurses. AORN Journal.2010;92(8):e1–e23.
8. Ball K. Compliance with surgical smoke evacuation guidelines: implications for practice. AORN Journal. 2010;92(2):142–149.
9. Ball K. Stamping out electrosurgery smoke. Outpatient Surgery. 11(12), 2010. available at http://www.outpatientsurgery.net/issues/2010/12/stamping-out-electrosurgery-smoke Accessed February 28, 2012
10. Ott DE. Smoke and particulate hazards during laparoscopic procedures. Surgical Services Management. 1997;3(3):11–13.
11. Venes D. Taber’s cyclopedic medical dictionary, ed 20. Philadelphia: F.A. Davis; 2005.
12. Wadlund DL. Laparoscopy: risks, benefits and complications. Nurs Clin North Am.2006;41(2):219–229.
13. Ball KA. Endoscopic surgery. St. Louis: Mosby; 1997.
14. Soper NJ, et al. Mastery of endoscopic and laparoscopic surgery, ed 2, Philadelphia: Lippincott Williams & Wilkins, 2004.
15. Gerges FJ, et al. Anesthesia or laparoscopy: a review. J Clin Anesthesia.2006;18(1):67–78.
16. Jobe BA, Hunter JG. Minimally invasive surgery. Brunicardi FC, et al. Schwartz’s principles of surgery, ed 8, New York: McGraw Hill, 2005.
17. Menes T, Spivak H. Laparoscopy: searching for the proper insufflations gas. Surgical Endoscopy.2000;14(11):1050–1058.
18. Abassi Z, et al. Adverse effects of pneumoperitoneum on renal function: involvement of the endothelin and nitric oxide systems. American Journal of Physiology: Regulatory, Integrative, & Comparative Physiology.2007;63(3):R842–R850.
19. Chandrakanth A, Talamini M. Laparoscopy and malignancy. Journal of Laparoendoscopic & Advanced Surgical Techniques. 2005;15(1):38–47.
20. Fletcher J, et al. Dissemination of melanoma cells within electrosurgery plume. The American Journal of Surgery. 1999;178(7):57–59.
21. Soper NJ, et al. Mastery of endoscopic and laparoscopic surgery, ed 2. Philadelphia: Lippincott Williams & Wilkins; 2004.
22. Henny CP, et al. Laparoscopic surgery: pitfalls due to anesthesia, positioning, and pneumoperitoneum. Surg Endosc.2005;19(9):1163–1171.