Cardiovascular system
Maternal physiologic adaptations
Antepartum period
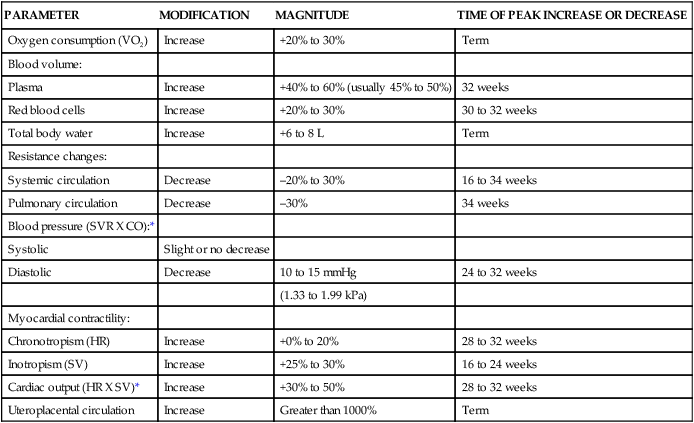
The major hemodynamic changes that occur during pregnancy are outlined in Table 9-1. These changes begin as early as 4 to 5 weeks of gestation and tend to plateau during the second or early third trimester.48 Cardiovascular and hemodynamic changes are due to hormonal influences, changes in other organ systems, and mechanical forces. The hemodynamic changes are partly the result of hormonal influences as well as of the development of the placental circulation and alterations in systemic vascular resistance (SVR). These changes include increases in total blood volume, plasma volume, red blood cell (RBC) volume, and cardiac output. Anatomic changes, such as the upward displacement of the diaphragm by the gravid uterus, shift the heart upward and laterally. There is slight cardiac enlargement on radiograph, and the left heart border is straightened. The size and position of the uterus, the strength of the abdominal muscles, and the configuration of the abdomen and thorax determine the extent of these changes.34
Table 9-1
Physiologic Changes of Pregnancy on the Cardiovascular System
| PARAMETER | MODIFICATION | MAGNITUDE | TIME OF PEAK INCREASE OR DECREASE |
| Oxygen consumption (VO2) | Increase | +20% to 30% | Term |
| Blood volume: | |||
| Plasma | Increase | +40% to 60% (usually 45% to 50%) | 32 weeks |
| Red blood cells | Increase | +20% to 30% | 30 to 32 weeks |
| Total body water | Increase | +6 to 8 L | Term |
| Resistance changes: | |||
| Systemic circulation | Decrease | –20% to 30% | 16 to 34 weeks |
| Pulmonary circulation | Decrease | –30% | 34 weeks |
| Blood pressure (SVR X CO):* | |||
| Systolic | Slight or no decrease | ||
| Diastolic | Decrease | 10 to 15 mmHg | 24 to 32 weeks |
| (1.33 to 1.99 kPa) | |||
| Myocardial contractility: | |||
| Chronotropism (HR) | Increase | +0% to 20% | 28 to 32 weeks |
| Inotropism (SV) | Increase | +25% to 30% | 16 to 24 weeks |
| Cardiac output (HR X SV)* | Increase | +30% to 50% | 28 to 32 weeks |
| Uteroplacental circulation | Increase | Greater than 1000% | Term |
CO, Cardiac output; HR, heart rate; SV, stroke volume, SVR, systemic vascular resistance.
Adapted from Gei, A.F. & Hankins, G.D. (2001). Cardiac disease and pregnancy. Obstet Gynecol Clin North Am, 28, 469.
Hemodynamic changes
Hemodynamic alterations in pregnancy include changes in blood volume, cardiac output, heart rate, systemic blood pressure, vascular resistance, and distribution of blood flow. Stroke volume, heart rate, and cardiac output increase significantly, whereas SVR, pulmonary vascular resistance (PVR), and colloid osmotic pressure decrease. Changes in blood, plasma, and RBC volume are discussed further in Chapter 8.
Total blood volume.
Total blood volume (TBV) is a combination of plasma volume and RBC volume, each of which increases during pregnancy. Circulating blood volume increases begin by at least 6 weeks’ gestation.111 Circulating blood volume increases by 30% to 40% (approximately 1½ L), with a usual range of 30% to 45%.40,111,115 TBV increases rapidly until midpregnancy and then more slowly during the latter half, peaking at 28 to 34 weeks, then plateaus or decreases slightly to term.27,81,111,135 Changes in blood volume are due to the increased steroid hormones, plasma renin activity, aldosterone, human placental lactogen, atrial natriuretic factor, and other mediators.18,48 Figure 8-1 illustrates changes in total blood volume and its component parts, plasma volume, and RBC volume.
The rise in blood volume correlates directly with fetal weight, supporting the concept of the placenta as an arteriovenous shunt in the maternal vascular compartment. Although the degree of hypervolemia varies from woman to woman, subsequent pregnancies in the same woman result in similar increases in circulating volume. Twin and other multiple pregnancies, however, result in greater increases in blood volume, imposing substantially greater demands on the cardiovascular system.111,135
Plasma volume.
About 75% of the TBV increase is in plasma volume. Plasma volume increases progressively from 6 to 8 weeks by approximately 45% to 50% (range, 40% to 60%) or about 1200 to 1600 mL above nonpregnant values 27,111 This change begins at 6 to 8 weeks and increases rapidly during the second trimester, followed by a slower but progressive increase that reaches its maximum of 4700 to 5200 mL at about 32 weeks.22,81,111 Alterations in blood and plasma volume are influenced by hormonal effects, nitric oxide mediated vasodilation, mechanical factors (blood flow in uteroplacental vessels) (see Chapter 8), and changes in the renal system and in fluid and electrolyte homeostasis (see Chapter 11).
Nitric oxide mediated vasodilation induces changes in the renin-angiotensin-aldosterone system, with increased sodium and water retention (see Chapter 11).111 Plasma renin activity and blood aldosterone levels are increased due to the action of estrogens, progesterone, and prostaglandins. An increase in plasma renin activity enhances sodium retention, thereby stimulating aldosterone secretion. Progesterone inhibits the action of aldosterone on the renal tubular cells, thus contributing to sodium retention and an increase in total body water. The degree of fluid retention is influenced by the increased distensibility of the vascular system and the uterine vein capacity present during pregnancy.27
Fluid distribution changes depending on body position. For example, the standing position or prolonged sitting is associated with the development of dependent edema. This is probably due to the trapping of blood in the legs and pelvis as the gravid uterus creates a mechanical impedance to blood flow through the inferior vena cava. This causes an increase in venous pressure in the lower extremities and a sharp rise in hydrostatic pressure in the microcirculation, with subsequent leakage of fluid from the vascular bed into the interstitium. The result is edema of the feet and ankles. Venous distensibility contributes to the decreased venous return to the heart.112
Red blood cell volume.
Red blood cell (RBC) production and thus volume increases throughout pregnancy to a level 20% to 30% higher than nonpregnant values.27,111 Intravascular expansion is mainly due to an increase in plasma volume; therefore hemodilution occurs. This physiologic anemia of pregnancy is reflected in a lower hematocrit and hemoglobin. These changes cannot be prevented with iron supplementation; however, women who are provided exogenous sources of iron do have higher hemoglobin levels in the third trimester than women not receiving supplements (see Figure 8-2). Changes in RBC volume are due to increased circulating erythropoietin and accelerated RBC production. The rise in erythropoietin in the last two trimesters is stimulated by progesterone, prolactin, and human placental lactogen.111 Changes in RBC volume and the role of iron are described further in Chapter 8.
Cardiac output and stroke volume.
Cardiac output, which is the product of heart rate times stroke volume, is one of the most significant hemodynamic changes encountered during pregnancy. Fifty percent of the increase occurs by 8 weeks’ gestation, mediated by changes in SVR.22,111 Cardiac output continues to rise more slowly until the third trimester to values, as measured in the left lateral recumbent position, 30% to 50% (usually 40% to 45%) greater than in nonpregnant women.1,18,25,27,111,115 A slight decline in cardiac output may be seen in late pregnancy due to the decrease in stroke volume near term.111 The increase in cardiac output is associated with an increase in venous return and greater right ventricular (RV) output, especially in the left lateral position.158
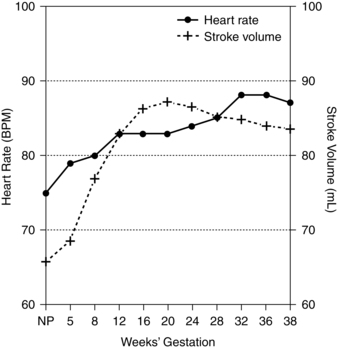
The increased cardiac output is due to changes in both stroke volume and heart rate. Changes in heart rate and stroke volume are reported by 5 weeks’ and 8 weeks’ gestation, respectively.111 The rise in cardiac output early in pregnancy is due primarily to an increase in stroke volume.1,60,111 Stroke volume increases progressively during the first and second trimesters, to a peak value of approximately 25% to 30% above nonpregnant values, peaking at 16 to 24 weeks.27,110 Stroke volume declines during the latter stages of pregnancy, and returns to values that are within the prepregnant range by term.22,27,111,158,162 The changes in stroke volume are likely due to increased ventricular muscle mass and end-diastolic volume changes.111 As pregnancy advances, the heart rate (see section on Heart Rate), which increases more slowly, becomes a more dominant factor in determining cardiac output.111 Figure 9-1 compares the changes in heart rate and stroke volume over gestation.
During pregnancy (especially the third trimester), the resting cardiac output fluctuates markedly with changes in body position.110,111,160 For example, a change from the left lateral recumbent position to supine can lead to a 25% to 30% decrease in cardiac output.160,162 Compression of the inferior vena cava by the uterus in the third trimester results in decreased venous return, stroke volume, and cardiac output.111 Heart rate changes do not necessarily occur with positional changes; therefore these changes in cardiac output are more likely due to a decrease in stroke volume.111,160
Twin, triplet, and other multiple pregnancies have a greater increase in cardiac output than do singleton pregnancies, possibly due to an increase in inotropy.78 The peak is greater, and the decline in cardiac output seen in late pregnancy is smaller. The cardiac output in multiple pregnancies is higher than that encountered in a singleton pregnancy by 20 weeks’ gestation and remains higher for the remainder of gestation.78,122
The increase in cardiac output during pregnancy is not related to the metabolic requirements of the mother or the fetus, or to the increase in maternal body mass. At the time the increase in cardiac output occurs, the fetus is relatively small. When fetal growth is accelerated (late in gestation), there is an actual decline in maternal cardiac output. There is, however, a progressive increase in maternal oxygen consumption, which may be due to the rising metabolic needs of the fetus as well as the demands of the maternal heart and respiratory muscles. Pregnancy at high altitudes is associated with less of an increase in cardiac output accompanied by a reduction in the usual expansion of maternal intravascular volume.79
The increased oxygen requirements are most likely the result of the contractility-promoting influences of estrogen and progesterone on heart and respiratory muscles and the development of the placental circulation. The placenta functions much like an arteriovenous shunt, with a concomitant decrease in peripheral vascular resistance. Therefore, despite the increase in cardiac output, there is a decrease in mean blood pressure due to a decrease in diastolic pressure. The concomitant increase in blood volume (either in time or magnitude) probably explains the increase in cardiac output. Both begin to rise as early as 6 to 8 weeks’ gestation, peaking toward the end of the second trimester, around 30% to 50% higher than prepregnant levels.110,111
Heart rate.
Heart rate is the determinant of cardiac output that has the widest range of values. This wide range provides stability to the circulatory system under a variety of circumstances (e.g., from rest to maximal exercise). Heart rate can be altered in order to maintain blood pressure if changes in vascular resistance or stroke volume are encountered. However, an increased heart rate is insufficient to increase cardiac output; it must be accompanied by an increase in venous return. Both heart rate and venous return are increased in pregnancy, contributing to the increase in cardiac output seen throughout gestation.110,111 The increased heart rate compensates for the decreased SVR and is due to increased myocardial α receptors due to estrogen.25,68
The maternal heart rate increases progressively during pregnancy, averaging 10 to 20 beats per minute higher (10% to 20% increase) by 32 weeks (see Figure 9-1).18,27,65,68,111 At term, the heart rate may return to near baseline levels in some women. Twin pregnancies have an earlier acceleration in heart rate, with a maximum increase 40% above the nonpregnant level near term.34
The increased heart rate results in an elevated myocardial oxygen requirement, which is probably not important in women without cardiac disease, but may become significant in pregnant women with underlying cardiovascular pathology. Beyond this, the increased resting heart rate decreases the maximal work capacity by diminishing the output increment that can be achieved during maximal exercise.110
Blood pressure.
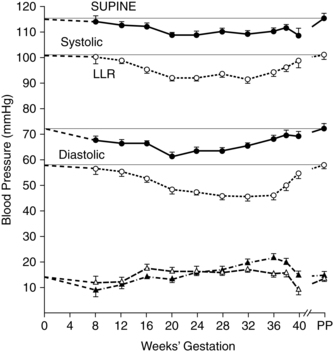
Although there are substantial increases in both blood volume and cardiac output during pregnancy, these changes are not associated with increases in either venous or arterial pressure since the increase in intravenous volume is balanced by the decreased SVR.18,111 In fact, blood pressure, especially diastolic pressure, is generally reported to decrease, reaching a nadir by midpregnancy.18,111 The initial decrease is thought to be due to a lag in compensation for changes in peripheral vascular resistance.111 The diastolic blood pressure decrease reaches a nadir at 24 to 32 weeks and then gradually returns to nonpregnant baseline values by term (Figure 9-2).23,27,111 The magnitude of blood pressure changes vary with the position of the woman during measurement. For example, arterial blood pressures are about 10 mmHg higher in sitting or standing than in a left lateral recumbent or supine position.111 Serial observations in a sitting or standing position show that as women advance through their pregnancies, systolic blood pressure remains either stable or decreases slightly, whereas diastolic pressure decreases an average of 10 to 15 mmHg (1.33 to 1.99 kPa).27,111 Mean arterial pressure decreases until midpregnancy, then increases to term.23 The reduction in blood pressure may be secondary to the vasodilatory effects of nitric oxide, which increases in pregnancy, as well as hormonal and other factors such as prostacyclin and relaxin that mediate a decrease in peripheral vascular resistance.123 Some investigators, have not found this midtrimester fall in blood pressure, but instead reported either no change or a slight rise from the first trimester until late pregnancy.116,151,165
Techniques and differences in the diastolic measurement point also influence blood pressure measurement. Therefore it is important to use a consistent method and maternal position to evaluate blood pressure.111,166 Conventional blood pressure techniques often lack standardization in terms of use of appropriate cuff size, equipment calibration, rounding off (to nearest 5 to 10 mmHg [0.66 to 1.33 kPa] versus the recommended 2 mmHg [0.26 kPa]), and method.57,66
Venous pressures during pregnancy also do not change significantly. Given the large increase in blood volume during pregnancy, an increase in venous pressure would be expected. The increased vascular capacitance and compliance seen during pregnancy explain this lack of change. These alterations are thought to be due to the effects of progesterone and endothelial-derived relaxant factors such as nitric oxide on blood vessel smooth muscle and collagen.4,111 Venous pressure below the uterus is increased, which may be caused by the increased capacitance encountered in the large pelvic veins and the veins distal to the uterus. The latter may affect venous return to the heart—especially in the upright position—due to regional pooling. The ability to sustain venous return is crucial in determining maternal exercise capacity (see “Exercise during Pregnancy”).110
Systemic vascular resistance.
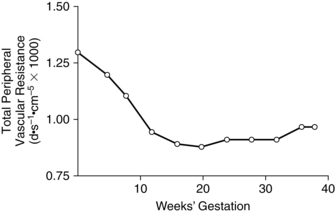
Changes in cardiac output during pregnancy are accomplished without an increase in arterial pressures because of the marked decrease in SVR, especially in peripheral vessels (reduction in peripheral vascular resistance).24,158 SVR (mean arterial pressure divided by cardiac output) is decreased 20% to 30% during pregnancy and parallels the decrease in blood pressure.65 SVR decreases by 5 weeks and usually reaches its lowest level by 16 to 34 weeks, then progressively increases to term (Figure 9-3).22,111
The decreased vascular resistance is due to softening of collagen fibers and hypertrophy of smooth muscle, systemic vasodilation due to the vasodilatory effects of progesterone and prostaglandins, remodeling of the maternal spiral arteries (see Chapter 3), fluid retention (leading to blood volume expansion), and the addition of the low-resistance uteroplacental circulation, which receives a large proportion of cardiac output.26,48,111 The decline in resistance is not limited to the uteroplacental circulation; rather, it is seen throughout the body.90 The decreased SVR is also mediated by endothelial prostacyclin, and endothelial-derived relaxant factors (e.g., nitric oxide) that enhance vasodilation.24,48,65,111,144 Decreased systemic and renal vascular tone occur very early in pregnancy and precede changes in blood volume.24 Thus the decrease in SVR may be the stimulus for changes in heart rate and stroke volume, and thus cardiac output, in early pregnancy, as well as sodium and water retention (see Chapter 11), and alterations in blood pressure.24,25,48,111 The hormonal activity of pregnancy also has a role in the reduction of SVR and contributes to changes in regional blood flow. Along with this, the increased heat production (from maternal, fetal, and placental metabolism) stimulates vasodilation of vessels (especially in heat-losing areas such as the hands) and further reductions in resistance.
Regional blood flow.
Much of the increased maternal cardiac output is distributed to the uteroplacental circuit. The increase in cardiac output above the needs of the uteroplacental circulation leads to increased flow to other organ systems, particularly the mammary glands, skin, and kidneys. This creates a reservoir that can be tapped as pregnancy progresses. Blood flow to the splanchnic bed and skeletal muscle perfusion may decrease.68
Mammary blood flow is increased. Clinically increased flow is evident by the engorgement that occurs early in pregnancy and the dilation of veins on the surface of the breasts. This dilation is usually accompanied by a sensation of heat and tingling. The effect of pregnancy on coronary artery blood flow is unknown. With the increased workload of the left ventricle (LV) (because of the increased cardiac output, blood volume, fetal growth, uterus enlargement, and body weight gain), it can be assumed that the coronary blood flow is increased to meet the cardiac myometrial demands. Cerebral blood flow is not significantly altered during pregnancy.111,123 Although absolute blood flow to the liver does not change significantly, the percentage of the cardiac output perfusing the liver decreases.111
Uterine blood flow.
The decline in uterine vascular resistance allows blood flow to the uterus to increase during gestation. At 10 weeks’ gestation the blood flow is approximately 50 mL/min, increasing to 200 mL/min by 28 weeks and 500 to 800 mL/min at term.111,115 Thus by term the uterus is receiving between 10% and 20% of the maternal cardiac output, versus 2% in nonpregnant women.111 The uterine spiral arteries undergo marked changes during pregnancy (see Chapter 3) that disrupt their muscular and elastic elements. As a result, the uteroplacental arteries are almost completely dilated and are no longer responsive to circulating pressor agents or influences of the autonomic nervous system.136 This results in a large pool of blood within the uterus to maintain fetoplacental blood flow and oxygenation.
Renal blood flow.
Renal blood flow (RBF) increases by the end of the first trimester and then decreases to term (see Chapter 11). The percentage of the cardiac output perfusing the kidneys does not change and remains around 18%. The glomerular filtration rate (GFR) increases 40% to 50% over nonpregnant levels and is due in large part to the increased RBF. The decrease in renal vascular resistance may be mediated by nitric oxide, prostacyclin (PGI2), and atrial natriuretic factor.90
Skin perfusion.
Skin perfusion increases significantly during pregnancy, with a slow but steady rise in perfusion up to 18 to 20 weeks’ gestation. This slow rise is followed by a sharp increase between 20 and 30 weeks, with no significant increase after that. The increased flow is measurable for up to 1 week postpartum.90 Clinically this increased flow may be accompanied by an increase in skin temperature and clammy hands, which are the result of dermal capillary dilation. Vascular spiders and palmar erythema can be seen in many women during pregnancy (see Chapter 14). The vasodilation may facilitate the dissipation of excessive heat created by fetal metabolism. Increased peripheral flow can also be seen in the mucous membranes of the nasal passages, explaining the nasal congestion that is common in pregnancy (see Chapter 15).90,123
Pulmonary blood flow.
Pulmonary blood flow increases secondary to increased circulating blood volume and increased cardiac output. This can be demonstrated on x-ray by increased vascular markings. The increase in pulmonary blood flow is balanced by a decrease in PVR.35 The 30% decrease in PVR is probably in response to hormonal stimulation, and is reflected in a lowered mean pulmonary artery pressure.35,65
Oxygen consumption
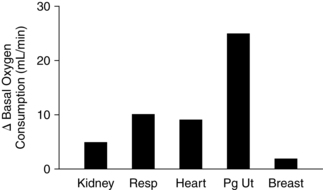
Oxygen consumption is a reflection of metabolic rate. During pregnancy there is a progressive increase in resting oxygen consumption, with a peak increase of 20% to 30% by term (Figure 9-4). This increase in oxygen consumption is due to the increased metabolic needs of the mother and the growing fetus.158 Oxygen consumption increases gradually over gestation, whereas cardiac output increases dramatically in the first and second trimesters. Because the resting cardiac output increases before there is a significant rise in maternal oxygen consumption, the arteriovenous oxygen difference decreases in early pregnancy, then gradually increases as oxygen consumption rises during pregnancy. This provides the uterus with well-oxygenated blood flow during the first trimester, before the completed development of the fetoplacental circulation.22,158
Physical changes
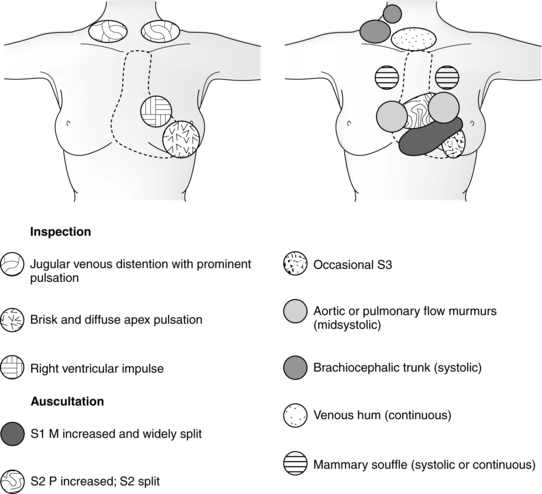
The physiologic hypervolemia of pregnancy results in alterations in the cardiac silhouette, chamber size, pressures, and electrocardiogram (ECG). These changes—along with the hemodynamic alterations—lead to changes in the physical findings encountered during cardiovascular assessment of the pregnant woman (Figure 9-5 and Table 9-2).
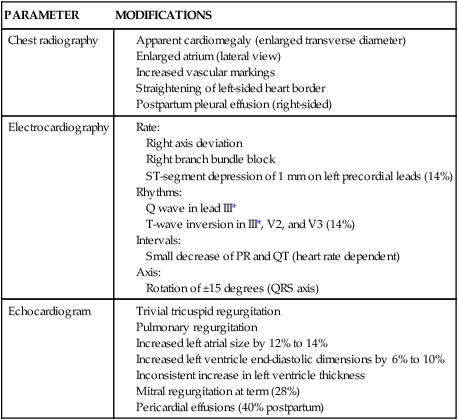
*Decrease or normalize with deep inspiration.
From Gei, A.F. & Hankins, G.D. (2001). Cardiac disease and pregnancy. Obstet Gynecol Clin North Am, 28, 473.
The heart and blood vessels undergo remodeling during pregnancy.68,158 All four chambers enlarge, with the greatest change seen in the left atrium.68 Cardiac ventricular wall muscle mass increases by 10% to 15% in the first trimester; end-diastolic volume increases in the second and third trimesters.27,111,158 These changes increase cardiac compliance, augmenting stroke volume and maintaining the ejection fraction.27,111,158 Increases in left atrial diameter parallel increases in blood volume, beginning in the first trimester and peaking around 30 weeks.111 Thus the pregnant woman has a physiologically dilated heart with increased compliance.111,158
The exact mechanism underlying changes in blood vessels is also unclear, but the result is an increase in aortic size, aortic compliance (evident by 5 weeks postmenstrual age), venous blood volume, and venous compliance.111,158 Compliance of the entire vasculature is increased due to softening of the collagen and smooth muscle hypertrophy.
As the uterus grows, the diaphragm is displaced upward and the heart shifts to a more horizontal position (see Figure 9-5).34,68,111 The LV point of maximum impact is easily palpated and may be shifted to the left. A right ventricle (RV) impulse can usually be palpated at the middle- to lower-left sternal border; this is the pulmonary trunk. Difficulty in localizing this point may be caused by the enlarged breast tissue.
Pulse pressure increases slightly in the third trimester due to changes in the differences between systolic and diastolic pressures.111 The arterial pulse is full, becoming sharp and jerky between 12 and 15 weeks. This finding remains until one week after delivery. Jugular vein pulsations are more readily seen, because vein distention appears around 20 weeks’ gestation. A venous hum may be associated with the rapid downward flow through the jugular veins. This is indicative of a rapid circulation and high cardiac output state.
Edema of the ankles and legs is a frequent finding during the latter part of pregnancy (see Chapter 14). As maternal age increases, edema is more common. An increase in capillary permeability and the fall in plasma colloid osmotic pressure, along with the increase in femoral venous pressure, are implicated in edema formation.125 Thigh-high support hose may reduce this edema by increasing SVR and reducing venous pooling.
Heart sounds.
Auscultation of the heart reveals an exaggerated split and loudness of both components of the first heart sounds (mitral and tricuspid valve closure) in most pregnant women. These changes can be heard for the first time between 12 and 20 weeks’ gestation and continue until 2 to 4 weeks postpartum. Nearly 90% of pregnant women will have a louder and more widely split first sound that is best heard at the left sternal border between the third and fifth intercostal spaces.111 This change is primarily due to the earlier closure of the mitral valve along with increased flow.111 Approximately one third of pregnant women have minor mitral regurgitation; pulmonic and tricuspid valve regurgitation is seen in most women during pregnancy.68
Aortic and pulmonic elements of the second heart sound do not change significantly during the first 30 weeks of gestation.111 During the last 10 weeks, persistent splitting that does not vary with respiration may be heard. This change may be due to the reduced diaphragmatic movement secondary to uterine size.90,125 A loud third heart sound is heard in up to 90% of pregnant women.53,111
Most women (92% to 95%) demonstrate a systolic murmur along the left sternal border, particularly during the last two trimesters, which is indicative of the increased cardiovascular load.18,68,90 This is considered an innocent murmur that is usually short and heard in early to mid-systole. Such murmurs are best auscultated along the left sternal border in the third intercostal space, although some are more prominent along the lower-left sternal border at the apex or in the aortic area. These murmurs have a musical quality and represent audible vibrations caused by ejection of blood from the RV into the pulmonary trunk or from the LV into the brachiocephalic arteries at the point of branching from the aortic arch.125 A systolic precordial murmur due to functional tricuspid regurgitation may also be heard.111 Systolic murmurs louder than grade 2/4 or any diastolic murmurs require further investigation. A third heart sound, venous hum, or mammary soufflé may be misinterpreted as a diastolic murmur.68,125
About 14% of women have murmurs of mammary vessel origin.111 Of these, 70% are continuous, whereas the other 30% are heard only during diastole. They are more common in lactating women but can be auscultated during pregnancy as well. Often unilateral and high pitched, these murmurs are best heard at the second to fourth intercostal space lateral to the left sternal border.68,125 They can be changed or obliterated by varying the pressure applied by the stethoscope. The mammary soufflé is an indication of the increased blood flow in the mammary vessels. The murmur disappears after the termination of lactation.125 An increase in arrhythmias is reported during pregnancy (see Arrhythmias on p. 260).45,56
Electrocardiograph and echocardiograph changes.
Changes in the ECG occur as the position of the heart shifts with the enlarging uterus and elevated diaphragm.27 These changes are usually minor and are summarized in Table 9-2. A small Q wave and inversion of the P wave are not uncommon. T-wave and ST-segment changes can also be seen.18,53 With descent of the fetus into the pelvis in late pregnancy, a right axis shift may be noted.18 As the heart size increases, the incidence of arrhythmias—usually in the form of supraventricular tachycardias—increases. Benign arrhythmias that were present before pregnancy may intensify or progress.
Echocardiography demonstrates an increase in left end-diastolic dimensions during the second and third trimesters. This is probably due to the expanded blood volume and increased filling during diastole. The increased stroke volume that accompanies this added volume maintains the end-systolic dimensions of the LV at nonpregnant levels. These findings are supported by cardiac catheterization data. The right ventricular diastolic pressure is slightly increased over the nonpregnant values. On the other hand, the RV systolic pressure is just below the nonpregnant values (25 to 30 mmHg [3.32 to 3.99 kPa]). This is probably due to the diminished mean pulmonary artery pressure and decreased PVR.53,111
Intrapartum period
During the intrapartum period, the repetitive, forceful contractions of the uterus as well as pain, anxiety, and apprehension can affect the cardiovascular system.90 Significant hemodynamic changes can be attributed to the pain and anxiety associated with labor and delivery (see Chapter 15), especially in primiparous women. This is due to the release of catecholamines and the increased systemic vascular tone. Each contraction contributes approximately 300 to 500 mL of additional volume to the circulation.27,60,111 This results in a significant increase in cardiac output, with a cumulative effect over the course of labor.1 A progressive rise in cardiac output is seen during the first stage of labor. Cardiac output rises further during the second stage and peaks immediately after delivery.111 Cardiac output increases 10% to 15% above pregnancy values during the first stage and is up to 50% greater in the second stage of labor.27,65,111,160 These increases in cardiac output are due to increases in both stroke volume and heart rate. Hemodynamic changes during a contraction lead to an improved venous return, transient tachycardia, and an increased circulating volume. The increase in pulmonary arterial-venous oxygen difference also supports this hypothesis, suggesting that there is a sudden influx of blood from the maternal vascular bed into the systemic circulation.
Other factors that contribute to the increase in cardiac output include sympathetic nervous system stimulation by pain and anxiety, position, method of delivery, and anesthesia.22,27 Pain and anxiety can be reduced by psychoprophylactic strategies as well as analgesia and anesthesia. Epidural or spinal anesthesia results in a sympathetic blockade that can lead to decreased blood pressure, hypotension, and decreased uteroplacental perfusion if maternal intravascular volume is not maintained.
In general, both systolic and diastolic blood pressure increase during contractions, returning to baseline levels between contractions, with greater increase seen in systolic (35 mmHg or 4.66 kPa) than diastolic values (25 mmHg or 3.32 kPa).111 Oxygen consumption gradually increases approximately 23%.22 Heart rate changes are variable, with increases or decreases occurring. SVR does not change.22 Labor may lead to an increase in not only the number but also the variety of arrhythmias. Arrhythmias seen during labor may include premature ventricular contractions, atrial premature beats, premature nodal contractions, sinus bradycardia, sinus tachycardia, and supraventricular tachycardia.122,138
Postpartum period
Delivery of the fetus, placenta, and amniotic fluid results in dramatic maternal hemodynamic alterations that can result in cardiovascular instability during the immediate postpartum period. These changes are due to the loss of blood at delivery and the body’s compensatory mechanisms. Up to 500 mL (10%) of blood may be lost in vaginal deliveries and 1000 mL (15% to 30%) with cesarean sections, although average loss is less. Despite this blood loss, cardiac output is significantly elevated above prelabor levels for 1 to 2 hours postpartum.128,129 The loss of blood is compensated for by an autotransfusion (influx of up to 500 mL of blood from the uteroplacental system into the maternal systemic circulation due to splanchnic vasoconstriction after placental delivery), increased central venous pressure, increased ventricular preload and increased cardiac output.60
Immediately after delivery, cardiac output is 60% to 80% higher than prelabor levels. However, within 10 to 15 minutes there is a sharp decline that stabilizes at prelabor values by 1 hour.111 Several factors may contribute to this transient increase in maternal cardiac output. These include reduction in gravid uterus pressure and improved venacaval blood flow, autotransfusion of uteroplacental blood back into maternal circulation, and decreased SVR and vascular capacitance due to contraction of the muscular uterus and absence of placental blood flow.90 Mobilization of extracellular fluid improves venous return to the heart, and is reflected in an increase in left atrial size at 1 to 3 days.111 The increased return to the heart contributes to the higher central venous pressure seen postpartum. Maternal hypervolemia acts as a protective mechanism for excessive blood loss at delivery (see Chapter 8).
During the first week after delivery, up to 2 L of body fluid is mobilized and excreted. This results in a 3-kg weight loss. Diuresis (in order to dissipate the increased extracellular fluid) occurs between day 2 and day 5.132 Without the diuresis of mobilized extracellular fluid, increased pulmonary wedge pressures and pulmonary edema can result.34,90 The latter may be encountered in women with preeclampsia or heart disease who do not undergo normal diuretic patterns.
Stroke volume and thus cardiac output remain elevated for at least 48 hours after delivery.132 This increase is probably due to increased venous return with loss of uterine blood flow and mobilization of interstitial fluid (see Chapter 11).111,132 Left atrial size increases over the first 1 to 3 days postpartum probably due to the increased venous return with mobilization of fluid.111 Cardiac output decreases by 30% by 2 weeks postpartum then gradually decreases to nonpregnant values by 6 to 12 weeks in most women but may last up to 24 weeks in some women.24,111,132
Most of the other cardiovascular system changes resolve by 6 to 8 weeks postpartum. Left atrial size and heart rate reach prepregnancy values by 10 days postpartum and LV size by 4 to 6 months.111 However in some women, stroke volume, cardiac output, end diastolic volume, and SVR may remain elevated for up to 12 to 24 weeks or longer.111,132 LV wall muscle mass gradually decreases over the first 6 months postpartum.68 The audible vibrations caused by ejection of blood from the RV into the pulmonary trunk or from the LV into the brachiocephalic arteries at the point of branching from the aortic arch usually disappear by postpartum day 8. For approximately 20% of women, the systolic murmur persists beyond 4 weeks postpartum. The intensity of the murmur does, however, decrease by day 8, even if it does not disappear.122,132
Clinical implications for the pregnant woman and her fetus
Arrhythmias
Many pregnant women experience rhythm disruptions that become more intense during the second and third trimesters.45,56 It is unclear how much of the reported increase is due to closer monitoring versus an actual change in frequency with pregnancy.27 Most of these arrhythmias are benign and are not indicative of heart disease. The most common (seen in 50% to 60%) are simple ventricular or atrial ectopy.45 The woman may describe skipped beats, momentary pressure in the neck or chest, or extra beats. These are usually representative of premature ventricular contractions and require no further treatment. Extra systoles or supraventricular tachycardia may also be seen in some women.18 The reason for the increase in arrhythmias may be related to the electrophysiologic effects of hormones, increased sympathetic nervous system activity, hemodynamic alterations, increased potassium, or in some cases, underlying cardiac disease.56 In addition, atrial stretching and estrogen may lower the threshold for arrhythmias.68 Most arrhythmias are neither life-threatening nor due to structural defects.27
Supine hypotensive syndrome
Orthostatic stress generated by changes in position (from recumbent to sitting to standing) is associated with acute hemodynamic changes. Blood pools in dependent vessels, which reduces venous return and decreases cardiac output and blood pressure with increasing orthostatic stress. Heart rate and systemic vascular resistance (SVR) increase; mean arterial pressure changes, however, are not significant. In addition, decreased baroreflex sensitivity during pregnancy leads to blunted reflex activation of the sympathetic nervous system and inadequate peripheral vasoconstriction.23
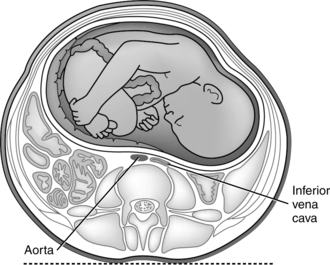
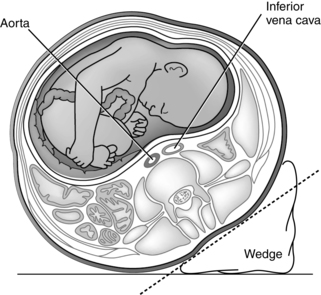
The gravid uterus is also associated with a significant amount of caval blood flow obstruction in the supine position. Approximately 90% of gravid women experience obstruction of the inferior vena cava in the supine position in late pregnancy, but rarely does this lead to hypotension or other symptoms. Late in pregnancy, but before the fetal presenting part becomes engaged, the uterus is mobile enough to fall back against the inferior vena cava in the supine position (Figure 9-6). This results in vena caval tamponade. Vascular compression may also be applied to the aorta and its branches. In most women, paravertebral collateral circulation develops during pregnancy. This, along with the dilated utero-ovarian circulation, permits blood flow from the legs and pelvis to bypass the vena cava.90 These changes are reversed in the left lateral position (see Figure 9-7), which displaces the uterus to the left and off the vena cava. In the supine position the aorta may also be compressed (Figure 9-6), which can also alter arterial blood pressure.34
Usually the fall in cardiac output due to a posture change is compensated for by an increase in peripheral resistance. This allows systemic blood pressure and heart rate to remain unchanged. However, up to 8% of pregnant women in the supine position may experience a significant decrease in heart rate and blood pressure, leading to symptoms of weakness, lightheadedness, nausea, dizziness, or syncope.111 This is referred to as supine hypotensive syndrome of pregnancy and is usually corrected when position is changed.34,111 These women may be more vulnerable due to inadequate paravertebral collateral blood supply.111
Exercise during pregnancy
Exercise has significant benefits for the mother and fetus. Regular aerobic exercise done two to three times per week maintains or improves maternal fitness during pregnancy.87 Fit women have been shown to have shorter labors and fewer perinatal complications.58,133 Concerns regarding exercise during pregnancy include the effects on the fetus and mother, including the risks of hyperthermia (see Chapter 20) and increased cardiac workload.
Physiologic responses to exercise include increased oxygen consumption, redistribution of blood, altered venous pooling, and changes in cardiac output and stroke volume. With submaximal exercise, minute ventilation, cardiac output, and heart rate are greater in the pregnant versus nonpregnant woman.65,107,121 Both exercise and pregnancy increase oxygen consumption and the need for energy substrate by different tissues. Respiratory system responses to exercise during pregnancy are discussed in Chapter 10.
Blood flow is redistributed during exercise, moving blood away from the viscera and to the skin and skeletal muscles. This leads to a reduction in utero-ovarian blood flow.28,169 Greater intensity and duration of exercise is associated with increased redistribution of blood from the uterus to the skin and muscles.62 The limitation in substrate delivery to the fetus could potentially have adverse effects resulting in fetal hypoxia; however, to cause such changes, the reduction in uterine blood flow has to exceed 50%. In healthy pregnant women, these occurrences would be rare, being most likely during strenuous, prolonged exercise.29,110
Therefore under normal conditions enough oxygen is thought to be available to the uterus to meet and exceed uterine and fetal demands. Compensatory mechanisms help maintain oxygen availability during maternal exercise.62 The decrease in placental blood flow is significantly less than the decrease in uterine blood flow.62 Blood flow is selectively distributed to the placenta at the expense of the myometrium.62 Regular sustained exercise during pregnancy has been reported to increase placental villous size and volume.121 The significance of this change is uncertain.
Maternal hematocrit rises during exercise. This increases maternal oxygen-carrying capacity, with a smaller reduction in oxygen availability–to–blood flow ratio. Uterine oxygen uptake increases during exercise in order to maintain a stable oxygen consumption level.62 Maternal ventricular performance is maintained with exercise. In response to strenuous exercise in early pregnancy, the LV adapts by increasing its contractile reserve; in late pregnancy, it adapts by increasing preload reserve.
Pregnancy increases the workload of the heart, although in most pregnancies the cardiac reserve is adequate to meet these demands. During early pregnancy, exercise is associated with further increases in stroke volume and cardiac output.65 As pregnancy progresses, the cardiac output changes with exercise are lower.121 These changes in later pregnancy may be due to increased peripheral pooling rather than a progressive decline in circulatory reserve as pregnancy progresses. Decreased exercise tolerance with fatigue and dyspnea is also noted in later pregnancy. Most studies show no change in the maximum heart rate during exercise with pregnancy, although some suggest the heart rate change may be slightly reduced due to a blunted sympathetic response.121
Exercise results in changes in epinephrine, norepinephrine, glucagon, cortisol, prolactin, and endorphin levels. Maternal exercise is also associated with a significant increase in circulating catecholamines. Under normal circumstances, the placenta is very efficient in metabolizing catecholamines, and only 10% to 15% reach the fetus. Elevated catecholamine levels could have an additional restrictive effect on the fetal circulation.110,121
The usual fetal response to maternal exercise is an increase in heart rate by 5 to 25 beats per minute.58,107,133 This increase may be due to decreased placental blood flow, stimulation of maternal vasoactive hormones, or exercise-induced uterine contractions.58,133 These changes are independent of either gestational age or intensity of maternal exercise. Immediately after exercise and during the next 5 minutes, the fetal heart rate (FHR) remains elevated regardless of the type of exercise. Within 15 minutes, the FHR drops to preexercise values in women engaged in mild to moderate exercise activities. For women engaged in strenuous activities, the FHR may not return to baseline for up to 30 minutes.58,110,133,164 The elevated FHR seen following exercise may also be a normal physiologic response to compensate for short periods of mild hypoxia.
In some women, transient fetal bradycardia has been reported after maximal exercise (heart rate greater than 180 beats per minute), possibly due to a rapid increase in catecholamines.144 These episodes of bradycardia did not compromise fetal outcome. An initial response of tachycardia may lead to bradycardia if hypoxia becomes prolonged and vagal stimulation occurs. There is also the possibility that these brief occurrences are within the normal realm of fetal reflex responses to major maternal hemodynamic and hormonal events.80,110 In most situations, fetal bradycardia was mild (100 to 119 beats per minute). Fetal reserve should be assessed if any abnormalities are suspected, and maternal cardiac as well as fetoplacental reserve should be the basis for determining exercise, work levels and activities during pregnancy.154
Regular exercise during pregnancy has many benefits including maintenance of fitness; improved cardiovascular function; control of blood pressure; improved metabolic efficiency, decreased backaches, fatigue, and shortness of breath; shorter labors; decreased clot formation and varicosities; feelings of wellness; prevention of excessive weight gain and fat deposition; decreased leg cramps; decreased leg edema; and more rapid postpartum recovery.4,27,28,30,58,62,74,107,113,133,156 Fetal benefits may include better growth, improved stress tolerance, and enhanced neurobehavioral maturation, with a leaner body composition at 5 years of age.30,31,107 Women with conditioning before pregnancy demonstrate improved metabolic effects with exercise during pregnancy compared to unconditioned women.28 Mild to moderate aerobic exercise (to 50% to 85% of maximum) has not been demonstrated to increase spontaneous abortions, but does decrease length of labor, number of cesarean births, fetal fat mass (and thus mean birth weight), and number of episodes of fetal distress.4,28,58,169 Non–weight-bearing exercise and low-intensity exercise do not appear to affect fetal growth.91 Maternal risks of exercise include musculoskeletal injury, risk of cardiovascular complications, hypoglycemia, and uterine contractions.62,107
The American College of Obstetricians and Gynecologists (ACOG) recommends moderate exercise of 30 minutes or more on most days of the week in the absence of complications.4 Sedentary women are encouraged to begin an exercise program; women with complications may begin an exercise program after medical evaluation.4 Suggested activities include swimming, biking, aerobic walking, stretching, and normal walking if the women cannot tolerate aerobic exercise. Weight-bearing exercises increase energy expenditure during pregnancy, whereas weight-supported exercises (biking, swimming) do not.121 Swimming and other aquatic exercises have particular benefits during pregnancy. Changes in heart rate and vital capacity are less while stroke volume is increased during exercise in water.80 Hydrostatic pressure moves extravascular fluid back into the vascular space, reducing fluid retention and enhancing diuresis.62 The water reduces thermal changes with exercise by acting as a thermoregulatory buffer around the woman.62
Vigorous exercise should not be performed in hot, humid weather or during a period of febrile illness, and body temperature should never exceed 40° C (104° F).4 Jumping, jerky movements, or deep flexion or extension of joints should be avoided because of the joint instability that occurs during pregnancy. Women should avoid sports with a high risk of blunt abdominal trauma.4 Gradual changes in position should be undertaken to avoid orthostatic stress and hypotension. Heart rate should be self-monitored during peak activity and should not exceed 70% of maximum. Heart rate and respiratory rate should return to resting rates within 15 minutes after exercise. Strenuous activity should be limited to 15 minutes. Caloric intake needs to be high enough to meet not only the demands of exercise but also of pregnancy.4
Studies on elite athletes with high-intensity exercise during pregnancy are limited, but there is some evidence of an increase in lighter (but not low-birth-weight) infants.4,91,127 However, after reviewing current studies, Pivarnik concluded that “data supporting adverse effects are suppositional. Data supporting the safety of training during pregnancy, although limited in quantity, are reassuring.”127
Maternal anatomic changes (weight gain, relaxation of ligaments and joints, progressive lordosis, and changes in center of gravity) of pregnancy often interfere with standard training routines in these athletes by the second trimester.58 ACOG guidelines state that “recreational and competitive athletes with uncomplicated pregnancies can remain active during pregnancy and should modify their usual routines as medically indicated” and recommend close medical supervision.4 Aerobic fitness during pregnancy does not decline significantly if women continue to exercise.107,127 Endurance trained athletes experience an increased blood volume expansion with increases in both RBC and plasma volume during pregnancy.127 Pregnancy-related changes in submaximal capacities may alter the woman’s athletic performance. The pregnant athlete may need to adjust her usual training heart rate upward to achieve the same amount of work as when she was not pregnant.127 If the woman continues resistance training, she should avoid supine exercises due to concerns about vena cava compression.127
At no time should exercise be continued if pain or contractions are experienced. The primary health care provider should be contacted if bleeding, dizziness, shortness of breath, palpitations, faintness, tachycardia, back pain, pubic pain, or difficulty in walking is experienced. Women designated as high risk for preterm labor need to receive special counseling regarding not only appropriate activity levels but also signs and symptoms of labor.4 Exercise programs for women with gestational diabetes mellitus have been shown to be a useful adjunct therapy and may also decrease the risk of developing this disorder.4,8,20,36,38 Exercise is also being examined as an intervention to reduce the risk of preeclampsia.43,106,170,171
Multiple pregnancy
The woman with a multiple pregnancy is subject to a higher risk of morbidity and mortality. There is an increased production of estriol, progesterone, and human chorionic somatomammotropin or placental lactogen (hPL) that results in an increased volume expansion and weight gain. Whereas plasma volume is increased by about 50% in single pregnancies, in twins there is an increase of up to 65%, with further elevations seen in women with higher order multiples.111 There is also a higher RBC volume, increased hemodilution of iron and folate, and an exaggerated physiologic anemia. The hemodilution may be due to a greater increase in total body volume or a greater demand for iron or folic acid.122
The increase in maternal cardiac output, stroke volume and heart rate are also greater in multiple pregnancies than that found in singletons.100 The change in cardiac output suggests decreased cardiovascular reserve. Characteristic changes in blood pressure with multiple pregnancy include a lowered diastolic pressure at 20 weeks (74% of women with twins have a diastolic pressure less than 80 mmHg [10.6 kPa]), which is followed by a greater rise in diastolic pressure between midpregnancy and delivery (95% experience a rise in diastolic pressure of 15 mmHg [1.99 kPa] or more).27,100,122
Mothers with multiple pregnancies are at greater risk for preterm labor, preeclampsia, placenta previa, placental abruption, anemia, hyperemesis gravidarum, and postpartum hemorrhage. The length of labor is similar in twin and single pregnancies, although with twins the active phase is longer and the latent phase is shorter. Labor may be more difficult, possibly due to uterine overdistention and an increased incidence of malpresentation. Blood loss is about double that in a single delivery, and hemorrhage is more frequent due to uterine atony and sudden decompression of the overdistended uterus. There is also a slightly higher incidence of vasa previa and placenta previa, increasing the possibility of hemorrhage at delivery. The risk of preeclampsia is greater in twin than in single pregnancies, possibly related to the larger placental mass found with twin pregnancies.122
Cardiac disease and pregnancy
Cardiac disease affects about 1% (range 0.1% to 4%) of pregnant women and accounts for 15% of pregnancy related to maternal morbidity.27,167 The incidence of pregnant women with cardiac disease is slowly increasing as improved management and technical capabilities for both the mother and fetus make it possible to sustain pregnancy in those who previously would have been advised to terminate or avoid pregnancy.173 Corrective surgery for infants and children with congenital heart disease has increased the number of these women who are now of childbearing age. In addition, the incidence of heart disease increases in women who wait until their forties or later to have children.
At delivery the risk for CHF is further increased by the increase in blood volume. Acute pulmonary edema may occur. Applying pressure to the abdomen can slow the blood return to the heart and facilitate cardiovascular stabilization. If the heart is unable to compensate for the decrease in SVR, the blood pressure falls. Maintenance of blood pressure can be attempted by administration of chronotropic and alpha pressor agents. Epidural and spinal anesthesia may further decrease SVR and should be used with caution in these patients. The increased cardiac load with the marked increase in cardiac output immediately after delivery may significantly increase ventricular filling pressures and stroke volume increasing the risk of clinical deterioration.35
Pregnancy is particularly dangerous and may be contraindicated for women who have Eisenmenger syndrome, peripartum cardiomyopathy, severe primary pulmonary hypertension, aortic stenosis, coarctation of the aorta, Marfan syndrome, and hemodynamically significant mitral stenosis (Table 9-3).18 The presence of a cardiac lesion involving pulmonary hypertension during pregnancy carries a risk of maternal mortality somewhere between 30% and 50%. Pregnancy may also aggravate preexisting cardiac conditions such that damage is extensive and recovery to prepregnancy levels is not possible. In addition, pregnancy may result in maternal heart disease (e.g., peripartum cardiomyopathy).18,27,35,67,119
Table 9-3
High Risk Maternal Cardiovascular Conditions
| DISORDER | ESTIMATED MATERNAL MORTALITY RATE (%) |
| Aortic valve stenosis | 10-20 |
| Coarctation of the aorta | 5 |
| Marfan syndrome | 10-20 |
| Peripartum cardiomyopathy | 15-60 |
| Severe pulmonary hypertension | 50 |
| Tetralogy of Fallot | 10 |
From Blanchard, D.G. & Shabetal, R. (2009). Cardiac disease. In R.K. Creasy, et al. (Eds.). Creasy & Resnik’s Maternal-fetal medicine: Principles and practice (6th ed.). Philadelphia: Saunders Elsevier, p. 798.
The fetus must also be monitored closely during pregnancy in a woman with cardiac disease. Fetal well-being and growth are dependent upon a continuous flow of well-oxygenated blood to the uterus. When this supply is reduced or interrupted or oxygenation is decreased, the fetus is at risk for altered growth and development and for increased mortality.35,54 In addition, the fetus is at increased risk (3% to 12% depending on the defect versus 0.8% in the general population) of being born with a congenital heart defect if either parent has congenital heart disease since this can be a multifactorial disorder (see Chapter 1).35 If the mother is the affected parent, she adds an environmental risk (altered hemodynamics during pregnancy) as well as a genetic risk for her infant.
All of these factors and the risk to the mother and fetus should be discussed in depth with couples before pregnancy. Preconception counseling allows the potential problems and treatment options to be explored and the woman’s current health status to be characterized. Surgical correction or palliative procedures are generally recommended to be done before pregnancy, followed by several months to a year of recovery time.18
Obstructive lesions
Mitral stenosis.
This lesion is caused almost exclusively by rheumatic heart disease. The stenotic valve restricts cardiac output, resulting in fatigue, which is the most common symptom. The obstruction of left atrial outflow leads to pressure elevations in the left atrium, pulmonary veins, and pulmonary capillaries, causing severe pulmonary congestion. Risk of death is greatest in the third trimester and postpartum.18 About one fourth of women experience their first symptoms with pregnancy.27
The increased blood volume, cardiac output, and heart rate of pregnancy, along with the fluid retention, may lead to an increase in symptoms, especially after 20 weeks.27 Signs of pulmonary congestion usually occur by 20 to 24 weeks as intravascular volumes begin to peak and stabilize around 30 weeks.27 The increase in cardiac output, plasma volume, and heart rate peak by this time, decrease diastolic filling time in the LV, and increase the pressure gradient across the mitral valve.27 An exacerbation may occur at the time of delivery due to increased pulmonary wedge pressure if there is prolonged tachycardia. The first 24 hours after delivery is a time of increased vulnerability in these women due to intravascular fluid shifts.27 The risk of maternal and neonatal complications is higher in women with abnormal functional capacity, cyanosis, or left-sided valve obstruction and is increased with maternal smoking, maternal age younger than 20 or older than 35 years, multiple pregnancy, and anticoagulant therapy.152
Management includes avoidance of the supine position and prophylaxis against rheumatic disease during pregnancy and against endocarditis during labor and delivery. If severe mitral stenosis is diagnosed, mitral commissurotomy is advised before conception.18 If symptoms develop during the course of pregnancy, activity restrictions to reduce the strain on the heart, sodium intake restrictions, diuretics, and digitalis may be considered. If symptoms of pulmonary congestion persist despite medical therapy, surgical intervention (either a balloon valvuloplasty or valve replacement) may be necessary. If thromboembolism occurs, anticoagulation therapy is needed.18,27
Mitral regurgitation.
Mitral regurgitation may be due to rheumatic heart disease; however, there are numerous other conditions that can lead to its development, including congenital heart disease, hypertension, ischemia, and idiopathic myocardial disease.18 Prolapse of the mitral valve during systole allows for regurgitation of blood back into the left atrium. Fatigue is the result. If severe, pulmonary congestion can occur. These women have a propensity for atrial fibrillation and left atrial thrombus formation. Individuals with mitral regurgitation may be asymptomatic for extended periods of time. In the woman with mild regurgitation, pregnancy is usually well tolerated, and the development of congestive heart failure (CHF) is rare.
Mitral valve prolapse.
In most situations, pregnancy is tolerated well in women with MVP, especially if there is no mitral valve regurgitation, with good maternal and fetal outcomes. The woman may experience an increase in palpitations, lightheadedness, dizziness, fainting with prolonged standing, arrhythmias, or chest pains that may require medical intervention with the use of β blockers.18
Left-to-right shunts
The magnitude of the left-to-right shunt is dependent upon the ratio of resistance in the systemic and pulmonary vascular circuits. During pregnancy, both circuits have a decline in resistance (see Table 9-1) so that there is usually no significant change if shunting occurs. If pulmonary vascular disease exists, the normal fall in pulmonary vascular resistance (PVR) may not occur. Cyanosis rarely occurs with these defects unless significant pulmonary hypertension or RV failure develops. Pregnancy does not seem to precipitate these events, however.
Atrial septal defect.
An uncorrected ASD is usually asymptomatic but may become symptomatic for the first time in pregnancy.18,27 The most common complications are arrhythmias, pulmonary hypertension, and heart failure. The hypervolemia of pregnancy increases the left-to-right shunt through the ASD, thereby creating an additional burden on the RV. This additional load is usually tolerated well by most women, although up to 15% fetal loss has been reported.27 If the additional load is not well tolerated, which usually occurs if the woman has pulmonary hypertension, chronic atrial fibrillation or RV dysfunction, RV failure occurs, leading to marked peripheral edema, atrial arrhythmias, pulmonary hypertension, and paradoxical systemic emboli across the septal defect.18,35
Ventricular septal defect.
A VSD can occur either as an isolated lesion or in conjunction with other congenital cardiac anomalies (such as tetralogy of Fallot, transposition of the great vessels, or coarctation of the aorta). The size of the defect determines the degree of shunting, tolerance to the additional burden of pregnancy, and prognosis. Small defects are usually tolerated well.18,27 Large VSDs often lead to CHF, arrhythmias, or development of pulmonary hypertension or Eisenmenger syndrome with increased maternal and fetal mortality.18,27 These women are often counseled to avoid becoming pregnant.18 A large VSD is often associated with some degree of aortic regurgitation, which contributes to the risk of CHF during pregnancy.
Patent ductus arteriosus.
A PDA is usually identified during infancy and is surgically corrected at that time. Therefore it is uncommon to find this type of defect in childbearing women. However, if it does exist, it is usually well tolerated during gestation, labor, and delivery, although closure before pregnancy is usually recommended.18 If the PDA is complicated by pulmonary hypertension, the prognosis is poorer.18
Right-to-left shunts
Eisenmenger syndrome.
This syndrome combines the presence of a congenital communication between systemic and pulmonary circulations with progressive pulmonary hypertension that leads to shunt reversal or bidirectional flow. It is often associated with VSD or PDA. During pregnancy the increased plasma volume increases the burden on the compromised heart.18 The decreased SVR increases the degree of right-to-left shunting, which reduces pulmonary perfusion, leading to hypoxemia and maternal and fetal compromise.18,35,60 Systemic hypotension leads to decreased RV filling pressures, which may be insufficient to perfuse the pulmonary bed, when high PVR exists. The result may be the onset of sudden and profound hypoxemia. Hemorrhage or complications of conduction anesthesia (hypovolemia) may precipitate hypoxemia and lead to death during the intrapartum period.18
The fetal and maternal mortality rate for pregnant women with Eisenmenger syndrome can be 50% when pulmonary hypertension is associated with a VSD.27 Death is usually from right ventricular failure with cardiogenic shock.27 Maternal mortality is greatest during parturition and early postpartum.18,169 Because of the high mortality rate, women are usually discouraged from becoming pregnant and pregnancy termination is usually recommended. If gestation continues, hospitalization, continuous oxygen administration, and the use of pulmonary vasodilators are recommended. During labor it is essential to maintain an adequate fluid load to maintain venous return and right ventricular filling.27 However, maintaining a preload edge to protect against unexpected blood loss may risk tipping the scales toward pulmonary edema. Only about one fourth of pregnancies in women with this disorder are term and approximately one third of the infants are growth restricted.169
Tetralogy of fallot (TOF).
This defect is the most common cause of right-to-left shunting. TOF includes a combination of VSD, pulmonary valve stenosis, RV hypertrophy, and rightward displacement of the aortic root. PVR is normal. Surgical correction usually occurs during infancy, although there may be residual shunting following correction. Pregnancy outcome is relatively good for those individuals who have undergone surgical correction who have good ventricular function and no significant right ventricular outflow tract obstruction.18,60,159 In pregnant women with uncorrected VSDs, the decrease in systemic vascular resistance (SVR) leads to increases in right-to-left shunting and cyanosis.27 This can be complicated by intrapartum blood loss, leading to systemic hypotension. Therefore careful monitoring of fluid status is warranted.18
Pulmonary artery hypertension.
Severe pulmonary hypertension associated with pregnancy carries a 50% maternal mortality rate and a 40% fetal mortality rate if the mother survives, although a recent study found a lower maternal mortality rate of 25%.16,18,27 Deterioration of cardiac function with right heart failure usually begins in the second trimester.35 Significant pulmonary hypertension is a contraindication to pregnancy, and pregnancy should be prevented or termination recommended if it occurs. If termination is not possible, physical activity should be curtailed and supine positioning avoided during late gestation. Careful monitoring of oxygenation and fluid status at the time of labor and delivery is essential in order to identify problems early and intervene appropriately.27
Marfan syndrome
Marfan syndrome is an autosomal dominant disorder of connective tissue whose clinical manifestations include skeletal, ocular, and cardiovascular abnormalities. Aortic dilation is one of these manifestations. Complications that can result include aneurysm formation and rupture, aortic dissection, and aortic regurgitation. Structural changes in the aortic wall due to high estrogen levels predispose pregnant women to aortic dissection. Rupture is more likely to occur in the third trimester or in the first stage of labor.18,159 Counseling regarding the risk of inheritance and the potential maternal complications should be given before conception if possible. If pregnancy occurs, the use of long-acting β blockers to decrease pulsatile pressure on the aortic wall is indicated, along with limitations on physical activity and preventing hypertensive complications.18
Peripartum cardiomyopathy
Peripartum cardiomyopathy is a form of dilated cardiomyopathy seen in women without a previous history of heart disease, which develops during the last month of pregnancy or up to 5 months postpartum.18,35 In dilated cardiomyopathy the cardiac chambers become significantly dilated with a hypokinetic LV and progressive decrease in LV function. Peripartum cardiomyopathy has a frequency of 1/3000 to 1/4000 live births with recurrence risk in subsequent pregnancies of up to 50%.18,35 This disorder has a variable and unpredictable clinical course. Generally 30% to 50% of women demonstrate partial recovery with persistence of some cardiac dysfunction; 20% develop deteriorating cardiac function to the point of needing a heart transplant.18,96 In women with partial recovery, cardiac function may improve over time.33 The cause of peripartum cardiomyopathy is unknown. It is not clear if the pregnancy/postpartum state causes the disorder or aggravates an underlying cardiomyopathic process.18,35,67 The risk of developing peripartum cardiomyopathy is higher with increased maternal age, chronic hypertension, multiple gestation, multiparity, and preeclampisa.35,67
Hypertensive disorders
Hypertensive disorders are one of the most common complications of pregnancy and a leading cause of maternal mortality.94,95 Hypertension during pregnancy is classified as (1) chronic hypertension, (2) preeclampsia, (3) preeclampsia superimposed on chronic hypertension, and (4) gestational hypertension. Definitions of each form are in Table 9-4.
Table 9-4
Classification of Hypertensive Disorders of Pregnancy
| CLASSIFICATION | DEFINITION AND DIAGNOSTIC CRITERIA |
| Preeclampsia | Hypertension with proteinuria |
| Hypertension: blood pressure >140 mmHg (18.62 kPa) systolic or 90 mmHg (11.97 kPa) diastolic after 20 weeks’ gestation in a woman normotensive before 20 weeks’ gestation | |
| Proteinuria: 300 mg/L (30 mg/dL) protein in a random specimen and excretion of 300 mg/24 hours | |
| Hypertension with other systemic symptoms of preeclampsia in the absence of proteinuria is highly suspicious of preeclampsia | |
| Chronic hypertension | Blood pressure 140/90 mmHg (18.62/11.97 kPa) or greater before the twentieth week of gestation or, if only diagnosed during pregnancy, persisting 6 weeks after pregnancy |
| Preeclampsia superimposed on chronic hypertension | Highly likely in women who develop new proteinuria or with preexisting hypertension and proteinuria who develop sudden increases in blood pressure or proteinuria, thrombocytopenia, or increases in hepatocellular enzymes |
| Gestational hypertension | Development of hypertension without other signs of preeclampsia |
Adapted from National High Blood Pressure Education Program. (2000). Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol, 183, 1.
Preeclampsia
Preeclampsia is a hypertensive, multisystem disorder characterized by hypertension and proteinuria that complicates 3% to 6% of all pregnancies.39,52,66,137 Other features may include central nervous system irritability and, at times, coagulation or liver function abnormalities. Changes in different organ systems with preeclampsia are summarized in Table 9-5. Women with preeclampsia may develop seizures (eclampsia) or a variant with abnormal liver function and thrombocytopenia known as the HELLP syndrome. The term HELLP comes from this syndrome’s characteristic clinical findings: hemolysis (H), elevated liver enzymes (EL), and a low platelet (LP) count. This syndrome occurs in 0.5% to 0.9% of all pregnancies, and in 10% to 20% of women with severe preeclampsia.82,150 Women with preeclampsia may be at risk of later cardiovascular disease.172
Table 9-5
Findings and Clinical Manifestations of Preeclampsia
| VASCULATURE OR SYSTEM | FINDINGS | CLINICAL MANIFESTATIONS |
| Cardiovascular | Increased cardiac output (CO) and systemic vasoconstriction | Systemic hypertension |
| Increased hydrostasis present | Generalized edema | |
| High CO and hypertension | Increased hemolysis | |
| Uteroplacental | Uteroplacental insufficiency | Fetal somatic growth deficiency; fetal hypoxemia and distress |
| Decidual ischemia | Abruptio placentae; placental infarcts | |
| Decidual thrombosis | Thrombocytopenia | |
| Renal | Decreased renal blood flow and glomerular filtration rate; endothelial damage | Proteinuria; elevated creatinine and decreased creatinine clearance; oliguria |
| High angiotensin II responsiveness in tubular vasculature | Elevated uric acid | |
| All of the above | Renal tubular necrosis and renal failure | |
| Cerebrovascular | Cerebral motor ischemia | Generalized grand mal seizures (eclampsia) |
| High cerebral perfusion pressure with regional edema | Cerebral hemorrhage | |
| Cerebral edema | Coma | |
| Regional ischemia | Central blindness; loss of speech | |
| Hepatic | Ischemic and hepatic cellular injury | Elevated liver enzymes |
| Mitochondrial injury | Intracellular fatty deposition | |
| Hematologic | Intravascular hemolysis | Schistocyte burr cells; elevated free hemoglobin and iron-decreased haptoglobin levels |
| Decidual thrombosis, release of fibrin degradation products | Thrombocytopenia; antiplatelet antibodies |
From Shah, D.M. (2011). Hypertensive disorder of pregnancy. In R.J. Martin, A.A. Fanaroff, & M.C. Walsh (Eds.). Fanaroff and Martin’s Neonatal-perinatal medicine: Diseases of the fetus and infant (9th ed.). Philadelphia: Mosby Elsevier, p. 280.
“Placental and maternal factors interact to develop preeclampsia. Originating in the placenta as a result of decreased trophoblast invasion, ischemia leads to oxidative stress and a continuum of events which adversely affect the maternal vasculature leading to hypertension and proteinuria.”115,p. 380 Preeclampsia is characterized by increased SVR, enhanced platelet aggregation, activation of the coagulation system, endothelial cell dysfunction, pathologic changes and decreased perfusion to most organ systems, especially the liver, kidney, brain, and placenta secondary to vasoconstriction; increased sensitivity to pressors, including angiotensin II; and a reduction in the usual increase in plasma volume due to loss of fluid from the vascular space.50,72,137,147,148 Factors predisposing to preeclampsia include conditions in which oxygen demand is increased (multiple pregnancy, molar pregnancy, or an edematous placenta) or conditions with decreased oxygen transfer (primipara, microvascular disease, chronic hypertension, diabetes, collagen disorders, or abnormal placentation).72,134,137,149 The net result of these factors is a direct or relative placental hypoperfusion. This suggests altered uteroplacental perfusion as an early event in the pathogenesis of preeclampsia, possibly due to oxidative stress and ischemia-reperfusion. In addition, many of these factors are also associated with increased fetal antigen load (i.e., multiple pregnancy, hydrops, molar pregnancy, or other increased placental mass) or inflammatory response (rheumatoid disease, infection) supporting an immunologic basis for preeclampsia.149 An immunologic basis is also supported by the greater frequency of preeclampsia in first pregnancies with limited exposure of the mother’s system to paternal sperm, and thus the paternal antigens expressed on fetal tissues, or in mutigravida women with new partners (see Chapter 13).138,147
Although the exact pathogenesis of preeclampsia is still unclear, “relative ischemia or hypoxia of the placenta resulting from defective progression of spiral artery remodeling and placental angiogenesis is thought to underlie preeclampsia.”115,p. 380 This is followed by maternal multisystem involvement with endothelial dysfunction.92,131,135,147 Thus inadequate remodeling of maternal uterine spiral arteries is an early pathogenic event.24,39,134,135,148,149 Normally extravillous trophoblast cells from the placenta migrate into the uterine spiral arteries in both the decidua and myometrium. These trophoblast cells remodel the maternal spiral arteries so that little vascular smooth muscle tissue, neural elements, and elastic matrix are left.10,147 The spiral arteries become large, flaccid uteroplacental vessels establishing a low-resistance circuit that enhances blood supply to the fetus and placenta. This remodeling process is described in Chapter 3. In preeclampsia the remodeling is incomplete and often confined to the decidual portion of the blood vessels (see Chapter 3), with 30% to 50% of the vessels in the myometrium and their adrenergic nerve supply left intact.46,134 The result is that the spiral arteries remain thick walled and muscular, leading to inadequate placental flow and thus reduced oxygen delivery to the placenta.
Alterations in implantation and remodeling of maternal spiral arteries may be secondary to maternal-fetal immune maladaptation at the maternal-fetal interface (see Chapter 13) due to ischemia-reperfusion leading to oxidative stress and vascular disease.147 An immunologic basis is supported by the increased incidence of classic preeclampsia in primiparous women or multiparous women with new partners.147,149 A similar lack of spiral artery change is seen without preeclampsia in some forms of fetal growth restriction and in some women with preterm labor. Development of preeclampsia seems to require decreased placental perfusion, with oxidative stress and endothelial activation. This may be due to genetic factors or maternal disease that alters maternal antioxidant enzymes or increases sensitivity to oxidative stress.
These changes within the placenta are thought to be linked to a maternal multisystem syndrome with endothelial dysfunction via “increased release of antiangiogenic factors sflt-1, endoglin, and syncytiotrophoblast membrane fragments into the maternal circulation. These may interact with or damage the maternal vasculature resulting in an inflammatory response and increased maternal vasculature resistance and vascular dysfunction in preeclampsia. Heightened oxidative/nitrative stress is seen in maternal vasculature and particularly in the placenta in preeclampsia.”115,p. 380 Oxidative stress secondary to hypoxia (due to altered perfusion) occurs at the maternal-fetal interface. Plasma lipoproteins, nonesterified fatty acids, and low-density lipoproteins are elevated in women with preeclampsia.157 These provide increased substrate for lipid peroxidation. Decreased placental perfusion may also increase lipid peroxidation with release of oxygen radicals without adequate counteracting antioxidant enzymes.39 This damages maternal vascular endothelium with activation of neutrophils and macrophages (inflammatory response). Women with preeclampsia have a greater rate of lipid peroxidation, release of reactive oxygen species (free radicals), and lower levels of antioxidants that may contribute to endothelial dysfunction.135,147
Release of antiangiogenic factors into maternal circulation by the oxidatively stressed placenta stimulate a generalized systemic inflammatory response leading to endothelial dysfunction.131 These antiangiogenic factors include sflt1, which is the soluble receptor for vascular endothelial growth factor (VEGF), and soluble endoglin, which is a co-receptor for transforming growth factor-β.72,115 These substances bind VEGF and placental growth factors, decreasing free levels of these factors, thus preventing their action on systemic endothelium, leading to endothelial dysfunction.3,72,84,103,115,131,149
Alterations in implantation and spiral artery remodeling decrease uteroplacental perfusion, placental ischemia, and alterations in maternal vascular.137 Placental ischemia stimulates release of cytokines that disrupt endothelial function by inducing structural and functional changes in endothelial cells, enhancing endothelin production and decreasing acetylcholine-induced vasodilation. The endothelium is a single layer of epithelium lining the blood vessels and in direct contact with blood. The endothelium releases factors to maintain vascular tone, enhance permeability, control plasma lipids, inhibit white blood cell adhesion and migration, inhibit platelet activation and aggregation, and inhibit smooth muscle proliferation and migration (to prevent atherosclerotic changes).1 Normally antithrombic, antiproliferative, vasodilating substances (e.g., nitric oxide, prostacyclin [or PGI2]) are in a homeostatic balance with vasoconstrictive, prothrombic, proliferative factors (e.g., thromboxane A2, endothelin, platelet-activating factor, superoxide, angiotensin II).
The balance within the uteroplacental circulation normally favors vasodilation. In women with preeclampsia, this balance is disrupted. Endothelial dysfunction leads to altered control of vascular tone (hypertension), increased glomerular permeability (proteinuria), altered procoagulant/anticoagulant ratio (coagulopathy), endothelial injury, and vasoconstriction (liver dysfunction).92 Markers of endothelial dysfunction found in women with preeclampsia include alteration in the procoagulant/anticoagulant ratio; increased fibronectin; enhanced platelet activation; alterations in vasomediators such as nitric oxide, endothelin, prostaglandins, and cytokines; factor VIII antigen; thrombomodulin; and VEGF.92
Substances such as cellular fibronectin, growth factors, vascular cell adhesion molecules, factor VIII, antigen, and other peptides released after endothelial injury are elevated in preeclampsia.115 The woman with preeclampsia has increased responsiveness to angiotensin II, leading to increased vascular resistance and eventually vasoconstriction, hypoxemia, and cell damage in various organs (see Table 9-5).147 This leads to further endothelial damage aggravated by oxidative stress.
Genetics plays a role in preeclampsia, but the exact pattern of inheritance is unclear. Preeclampsia is more common in daughters of women who had preeclampsia and in pregnancies fathered by sons of women with preeclampsia.72,135,137 Models of inheritance that have been proposed include a maternal dominant gene with reduced penetrance, maternal and fetal gene interaction, mitochondrial inheritance, or a gene-environment interaction increasing susceptibility.160 Preeclampsia has been associated with loci on chromosomes 2p, 2q, 4q, 9, and 10 in various studies.137,160
Summary
The cardiovascular changes associated with pregnancy are significant although well tolerated by most women. Maternal and fetal risks occur when underlying cardiovascular or pulmonary disease processes are compounded with the changes incurred during pregnancy. Cardiovascular problems increase maternal morbidity and mortality and can compromise the health and well-being of the fetus. Careful assessment and ongoing monitoring of cardiovascular status throughout the prenatal, intrapartum, and postpartum periods are essential for early identification and prompt intervention, thereby improving maternal and neonatal outcomes. Table 9-6 summarizes clinical implications related to the cardiovascular system during pregnancy.
Table 9-6
Recommendations for Clinical Practice Related to the Cardiovascular System in Pregnant Women
Recognize usual cardiovascular and hemodynamic patterns of change during pregnancy (pp. 252-259, Table 9-1).
Assess maternal cardiovascular changes throughout pregnancy (pp. 252-259).
Counsel women on normal cardiovascular changes and anticipated symptoms associated with those changes (pp. 252-259).
Counsel women in the third trimester to use the lateral recumbent position (pp. 261-262 and Figures 9-6 and 9-7).
Assess and closely monitor women at risk for and with preeclampsia throughout pregnancy (pp. 267-269).
Encourage moderate exercise on a regular basis during pregnancy (pp. 261-263).
Encourage sedentary women to begin an exercise program (pp. 261-263).
Counsel women with complications to be medically evaluated before beginning an exercise program (pp. 262-263).
Recommend interval-type exercises rather than long continuous exercise (p. 263).
Provide counseling and regularly evaluate pregnant women who start a progressive exercise program during pregnancy (pp. 262-263).
Teach women self-uterine palpation to assess for uterine contractions during exercise (p. 263).
Advise cessation of exercise if contractions, pain or bleeding occurs (p. 263).
Recognize usual changes in heart sounds and rhythms during pregnancy. (pp. 258-260).
Monitor vascular volume status carefully during the intrapartum and postpartum periods (pp. 259-260).
Evaluate blood loss and fundal tone postpartum (pp. 260-261).
Monitor for signs of congestive heart failure in women with cardiac disease, especially in the second half of pregnancy and during the intrapartum and immediate postpartum periods (pp. 255, 264-266).
Assess maternal activity tolerance in women with cardiac disease and discuss management of daily activities (pp. 263-266).
Teach women with cardiac disease the signs and symptoms of cardiovascular decompensation (pp. 263-266).