Chapter 22 Cardiovascular Disease
Coronary artery disease
1. What percent of adult patients undergoing surgery are estimated to have, or be at risk for, coronary artery disease?
2. What are some components of a routine preoperative cardiac evaluation? What are some more specialized methods of cardiac evaluation? What is the ultimate purpose of a preoperative cardiac evaluation?
3. What are some important aspects of the preoperative history taken from patients with coronary artery disease with respect to their cardiac status?
4. What are some coexisting noncardiac diseases that are frequently present in patients with coronary artery disease?
5. By what percent can a major coronary artery be stenosed in an asymptomatic patient?
6. What is the best indicator for a patient’s cardiac reserve?
7. When is angina pectoris considered “stable”?
8. When is angina pectoris considered “unstable”? What is the clinical implication of unstable angina?
9. What is it likely an indication of when dyspnea follows the onset of angina pectoris?
10. How does angina pectoris due to spasm of the coronary arteries differ from classic angina pectoris?
11. What is silent myocardial ischemia?
12. What is the most common symptom of angina in men and women?
13. Approximately what percent of myocardial ischemic episodes are not associated with angina pectoris? Approximately what percent of myocardial infarctions are not associated with angina pectoris?
14. Is hypertension or tachycardia more likely to result in myocardial ischemia in the patient with coronary artery disease? What is the physiologic explanation for this?
15. What is the basis for the common recommendation that elective surgery be delayed until 6 months or more after a prior myocardial infarction?
16. What is the approximate incidence of perioperative myocardial infarction 6 months after a myocardial infarction? What is the approximate incidence of perioperative myocardial infarction in patients who have not had a prior myocardial infarction?
17. What time period after surgery do most perioperative myocardial infarctions occur?
18. What are some cardiac medications that patients with coronary artery disease are likely to be taking? What is the recommendation regarding the patient’s preoperative medicine regimen with regard to their regular cardiac medicines?
19. What information can be gained from a preoperative electrocardiogram?
20. How might myocardial ischemia appear on the electrocardiogram?
21. Complete the following table:
| Electrocardiogram Lead | Coronary Artery Responsible for Myocardial Ischemia | Area of Myocardium That May Be Involved |
|---|---|---|
| II, III, Avf | ||
| V3-V5 | ||
| I, aVL |
22. Name some determinants of myocardial oxygen requirements and delivery.
23. What are some intraoperative goals for the anesthesiologist in an attempt to decrease the risk of myocardial ischemia in patients at risk?
24. What is the difference between risk stratification and risk reduction?
25. What are the risks of recent percutaneous coronary angioplasty in surgical patients and how do they differ with bare metal versus drug eluting stents?
26. What are two potential benefits of administering premedication preoperatively to patients with coronary artery disease?
27. How should anesthesia be induced in patients at risk for myocardial ischemia?
28. Why is there an increased risk of myocardial ischemia during direct laryngoscopy? What are some things the anesthesiologist may do during this time to minimize this risk?
29. What are some methods of maintenance of anesthesia that may be employed by the anesthesiologist for the patient with coronary artery disease?
30. What is coronary artery steal syndrome? What is its clinical significance?
31. What is a concern regarding the administration of a regional anesthetic to patients with coronary artery disease?
32. What are some considerations an anesthesiologist should take when selecting a neuromuscular blocking drug for patients with coronary artery disease? What is unique about pancuronium in this situation?
33. How should neuromuscular blockade be reversed in patients with coronary artery disease?
34. What are some factors that influence the intensity of intraoperative monitoring by the anesthesiologist?
35. When might an intraoperative pulmonary artery catheter be useful? What information does it provide?
36. What is some information that may be provided by an intraoperative transesophageal echocardiogram?
37. What are some treatment options when myocardial ischemia is detected intraoperatively?
38. What is the problem with decreases in body temperature that may occur intraoperatively in patients with coronary artery disease?
39. Why is it important to monitor heart rate in the patient with coronary artery disease?
Valvular heart disease
40. What information can be gained from Doppler echocardiography in patients with valvular heart disease?
41. How should anesthetic drugs and neuromuscular blocking drugs be selected for the patient with valvular heart disease?
42. When is it important to administer antibiotics to patients with known valvular heart disease?
43. What is mitral stenosis? How does it affect left atrial and pulmonary venous pressures? At what chronic left atrial pressure is an increase in pulmonary vascular resistance likely to be seen?
44. What is the most common cause of mitral stenosis? How does it present?
45. Why are patients with mitral stenosis at an increased risk of atrial fibrillation?
46. Why are patients with mitral stenosis at an increased risk of thrombus formation in the left atrium?
47. What are some anesthetic considerations for patients with mitral stenosis?
48. How can the maintenance of anesthesia be achieved in patients with mitral stenosis?
49. How might the adequacy of intravascular fluid replacement be monitored in patients with mitral stenosis? Why is this important?
50. Why might the mechanical support of ventilation be required postoperatively in patients with mitral stenosis?
51. What is mitral regurgitation? How is mitral regurgitation reflected on the recording of pulmonary artery occlusion pressure tracings?
52. What is the most common cause of mitral regurgitation? What other pathologic process is often present under these circumstances? What are some other causes of mitral regurgitation?
53. What are some anesthetic considerations for patients with mitral regurgitation?
54. How can the maintenance of anesthesia be achieved in patients with mitral regurgitation?
55. What is aortic stenosis? How is the severity of aortic stenosis estimated? What is considered to be hemodynamically significant aortic stenosis?
56. Name at least two causes of aortic stenosis. What is the natural course of aortic stenosis?
57. Why might patients with aortic stenosis have angina pectoris despite the absence of coronary artery disease?
58. How is aortic stenosis diagnosed on cardiac auscultation? Why is it important for the anesthesiologist to rule out aortic stenosis by auscultation preoperatively?
59. What are some anesthetic considerations for the patient with aortic stenosis?
60. What would result from tachycardia, bradycardia, or decreases in systemic vascular resistance in the patient with aortic stenosis?
61. How can the maintenance of anesthesia be achieved in patients with aortic stenosis?
62. How should the intravascular fluid status be managed intraoperatively in patients with aortic stenosis?
63. In patients with chronic aortic stenosis, why might the pulmonary artery occlusion pressure not be reflective of the left ventricular end-diastolic volume?
64. How effective are external cardiac compressions in patients with aortic stenosis during cardiopulmonary arrest?
65. What is aortic regurgitation? What is the effect of chronic aortic regurgitation on the left ventricle?
66. What is acute aortic regurgitation most likely due to? What is chronic aortic regurgitation most likely due to?
67. Why might a patient with aortic regurgitation have angina pectoris despite the absence of coronary artery disease?
68. What are the goals for the anesthetic management of aortic regurgitation? The anesthetic management of aortic regurgitation resembles the anesthetic management for which other valvular disease?
69. What is mitral valve prolapse? What percent of the adult population is estimated to have mitral valve prolapse?
70. What are some other conditions associated with mitral valve prolapse?
71. What symptoms do most patients with mitral valve prolapse have?
72. What are some potential complications of mitral valve prolapse?
73. What is the goal of the maintenance of anesthesia in patients with mitral valve prolapse? How should the intravascular fluid volume status be managed in patients with mitral valve prolapse?
74. What is the potential problem with regional anesthesia in patients with mitral valve prolapse?
Disturbances of cardiac conduction and rhythm
75. What are some tools available to the clinician for the diagnosis of disturbances in cardiac conduction and rhythm?
76. What are some types of conduction defects? Are conduction defects above or below the atrioventricular node usually permanent?
77. Is the placement of a prophylactic artificial cardiac pacemaker before surgery indicated in a patient with a bifascicular block? Why or why not? What is the theoretical concern?
78. How is third-degree atrioventricular heart block treated? What are the various methods by which this can be accomplished? How can third-degree heart block be treated pharmacologically?
79. What is sick sinus syndrome? How does it present? How is it treated?
80. What are ventricular premature beats? What are the hallmark features of a ventricular premature beat on an electrocardiogram?
81. When do premature ventricular beats warrant treatment? How are they treated under these circumstances?
82. What may be some causes of ventricular premature beats?
83. When is ventricular tachycardia diagnosed? How can it be treated?
84. What are preexcitation syndromes?
85. What is Wolff-Parkinson-White (WPW) syndrome? What is the incidence of WPW syndrome in the general population? How is it characterized on the electrocardiogram?
86. What is the most common cardiac dysrhythmia associated with WPW syndrome? How can it be treated?
87. What is the goal of the anesthetic management of a patient with WPW syndrome?
88. What are the various methods by which paroxysmal atrial tachycardia or fibrillation may be treated in the perioperative period in patients with WPW syndrome?
89. What is prolonged QT interval syndrome? What adverse events are associated with a prolonged QT interval? How can they be treated pharmacologically?
90. What is a congenital cause of prolonged QT interval syndrome? How is a stellate ganglion block thought to work for this?
91. What is the goal of the anesthetic management of a patient with a chronically prolonged QT interval?
Artificial cardiac pacemakers
92. What should be included in the preoperative evaluation of the patient with an artificial cardiac pacemaker?
93. How should the pacemaker be evaluated by the anesthesiologist preoperatively?
94. What intraoperative monitoring is important in a patient with an artificial cardiac pacemaker?
95. What can occur if the ground plate for electrocautery is placed too near the pulse generator of the artificial cardiac pacemaker?
96. How is the selection of drugs or anesthetic techniques altered by the presence of an artificial cardiac pacemaker in a patient?
97. Why should a magnet be kept in the operating room intraoperatively for a patient with an artificial cardiac pacemaker undergoing anesthesia?
98. What are some causes of temporary pacemaker malfunction? When is placement of a pulmonary artery catheter in a patient with an artificial cardiac pacemaker a risk?
Essential hypertension
99. What is the definition of essential hypertension? What is the benefit of the long-term treatment of patients with essential hypertension?
100. What should be included in the preoperative evaluation of a patient with essential hypertension?
101. How should blood pressure medications be managed in the perioperative period in the patient with essential hypertension?
102. What other medical problems are frequently seen in patients with essential hypertension? Approximately what percent of patients with peripheral vascular disease can be assumed to have 50% or greater stenosis of one or more coronary arteries even in the absence of symptoms?
103. How is the curve for the autoregulation of cerebral blood flow altered in patients with essential hypertension?
104. What is the value of treating essential hypertension in patients before an elective procedure?
105. How do patients with essential hypertension frequently respond physiologically to the induction of anesthesia with intravenous medications? Why is this thought to occur?
106. How do patients with essential hypertension frequently respond physiologically to direct laryngoscopy? What are these patients at risk of during this time? How can this response be attenuated?
107. What is the goal for the anesthetic management of patients with essential hypertension?
108. How can the maintenance of anesthesia in patients with essential hypertension be achieved?
109. How might intraoperative hypotension be managed by the anesthesiologist in patients with essential hypertension?
110. What is the potential problem with regional anesthesia in patients with essential hypertension?
111. How frequently does hypertension occur in the early postoperative period in patients with essential hypertension? How can it be managed?
Congestive heart failure
112. What is the correlation between congestive heart failure and postoperative morbidity? What does this suggest for the patient scheduled for elective surgery in the presence of congestive heart failure?
113. What is the goal of the anesthetic management of patients with congestive heart failure who are undergoing urgent or emergent surgery? What medicines may be useful to achieve this?
114. How does positive-pressure ventilation of the lungs affect patients in congestive heart failure?
115. For major surgery in patients with congestive heart failure, what monitoring may be necessary?
116. For peripheral surgery in patients with congestive heart failure, can regional anesthesia be selected as an anesthetic option?
Hypertrophic cardiomyopathy
117. What is another name for hypertrophic cardiomyopathy? What pathophysiology defines hypertrophic cardiomyopathy? What is the stroke volume in patients with hypertrophic cardiomyopathy?
118. What is the goal of the anesthetic management of patients with hypertrophic cardiomyopathy?
119. How can intraoperative hypotension be treated in patients with hypertrophic cardiomyopathy?
120. How can intraoperative hypertension be treated in patients with hypertrophic cardiomyopathy?
121. What is the problem with using β agonists for the treatment of hypotension or using nitrates for the treatment of hypertension in patients with hypertrophic cardiomyopathy?
Cor pulmonale
123. What are some signs and symptoms associated with cor pulmonale?
124. What are some treatment methods for cor pulmonale?
125. What is the recommendation for the patient with cor pulmonale who is scheduled for an elective surgical procedure?
126. What is the goal of the anesthetic management of patients with cor pulmonale? How can this be achieved?
127. What is the advantage of monitoring pulmonary artery pressure during surgery in patients with cor pulmonale?
Cardiac tamponade
128. What is cardiac tamponade?
129. Name some manifestations of cardiac tamponade.
130. What is the treatment for cardiac tamponade? What are some temporizing measures for patients with cardiac tamponade awaiting definitive treatment?
131. What is the goal of the anesthetic management of cardiac tamponade?
132. What effect can the induction of anesthesia and positive-pressure ventilation of the lungs have on patients with cardiac tamponade?
133. What is the recommendation for anesthesia in patients with cardiac tamponade?
134. What pharmacologic agents may be useful in patients with cardiac tamponade?
Aneurysms of the aorta
135. What is the most frequent cause of aortic aneurysms? Do most aortic aneurysms involve the thoracic or abdominal aorta?
136. What is a dissecting aneurysm?
137. When is elective resection of an abdominal aortic aneurysm recommended?
138. What are some medical problems frequently associated with aortic aneurysms?
139. What is the goal of the anesthetic management of patients undergoing resection of an abdominal aortic aneurysm? What monitoring is warranted in these procedures?
140. When are patients with coronary artery disease especially at risk of myocardial ischemia during surgery for resection of an aortic aneurysm?
141. How should intraoperative fluids be managed during surgery for resection of an aortic aneurysm?
142. Why does hypotension frequently accompany unclamping of the abdominal aorta during surgery for the resection of an aortic aneurysm? What are some methods for minimizing the hypotension?
143. What are some concerns regarding renal function in patients undergoing aortic aneurysm repair?
144. What are some concerns regarding spinal cord function in patients undergoing aortic aneurysm repair?
Cardiopulmonary bypass
145. How is blood drained from the venae cavae during cardiopulmonary bypass?
146. What are two different types of pumps that are used to return blood to the arterial system during cardiopulmonary bypass? Which results in less trauma to blood?
147. How is blood kept from entering the heart from the superior and inferior venae cavae during cardiopulmonary bypass for mitral valve or intracardiac surgery?
148. Under what conditions does the aorta need to be cross-clamped distal to the aortic valve and proximal to the inflow cannula during cardiopulmonary bypass?
149. How can venous drainage from the inferior and superior venae cavae during cardiopulmonary bypass be facilitated?
150. What is the required cardiac index delivered by the roller pump on the cardiopulmonary bypass machine dependent upon? What approximate cardiac index is usually sufficient?
151. What is the advantage of low flows during cardiopulmonary bypass?
152. What are two different types of oxygenators that are used to oxygenate blood that is returning to the arterial system during cardiopulmonary bypass?
153. What is the advantage of a bubble oxygenator? What is the disadvantage of a bubble oxygenator?
154. What is the advantage of a membrane oxygenator? What is the disadvantage of a membrane oxygenator?
155. How can the patient’s body be heated or cooled by the cardiopulmonary bypass machine?
156. How is blood loss from the field recirculated to the patient during cardiopulmonary bypass?
157. What is a problem with the cardiotomy suction used during cardiopulmonary bypass?
158. Why might the left ventricle need a vent during cardiopulmonary bypass? How might this be achieved?
159. How are systemic emboli from cellular debris prevented from occurring during cardiopulmonary bypass?
160. What does priming of the cardiopulmonary bypass system refer to? What is the cardiopulmonary bypass system primed with?
161. What is the patient’s hematocrit maintained at during cardiopulmonary bypass? Why is it important to hemodilute the patient’s blood during cardiopulmonary bypass?
162. Why is it important to remove all air from the cardiopulmonary bypass system during cardiopulmonary bypass?
163. Why is heparin-induced anticoagulation of the patient’s blood necessary during cardiopulmonary bypass? What dose of heparin is usually administered? How is the adequacy of anticoagulation confirmed?
164. What are some explanations for the low mean arterial pressure often seen after the institution of cardiopulmonary bypass? What blood pressure is typically considered acceptable?
165. Why does blood pressure slowly rise spontaneously after some time on cardiopulmonary bypass?
166. What are the dangers of hypertension while on cardiopulmonary bypass? How can hypertension under these circumstances be treated?
167. What are some methods by which the adequacy of tissue perfusion during cardiopulmonary bypass can be evaluated?
168. Why is diuresis induced during cardiopulmonary bypass?
169. What may be the cause of an increasing central venous pressure with or without facial edema while on cardiopulmonary bypass? How can this be confirmed?
170. What may be the cause of increasing abdominal distention while on cardiopulmonary bypass?
171. What are some complications of extracorporeal circulatory support or cardiopulmonary bypass?
172. How should ventilation of the lungs be managed during cardiopulmonary bypass?
173. What is the goal of myocardial preservation during cardiopulmonary bypass? What are some methods by which this can be achieved?
174. What is the oxygen consumption of a normally contracting heart at 30° C? What is the oxygen consumption of a fibrillating heart at 22° C? What is the oxygen consumption of an electromechanically quiet heart at 22° C?
175. How is the effectiveness of cold cardioplegia of the heart measured?
176. What are two potential negative effects of intramyocardial hyperkalemia due to cold cardioplegia after cardiopulmonary bypass? How can they be treated?
177. What are two potential sources for systemic hyperkalemia during cardiopulmonary bypass? How can the hyperkalemia be treated if it were to persist at the conclusion of cardiopulmonary bypass?
178. Why might supplemental intravenous anesthetics be administered during cardiopulmonary bypass?
179. Why might supplemental neuromuscular blocking drugs be administered during cardiopulmonary bypass?
180. Is supplemental anesthesia routinely required during rewarming after the conclusion of cardiopulmonary bypass?
181. What conditions in the patient must be present for cardiopulmonary bypass to be discontinued?
182. When are the aortic and vena cava cannulae removed after cardiopulmonary bypass?
183. What are some potential problems associated with persistent hypothermia after cardiopulmonary bypass?
184. What special precautions must be taken before discontinuing cardiopulmonary bypass in patients who have had the left side of the heart opened, as during valve replacement surgery? What is the potential risk?
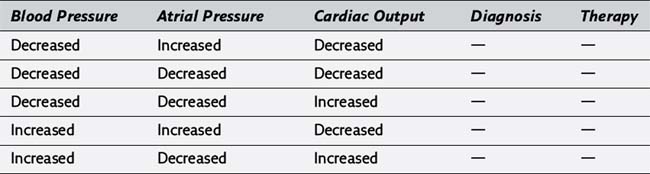
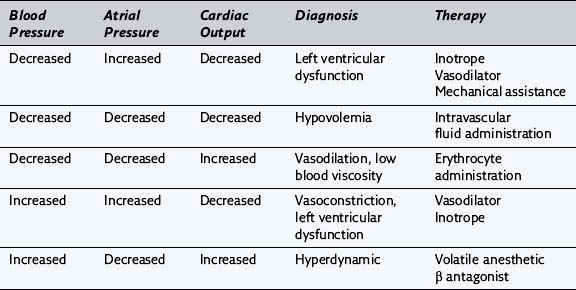
185. For each of the following situations, please complete the diagnosis and appropriate therapy:
186. Why might a patient have posterior papillary muscle dysfunction after cardiopulmonary bypass? How would this be manifest on the pulmonary artery occlusion pressure tracing?
187. What is a mechanical addition to the pharmacologic support of cardiac output in patients with a poor cardiac output after cardiopulmonary bypass? How does it work? What physiologic alterations may interfere with its efficacy?
188. When is protamine administered after cardiopulmonary bypass? Why?
189. What are some possible side effects of protamine administration?
190. What does the perfusionist do with blood and fluid that remains in the cardiopulmonary bypass circuit after cardiopulmonary bypass?
191. Why might there be a gradient between central aortic and radial artery blood pressures in the early period after cardiopulmonary bypass? How long can this effect persist?
Answers*
Coronary artery disease
1. It is estimated that 40% of adult patients undergoing surgery have, or are at risk for, coronary artery disease. (384)
2. Components of a routine preoperative cardiac evaluation include the history and physical examination, evaluation of the patient’s electrocardiogram, and reviewing or ordering more specialized procedures. Specialized methods of cardiac evaluation include a Holter monitor, exercise electrocardiogram, echocardiogram, radioisotope imaging, cardiac catheterization, and angiography. The ultimate purpose of a preoperative cardiac evaluation is to assess the patient’s risk of an adverse perioperative cardiac event, to determine whether the patient is in optimal medical condition for surgery, and to reduce operative risk. (384)
3. Important aspects of the preoperative history taken from patients with coronary artery disease with respect to their cardiac status include their exercise tolerance, characteristics of their angina, and the presence of a previous myocardial infarction. It is also important to learn what cardiac medicines the patient may be taking and what the potential interactions of these are with anesthetics that may be administered for surgery. (384)
4. Noncardiac diseases that are frequently present in patients with coronary artery disease include peripheral vascular disease, chronic obstructive pulmonary disease, renal dysfunction, chronic hypertension, and diabetes mellitus. (384)
5. A major coronary artery can be stenosed by as much as 50% to 70% in an asymptomatic patient. (384)
6. The best indicator for a patient’s cardiac reserve is by evaluation of their exercise tolerance. A limited exercise tolerance in the absence of significant pulmonary disease gives evidence of a decrease in a patient’s cardiac reserve. Alternatively, the cardiac reserve of a patient who is able to climb up two to three flights of stairs without stopping is probably adequate. (384)
7. Angina pectoris is considered “stable” when there has been no change in the patient’s anginal symptoms for at least 60 days. Factors related to the angina that should be evaluated include the precipitating factors, frequency, and duration. (384)
8. Angina pectoris is considered “unstable” when there has been a change in the patient’s anginal symptoms. Changes that should be evaluated include the degree of activity a patient can do before the onset of angina and the duration of each anginal episode. Another symptom of unstable angina is chest pain occurring at rest. The clinical implication of unstable angina is that the patient may be at risk of an impending myocardial infarction. (384)
9. Dyspnea after the onset of angina pectoris is likely an indication of acute left ventricular dysfunction due to myocardial ischemia and acute, transient cardiac failure. (384)
10. Angina pectoris due to spasm of the coronary arteries differs from classic angina pectoris in that the pain may occur at rest but may not occur during periods of exertion. Angina of this type is associated with ST segment changes on the electrocardiogram. This type of angina is referred to as Prinzmetal’s or variant angina. (384)
11. Silent myocardial ischemia is myocardial ischemia that occurs in the absence of angina. This type of angina is more common in patients with diabetes mellitus and carries the same prognosis as myocardial ischemia associated with angina. (384)
12. The most common symptom of angina in men is dyspnea on exertion. Shortness of breath with climbing stairs is very common. Walking on a flat surface does not seem to be sufficient to elicit shortness of breath until the symptoms are severe. Waking from sleep with angina is also a symptom of severe angina. Women most commonly complain of nonspecific fatigue, making identification of angina more difficult. (384)
13. Approximately 70% of myocardial ischemic episodes are not associated with angina pectoris, and myocardial infarctions are not associated with angina pectoris approximately 15% of the time. (384)
14. Tachycardia is more likely than hypertension to result in myocardial ischemia in the patient with coronary artery disease secondary to an increased oxygen consumption with a decreased duration for coronary blood flow to the left ventricle. Tachycardia results in an increased myocardial oxygen requirement as oxygen consumption is per beat combined with a decreased myocardial perfusion time. Myocardial perfusion to the left ventricle, and thus myocardial oxygen supply, occurs during diastole. Hypertension, on the other hand, leads to an increased myocardial oxygen requirement, but it is also a simultaneous increase in myocardial perfusion. (384)
15. The basis for the common recommendation that elective surgery be delayed until 6 months after a prior myocardial infarction is based on numerous epidemiologic studies. These studies have shown that there is a 5% to 86% reinfarction rate in the perioperative period if previous myocardial infarction preceded the surgical procedure by less than 6 months. This rate of myocardial infarction is 1.5 to 10 times higher than if more than 6 months separated the previous myocardial infarction and the surgical procedure. (385)
16. The approximate incidence of perioperative myocardial infarction 6 months or more after a myocardial infarction is 5% to 6%, whereas the approximate incidence of perioperative myocardial infarction in patients who have not had a prior myocardial infarction is 0.13%. (385, Table 25-1)
17. Most perioperative myocardial infarctions occur in the first 48 to 72 hours postoperatively. (385)
18. Cardiac medications that patients with coronary artery disease are likely to be taking include β antagonists, nitrates, calcium channel blockers, antihypertensives, and diuretics. The recommendation is that patients continue taking their regular cardiac medicines throughout the perioperative period. (385)
19. Preoperative electrocardiograms may provide evidence of myocardial ischemia, prior myocardial infarction, cardiac hypertrophy, abnormal cardiac rhythm or conduction disturbances, and electrolyte abnormalities. (386)
20. Myocardial ischemia may appear as ST segment changes or T wave changes on an electrocardiogram. (386)
| Electrocardiogram Lead | Coronary Artery Responsible for Myocardial Ischemia | Area of Myocardium That May Be Involved |
|---|---|---|
| II, III, aVF | Right coronary artery | Right atrium, atrioventricular node, right ventricle |
| V3-V5 | Left anterior descending coronary artery | Anterolateral portion of left ventricle |
| I, aVL | Circumflex coronary artery | Lateral aspects of the left ventricle |
22. Determinants of myocardial oxygen requirements and delivery are related to factors that affect myocardial oxygen supply or myocardial oxygen demand. Myocardial oxygen supply is decreased by tachycardia, hypotension, increased preload, hypocapnia, coronary artery spasm, anemia, and hypoxemia. Myocardial oxygen demand is increased by tachycardia, increased wall tension, and increased myocardial contractility. A goal of the anesthetic management of patients with coronary artery disease is maintenance of the balance between myocardial oxygen supply and demand to minimize the risk of myocardial ischemia. (389, Table 25-4)
23. In an attempt to decrease the risk of a perioperative myocardial infarction in patients at risk, the anesthesiologist should attempt to maintain stable patient hemodynamics. In general, the desired hemodynamics to minimize the risk of intraoperative ischemia include slower heart rates, lower filling pressures, and normal systolic blood pressures. A common recommendation for patients at risk of myocardial ischemia is that heart rate and blood pressure be maintained within 20% of awake values intraoperatively. Even so, approximately 50% of all new perioperative myocardial ischemic episodes are not preceded by or associated with changes in heart rate or blood pressure. Nevertheless, the anesthesiologist may choose to closely monitor the patient’s more limited hemodynamic status using invasive monitors to achieve these goals. He or she should also be prepared to intervene quickly with pharmacologic interventions should they become necessary. (389)
24. Risk stratification is the identification of risk factors in patients that lead to the determination of preoperative risk. Risk stratification does not actually decrease risk, it simply identifies it. Risk reduction requires changing the care provided to the patient either through medications such as the administration of perioperative blockade, or through an alteration in the anesthetic or surgical plan. (386-388, Table 25-2)
25. Patients with recent intracoronary stents have an increased risk of myocardial infarction and death if platelet inhibitors are withdrawn for surgery. Patients with bare metal stents likely require 3 or more months of antiplatelet therapy and those with drug eluting stents may require a year or more before risk is acceptable to discontinue platelet inhibitors for surgery. Many patients who have had percutaneous intervention should be operated on while on aspirin if surgical conditions allow. (388)
26. Two benefits of administering premedication preoperatively to patients with coronary artery disease are the decrease in the secretion of potentially harmful catecholamines and the potential to prevent the increase in myocardial oxygen requirements that may occur with tachycardia and hypertension related to anxiety. (390)
27. The induction of anesthesia in patients at risk for myocardial ischemia is typically achieved with great care. The patient’s standard daily medications should be reviewed and administered if there are no specific contraindications. Patients on β-blockers should receive them. A preinduction intraarterial line may help recognize hemodynamic perturbations reducing risk. Infusions of phenylephrine are helpful to reduce hypotension on induction. Careful administration of intravenous induction agents, narcotics, and inhaled agents, combined with monitoring and careful vasoconstrictor use, are essential. It is important to avoid tachycardia with consummate increases in myocardial oxygen requirements. (390)
28. Direct laryngoscopy is associated with an increased risk of myocardial ischemia because it often produces intense sympathetic nervous system stimulation leading to tachycardia and hypertension. To minimize this risk, there must be adequate levels of anesthesia to suppress sympathetic nervous system stimulation. Volatile anesthetics, intravenous anesthetics other than ketamine, opioids, and lidocaine may all be used to blunt the response to direct laryngoscopy. β antagonists may be administered before induction to attenuate the increase in heart rate and blood pressure that can occur. (390)
29. The maintenance of anesthesia for the patient with coronary artery disease may be achieved through the administration of volatile anesthetics, propofol, dexmedetomidine, and opioids, with or without nitrous oxide. (391)
30. Coronary artery steal syndrome is a theoretical risk in which administration of a coronary artery vasodilator to a patient with coronary artery disease could result in diversion of blood flow from the ischemic areas, in which stenotic coronary arteries are maximally dilated, to areas in which the coronary arteries are patent and able to vasodilate. Isoflurane, of all the volatile anesthetics, is the most potent coronary vasodilator. It was once thought that isoflurane is the volatile anesthetic that is most likely to result in this syndrome. Clinically, however, the administration of isoflurane to patients with coronary artery disease has not been shown to increase the risk of myocardial ischemia through the coronary artery steal syndrome. (391)
31. The administration of a regional anesthetic to patients with coronary artery disease can result in hypotension, which may in turn lead to decreased blood flow through pressure-dependent stenosed coronary arteries. For this reason it is important for the anesthesiologist to be prepared to treat decreases in blood pressure with induction of any anesthetic. An advantage of regional anesthesia for patients with coronary artery disease is that the anesthesiologist may continue to monitor the patient for symptoms of angina and treat them accordingly. (391)
32. Considerations in the selection of a neuromuscular blocking drug for patients with coronary artery disease should take into account the effects of the neuromuscular blocking drug on the cardiovascular system. For example, a neuromuscular blocking drug that may lower blood pressure through the release of histamine should be administered slowly to minimize those effects. Pancuronium causes mild increases in heart rate and blood pressure that may or may not be beneficial, depending on the status of the patient. (391)
33. Neuromuscular blockade may be reversed in patients with coronary artery disease in the usual manner with an anticholinesterase-anticholinergic drug combination. Care should be taken to avoid tachycardia and subsequent myocardial ischemia with reversal. Glycopyrrolate has less of a chronotropic effect on the heart, but either glycopyrrolate or atropine is acceptable for the reversal of neuromuscular blockade. Alternatively, avoiding reversal by appropriate timing and choice of nondepolarizing muscle relaxants can reduce the risk of tachycardia and other side effects of nondepolarizing muscle relaxant reversal. (392)
34. The intensity of intraoperative monitoring the anesthesiologist chooses to implement for a surgical procedure in a patient with coronary artery disease is influenced by the type of procedure the patient is undergoing, the severity of the patient’s disease, the choice of anesthetic technique, and a risk-benefit analysis of each type of potential monitoring. (392)
35. While no clinical benefit has been shown of the use of a pulmonary artery catheter, it may be useful in patients with poor left ventricular function, valvular heart disease, a recent myocardial infarction, or pulmonary vascular disease, in situations of massive trauma, or in major vascular surgery. Information provided by a pulmonary artery catheter includes more accurate assessment of cardiac filling pressures than a central venous monitor in the presence of pulmonary vascular disease, left-sided heart dysfunction, or potential left-sided heart dysfunction due to myocardial ischemia. The pulmonary artery catheter can be used to measure cardiac output and calculate systemic vascular resistance. (392)
36. Information provided by an intraoperative transesophageal echocardiogram includes both functional and anatomic information including early detection of myocardial ischemia through the presence of new onset regional wall motion abnormalities, an assessment of the intravascular fluid volume status of the patient, an estimation of the cardiac output, an estimation of left ventricular afterload, and an evaluation of the cardiac valves. (392)
37. The detection of intraoperative myocardial ischemia should promptly lead to the treatment of any hemodynamic alterations in an attempt to increase myocardial oxygen supply while decreasing myocardial oxygen demand. Tachycardia may be treated with a β-adrenergic antagonist. These drugs decrease the demand of the myocardium for oxygen through its effects of decreases in heart rate and myocardial contractility. Administration of any medication should be judicious in patients with left ventricular dysfunction. Hypertension may be treated with a nitrate. Nitroglycerin may also be used in a situation in which there are ischemic changes on the electrocardiogram but blood pressure remains normal to high. Intravenous nitroglycerin administration may lead to reflex tachycardia. Hypotension may be treated with a sympathomimetic drug and intravascular fluids. (392)
38. Decreases in body temperature that may occur intraoperatively in patients with coronary artery disease can result in shivering on awakening. Shivering can significantly increase myocardial and systemic oxygen requirements and can be especially detrimental to patients with coronary artery disease because it is often accompanied by tachycardia. (392)
39. It is important to control heart rate to avoid myocardial ischemia. Control of pain, stress, volume status, and administration of antiischemic agents is essential in the patient with coronary artery disease. Tachycardia from any cause (including pain, hypovolemia, atrial fibrillation, and stress) increases myocardial oxygen requirements and is detrimental to the patient with coronary artery disease. (392)
Valvular heart disease
40. Information that can be gained from Doppler echocardiography in patients with valvular heart disease includes the significance of cardiac murmurs, hemodynamic abnormalities, transvalvular pressure gradients, the orifice area of the cardiac valve, and the evaluation of prosthetic valve function. (393, Table 25-5)
41. Anesthetic drugs and neuromuscular blocking drugs should be selected for the patient with valvular heart disease based on the effects they may have on cardiac rhythm, heart rate, blood pressure, systemic vascular resistance, and pulmonary vascular resistance. The objective is to choose anesthetic drugs and neuromuscular blocking drugs that will not compromise cardiac output with their administration. (393)
42. Antibiotics used to be administered to patients with known valvular heart disease prophylactically to protect the patient from infective endocarditis. Administration of prophylactic antibiotics is now only recommended for patients with congenital heart disease, prosthetic heart valves, patients with a history of infective endocarditis, or heart transplant patients with a developing cardiac valvulopathy. Prophylaxis is recommended to minimize the risk of infection from a bacteremic event, such as surgical or dental procedures. Bacteremia does not seem to occur with orotracheal intubation, but it may occur with nasotracheal intubation independent of any surgical event. There are recommended prophylaxis regimens that vary depending on the site of surgery, mechanism of administration, and any history of allergies to antibiotics the patient may have. (393, Table 13-8)
43. Mitral stenosis is a mechanical obstruction to left ventricular diastolic filling secondary to a decrease in the orifice of the mitral valve. Measurement of the mitral valve area provides for the best indication of the severity of the disease. Mitral stenosis is classified as severe when the mitral valve area is less than 1 cm2. Left atrial and pulmonary venous pressures are increased in patients with mitral stenosis. An increase in pulmonary vascular resistance is likely to be seen when the left atrial pressure is higher than 25 mm Hg on a chronic basis. (393)
44. The most common etiology for mitral stenosis is rheumatic heart disease. The mitral valve leaflets often fuse, scar, and fibrose during the healing process of acute rheumatic carditis. Mitral stenosis presents after a prolonged course of development, usually about 20 years after the initial episode of rheumatic fever. Often the disease presents with atrial fibrillation or when there is an increased demand for cardiac output, as may occur during pregnancy or exercise. Patients with mitral stenosis may have recurrent episodes of pulmonary edema, dyspnea, paroxysmal nocturnal dyspnea, chest pains, palpitations, and fatigue. (393)
45. Patients with mitral stenosis are at an increased risk of atrial fibrillation secondary to the distention of the left atrium. (393)
46. Patients with mitral stenosis are at an increased risk of thrombus formation in the left atrium because of the stasis of blood in that heart chamber. Thrombi in the left atrium may be ejected from the heart as systemic emboli. (393)
47. Considerations for the anesthetic management of patients with mitral stenosis include maintenance of a normal sinus rhythm and heart rate, maintenance of a normal intravascular fluid volume, and the avoidance of increases in pulmonary vascular resistance. Patients with mitral stenosis have a greater reliance on atrial contraction for left ventricular filling. Alterations from sinus rhythm should be promptly treated chemically or with cardioversion. Tachycardia and bradycardia may both result in decreases in left ventricular filling. The intravascular fluid volume should be maintained at near-normal or maximally tolerated levels, while avoiding pulmonary edema. Increases in pulmonary vascular resistance and pulmonary hypertension may place the patient at an increased risk for pulmonary artery rupture with placement of a pulmonary artery catheter and repeated wedge pressure measurements. Care should be taken to avoid overtransfusion or the head-down position in these patients. Arterial hypoxemia or hypercarbia may exacerbate pulmonary hypertension and precipitate right ventricular failure and should be avoided. Central venous pressure monitoring may be useful to detect changes in the right ventricular pressure. (393)
48. The maintenance of anesthesia can be achieved in patients with mitral stenosis through the administration of volatile anesthetics, nitrous oxide, and opioids. Of greater importance is the management of the cardiovascular effects of these drugs to achieve the goal of the anesthetic management of patients with mitral stenosis and treatment of the unfavorable effects of these drugs, accordingly. For example, pancuronium may not be an appropriate choice for neuromuscular blockade in patients with mitral stenosis secondary to the increased speed of transmission of cardiac impulses through the atrioventricular node that result from this drug. This increased speed of transmission may be detrimental to patients prone to atrial fibrillation. Likewise, the administration of ketamine to these patients should be avoided. The increase in pulmonary vascular resistance that is associated with nitrous oxide is not usually sufficient enough to detract from its utility in patients with mitral stenosis. Drugs that are being administered for heart rate control should be continued throughout the perioperative period. (393-394)
49. Intraoperative monitoring of the right atrial pressure may be useful in assessing the adequacy of intravascular fluid replacement in patients with mitral stenosis. The monitoring of intraoperative fluid therapy in these patients is important because they are prone to intravascular fluid overload, leading to right heart failure and pulmonary edema. (394)
50. The mechanical support of ventilation may be required postoperatively in patients with mitral stenosis because they are susceptible to developing pulmonary edema and right-sided heart failure. This may be especially true in patients with mitral stenosis after major thoracic or abdominal surgery. (394)
51. Mitral regurgitation occurs as a result of an incompetent mitral valve. Physiologically, there is left atrial overload and a decreased left ventricular stroke volume in these patients. When mitral regurgitation develops over time, left ventricular dilation and left ventricular hypertrophy develop to maintain the left ventricular stroke volume. With progression of the disease, however, congestive heart failure can occur. Patients with chronic mitral regurgitation are frequently in atrial fibrillation. Acute mitral regurgitation results in acute increases in left atrial pressure and pulmonary artery pressures and can present as pulmonary congestion, pulmonary hypertension, and right-sided heart failure. Measurement of the regurgitant fraction provides for an estimate of the severity of the disease. For instance, a regurgitant fraction of 0.6 or greater is typically associated with congestive heart failure. A recording of pulmonary artery occlusion pressure tracings in a patient with mitral regurgitation would show prominent v waves that are characteristic of mitral regurgitation. (394, Figure 19-2)
52. The most common cause of mitral regurgitation is rheumatic heart disease. When mitral regurgitation is secondary to rheumatic heart disease it is often chronic, is accompanied by mitral stenosis, and progresses over years. The most common cause of isolated mitral regurgitation is papillary muscle dysfunction, which is usually acute in onset with a corresponding acute onset of symptoms. Papillary muscle dysfunction usually occurs after a myocardial infarction or after rupture of the chordae tendineae secondary to infective endocarditis. (394)
53. Considerations for the anesthetic management of patients with mitral regurgitation include the avoidance of sudden decreases in heart rate, the avoidance of sudden increases in systemic vascular resistance, and minimizing drug-induced myocardial depression, because each of these will increase regurgitant flow. The size of the v wave on the pulmonary artery catheter tracing may be monitored as a reflection of mitral regurgitant flow. (394, Table 25-7)
54. The maintenance of anesthesia in patients with mitral regurgitation can be achieved through the administration of a volatile anesthetic, nitrous oxide, and an opioid. Of greater importance is the management of the cardiovascular effects of these drugs to achieve the goal of the anesthetic management of patients with mitral regurgitation and treatment of the unfavorable effects of these drugs accordingly. The goals include maintenance of normal to increased heart rate, normal to reduced systemic vascular resistance, and myocardial contractility. Nondepolarizing neuromuscular blocking drugs, including pancuronium, may be safely used in patients with mitral regurgitation. The increase in heart rate that can result from the administration of pancuronium can be beneficial to patients with mitral regurgitation. (395)
55. Aortic stenosis is the mechanical obstruction to the ejection of blood from the left ventricle secondary to a decrease in the orifice of the aortic valve. Increased left ventricular systolic pressure necessarily results from the chronic attempt of this chamber to maintain an adequate stroke volume through a narrowed aortic valve in aortic stenosis. The increased thickness of the left ventricular wall that is often seen in patients with aortic stenosis occurs in response to chronically increased intraventricular pressures. The severity of aortic stenosis is estimated by the degree of stenosis of the valve. A pressure gradient across the aortic valve that is in excess of 50 mm Hg is considered hemodynamically significant aortic stenosis. (395)
56. Two causes of aortic stenosis are rheumatic heart disease and the progressive calcification and stenosis of a congenitally abnormal valve. The congenital valve abnormality most often associated with aortic stenosis is a bicuspid valve. The natural course of aortic stenosis is one of an insidious, long progression of asymptomatic disease before the onset of symptoms. Symptoms may include angina, syncope, dyspnea on exertion, and congestive heart failure. (395)
57. Patients with aortic stenosis may have angina pectoris, which typically occurs with exertion despite the absence of coronary artery disease. Myocardial ischemia and angina occurs because of an increased demand for and decreased supply of myocardial oxygen. The increase in myocardial oxygen demand is due to left ventricular hypertrophy combined with increased left ventricular pressures and increased myocardial work. The decrease in myocardial oxygen delivery results from compression of subendocardial coronary blood vessels by increased left ventricular systolic pressures, as well as the gradient in pressure from the left ventricle to the coronary ostia caused by the stenotic valve. (395)
58. A systolic murmur heard best in the second right intercostal space characterizes the murmur of aortic stenosis heard on cardiac auscultation. It is important for the anesthesiologist to rule out aortic stenosis by auscultation preoperatively to best manage the patient. For instance, a precipitous drop in systemic vascular resistance, as may occur with a regional anesthetic or induction of general anesthesia, could be lethal to the patient with aortic stenosis. (395)
59. Consideration for the anesthetic management of a patient with aortic stenosis includes the maintenance of a stable blood pressure. Avoiding hypotension and tachycardia are critical. The goal of the anesthetic management of patients with aortic stenosis is the maintenance of normal sinus rhythm, normal heart rates, and normal myocardial contractility, while avoiding sudden decreases in systemic vascular resistance, or hypovolemia. (395, Table 25-8)
60. Tachycardia in the patient with aortic stenosis increases oxygen consumption and decreases coronary filling time by shortening diastole. Tachycardia also decreases the amount of time for left ventricular filling, leading to a decrease in the stroke volume. Bradycardia can lead to an acute overdistention of the left ventricle in these patients. Sinus rhythm is desired because the contribution of the atrial contraction to left ventricular filling is greater in these patients. Decreases in systemic vascular resistance in patients with aortic stenosis can lead to decreases in coronary blood flow with myocardial ischemia, with rapid ventricular decompensation and death. (395)
61. The maintenance of anesthesia in patients with aortic stenosis can be achieved through the administration of narcotics, volatile agents, or intravenous anesthetics. Volatile anesthetics administered carefully to avoid excessive decreases in systemic vascular resistance and tachycardia are common. The most important point in patients with aortic stenosis is to carefully manage hemodynamics to avoid hypotension, tachycardia, myocardial ischemia, and ventricular dysfunction. Intraarterial pressure monitoring prior to the induction of anesthesia is mandatory, as is the availability of vasoconstrictors, both bolus and infusion, such as phenylephrine. (396)
62. Management of the intravascular fluid status of patients with aortic stenosis should be geared toward the maintenance of an adequate intravascular volume through the prompt, liberal correction of blood and fluid losses. (396)
63. The pulmonary artery occlusion pressure may not be reflective of the left ventricular end-diastolic volume in patients with chronic aortic stenosis secondary to the decrease in left ventricular compliance seen in these patients. (396)
64. External cardiac compressions administered during cardiopulmonary arrest are not effective in patients with aortic stenosis because of the greater pressures that are necessary to create forward flow through the stenosed aortic valve. (396)
65. Aortic regurgitation results from an incompetent aortic valve. Patients with aortic regurgitation have a decreased left ventricular stroke volume due to regurgitation of part of the ejected stroke volume from the aorta back into the left ventricle. This places an increased volume load on the left ventricle. Chronic aortic regurgitation results in eccentric hypertrophy of the left ventricle in an attempt to compensate for the regurgitation by increasing the stroke volume. Symptoms may include dyspnea, fatigue, and palpitations. (396)
66. Acute aortic regurgitation is most likely due to infective endocarditis, trauma, connective tissue disease, or a dissecting thoracic aortic aneurysm. Chronic aortic regurgitation is most likely due to prior rheumatic fever, but it may also be due to hypertension, syphilis, and other causes. (396)
67. Angina pectoris despite the absence of coronary artery disease in a patient with aortic regurgitation may occur as a result of increased myocardial oxygen requirements in the presence of a decreased supply. The increase in myocardial oxygen requirements is due to left ventricular hypertrophy. The decrease in myocardial oxygen supply is due to a decrease in aortic diastolic pressure, which decreases coronary blood flow. Coronary blood flow to the left ventricle occurs during diastole, so lower diastolic pressures compromise it. Angina resulting from aortic regurgitation is typically a late and dismal sign. (396)
68. The anesthetic management of aortic regurgitation resembles the anesthetic management for mitral regurgitation. Considerations for the anesthetic management of patients with aortic regurgitation include the avoidance of sudden decreases in heart rate, the avoidance of sudden increases in systemic vascular resistance, and minimizing drug-induced myocardial depression. (396)
69. Mitral valve prolapse is a valvular disease in which the valve prolapses into the left atrium during contraction of the left ventricle. Valve prolapse is caused by an abnormality of the valve support structure. Mitral valve prolapse on cardiac auscultation is characterized by a systolic murmur with a clicking sound. It has been estimated that 5% to 15% of the adult population has mitral valve prolapse, also called click-murmur syndrome. Currently, this estimate is believed to be higher than the true prevalence. The diagnosis of mitral valve prolapse can be confirmed through echocardiography. (396)
70. Mitral valve prolapse is associated with atrial secundum defects, von Willebrand syndrome, and polycystic kidney disease, as well as with musculoskeletal abnormalities such as Marfan syndrome, pectus excavatum, and kyphoscoliosis. Females are more likely than males to have mitral valve prolapse. (396)
71. Patients with mitral valve prolapse typically are asymptomatic. Symptoms that can be associated with mitral valve prolapse include palpitations, dyspnea, atypical chest pain, dizziness, and syncope. (396)
72. Potential complications of mitral valve prolapse include mitral regurgitation, infective endocarditis, transient cerebral ischemic events, cardiac dysrhythmias, and sudden death. Sudden death is extremely rare, however. Cardiac dysrhythmias associated with atrioventricular bypass tracts and preexcitation syndromes are fairly common in these patients. Transient cerebral ischemic events may lead to the prescription of aspirin or anticoagulants for patients with mitral valve prolapse. (395-396)
73. The maintenance of anesthesia in patients with mitral valve prolapse should be geared toward the avoidance of cardiac emptying. Cardiac emptying results in increased prolapse of the mitral valve into the left atrium. Avoidance of sympathetic nervous system stimulation, decreases in systemic vascular resistance, and the performance of surgery with patients in the head-up or sitting position will all minimize cardiac emptying. The intravascular fluid volume of the patient should be maintained at normal or high normal for the same reason. Hypotension in patients with mitral valve prolapse can be treated with phenylephrine. Cardiac dysrhythmias that occur intraoperatively should be promptly treated. Ketamine is not recommended in patients with mitral valve prolapse because of its propensity to increase myocardial contractility and heart rate. (397)
74. The potential problem with regional anesthesia in patients with mitral valve prolapse is the decrease in systemic vascular resistance that can be detrimental to these patients. Appropriate monitoring can make regional anesthesia the preferred anesthetic approach for some surgical patients with valvular heart disease. (397)
Disturbances of cardiac conduction and rhythm
75. Tools available to the clinician for the diagnosis of disturbances in cardiac conduction and rhythm include an electrocardiogram, Holter monitoring, or an electrophysiology (EP) study. A Holter monitor is an ambulatory electrocardiogram that can be worn for days to document the occurrence of cardiac dysrhythmias and to assess the efficacy of treatment interventions. (397)
76. The conduction system of the heart includes the sinoatrial node, atrioventricular node, the bundle of His, and Purkinje fibers of the right and left bundle branches. Types of conduction defects include sinus node block, atrioventricular conduction defects, and intraventricular conduction defects. Atrioventricular conduction defects are classified as first-, second-, or third-degree heart blocks. Intraventricular conduction defects include right bundle branch block, left bundle branch block, and left fascicular hemiblock. Heart block below the atrioventricular node is usually progressive and permanent, whereas heart block above the atrioventricular node is usually transient and benign. (397, Table 25-10)
77. The placement of a prophylactic artificial cardiac pacemaker before surgery is not indicated in a patient with a bifascicular block. The theoretical concern in preoperative patients with a bifascicular block is that the single remaining intact fascicle will become compromised by perioperative events, such as changes in hemodynamics, oxygenation, or electrolytes. This would lead to acute third-degree atrioventricular heart block. Third-degree atrioventricular block is also referred to as complete heart block because all the electrical activity from the atria fails to be conducted to the ventricles. Ventricular contractions in patients with third-degree atrioventricular block occur at a rate of about 40 beats per minute, typically too slow to maintain an adequate cardiac output. Fortunately, there is no evidence that bifascicular blocks proceed to third-degree atrioventricular block with enough consistency to warrant the prophylactic placement of a pacemaker. (398)
78. Third-degree atrioventricular heart block is treated by the placement of an artificial cardiac pacemaker. There are various methods by which this can be accomplished. An endocardial pacemaker lead may be inserted intravenously, an epicardial or myocardial lead may be placed by the subcostal approach, or noninvasive transcutaneous cardiac pacing can be started. The pharmacologic treatment of third-degree heart block involves a continuous infusion of isoproterenol, which can act as a medical pacemaker until artificial electrical cardiac pacing is implemented. (398)
79. Sick sinus syndrome occurs as a result of degenerative changes in the sinoatrial node and is associated with an inappropriate sinus bradycardia. In sick sinus syndrome, rapid heart rates inhibit the normal pacemaker activity of the sinoatrial node and lead to periods of asystole. Sick sinus syndrome therefore usually presents as bradycardia with episodes of supraventricular tachycardia. Treatment is by administering medicines to control tachycardia. When these medicines result in bradycardia, medical management is said to have failed and artificial cardiac pacemakers become the next line of treatment. Patients with sick sinus syndrome may be at a high risk for pulmonary embolism and may therefore be started on anticoagulants. (398)
80. Ventricular premature beats occur as a result of ectopic pacemaker activity at a level below the atrioventricular node. The premature ventricular contraction then spreads through the ventricular conducting system. The premature ventricular contraction often blocks the sinoatrial node’s subsequent depolarization, leading to a characteristic pause until the next normal sinus beat is generated. The hallmark features of a ventricular premature beat on an electrocardiogram are representative of the aberrant conduction associated with the ventricular contraction. They include a premature occurrence, the absence of a P wave preceding the QRS complex, a wide and bizarre appearing QRS complex, an inverted T wave, and a compensatory pause after the premature beat. (398)
81. Premature ventricular beats warrant treatment when they occur more frequently than six times a minute, are multifocal, occur in a train of three or more, or take place during the ascending limb of the T wave, that is, during the refractory period of the ventricle. Treatment is typically with lidocaine at a dose of 1 to 2 mg/kg. Recurrent premature ventricular beats can be treated with a lidocaine infusion. Additional therapy, if necessary, may include amiodarone, β antagonists, bretylium, procainamide, quinidine, verapamil, or overdrive pacing. A search for an underlying cause of the premature beats should be the primary goal. (398)
82. Causes of ventricular premature beats include myocardial ischemia, arterial hypoxemia, hypercarbia, hypertension, hypokalemia, and mechanical irritation of the ventricles. (398)
83. Ventricular tachycardia may be diagnosed with the appearance of three or more consecutive, wide QRS complexes on the electrocardiogram occurring at an effective heart rate higher than 120 beats per minute. The QRS complexes must be greater than 0.12 second. The P waves have no fixed relationship to the QRS complex because the beat originates in the ventricle. The onset of ventricular tachycardia can be life threatening. Ventricular tachycardia should be treated with intravenous amiodarone as a bolus followed by an infusion if the patient is hemodynamically stable. Hemodynamic instability, loss of consciousness, or myocardial ischemia should prompt immediate electrical cardioversion. (398)
84. Preexcitation syndromes are defined as an activation of a portion of the ventricles by cardiac impulses that have originated in the atria but were conducted to the ventricles by an accessory conduction pathway. Activation of the ventricles during this syndrome occurs sooner than it otherwise would have because of the accessory pathway, making the QRS complex appear sooner than it would have if sinus rhythm were maintained. (398)
85. WPW syndrome is the most commonly occurring preexcitation syndrome. The incidence of this syndrome is 0.3% in the general population. These patients may have sporadic supraventricular tachycardia or atrial fibrillation. In extreme cases, the rapid heart rate may be associated with syncope or congestive heart failure. On the electrocardiogram, WPW syndrome is characterized by a short P–R interval and a wide QRS complex. There is also a characteristic delta wave that appears on the electrocardiogram. The delta wave, together with the QRS complex, represents the composite of cardiac impulses conducted by both the normal and accessory pathways. (398)
86. The most common cardiac dysrhythmia associated with WPW syndrome is paroxysmal atrial tachycardia. WPW syndrome is most frequently treated by catheter ablation of the accessory pathway. Identification of the accessory pathway is accomplished by electrophysiologic mapping. (398)
87. The goal of the anesthetic management of a patient with WPW syndrome is the avoidance of any events, such as anxiety or drugs, that can result in sympathetic nervous system stimulation. Any cardiac antidysrhythmic drugs should be continued throughout the perioperative period. An adequate depth of anesthesia should be achieved before direct laryngoscopy to ensure that the patient does not respond to the noxious stimulus with sympathetic nervous system activity, placing the patient at an increased risk of tachyarrhythmias. Reduction in the stimulation of laryngoscopy can be achieved with adequate doses of an intravenous induction agent such as propofol, thiopental, benzodiazepines, opioids, β-blockers, or with a bolus of lidocaine just before direct laryngoscopy. Ketamine is not recommended as it stimulates the sympathetic nervous system. The duration of laryngoscopy should also be as short as possible. (398)
88. Methods for the treatment of paroxysmal atrial tachycardia or fibrillation that can occur in the perioperative period in patients with WPW syndrome include the administration of adenosine or procainamide. Adenosine acts by prolonging the refractory period of the atrioventricular node, whereas procainamide acts by increasing the refractory period of the accessory pathways. β-adrenergic antagonists can be used to control the heart rate. When the tachydysrhythmias become life threatening, emergent electrical cardioversion is indicated. Of note, drugs such as verapamil and digitalis may actually result in an increase in ventricular response during the dysrhythmia by accelerating the conduction in the accessory atrioventricular pathway. (399)
89. Prolonged QT interval syndrome can be congenital or acquired. Acquired prolonged QT interval syndrome can be due to quinidine, tricyclic antidepressants, subarachnoid hemorrhage, hypokalemia, hypocalcemia, or hypomagnesemia. It may also present in the postoperative period after right radical neck dissection. The diagnosis of prolonged QT interval syndrome is made when the QT interval is chronically greater than 0.44 second. Adverse events that are associated with a prolonged QT interval include ventricular dysrhythmias, syncope, and sudden death. The pharmacologic treatment of a chronically prolonged QT interval can include β antagonists or a left stellate ganglion block. These treatments are empirical. (399)
90. A congenital cause of a prolonged QT interval is thought to be due to an imbalance of autonomic innervation to the heart caused by decreases in right cardiac sympathetic nerve activity. A left stellate ganglion block is thought to work by decreasing left cardiac sympathetic nerve activity, thereby balancing the autonomic innervation to the heart. (399)
91. The goal of the anesthetic management of a patient with a chronically prolonged QT interval includes the avoidance of any events or drugs that are likely to cause sympathetic nervous system stimulation. General anesthesia has triggered life-threatening ventricular dysrhythmia and cardiac arrest in patients with this syndrome. β-adrenergic blockade may be instituted preoperatively to minimize this risk. Although thiopental prolongs the QT interval in normal patients, it has been used for the induction of anesthesia in patients with the syndrome without any problems. Direct laryngoscopy should be performed with the patient deeply anesthetized. Should acute ventricular dysrhythmias occur, they can be treated with a β antagonist. Procainamide and quinidine are both known to prolong the QT interval in normal patients and should probably be avoided. Lidocaine, which also prolongs the QT interval in normal patients, has been used to successfully treat ventricular dysrhythmias in these patients. Electrical cardioversion may be necessary in the event of dysrhythmias that become life threatening. (399)
Artificial cardiac pacemakers
92. The preoperative evaluation of a patient with an artificial cardiac pacemaker should include an understanding of the underlying cardiac condition that required placement of the pacemaker and an assessment of the current function of the pacemaker, brand, model, make, and magnet mode. (399)
93. A pacemaker should be evaluated by the anesthesiologist preoperatively so that the anesthesiologist has a good understanding of the pacemaker and its programming. For instance, the anesthesiologist should be aware of what the default rhythm is (should the pacemaker not capture), the type of pacemaker, the chamber paced, the chamber sensed, how to detect deterioration in battery function, who can reprogram the pacemaker, and the current rate and sensitivity settings of the pacemaker and magnet mode. A discussion with the electrophysiology service or the pacemaker company representative can quickly resolve any issues. (399)
94. Intraoperative monitoring that is important in a patient with an artificial cardiac pacemaker includes the electrocardiogram, pulse oximeter, and possibly an intraarterial catheter. Intraarterial catheters or a pulse oximeter that are not affected by electrocautery may allow for diagnosis of interference of the pacemaker by electrocautery. In a patient with third-degree heart block and no escape rhythm, intraarterial catheters can be quite helpful. Inhibition of the pacemaker by electrocautery may lead to pacemaker inhibition and asystole in patients with third-degree heart block. The intraarterial catheter or pulse oximeter provides a measure of blood flow and cardiac output during that period allowing rapid diagnosis of the interference between the electrocautery and the pacemaker. (399)
95. If the ground plate for the electrocautery is placed too near the pulse generator of the artificial cardiac pacemaker, there could be electromagnetic interference that is interpreted as spontaneous cardiac activity by the pacemaker. This interference may result in asystole due to an inhibition of pulse generator activity by the pacemaker. The ground plate should be placed as far away as possible from the pulse generator but at least 15 cm away. Other potential sources of mechanical interference include electroconvulsive shock therapy, succinylcholine-induced fasciculations, and myoclonic movements. (399)
96. The selection of drugs or anesthetic techniques for a patient should not be altered by the presence of an artificial cardiac pacemaker. However, patients with pacemakers or implantable cardiac defibrillators have an increased risk of coronary artery disease and ventricular dysfunction and should be monitored and anesthetized with added caution. (399)
97. A “pacemaker” magnet should be kept in the operating room intraoperatively for patients with artificial cardiac pacemakers to convert the pacemaker modes to an asynchronous mode, or fixed rate, should it become necessary. For instance, if the patient’s pacemaker stops functioning intraoperatively, placement of an external converter magnet over the pulse generator may convert the pacemaker to an asynchronous mode. The function of the magnet should be reviewed by the anesthesiologist before surgery. (399)
98. The most common cause of temporary pacemaker malfunction is the disruption of contact between the pacemaker electrode wires and the endocardium. Some causes of this disruption include muscular exertion, blunt trauma, cardioversion, and positive-pressure ventilation. When this occurs, pacemaker spikes will continue to be seen on the electrocardiogram, although there is no myocardial activity or pulse. Placement of a pulmonary artery catheter in a patient with an artificial cardiac pacemaker may disrupt the placement of transvenous endocardial electrodes if they have been placed in the 2 weeks preceding the procedure. (400)
Essential hypertension
99. Essential hypertension has been defined as a sustained elevated blood pressure on more than one reading without any known cause. Systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 90 mm Hg have been arbitrarily defined as the limits at which hypertension begins. The benefits of the long-term treatment of patients with essential hypertension include decreases in the incidence of cerebrovascular accidents, congestive heart failure, and renal disease. (400)
100. The preoperative evaluation of a patient with essential hypertension should include a determination of the adequacy of blood pressure control, a review of the pharmacology of the antihypertensive drugs, and an evaluation of effects of the hypertension on other organs. (400)
101. Antihypertensives include angiotensin-converting enzyme inhibitors, calcium channel blockers, β-adrenergic antagonists, diuretics, and vasodilators. It is generally recommended that blood pressure medications be administered on their routine schedule in the perioperative period in the patient with essential hypertension. This includes medicines on the morning of the surgical procedure. Withdrawal of medications in the perioperative period can lead to an increase in complications and should be avoided. (400)
102. Medical problems that are frequently seen in patients with essential hypertension include congestive heart failure, coronary artery disease, cerebral ischemia, renal dysfunction, and peripheral vascular disease. Approximately 50% of patients with peripheral vascular disease can be assumed to have 50% or greater stenosis of one or more coronary arteries, even in the absence of symptoms. (400)
103. The curve for the autoregulation of cerebral blood flow in patients with essential hypertension is shifted to the right, such that autoregulation occurs at a higher pressure than it would for a normotensive patient. This implies that the same degree of absolute hypotension in patients with a history of hypertension may be more harmful than the same blood pressure would be for a normotensive patient. Thus maintenance of blood pressure in the perioperative period should be relative to what the preoperative resting blood pressure is specific to that patient. (400)
104. Treating essential hypertension in patients before an elective procedure has been shown to be beneficial in decreasing the risk of intraoperative hypotension and myocardial ischemia. There have been multiple studies conducted regarding this topic. Studies have also shown that there is not an increased incidence of cardiac complications in hypertensive patients in the perioperative period as long as the diastolic blood pressure was not higher than 110 mm Hg preoperatively. (400)
105. Patients with essential hypertension frequently respond to the induction of anesthesia with an exaggerated decrease in blood pressure. This hypotension is thought to occur as a result of an unmasking of a decreased intravascular fluid volume status. (400-401)
106. Patients with essential hypertension are especially likely to respond to direct laryngoscopy with exaggerated increases in blood pressure, placing them at risk of myocardial ischemia. This response can be attenuated with adequate levels of anesthesia. It must be done with caution in hypertensive patients, because an excessive depth of anesthesia may produce hypotension in these patients as well. Other methods may be used to attenuate the sympathetic nervous system response to direct laryngoscopy and the associated exaggerated hypertension. For instance, esmolol or lidocaine may be administered just before direct laryngoscopy. In addition, the duration of direct laryngoscopy should be minimized. (401)
107. The goal for the anesthetic management of patients with essential hypertension is to minimize the fluctuations in blood pressure characteristic of these patients with anesthetics and antihypertensive medications as appropriate. The patient should also be continually monitored for evidence of myocardial ischemia via a continuous electrocardiogram. (401, Table 25-11)
108. The maintenance of anesthesia in patients with essential hypertension can be achieved through the administration of a volatile anesthetic in conjunction with an opioid, intravenous agents, or nitrous oxide. An anesthetic dose sufficient to attenuate hypertensive responses to surgical stimulation should be administered. The maintenance of normotension intraoperatively may also require the administration of other medicines, such as β antagonists, vasoconstrictors, or nitrates. (401)
109. Intraoperative hypotension in patients with essential hypertension can be managed by the administration of intravenous fluids, by decreasing the concentration of volatile anesthetics, and by the administration of vasopressors as necessary. (401-402)
110. Regional anesthesia is frequently an excellent choice in appropriate surgical cases for patients with cardiovascular disease. The administration of regional anesthesia in patients with essential hypertension has the theoretical problem of causing excessive decreases in systemic blood pressure. Hypotension can occur secondary to vasodilation associated with the sympathetic nervous system blockade in combination with the decreased intravascular fluid volume status often seen in patients with chronic hypertension. The anesthesiologist must be prepared to support the blood pressure as necessary when a regional anesthetic is administered to these patients. (401)
111. Hypertension occurs frequently in the early postoperative period in patients with a diagnosis of essential hypertension. Hypertension secondary to inadequate pain control should be considered. If hypertension persists despite adequate analgesia, the administration of additional doses of antihypertensives is likely necessary. (402)
Congestive heart failure
112. Congestive heart failure is highly correlated with postoperative morbidity. In fact, preoperative congestive heart failure is the single greatest preoperative risk factor for predicting postoperative morbidity. This suggests that the preoperative patient with congestive heart failure scheduled for elective surgery should not have his or her surgery until treatment of the congestive heart failure can be instituted and the patient’s medical status optimized. (402)
113. The goal of the anesthetic management of patients with congestive heart failure who are undergoing urgent or emergent surgery is the optimization of cardiac output. Optimal cardiac output in patients with congestive heart failure undergoing anesthesia may be best achieved with careful hemodynamic management. The patient’s chronic medications should be given preoperatively if there are no specific contraindications. Volatile anesthetics may produce a dose-dependent depression of cardiac muscle function that is greater in patients with congestive heart failure than in patients without congestive heart failure. In addition, the maintenance of myocardial contractility may necessitate the continuous administration of β-adrenergic agonists and vasoactive agents in the perioperative period. Avoidance of the administration of β-adrenergic agonists, if possible, may reduce overall risk. (403)
114. Positive-pressure ventilation of the lungs of patients in congestive heart failure may be beneficial because of its effect of decreasing pulmonary vascular congestion and improvement in arterial oxygenation. (403)
115. Monitors in the patient with congestive heart failure undergoing major surgery include an intraarterial catheter. Although there is no evidence to support the use of pulmonary artery catheters to monitor central filling pressures and cardiac output, they can be used to monitor the effects of inotropic, vasoactive agents, and volume status. Transesophageal echocardiography may be more useful than pulmonary artery catheterization but is not required. Monitors chosen should be influenced by the patient’s medical status, risk-benefit ratios, and the surgical procedure. (403)
116. Regional anesthesia for peripheral surgery in patients with congestive heart failure can be administered safely but is not proven to have reliably better outcomes than general anesthesia for these patients. Mild decreases in the systemic vascular resistance produced by an epidural or spinal anesthetic may provide for improvement in cardiac output in the patient with congestive heart failure. Greater decreases in the systemic vascular resistance should be avoided if possible. (403)
Hypertrophic cardiomyopathy
117. Hypertrophic cardiomyopathy is a genetically transmitted disease also known as idiopathic hypertrophic subaortic stenosis. The pathology that defines hypertrophic cardiomyopathy is the obstruction to left ventricular outflow produced by asymmetric hypertrophy of the intraventricular septal muscle. As a result of the obstruction to ventricular outflow, left ventricular hypertrophy develops to the degree that the volume of the left chamber is decreased. As the disease advances, the increased muscle mass in the subaortic region can lead to complete obstruction of left ventricular outflow. The stroke volume in patients with hypertrophic cardiomyopathy remains normal despite the physiologic changes. The normal stroke volume is reflective of the hypercontractile state of the myocardium. (403)
118. The goal of the anesthetic management of patients with hypertrophic cardiomyopathy is geared toward decreasing the pressure gradient across the obstructed left ventricular outflow tract. There are several methods of decreasing the left ventricular outflow obstruction in patients with hypertrophic cardiomyopathy. These include decreasing myocardial contractility, as with the administration of β antagonists; increasing the dose of volatile agents; increasing preload with increased intravascular fluid volume; and increasing afterload with α-adrenergic stimulation as is produced by phenylephrine. (403)
119. Intraoperative hypotension in patients with hypertrophic cardiomyopathy can be treated with the administration of intravascular fluids as well as phenylephrine. (404)
120. Intraoperative hypertension in patients with hypertrophic cardiomyopathy can be treated with the administration of increased concentrations of volatile anesthetics. (404)
121. The administration of β agonists for the treatment of hypotension in patients with hypertrophic cardiomyopathy can result in an increase in myocardial contractility and a corresponding increase in left ventricular outflow obstruction. Likewise, the administration of nitrates such as nitroprusside or nitroglycerin to these patients can increase left ventricular outflow obstruction by decreasing systemic vascular resistance, making them an unlikely choice for the treatment of hypertension. (404)
Cor pulmonale
122. Cor pulmonale is right ventricular hypertrophy and cardiac dysfunction that occurs as a result of pulmonary hypertension. The most likely cause of cor pulmonale is chronic obstructive pulmonary disease with associated chronic arterial hypoxemia leading to chronic pulmonary vascular vasoconstriction. Vascular smooth muscle hypertrophy and permanently increased pulmonary vascular resistance results from sustained pulmonary vascular vasoconstriction. When systemic acidosis is also present, there is a synergistic effect between arterial hypoxemia and acidosis on the pulmonary vasculature. In general, when the cause of the increased pulmonary vasculature resistance is due to arterial hypoxemia from chronic obstructive pulmonary disease, the prognosis is somewhat favorable if the arterial hypoxemia can be reversed with the administration of oxygen. Other causes of increased pulmonary vascular resistance leading to cor pulmonale, such as primary pulmonary hypertension or pulmonary fibrosis, have less favorable outcomes. (404)
123. Symptoms of cor pulmonale are often masked by the symptoms associated with the existence of coexisting chronic obstructive pulmonary disease. As the right ventricle becomes increasingly impaired, patients may experience syncope with exertion. Patients may also have chronic dependent edema, an enlarged liver, ascites, and dilated neck veins. On the lateral chest radiograph, right ventricular hypertrophy may present as a decrease in the retrosternal space. There may also be a decrease in pulmonary vascular markings. Right ventricular hypertrophy on the electrocardiogram would show peaked P waves in leads II, III, and aVF. Often there will also be right-axis deviation. On right-sided heart catheterization, the mean pulmonary artery pressure will be elevated, whereas the pulmonary artery occlusion pressure is normal. Pulmonary hypertension is considered mild with mean pulmonary artery pressure between 20 and 35 mm Hg and moderate when the pressure is greater than 35 mm Hg. (404)
124. The treatment of cor pulmonale is directed toward decreasing right ventricular work by decreasing the pulmonary vascular resistance. This reduction in pulmonary vascular resistance may be achieved through the correction of the patient’s pH and through the administration of oxygen to reverse arterial hypoxemia if possible. Diuretics may also be administered for patients with congestive heart failure. Nitroglycerin administration may result in lowering of the pulmonary artery pressure and a decrease in pulmonary vascular resistance. Pulmonary vasodilation with prostaglandins, endothelial receptor antagonists, inhaled nitric oxide, type 5 phosphodiesterase inhibitors, or soluble guanylate cyclase inhibitors has been tried with variable success. (404)
125. Just as in any other patient, the patient with cor pulmonale who is scheduled for an elective surgical procedure should be medically optimized before the procedure. Any pulmonary infections should be treated. Patients should have any bronchospasm reversed, be well hydrated, and have their electrolytes evaluated and corrected if necessary. (404)
126. The goal of the anesthetic management of patients with cor pulmonale is the avoidance of events or drugs that could result in an increase in pulmonary vascular resistance, thereby worsening right ventricular failure. Events that may result in an increase in pulmonary vascular resistance include arterial hypoxemia, hypercapnia, acidosis, and decreases in body temperature. An abrupt or significant decrease in the systemic vascular resistance should be avoided. Nitrous oxide should be avoided because of its potential for increasing pulmonary vascular resistance. Although positive-pressure ventilation may increase pulmonary vascular resistance, its potential benefit for improving arterial oxygenation likely outweighs its risk. An intraarterial catheter allows for arterial blood gas analysis to assess the effects of any interventions on the patient’s arterial oxygenation and pH and should be considered essential. (404)
127. The advantage of monitoring pulmonary artery and central venous pressure during surgery in patients with cor pulmonale is the ability of the anesthesiologist to assess the hemodynamic effects of the surgical procedure and optimize pharmacologic and hemodynamic management. (404)
Cardiac tamponade
128. Cardiac tamponade occurs as a result of increased intrapericardial pressure from the accumulation of fluid in the pericardial space. The increase in pericardial pressures causes a decrease in compliance of the right ventricle, reducing right ventricular filling, stroke volume, and cardiac output, thus causing hypotension. The decrease in stroke volume results in sympathetic nervous system activation in an attempt to maintain cardiac output. Cardiac output and systemic blood pressure in these patients become dependent on heart rate and on a central venous pressure that exceeds the right ventricular end-diastolic pressure. (404)
129. Manifestations of cardiac tamponade include hypotension, tachycardia, vasoconstriction, equalization of diastolic filling pressures, increased central venous pressure, and a fixed stroke volume. These patients also have pulsus paradoxus. Pulsus paradoxus is a decrease in the arterial blood pressure by greater than 10 mm Hg during inspiration. This change in pressure is the opposite of what would be expected in normal patients and reflects the decrease in ventricular stroke volume that occurs with inspiration. On the chest radiograph, there may be a change in the cardiac silhouette when 250 mL or greater of fluid has accumulated in the pericardial space. Decreased voltages through all leads may be seen in patients with cardiac tamponade. Cardiac tamponade is best diagnosed through echocardiography. (404)
130. The definitive treatment of cardiac tamponade is the drainage of the pericardial fluid. Drainage can be achieved either percutaneously or by a pericardiotomy in the operating room under general or local anesthesia. Temporizing measures until definitive treatment include the expansion of the intravascular fluid volume; the administration of agents that will increase myocardial contractility, such as epinephrine, norepinephrine, or dopamine; and the correction of metabolic acidosis, which may depress myocardial contractility. Definitive care requires drainage of the pericardial fluid and may be life saving. (404)
131. Prior to the induction of general anesthesia, the patient should be prepped and draped and the surgeons scrubbed and ready to make an incision. Immediate hemodynamic collapse may occur with induction of general anesthesia that can only be resolved with surgical relief of the pericardial tamponade. The goal of the anesthetic management of patients with cardiac tamponade is the avoidance of events or drugs that could result in a decrease in cardiac output. Myocardial contractility, arterial blood pressure, increased heart rate, and venous return must all be maintained. Rapid surgical drainage of the tamponade is critical to avoid hemodynamic collapse and death. (405)
132. The induction of anesthesia and positive-pressure ventilation of the lungs of patients with cardiac tamponade can result in profound, irreversible hypotension. Hypotension that occurs in response to positive-pressure ventilation of the lungs of these patients results from anesthetic-induced peripheral vasodilation, direct myocardial depression, and decreases in venous return from positive intrathoracic pressure. The recommendation for patients with cardiac tamponade is that, if at all possible, percutaneous pericardiocentesis be performed under local anesthesia to relieve some of the tamponade before the induction of anesthesia. This drainage should be done in the operating room with the patient spontaneously breathing and continually monitored. If time permits, monitors may include an intraarterial catheter for continuous arterial blood pressure monitoring and a central venous catheter to monitor central venous pressures. The ability to immediately surgically drain the pericardium should be established prior to the induction of general anesthesia. (404-405)
133. The recommendation for anesthesia in patients with cardiac tamponade is that percutaneous pericardiocentesis be performed under local anesthesia before the induction of anesthesia. If that is not possible, an awake orotracheal intubation should be considered. The patient should have the urgency of the need to perform the procedure explained, and the airway should be anesthetized topically. After confirmed orotracheal intubation, the patient can then be gently sedated while still spontaneously ventilating the lungs. Small doses of ketamine may be administered to the patient to provide analgesia and sedation during pericardiocentesis. The induction of anesthesia and positive-pressure ventilation if required for further surgical exploration should not be instituted until immediate drainage of the pericardial space can be achieved. (404-405)
134. Sympathetic nervous system stimulants, such as epinephrine, norepinephrine, dopamine, dobutamine, or isoproterenol, administered as continuous infusions may be useful in patients with cardiac tamponade although they will not preclude or prevent hemodynamic collapse and cardiac arrest with loss of ventricular filling. The pericardium must be drained to allow venous filling and cardiac output. (405)
Aneurysms of the aorta
135. Most aortic aneurysms involve the abdominal aorta. About 95% of abdominal aortic aneurysms are due to atherosclerosis, in contrast to about 50% of thoracic aortic aneurysms. Other causes of aortic aneurysms include trauma, mycotic infection, connective tissue disorders such as Marfan syndrome, and syphilis. Only about 0.5% of abdominal aortic aneurysms extend into the renal arteries. (405)
136. A dissecting aneurysm occurs when a tear in the intima of the aorta separates the layers of the wall of the aorta. Blood is then allowed to enter and penetrate between the intima on one side and the media and adventitia layers on the other, creating a false lumen. The dissection can then reenter the true lumen through another tear in the intima, or it may rupture through the adventitia. Acute dissection may present as excruciating chest pain and patients may appear to be in shock. Peripheral pulses may be difficult to palpate. The treatment for aortic dissection is either surgical excision, usually followed by placement of a graft, or endovascular graft placement. Short-term management until a definitive treatment can take place may include decreasing blood pressure to the lowest acceptable level and the relief of pain. (405)
137. The risk of rupture of an abdominal aortic aneurysm is best predicted by the diameter of the aneurysm, as well as its rate of expansion. Elective resection of an abdominal aortic aneurysm is recommended when the diameter of the aneurysm is estimated to be 5 cm or greater. This recommendation is made based on the dramatic increase in the likelihood of spontaneous rupture of the aneurysm when the size of the aneurysm exceeds 5 cm. Abdominal aortic aneurysms with a diameter less than 5 cm are typically followed with serial measurements to evaluate their rate of expansion. If the aneurysm expands by more than 0.5 cm in a 6-month period, or if the patient becomes symptomatic, surgical repair is recommended. (405)
138. Medical problems frequently associated with aortic aneurysms include hypertension, diabetes mellitus, ischemic heart disease, and atherosclerosis. (405)
139. The goal of the anesthetic management of patients undergoing resection of an abdominal aortic aneurysm is aimed toward the maintenance of cardiovascular and hemodynamic parameters at or near normal. Aggressive intraoperative monitoring is necessary to achieve this goal. Patients should have their intraarterial blood pressure closely monitored throughout the case. Perioperative administration of β-adrenergic antagonists, statins, and aspirin may reduce cardiac risk. (405)
140. Patients with coronary artery disease are especially at risk of myocardial ischemia during surgery for resection of an aortic aneurysm during cross-clamping of the aorta. Cross-clamping of the aorta at the suprarenal or supraceliac level creates the greatest increase in systemic vascular resistance and central venous pressure, and a decrease in cardiac output. On the pulmonary artery catheter, cross-clamping of the aorta would result in an increase in the pulmonary artery occlusion pressure. In contrast, cross-clamping the aorta below the level of the renal arteries creates minimal hemodynamic changes. The hemodynamic response to aortic cross-clamping is influenced by the patient’s cardiac status, intravascular fluid volume, and anesthetic drugs and technique. Management of the patient during aortic cross-clamping should be aimed toward decreasing the systolic blood pressure and cardiac filling pressures. Pharmacologic agents that could be administered might include inhaled anesthetic agents, nitroprusside, or nitroglycerin. (405)
141. Intraoperative fluid management during surgery for resection of an aortic aneurysm is best guided by the data obtained from hemodynamic monitors, either from arterial pulse pressure variation, stroke volume variation, central venous and pulmonary arterial pressures, or transesophageal echocardiography. Optimal fluid management can be administered with appropriate hemodynamic monitoring. (405)
142. Hypotension frequently accompanies unclamping of the abdominal aorta during the resection of an aortic aneurysm. The hypotension is believed to occur as a result of the sudden increase in venous capacitance that accompanies unclamping. Even when the aortic cross-clamp is infrarenal, unclamping can result in a decrease in the systolic blood pressure by about 40 mm Hg. Methods for minimizing the hypotension include adequate volume replacement before unclamping and the gradual removal of the aortic cross-clamp to allow time for the pooled venous blood to circulate. It is prudent to use only short-acting vasodilators, such as inhaled agents, nitroprusside, or nitroglycerin, to treat increases in the systemic vascular resistance during aortic cross-clamping so that their effects can be reversed with titration or by discontinuation before unclamping of the aorta. The systemic vascular resistance may be increased after unclamping with the administration of phenylephrine if necessary. (405)
143. Renal function may become impaired postoperatively to the extent that hemodialysis is required after aortic aneurysm repair, particularly if the aortic cross-clamp was suprarenal. Coexisting renal disease appears to place the patient at the greatest risk of postoperative renal dysfunction, but other risk factors include the duration of aortic cross-clamp time, thrombotic or embolic interruption of renal blood flow, hypovolemia, and hypotension. Hypothermia may protect the kidneys during periods of ischemia. In an effort to decrease the risk of postoperative renal dysfunction, the intravascular fluid volume should be maintained and the urine output should be closely monitored during aortic aneurysm repair. Mannitol is frequently given just before aortic cross-clamping to facilitate diuresis and maintain glomerular function. A loop diuretic may be also be administered in selected cases if the urine output is unsatisfactory. Alternatively, a continuous dopamine infusion at low doses may be started to dilate renal blood vessels to maintain renal blood flow and urine output. Unfortunately, none of these therapies have been definitively demonstrated to have efficacy for the prevention of renal dysfunction. (405)
144. Spinal cord ischemia and paraplegia can occur after supraceliac aortic cross-clamping for thoracic aortic aneurysm repair. The mechanism for the ischemia is most likely due to an interruption of a portion of the blood supply to the spinal cord. The spinal cord blood supply is from two posterior arteries and one anterior spinal artery. The greatest contributor to the blood supply of the anterior spinal artery is the artery of Adamkiewicz, whose origin is between T9 and T12 in 75% of patients. The anterior spinal artery supplies the motor tracts in the spinal cord. Mechanisms employed to reduce the risk of spinal cord ischemia include cerebrospinal fluid drainage, intrathecal papaverine injection, naloxone administration, barbiturate administration, hypothermia, and partial bypass. Hypothermia is believed to be the most effective method of neuroprotection through its effects of decreasing oxygen requirements. Cerebrospinal fluid drainage is thought to improve spinal cord perfusion because the spinal cord perfusion pressure is the distal mean aortic pressure minus the cerebrospinal fluid pressure. Cerebrospinal fluid drainage to improve spinal cord perfusion during aortic aneurysm repair remains controversial. (405)
Cardiopulmonary bypass
145. Blood is drained from the venae cavae during cardiopulmonary bypass by siphon action caused by gravity. The blood then passes from the cardiotomy reservoir, through a pump, a heat exchanger, an oxygenator, and a filter before it is returned to the arterial system. (406, Figure 25-3)
146. The two different types of pumps that are used to return blood to the arterial system during cardiopulmonary bypass are the roller pump and the centrifugal pump. The roller pump works by compressing the tubing that contains the fluid between a roller and curved metal back plate, producing flow. In contrast, the centrifugal pump produces flow and less trauma to blood. The flow rate generated by the centrifugal pump is affected by the resistance of the tubing and the patient’s systemic vascular resistance. (406-407)
147. Blood is kept from entering the heart from the superior and inferior venae cavae during cardiopulmonary bypass for open heart or intracardiac surgery by placing both a superior and inferior vena caval cannulae, as well as ligatures placed around the superior and inferior vena cava proximal to the cannulae, thereby occluding the cava and preventing flow into the heart. All returning blood from the patient’s venous system enters the cardiopulmonary bypass machine via the two large cannulae, which are placed in the superior and inferior venae cavae. (406-407)
148. The aorta is cross-clamped distal to the aortic valve and proximal to the inflow cannula during cardiopulmonary bypass to allow cardioplegic arrest. An incompetent aortic valve, in an arrested heart, without this cross-clamp, would allow blood to flow retrograde from the aorta into the heart, stretching the myocardium, and causing permanent muscle injury and ventricular dysfunction. (407)
149. Venous drainage from the inferior and superior venae cavae during cardiopulmonary bypass, which is achieved by gravity, can be facilitated by raising the operating table to a higher level, creating a larger vertical distance between the operating room table and the cardiopulmonary bypass machine or by adding negative pressure or suction to the cardiotomy reservoir. (407)
150. The required cardiac index that is delivered to the patient by the roller pump on the cardiopulmonary bypass machine depends on the patient’s body temperature and oxygen consumption. A cardiac index of 2 to 2.4 L/min per meter squared is usually sufficient in the normothermic or slightly hypothermic patient. Flows of about half these levels have also been used without adverse effects. (407)
151. The advantage of low flows during cardiopulmonary bypass is that less trauma is sustained to the blood. Less noncoronary collateral blood flow returns to the heart as well, which may lead to better myocardial protection because less warm blood is entering the heart and counteracting the cold myocardial preservation solutions. (407)
152. Two different types of oxygenators that are used to oxygenate blood that is returning to the arterial system during cardiopulmonary bypass are a bubble oxygenator and a membrane oxygenator, although bubble oxygenators are rarely used at the present time. (407)
153. Advantages of a bubble oxygenator over a membrane oxygenator include its relative simplicity and lower cost. Disadvantages of a bubble oxygenator include an increase in the amount of turbulence and foaming it produces, and denaturing of blood proteins by direct contact with gas, resulting in trauma to blood that increases with the duration of the bypass time. Bubble oxygenators are rarely used in the current era. (407)
154. An advantage of a membrane oxygenator includes the relatively less trauma produced to the blood than that produced by a bubble oxygenator by avoiding direct exposure of blood to gas. Disadvantages of a membrane oxygenator include its increased complexity and cost. (408)
155. In addition to the usual methods of heating a patient’s body intraoperatively, the body can also be heated or cooled during cardiopulmonary bypass through the use of heat exchangers that are incorporated into the extracorporeal circuit. These heat exchangers are able to heat or cool blood as it circulates through the extracorporeal circuit via a countercurrent flow system. (408)
156. Blood loss from the field can be recirculated to the patient during cardiopulmonary bypass by having the blood that is suctioned return to a cardiotomy reservoir. In the cardiotomy reservoir the blood is filtered, defoamed, and returned to the oxygenator. The blood is then recirculated to the patient after oxygenation. (408)
157. A problem with the cardiotomy suction used during cardiopulmonary bypass is that it is a significant contributor to the hemolysis and particulate emboli that occurs during cardiopulmonary bypass. (408)
158. Venting of the left ventricle may be necessary to prevent harmful left ventricular distention during cardiac surgery in which the heart is not opened. Persistent left ventricular distention may lead to permanent damage to the myocardial contractile elements. There are at least three reasons why the left ventricle might need a vent during cardiopulmonary bypass. First, incompetence of the aortic valve can lead to the retrograde flow of blood from the aorta to the heart. Second, there may be a large degree of blood flow from the coronary sinus and bronchial circulation back to the heart. Third, surgical positioning may result in backward flow from the aorta into the heart, or from the heart into the pulmonary veins. Finally, venting of the ventricle or pulmonary artery can help reduce risks of elevated pulmonary artery pressures during cardiopulmonary bypass. Venting of the left ventricle is achieved through the placement of a catheter into the left ventricle, usually through a pulmonary vein or via the left atrium. (407)
159. Systemic emboli from cellular debris are prevented from occurring during cardiopulmonary bypass through the use of filters that are incorporated into the extracorporeal circuit in the cardiotomy reservoir and downstream after the oxygenator. (408)
160. Priming of the cardiopulmonary bypass system refers to the filling of the tubing of the cardiopulmonary bypass system with fluid and sometimes blood. The fluid consists of an osmotically active substance, an osmotic diuretic, an antibiotic, and electrolyte supplements. Blood is added if the patient’s hematocrit necessitates it. (408)
161. Hemodilution of the patient’s blood to a hematocrit of 20 to 25 during cardiopulmonary bypass lessens the viscosity of the blood. This decrease is important to facilitate circulation through the small vessels during hypothermia. (408)
162. It is essential to remove all air from the cardiopulmonary bypass system during cardiopulmonary bypass to prevent the pumping of air into the arterial system of the patient. (408)
163. Heparin-induced anticoagulation of the patient is mandatory before the institution of cardiopulmonary bypass to prevent patient death through clotting of the blood both in the patient and in the cardiopulmonary bypass machine. The dose of heparin that is usually administered is 300 to 400 units/kg. The adequacy of anticoagulation must be confirmed before the placement of the venous and aortic cannulae used for cardiopulmonary bypass. The adequacy of anticoagulation is usually confirmed by evaluating the activated clotting time, which should remain longer than 450 seconds throughout the course of cardiopulmonary bypass. The activated clotting time should be evaluated periodically during the course of cardiopulmonary bypass and additional heparin administered as necessary. After the cannulae are removed from the patient and cardiopulmonary bypass terminated, the effects of heparin may be reversed with the administration of protamine. The activated clotting time should return to baseline, typically between 90 to 120 seconds, after the administration of protamine. (408)
164. The low mean arterial pressure often seen after the institution of cardiopulmonary bypass is believed to be due to the peripheral vasodilation caused by the decreased viscosity, decreased temperature, and low oxygen content of infused priming solution. What pressures during cardiopulmonary bypass are sufficient to allow for coronary and cerebral perfusion is a subject of great debate. Most institutions prefer the mean arterial pressures to be about 50 mm Hg and to administer phenylephrine if blood pressure support is needed. Other institutions allow the mean arterial blood pressure to decrease to 40 mm Hg without any adverse effect. The perfusion pressures for extracorporeal circulatory support are even lower in infants and children. (408)
165. Blood pressure slowly rises spontaneously after some time on cardiopulmonary bypass as a result of vasoconstriction. The vasoconstriction may be in response to stimulation of the sympathetic nervous system or the renin-angiotensin system. It may also be an indication of inadequate perfusion to some tissues. (408)
166. The potential dangers of hypertension while on cardiopulmonary bypass include aortic dissection, intracerebral hemorrhage, and an increase in coronary and bronchial artery circulation, leading to increased return of warm blood to the heart during a time of cold cardioplegia. Hypertension under these circumstances can be treated by decreasing the systemic vascular resistance with a nitrate or a volatile anesthetic. Vaporizers have been incorporated into the cardiopulmonary bypass circuit for the administration of a volatile anesthetic to patients while on cardiopulmonary bypass. (409)
167. The patient can be monitored for adequate tissue perfusion duringa guide to renal perfusion. A renal output of 1 mL/kg each hour is considered sufficient. Second, the patient’s acid-base status can be monitored for any evidence of a progressive metabolic acidosis. Third, the patient’s mixed venous oxygen tension can be monitored for evidence of excessive oxygen extraction. A mixed venous PO2 lower than 40 mm Hg is generally regarded as evidence of inadequate tissue perfusion. Finally, the nasopharyngeal temperature can be compared with a core temperature such as the bladder, rectum, skeletal muscle, or skin temperature. Whereas bladder temperature represents core temperature, the nasopharyngeal temperature is an indicator of perfusion to the brain. The greater the discrepancy between these two, the greater the indication of poor cerebral perfusion. Cerebral oximetry using infrared light can monitor adequacy of cerebral perfusion and may reduce risk of central nervous system injury. (409)
168. Diuresis is induced during cardiopulmonary bypass due to the inclusion of mannitol in the priming solution, hypothermia which interferes with renal tubular absorption, and well-perfused renal glomeruli with blood low in oncotic pressure due to hemodilution. For this reason during cardiopulmonary bypass the minimally acceptable urine output is 1 mL/kg/hr. Adequate urine output is important for the excretion of potassium administered in the cardioplegia solution. When the urine output is less than desired, a mechanical obstruction to urine flow in the catheter should be considered before instituting methods to increase the urine output. (409)
169. Causes of an increasing central venous pressure with or without facial edema while on cardiopulmonary bypass include aortic cannula flow into the carotid artery, obstruction of the superior vena cava cannula, and obstruction of jugular venous drainage by either a cannula, head position, or neck compression. Inadequate venous return from the patient to the cardiopulmonary bypass machine is an indication that one of these may have occurred. (409)
170. Causes of increasing abdominal distention while on cardiopulmonary bypass include obstruction of the inferior vena cava cannula, intraabdominal hemorrhage or ascites, or gastrointestinal distention by gas or fluid. (409)
171. The most serious complications of cardiopulmonary bypass include aortic dissection, carotid artery dissection, air in the aortic inflow tubing, and clotting of the bypass circuit. (409)
172. Ventilation of the lungs of a patient is not necessary during cardiopulmonary bypass. The lungs can be ventilated with oxygen during partial cardiopulmonary bypass, or when there is partial pulmonary blood flow. Evidence of pulmonary blood flow is seen as pulsatile pulmonary artery flow on the pulmonary artery catheter tracing. (409)
173. The goal of myocardial preservation during cardiopulmonary bypass is the minimization of the effects of ischemia on the heart. Myocardial protection is achieved in a variety of ways, all of which are aimed toward reducing the myocardial oxygen requirement of the heart during that period. Myocardial cooling can be achieved by hypothermic cardiopulmonary bypass, by the direct placement of ice on the epicardium, through pericardial irrigation with iced fluid, and by the intracoronary infusion of a cold cardioplegia solution. Myocardial arrest is achieved by the infusion of cardioplegia solution containing potassium both through a cannula at the aortic root and by retrograde flow through the coronary sinuses. The potassium causes cessation of electrical and mechanical cardiac activity by blocking the initial phase of myocardial depolarization. The prevention of myocardial rewarming during cardiopulmonary bypass can be achieved by placing a vent in the left ventricle or by placing a cross-clamp in the aorta distal to the aortic valve. (409)
174. The oxygen consumption of a normally contracting heart at 30° C is 8 to 10 mL/100 g of heart muscle in a minute. The oxygen consumption of a fibrillating heart at 22° C is 2 mL/100 g per minute, and the oxygen consumption of an electromechanically quiet heart at 22° C is approximately 0.3 mL/100 g per minute. (409)
175. The effectiveness of cold cardioplegia of the heart can be measured by placing a temperature probe in the left ventricle and directly measuring the temperature of the heart. The absence of any electrical activity on the heart is also a good indication that the heart muscle is effectively quiescent. (409)
176. Two potential negative effects of the intramyocardial hyperkalemia of the cold cardioplegia solutions after cardiopulmonary bypass are decreased myocardial contractility and an increased incidence of atrioventricular heart block while coming off cardiopulmonary bypass. These can both be treated with the administration of calcium and, if necessary, insulin with or without glucose. In addition, the atrioventricular block can be treated through the use of an artificial cardiac pacemaker. The pacemaker is usually only needed temporarily because the atrioventricular block typically only lasts for 1 to 2 hours after discontinuing cardiopulmonary bypass. (409)
177. Two potential sources for systemic hyperkalemia during cardiopulmonary bypass are the recirculation of cardioplegia solution that has drained into the blood and decreased renal function. Hyperkalemia that persists at the conclusion of cardiopulmonary bypass can be treated with the administration of insulin and glucose in addition to calcium, or by ultrafiltration on the extracorporeal bypass circuit, or by administration of diuretics such as furosemide. (410)
178. Although there is a decreased minimum alveolar concentration (MAC) under hypothermic conditions, the decrease in MAC may not be sufficient to offset the sudden dilution of anesthetics that occurs when the patient is placed on cardiopulmonary bypass. For this reason, supplemental intravenous anesthetics may be needed during cardiopulmonary bypass in some cases to ensure an adequate depth of anesthesia. (410)
179. There is a sudden dilution of the neuromuscular blocking drug level that occurs when the patient is placed on cardiopulmonary bypass. Supplemental neuromuscular blocking drug should be administered just prior to the initiation of extracorporeal circulatory support or cardiopulmonary bypass to ensure there is a neuromuscular blocking drug level sufficient to prevent patient movement during this important portion of the procedure. (410)
180. Although supplemental anesthesia is not routinely required during rewarming after the conclusion of cardiopulmonary bypass, it is important that the anesthesiologist be aware that the rewarming patient could be returning to consciousness in a paralyzed state. Low-dose inhaled agents reduce the risk of intraoperative awareness. There are a number of choices for post bypass anesthesia, including dexmedetomidine, propofol, opiates, benzodiazepines, volatile agents, or a combination of agents. Intravenous infusions allowing continued sedation into the intensive care unit are commonly chosen. (410)
181. Conditions in the patient that must be present for the discontinuation of cardiopulmonary bypass include hemodynamic stability; normothermia; the venting of all arterial air; an adequate cardiac rate, rhythm, and output; normal acid-base status and electrolyte levels; ventilation of the lungs; and an adequate intravascular status and hematocrit. (410, Table 25-15)
182. The aortic and vena cava cannulae are removed after an adequate blood pressure and cardiac output have been maintained by the heart for several minutes. For optimal safety, the ability to rapidly reestablish cardiopulmonary bypass should be maintained for some time after the discontinuation of cardiopulmonary bypass. (412)
183. Potential problems associated with persistent hypothermia after cardiopulmonary bypass include coagulopathy, hypertension, tachycardia and sympathetic nervous system stimulation, shivering, metabolic acidosis, and difficulty in defibrillating the heart and maintaining a normal cardiac rhythm. The effects of persistent hypothermia on the heart are particularly evident at temperatures less than 34° C. Rewarming a patient’s body can be achieved more rapidly after systemic vasodilation through the administration of a vasodilator, such as nitroprusside, or a volatile anesthetic. (410)
184. A special precaution that must be taken before discontinuing cardiopulmonary bypass in patients who have had the heart opened, as during valve replacement surgery, is the venting of all air from the heart. This can be accomplished by surgical massage of the left atrium and ventricle. In addition, rotating the table from side-to-side simultaneous with the maintenance of positive-pressure ventilation of the lungs and placement of the patient’s head at a lower level than the heart may assist in venting any air from the heart. Positive pressure of the lungs should be maintained during the removal of the left ventricle vent cannula. The avoidance of nitrous oxide administration after cardiopulmonary bypass might minimize the potential increase in the size of microemboli that may have occurred. The potential risk of air remaining in the heart at the conclusion of cardiopulmonary bypass is the embolization of air to the arterial circulation, especially the coronary and cerebral circulations. Air is most likely to embolize from the heart after cardiopulmonary bypass during manipulation of the heart and alterations in the anatomy, closure of the sternum, and movement of the patient. (410-412)
186. Posterior papillary muscle dysfunction after cardiopulmonary bypass can occur as a result of inadequate cooling of the posterior myocardium during cardiopulmonary bypass. The posterior myocardium is the portion of the heart muscle that is most vulnerable to inadvertent warming of the heart from the return of blood from the coronary and bronchial circulations, as well as any potential blood that flows retrograde via an incompetent aortic valve. Posterior papillary muscle function impairment would manifest as mitral regurgitation. In addition, prominent v waves would be evident on the pulmonary artery occlusion pressure tracing. (411)
187. In some situations the cardiac output of the patient on discontinuation of cardiopulmonary bypass is inadequate because of either poor myocardial function or refractory myocardial ischemia. Under these circumstances the intraaortic balloon pump is a mechanical addition to the pharmacologic support of cardiac output. An intraaortic balloon pump is a balloon that is 25 cm long and mounted on a long plastic catheter. The pump is advanced from the left femoral artery to the aorta. The pump inflates and deflates timed to the electrocardiogram. Inflation of the balloon, which occurs during diastole, increases the diastolic blood pressure and increases the coronary perfusion pressure gradient. Deflation of the balloon occurs just before systole. This allows for a reduction of afterload and the pressure against which the heart has to pump. Overall the intraaortic balloon pump increases coronary blood flow by increasing diastolic pressure while decreasing the work of the myocardium and thus the myocardial oxygen requirement. The efficacy of the balloon pump is altered by rapid heart rates, cardiac dysrhythmias, and aortic insufficiency. (411-412)
188. Protamine administration after termination of extracorporeal circulatory support, cardiopulmonary bypass, should take place prior the removal of the aortic cannulae to allow rapid return to extracorporeal circulatory support in the case of severe protamine reaction. If a protamine reaction occurs and return to extracorporeal circulatory support is required, additional heparin in doses of 300 to 400 units/kg may be required depending on the dose of protamine administered. Protamine is administered to reverse the anticoagulant effects produced by heparin. (412)
189. Side effects associated with the administration of protamine include hypotension due to vasodilation, myocardial depression, pulmonary hypertension, histamine release, and, rarely, anaphylactic or anaphylactoid reactions. Anaphylactic and anaphylactoid reactions can be associated with bronchospasm and pulmonary edema. These potential effects of protamine call for the careful, slow titration of protamine administration. (412)
190. Blood and fluid that remain in the cardiopulmonary bypass circuit after cardiopulmonary bypass are washed, collected, and placed in plastic bags by the perfusionist. The blood and fluid can then be administered to the patient. (412)
191. A gradient between central aortic and radial artery blood pressures can exist in the early period after cardiopulmonary bypass. Although the exact mechanism for this is not known, it is believed to be due to vasoconstriction in the extremity. The discrepancy can be determined by the surgical placement of a needle in the aorta and transduction of the pressure. Although the duration of this effect is typically only about 60 minutes, a femoral artery catheter can be placed for the transduction of arterial pressure if the discrepancy is large. (412)