Cardiovascular and Pulmonary Manifestations of Systemic Conditions
To maximize the effectiveness of their treatment prescriptions, physical therapists need to be able to predict the impact of systemic conditions on oxygen transport in a given patient.1 Complications due to systemic conditions are increasingly prevalent. This may reflect both the aging of the population and the improved survival rate and prognosis of patients with multisystem comorbidity. In addition, it is becoming more common for physical therapists to treat patients without referral, in which case no referring practitioner is alerting the physical therapist about the presence and significance of underlying systemic conditions. In their role as clinical exercise physiologists, physical therapists must be able to identify all factors that compromise or threaten oxygen transport so that treatment interventions can be prescribed effectively and with minimal risk. In addition, untoward exercise responses need to be explained; thus the physical therapist needs a comprehensive understanding of how morbidity affects oxygen transport in general. In some instances, untoward exercise responses may alert the physical therapist to some previously undetected problem warranting referral to the patient’s physician.
Diagnosis and Assessment
Oxygen transport can be affected by dysfunction of virtually any one of the major organ systems of the body (Box 6-1). The pulmonary and pleural complications of heart disease and the cardiac complications of pulmonary disease are usually predictable and therefore are most readily detected clinically. However, the cardiovascular and pulmonary complications of conditions affecting other organ systems can be more subtle, if not more devastating.
Cardiovascular Conditions
The pulmonary complications of heart disease and the cardiac complications of pulmonary disease have been well documented for several decades.2 A mechanically inefficient heart disrupts the normal forward propulsion of deoxygenated and oxygenated blood to and from the lungs. Because the right and left sides of the heart function in series, a problem on one side inevitably has some effect, which can lead to a problem, on the other side. For these reasons, the heart and lungs should be thought of as a single functioning unit. Disruption of the cardiovascular and pulmonary circuit leads to the backlogging of blood and an increased volume of blood in the capacitance vessels or the veins. Right heart failure contributes to increased central venous pressure (i.e., right atrial pressure) and, if sufficiently severe, leads to bilateral peripheral edema in the dependent body parts. Because blood is not forwarded to the lungs adequately, hypoxemia can result. In turn, hypoxic vasoconstriction of the pulmonary circulation leads to increased pulmonary vascular resistance and, hence, to increased right ventricular afterload and work.
In left heart failure, the normal pattern of increased ventilation to the bases may be reversed (i.e., the apices of the lungs may be better ventilated).3 If pulmonary edema complicates the clinical picture, the alveoli become flooded, resulting in reduced ventilation and ventilation and perfusion mismatching. The alveolar-arterial oxygen (A-aO2) gradient is then increased, diffusing capacity is decreased, and arterial partial pressure of oxygen (PaO2) is decreased. Lung compliance is inversely related to pulmonary artery pressures and interstitial fluid accumulation.4 The net effect of these abnormalities is both obstructive and restrictive pathophysiological patterns of lung dysfunction (i.e., reduced forced expiratory volumes and vital capacity and an overall increase in the work of breathing).
Pulmonary Conditions
Pleural complications can arise from lung disease as well as from heart disease. Both heart and lung function can be compromised by altered fluid balance in the pleurae. Fluid balance in the pleural space is comparable, in terms of its regulation, to that in the alveolar space. Both are determined by Starling forces. Specifically, hydrostatic pressure pushes fluid into the pleural space while oncotic pressure counters the effect of these forces. The net effect of these filtration and absorption forces is a minimal net filtration pressure. When the balance of these forces is disrupted, heart and lung function can be threatened. Excessive fluid floods the space, usually reflecting both excessive hydrostatic pressure and diminished oncotic pressure. The lymphatic vessels become overwhelmed and are unable to keep the pleural space dry. Pleural fluid accumulates and either displaces lung tissue (in small to moderate effusions) or restricts the opening of adjacent alveolar sacs, causing atelectasis (severe effusions),5 which, if sufficiently severe, may restrict cardiac filling. Pleural fluid accumulation poses a unique threat to oxygen transport as a result of its direct physical effect on the lungs, heart, or both, so it warrants special attention by physical therapists.
Pulmonary lymphatics control fluid balance within the lung parenchyma. Lymphatic vessels arise within the pleurae and not within the alveolar capillary space. They drain fluid from the interlobular septae and subpleural areas into the hilar vessels and the primary tracheobronchial lymph nodes. Problems arising within the heart or lungs can contribute to imbalances in the major lymphatic inflow and outflow channels. This contributes to fluid accumulation, stagnation, and physical compression of the myocardium and lungs.6
Musculoskeletal Conditions
Musculoskeletal conditions impact cardiovascular and pulmonary function secondary to their effects on muscle (in particular, the diaphragm, muscles of the chest wall, oropharynx, larynx, and abdomen) and bones and joints (e.g., arthritis, ankylosing spondylitis, kyphoscoliosis, and deformities secondary to neuromuscular diseases and chronic lung diseases) (Table 6-1). Additional effects are imposed by inactivity (i.e., muscle wasting, joint rigidity, and deformity). Increased joint rigidity limits the amount of physical activity a patient may perform, which contributes to cardiovascular and pulmonary compromise, in addition to the local effect of increased chest wall rigidity and compromised bucket-handle and pump-handle motions. The normal three-dimensional movement of the chest wall and normal pulmonary circulation and lymphatic function are compromised (Chapter 23). The cardiovascular and pulmonary manifestations of musculoskeletal conditions are summarized in Table 6-1.
Table 6-1
Cardiovascular and Pulmonary Manifestations of Musculoskeletal Conditions
| Impairment | Factors that Potentially Contribute to Impairment | |
| General Manifestations | Decreased alveolar ventilation | Altered respiratory mechanics |
| Chest wall rigidity | ||
| Decreased chest wall excursion | ||
| Impaired mucociliary transport | Airflow obstruction | |
| Pulmonary restriction | ||
| Atelectasis | ||
| Inspissated secretions | ||
| Increased work of breathing | Inefficient breathing pattern | |
| Increased work of the heart | Inefficient breathing pattern | |
| Constrictive pericarditis | ||
| Decreased aerobic capacity | ||
| Manifestations in Specific Conditions | Rheumatoid arthritis | Pleuritis |
| Diffuse interstitial pneumonitis and fibrosis | ||
| Pulmonary arteritis | ||
| Bronchiolitis | ||
| Pleural effusions | ||
| Ankylosing spondylitis | Upper lobe fibrobullous disease | |
| Chest wall restriction |
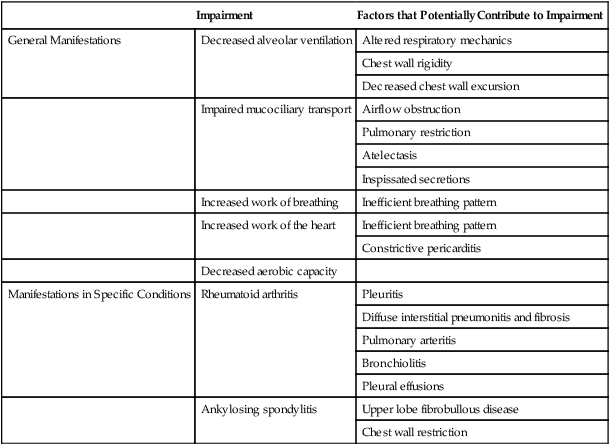
The cardiovascular and pulmonary deficits associated with musculoskeletal disorders of the chest wall include reduced and potentially asymmetric lung volumes consistent with pulmonary restriction, reduced flow rates, reduced inspiratory and expiratory pressures, atelectasis, dynamic airway compression, ventilation-perfusion mismatching, inefficient breathing pattern, impaired cough and gag reflexes, increased risk for aspiration, increased risk for obstruction secondary to impaired mucociliary clearance, restricted mobility, compression of mediastinal structures and heart, and impaired lymphatic drainage, which depends on normal expiratory and inspiratory cycles.7 Osteoporotic fractures also compromise normal chest wall configuration, hence, pulmonary function.8
Autoimmune conditions that have musculoskeletal manifestations can be associated with systemic conditions. Rheumatoid arthritis, for example, is associated with accelerated atherosclerosis.9
Connective Tissue Conditions
Connective tissue, or collagen, vascular disorders (e.g., scleroderma, systemic lupus erythematosus, and systemic sclerosis) invariably affect the cardiovascular and pulmonary systems (Box 6-2).10 Inflammation and tissue injury can affect the airway, lung parenchyma, pulmonary vasculature, pleurae, respiratory muscles, heart, and pericardium. Shrinking-lung syndrome associated with chronic connective tissue changes is a feature of advanced disease and is characterized by loss of alveolar surface area, diffusion capacity, and lung volumes. Fibrotic changes increase the elasticity of the lung parenchyma and reduce lung compliance, thereby increasing the work of breathing. These changes are comparable to those in idiopathic pulmonary fibrosis. Both the electrical conduction system of the heart and its mechanical behavior are adversely affected by systemic connective tissue changes. Furthermore, connective tissue changes in the skin can lead to chest wall restriction. Renal-pulmonary syndrome has been associated with connective tissue conditions.11 The cardiovascular and pulmonary manifestations of connective tissue conditions are summarized in Box 6-2.
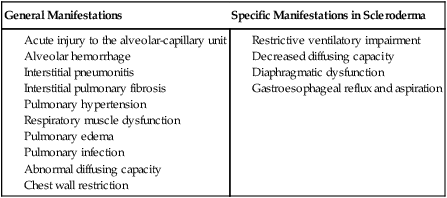
Connective tissue conditions contribute to blood vessel changes and have been implicated in secondary pulmonary arterial hypertension.12,13
Neurological Conditions
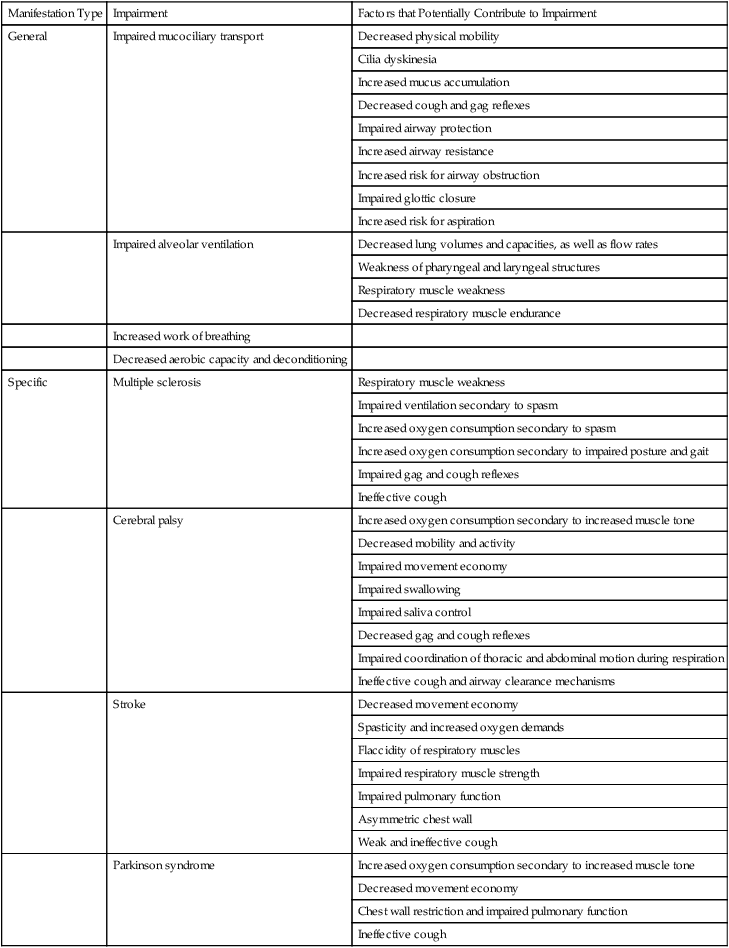
Cardiovascular and pulmonary consequences of neurological conditions reflect the pathophysiological mechanisms involved.14 There are three basic patterns of pathology: dysfunction of the central nervous system (CNS), dysfunction of the peripheral nervous system, and dysfunction of the autonomic nervous system. The cardiovascular and pulmonary manifestations of neurological conditions are summarized in Table 6-2.
Table 6-2
Cardiovascular and Pulmonary Manifestations of Neurological Conditions
| Manifestation Type | Impairment | Factors that Potentially Contribute to Impairment |
| General | Impaired mucociliary transport | Decreased physical mobility |
| Cilia dyskinesia | ||
| Increased mucus accumulation | ||
| Decreased cough and gag reflexes | ||
| Impaired airway protection | ||
| Increased airway resistance | ||
| Increased risk for airway obstruction | ||
| Impaired glottic closure | ||
| Increased risk for aspiration | ||
| Impaired alveolar ventilation | Decreased lung volumes and capacities, as well as flow rates | |
| Weakness of pharyngeal and laryngeal structures | ||
| Respiratory muscle weakness | ||
| Decreased respiratory muscle endurance | ||
| Increased work of breathing | ||
| Decreased aerobic capacity and deconditioning | ||
| Specific | Multiple sclerosis | Respiratory muscle weakness |
| Impaired ventilation secondary to spasm | ||
| Increased oxygen consumption secondary to spasm | ||
| Increased oxygen consumption secondary to impaired posture and gait | ||
| Impaired gag and cough reflexes | ||
| Ineffective cough | ||
| Cerebral palsy | Increased oxygen consumption secondary to increased muscle tone | |
| Decreased mobility and activity | ||
| Impaired movement economy | ||
| Impaired swallowing | ||
| Impaired saliva control | ||
| Decreased gag and cough reflexes | ||
| Impaired coordination of thoracic and abdominal motion during respiration | ||
| Ineffective cough and airway clearance mechanisms | ||
| Stroke | Decreased movement economy | |
| Spasticity and increased oxygen demands | ||
| Flaccidity of respiratory muscles | ||
| Impaired respiratory muscle strength | ||
| Impaired pulmonary function | ||
| Asymmetric chest wall | ||
| Weak and ineffective cough | ||
| Parkinson syndrome | Increased oxygen consumption secondary to increased muscle tone | |
| Decreased movement economy | ||
| Chest wall restriction and impaired pulmonary function | ||
| Ineffective cough |
Dysfunction of the Central Nervous System
Activity of the central generator is affected by arousal and the general alerting reaction of the reticular activating system. In addition, breathing control is influenced by the hypothalamus, orbital cortex, forebrain, and amygdala.15
Insult to the CNS can result in cardiovascular responses that precipitate neurogenic pulmonary edema. Such responses include systemic hypertension, pulmonary hypertension, intracranial hypertension, and bradycardia. The medulla may mediate the cardiovascular responses associated with neurogenic pulmonary edema. Marked sympathetic stimulation, catecholamine release, and vagotonia appear to precipitate neurogenic pulmonary edema and the resulting leaking of the alveolar capillary membrane and alveolar flooding.16
Demyelinating diseases such as multiple sclerosis result in progressive deterioration of neuromuscular function. The muscles of respiration become increasingly involved, resulting in respiratory insufficiency due to weakness.17,18 In addition, with increasing debility, cardiovascular and pulmonary conditioning is reduced. Weakness of the pharyngeal musculature contributes to loss of airway protection in addition to the loss of cough and gag reflexes. Aspiration is problematic for these patients as the disease advances.
Patients with stroke may have central dysfunction that affects cardiovascular and pulmonary regulation and function, including reduced electrical activity of the respiratory muscles or peripheral dysfunction such that weakness, spasticity, impaired biomechanics, and gait directly affect respiratory muscle function and chest wall excursion. Hemiparesis of the diaphragm on the affected side can occur. Abdominal muscle weakness contributes to impaired cough effectiveness. Pharyngeal weakness contributes to sleep apnea in these patients. The common clinical presentation of unilateral dysfunction leads to a posture characterized by listing to the affected side when recumbent or sitting and during ambulation. This posture impairs ventilation and chest wall expansion on the affected side. Abdominal muscle dysfunction directly affects intraabdominal pressure and the efficiency of diaphragmatic descent during contraction (i.e., should the abdominal muscles become flaccid, the efficiency of diaphragmatic contraction is reduced). Lung volumes and flow rates are reduced proportionately, causing a restrictive pattern of lung function. Muscle spasticity contributes to chest wall restriction and increased metabolic cost. Reduced activity and exercise around the time of the stroke contribute to reduced cardiovascular and pulmonary conditioning and capacity of the oxygen transport system. A high proportion of patients with strokes are hypertensive and older, a population with a higher prevalence of heart disease and atherosclerosis.19,20
Cerebral palsy is associated with increased muscle tone, which correspondingly increases basal and exercise oxygen consumption, resulting in increased oxygen demands. Even though the demands are increased in this condition, oxygen delivery is compromised. For example, thoracic deformity and spasm of the muscles of the chest wall and abdomen impair the breathing pattern and its efficiency. Minimizing secondary scoliosis is a priority.21 The airways of patients with cerebral palsy are vulnerable to obstruction because of poor gag, cough, and swallowing reflexes. In addition, these patients often have poor saliva control, which further increases the risk for aspiration. Mental retardation often complicates the presentation of cerebral palsy and prevents these patients from responding adequately to their hydration needs and being able to cooperate with life-preserving treatments. These patients may harbor numerous microorganisms, adding further to their general risk for infection. Of clinical importance is the fact that pulmonary function can be substantially improved with adaptive seating and postural control.22
Patients with Parkinson syndrome also exhibit cardiovascular and pulmonary deficits.23 Oxygen demand is increased commensurate with increased muscle tone. Patients with Parkinson syndrome have reduced cardiovascular and pulmonary conditioning levels as a result of compromised agility and ability to be independently mobile. These patients have a restrictive pattern of lung disease in which most lung volumes and capacities are reduced. Chest wall rigidity impairs the normal pump-handle and bucket-handle movements, so breathing efficiency is reduced. The energy cost of breathing is correspondingly increased. Respiratory insufficiency in Parkinson syndrome likely reflects increases in tone of the respiratory muscles, chest wall rigidity, and parasympathetic tone and resulting airway obstruction. Parkinson syndrome can be associated with cardiac sympathetic denervation,24 which has implications for the assessment, monitoring during exercise, and exercise prescription.
Patients with a history of poliomyelitis, with or without cardiovascular and pulmonary complications at onset, may exhibit pulmonary limitation several decades later.25,26 These individuals are at risk for developing respiratory insufficiency as a result of respiratory and abdominal muscle weakness, chest wall deformity, minor infection, and periods of relative immobility; or secondary to medical interventions, such as anesthesia and sedation. Further, because of prolonged reduced physical activity, patients with chronic poliomyelitis may be overweight.
Spinal cord lesions have a variable effect on cardiovascular and pulmonary function, depending on the level of the lesion. Cervical lesions result in a high mortality rate due to cardiovascular and pulmonary complications. All lung volumes are diminished with the exception of total lung capacity (TLC), which over time returns to normal; tidal volume (TV), which is usually preserved (10% of TLC); and residual volume (RV), which is increased.27 Patients with quadriplegia tend to have a greater diaphragmatic contribution to tidal ventilation than people without such injury. In addition, these patients have impaired cough as a result of the loss of innervation and paresis of the diaphragm, in some cases, and of abdominal and intercostal muscles. The contribution of accessory muscle activity to ventilation varies considerably. In quadriplegia in which only the accessory muscles and diaphragm have been spared, platypnea (increased dyspnea when upright) may occur.28 In the upright position, the diaphragm is flattened and less efficient when moved downward by reduced abdominal pressures.
Dysfunction of the Autonomic Nervous System
Cardiovascular and pulmonary consequences of disorders of the autonomic nervous system have been documented. Of those conditions with an autonomic component, autonomic neuropathies, diabetes, and alcoholism have been the most thoroughly studied. Multiple-system atrophy accompanies autonomic failure affecting multiple systems. Because of the anatomic proximity of the autonomic, respiratory, and hypnogenic neurons and the degeneration of these structures in this condition, dysfunction of the respiratory control mechanisms parallels autonomic and somatic dysfunction. The respiratory dysrhythmias that are seen in multiple-system atrophy include central and upper airway obstruction; irregular rate, rhythm, and amplitude of respiration, with or without oxygen desaturation; transient uncoupling of the intercostal and diaphragmatic muscle activity; prolonged periods of apnea; Cheyne-Stokes respiration; inspiratory gasps; and transient sudden respiratory arrest.29
Patients with diabetes and autonomic neuropathies exhibit variable cardiovascular and pulmonary dysfunction. Postural hypotension is a complication of diabetic autonomic neuropathy and efferent sympathetic vasomotor denervation. Norepinephrine levels are generally reduced in these patients. The splanchnic and peripheral circulations fail to constrict in response to standing, so cardiac output falls. The postural effect is exacerbated by reduced cardiac acceleration in patients with diabetes. Insulin has been associated with cardiovascular effects, including reduced plasma volume, increased peripheral blood flow secondary to vasodilation, and increased heart rate. In the presence of autonomic neuropathies, insulin can induce postural hypotension. Diabetic diarrhea secondary to abnormal gut motility can contribute to fluid loss and its sequelae. Cardiovascular and pulmonary changes associated with autonomic neuropathy secondary to diabetes include altered ventilatory responses to hypoxia and hypercapnia, altered respiratory pattern and apneic episodes during sleep, altered bronchial reactivity, and impaired cough.29 Impaired peripheral perfusion and tissue nutrition are also consequences of diabetes and autonomic neuropathies, which will be described later in this chapter (see Endocrine Conditions).
Gastrointestinal Conditions
The cardiovascular and pulmonary manifestations of gastrointestinal (GI) dysfunction are summarized in Table 6-3. Inflammatory bowel disease and pancreatitis are principal examples of GI dysfunction affecting cardiovascular and pulmonary function. Aspiration is a significant cause of morbidity and mortality in patients with GI dysfunction, so it should be prevented or detected early. The pathophysiology, management, and outcome depend on the nature of the aspirate. Several predisposing factors produce aspiration pneumonia, including a decreased level of consciousness, disorders of pharyngeal and esophageal motility, altered anatomy, disorders of gastric and intestinal motility, and iatrogenic factors such as surgery, nasogastric intubation, and general anesthesia.
Table 6-3
Cardiovascular and Pulmonary Manifestations of GI Conditions
| Impairment | Factors that Potentially Contribute to Impairment |
| Risk for aspiration | Gastroesophageal reflux |
| Increased airway resistance | Bronchospasm |
| Decreased lung volumes | Elevated hemidiaphragms |
| Compression atelectasis | |
| Arterial hypoxemia | Alveolar capillary leak and V/Q mismatch |
| Alveolar hemorrhage and consolidation worsen shunt | |
| Increased pulmonary vascular resistance |
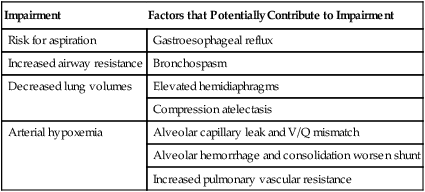
Inflammatory bowel disease can lead to the following cardiovascular and pulmonary pathologies: vasculitis, fibrosis, granulomatous disease, and pulmonary thromboembolism. Bronchitis and bronchiectasis have also been associated with inflammatory bowel disease; however, their occurrence is not related to disease severity or therapy. Biopsy specimens have shown basement membrane thickening, thickening of the epithelium, and infiltration of the underlying connective tissue with inflammatory cells.30
Hepatic Conditions
Both acute and chronic liver conditions can predispose a patient to cardiovascular and pulmonary and cardiovascular complications (Box 6-3). Hepatic dysfunction can lead to hypoxemia secondary to intrapulmonary vascular dilation and noncardiogenic pulmonary edema. Hepatopulmonary syndrome, hallmarked by intrapulmonary vascular dilation, produces both diffusion and perfusion defects in the lungs and is the principal reason for severe hypoxemia.31
Such pulmonary vasodilation is associated with excess perfusion with respect to ventilation.32 Arterial oxygenation may range from an asymptomatic increase in alveolar-arterial oxygen pressure difference to extreme breathlessness secondary to severe hypoxemia. Of relevance to the physical therapist is that oxygenation in these patients is frequently worse when the patient is standing compared with lying supine (orthodeoxia). In severe cases, pulmonary edema may occur secondarily to hepatic encephalopathy and cerebral edema.33
The liver is a primary organ for the production of procoagulants and anticoagulants in the blood, both of which are carefully regulated to maintain optimal balance. Liver dysfunction can disrupt the production of these complementary agents and lead to a tendency to bleed or clot. Disseminated intravascular coagulation refers to an extreme imbalance between these vasoactive agents in which bleeding and clotting problems occur simultaneously, resulting in serious consequences to the patient. With respect to central dysfunction, recently impaired myocardial contractility has been reported in patients with cirrhosis.34
Renal Conditions
Cardiovascular and pulmonary complications can result from renal dysfunction and from the category of disorders referred to as renal-pulmonary syndromes (Box 6-4).35,36 The pathophysiological characteristics of these disorders include alveolar hemorrhage, interstitial and alveolar inflammation, and dysfunction of the pulmonary vasculature. Pulmonary function testing may detect both obstructive and restrictive abnormalities as a result of bronchial complications and as a result of inflammation and hemorrhage, respectively.
In systemic illness, pathology of the lungs and kidneys often coexist. Physical therapy warrants being aggressive, given the course of the renal-pulmonary syndromes and the potential for relapses and irreversible organ damage. Notwithstanding the serious medical implications of renal dysfunction, even simple measures such as dietary salt restriction need to be advocated, even for patients on dialysis.37
Hematological Conditions
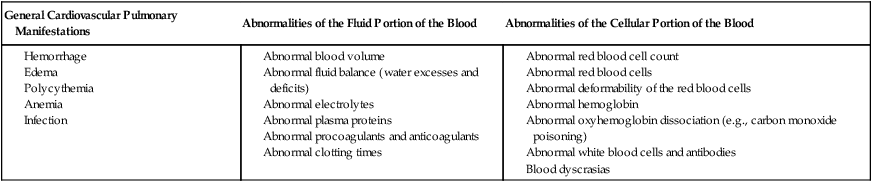
Hematological disorders that can manifest cardiovascular and pulmonary symptoms include abnormalities of the fluid and cellular components of the blood, as well as coagulopathies.38 Cardiovascular and pulmonary manifestations of hematological conditions are summarized in Box 6-5. The primary underlying mechanisms by which these conditions disrupt gas exchange include hemorrhage, infection, edema, anemia, fibrosis, and malignancies.
Of the erythrocyte disorders, sickle cell anemia is probably the most common, and its manifestations largely reflect erythrocyte rigidity and vasoocclusion.39 Acute chest infection and thrombosis leading to pulmonary infarction may occur with clinical symptoms such as fever, pleuritic chest pain, cough, and pulmonary infiltrates. The pulmonary function of patients with sickle cell anemia is abnormal; there is likely to be a reduction in TLC, vital capacity, diffusing capacity, arterial oxygen tensions, and exercise capacity.
Endocrine Conditions
Endocrine and metabolic disorders can be associated with cardiovascular and pulmonary complications (Box 6-6).6,40 Disorders of the thyroid gland, pancreas (diabetes mellitus), and adrenal glands are examples that are often seen clinically. Thyroid hormone influences surfactant synthesis and the central drive to breathe. Hypothyroidism can lead to sleep apnea, pleural effusions secondary to altered capillary permeability, and pericardial effusions. Decreases in vital capacity have also been reported secondary to muscle weakness. Individuals with hypothyroidism have exercise intolerance.41 Hyperthyroidism increases cellular oxidative metabolism (the metabolic rate), leading to an increase in oxygen consumption and CO2 production and an overall increase in minute ventilation.41 Vital capacity, lung compliance, and diffusion capacity can be reduced. In addition, maximal inspiratory and expiratory pressures can be reduced secondary to muscle weakness. In thyroid dysfunction, cardiorespiratory function can be normal at rest, but dysfunction can be unmasked during exercise stress.
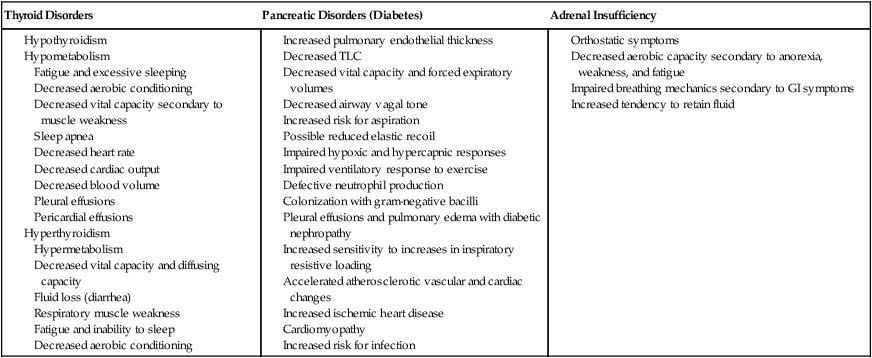
Individuals with diabetes may lose their pulmonary reserve capacity.42 Although this may present as a subclinical finding, such loss can manifest with stress, aging, chronic hypoxia secondary to lung disease, or volume overload secondary to cardiac and renal failure.
People with diabetes are prone to aspiration and respiratory infections. Late complications of diabetic neuropathy include renal failure, which may be accompanied by pleural effusions and pulmonary edema. Autonomic neuropathy may affect vagal activity and airway tone and the vasomotor tone of the blood vessels. Ischemic heart disease and cardiomyopathies are common in patients with diabetes and may cause congestive heart failure and cardiogenic pulmonary edema. Patients with diabetes have been reported to have reduced sensitivity to inspiratory loading,43 which may impair their subjective responses to exercise. Peripheral perfusion can be compromised, resulting in diabetes. Limbs are endangered by reduced perfusion and reduced sensation. Severe cases result in limb ischemia, gangrene, and surgical amputation. Infection rates are high in patients with diabetes.
Adrenal insufficiency can also compromise oxygen transport.40 Reduced aerobic capacity results from symptoms of weakness and fatigue and associated muscle and joint complaints. Orthostatic intolerance primarily reflects reduced inotropic capacity of the heart and reduced systemic vascular resistance.
Immunological Conditions
Congenital and acquired defects in the immunological status of the lungs lead to cardiovascular and pulmonary dysfunction, including inflammation and infection. Acquired immunodeficiency syndrome (AIDS), an example of a primary disorder of cell-mediated immunity, has reached epidemic proportions over the past 20 years. This syndrome leads to lymphocyte death. The most serious pulmonary infection associated with AIDS is Pneumocystis carinii pneumonia.44 The fact that a patient with the human immunodeficiency virus (HIV) is likely to have recurrent pneumonia suggests that the infecting organisms persist in the lung despite treatment.45 The clinical presentation includes diffuse pulmonary infiltrates, cough, dyspnea, and hemoptysis. These patients can also have symptoms of upper airway obstruction.
Nutritional Disorders
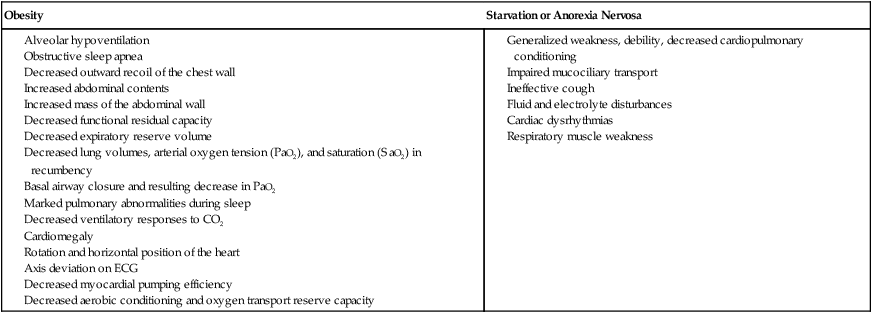
The two most common nutritional disorders seen in Western countries are obesity and anorexia nervosa, which is akin to starvation. Two-thirds of the U.S. population is now categorized as being overweight and at risk for its sequelae. Obesity contributes in several ways to impaired oxygen transport.46 Alveolar hypoventilation and impaired PaO2 and gas exchange result from chest wall restriction. Systemic and pulmonary blood pressures are increased. In addition, large abdomens and abdominal contents impinge on diaphragmatic motion and can restrict diaphragmatic descent. This can lead to compression of the dependent lung fields. Obesity is associated with elevated C-reactive protein, a marker for inflammation and elevated cardiovascular health risk.47 Recumbency can induce respiratory insufficiency (positional respiratory failure). Individuals who are obese have a higher incidence of snoring and obstructive sleep apnea secondary to weakness and increased compliance of the postpharyngeal structures. In addition, these patients often have weak, ineffectual coughs resulting from expiratory muscle weakness and the mechanical inefficiency of these muscles. The work of breathing is markedly increased. Furthermore, in chronic cases, reactive pulmonary vasoconstriction in response to chronic hypoxemia contributes to right ventricular insufficiency and increased work of the heart and cardiomegaly. Individuals who are obese tend to be less active and aerobically deconditioned, with reduced efficiency of oxygen transport overall and increased risk for thromboemboli. Hypertension is also a serious sequela of obesity.
In addition to the direct effects obesity has on oxygen transport, central obesity has been strongly linked with metabolic syndrome. Additional risk factors include increased blood pressure, dyslipidemia (raised triglycerides and lowered high-density lipoprotein cholesterol), increased fasting glucose and type 2 diabetes mellitus.48 In any patient who is overweight, these risk factors need to be assessed, not only so that treatment can be prescribed accordingly, but to facilitate referral of the patient to other health care professionals, if necessary. Abdominal fat, although associated with aging, is more closely linked with health risk than subcutaneous fat. Conventional measures of body composition may not estimate health risk sufficiently (e.g., insulin resistance and cardiovascular disease).49
The major cardiovascular and pulmonary manifestations of anorexia nervosa relate to generalized weakness and reduced endurance of all muscles, including the respiratory muscles. Cough effectiveness is correspondingly compromised. Oxygen transport reserve is minimal. Because of poor nutrition and fluid intake, the patient is at risk for anemia, fluid and electrolyte imbalances, and cardiac dysrhythmias.50 Common manifestations of nutritional disorders are summarized in Box 6-7.