Cardiovascular and Pulmonary Anatomy
The heart lies in series with the lungs, constituting the cardiovascular and pulmonary unit, the central component of the oxygen transport pathway.1,2 Virtually all the blood returned to the right side of the heart passes through the lungs and is delivered to the left side of the heart for ejection into the systemic, coronary, and bronchopulmonary circulations. Because of this interrelationship, changes in lung function can exert changes in heart function and vice versa. A detailed understanding of the anatomy of the heart and lungs and how these organs work synergistically is essential to the practice of cardiovascular and pulmonary physical therapy.
This chapter presents the anatomy of the cardiovascular and pulmonary systems, including the skeletal features of the thoracic cavity; muscles of respiration; anatomy of the tracheobronchial tree; lung parenchyma; basic anatomy of the heart; and peripheral, pulmonary, and lymphatic circulations.3–11
Thorax
The bony thorax covers and protects the principal organs of respiration and circulation as well as the liver and the stomach (Fig. 3-1). The anterior surface is formed by the sternum and the costal cartilage. The lateral surfaces are formed by the ribs. The posterior surface is formed by the 12 thoracic vertebrae and the posterior part of the 12 ribs. At birth, the thorax is nearly circular, but during childhood and adolescence it becomes more elliptical. In adulthood, the transverse diameter of the chest wall is greater than the anteroposterior diameter.
Movements of the Thorax
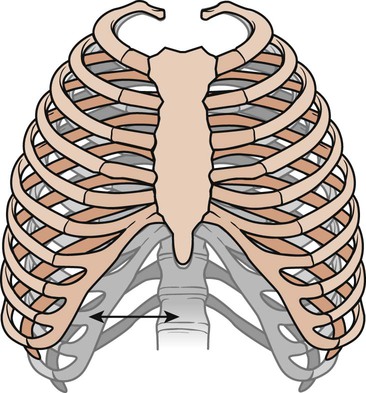
The frequency of movement of the bony thorax joints is greater than that of almost any other combination of joints in the body. Two types of movements have been described: the pump-handle movement and the bucket-handle movement (Fig. 3-2).12 The upper ribs are limited in their ability to move. Each pair swings like a pump handle, with elevation thrusting the sternum forward. This forward movement increases the anteroposterior diameter and the depth of the thorax and is called the pump-handle movement. In the lower ribs, there is little anteroposterior movement. During inspiration, the ribs swing outward and upward, each rib helps to elevate the rib above it. This bucket-handle movement increases the transverse diameter of the thoracic cage. Thus during inspiration, the thorax increases its volume by increasing its anteroposterior and transverse diameters.
Muscles of Respiration
Inspiration
Diaphragm
The diaphragm is a large, dome-shaped muscle that separates the thoracic and abdominal cavities. Its upper surface supports the pericardium (with which it is partially blended), heart, pleurae, and lungs. Its lower surface is almost completely covered by the peritoneum and overlies the liver, kidneys, suprarenal glands, stomach, and spleen (Fig. 3-3). This large muscle can be divided into right and left halves. Each half is made up of three parts: sternal, lumbar, and costal. These three parts insert into the central tendon, which lies just below the heart. The sternal part arises from the back of the xiphoid process and descends to the central tendon. On each side is a small gap, the sternocostal triangle, which is located between the sternal and costal parts. It transmits the superior epigastric vessels and is often the site of diaphragmatic hernias. The costal parts form the right and left domes. They arise from the inner surfaces of the lower four ribs and the lower six costal cartilages, then interdigitate and transverse the abdomen to insert into the anterolateral part of the central tendon, the central part of the diaphragm. The lumbar part arises from the bodies of the upper lumbar vertebrae and extends upward to the central tendon. The central tendon is a thin, strong aponeurosis situated near the center of the muscle, somewhat closer to the front of the body. It resembles a trefoil leaf, with its three divisions or leaflets. The right leaflet is the largest, the middle is the next largest, and the left leaflet is the smallest.
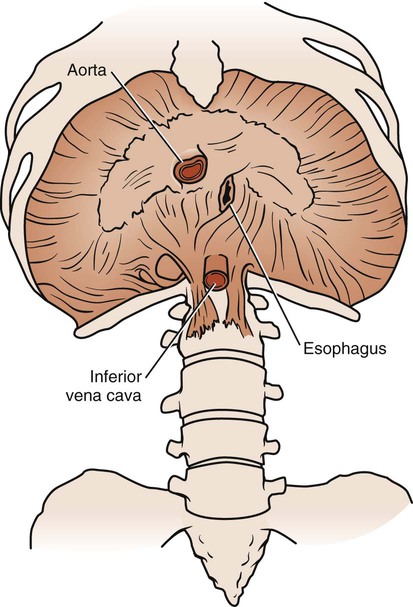
Major vessels traverse the diaphragm through one of three openings (see Fig. 3-3). The vena caval opening is located to the right of the midline in the central tendon and contains branches of the right phrenic nerve and the inferior vena cava. The esophageal opening is located to the left of the midline and contains the esophagus, the vagal nerve trunks, and branches of the gastric vessels. The aortic opening is located in the midline and contains the aorta, the thoracic duct, and sometimes the azygos vein. The diaphragm is also pierced by branches of the left phrenic nerve, small veins, and lymph vessels.
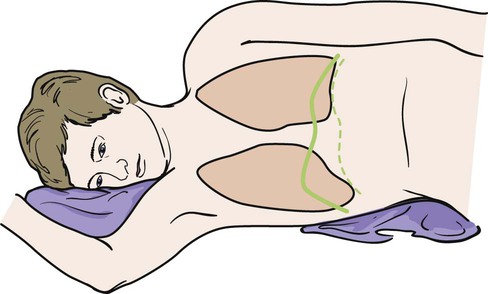
The position of the diaphragm and its range of movement vary with posture, the degree of distention of the stomach, size of the intestines, size of the liver, and obesity. Average movement of the diaphragm in quiet respiration is 12.5 mm on the right and 12 mm on the left. This can increase to a maximum of 30 mm on the right and 28 mm on the left during increased ventilation. An individual’s posture determines the position of the diaphragm. In the supine position, the resting level of the diaphragm rises. The greatest respiratory excursions during normal breathing occur in this position; however, the lung volumes are decreased because of the elevated position of the abdominal organs within the thoracic cavity. In a sitting or upright position, the dome of the diaphragm is pulled down by the abdominal organs, allowing a larger lung volume. For this reason, individuals who are short of breath are more comfortable sitting than reclining. In a side-lying position, the dome of the diaphragm on the lower side rises farther into the thorax than the dome on the upper side (Fig. 3-4). The abdominal organs tend to be displaced forward in a side-lying position, allowing greater excursion of the dome on the lower side. In contrast, the upper side moves little with respiration. On radiograph, the position of the diaphragm can indicate whether the film was taken during inspiration or expiration and may also indicate pathology in the lungs, pleurae, or abdomen.
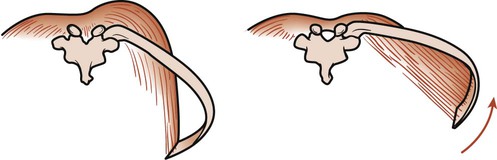
Contraction of the diaphragm increases the thoracic volume vertically and transversely. The central tendon is drawn down by the diaphragm as it contracts. As the dome descends, abdominal organs are pushed forward as far as the abdominal walls will allow. When the dome can descend no farther, the costal fibers of the diaphragm contract to increase the thoracic diameter of the thorax. This occurs because the fibers of the costal part of the diaphragm run vertically from their attachment at the costal margin. Thus contraction of these fibers elevates and everts the ribs (Fig. 3-5). If the diaphragm is in a low position, it will change the angle of pull of the muscle’s costal fibers. Contraction of these fibers creates a horizontal pull, which causes the lateral diameter to become smaller as the ribs are pulled in toward the central tendon.
As the diaphragm descends, it compresses the abdominal organs, increasing intraabdominal pressure. At the same time, the intrathoracic pressure decreases as the lung volume is increased by the descending diaphragm. Inspiratory airflow occurs as a result of this decrease in intrathoracic pressure (see Chapter 4). The pressure gradient between the abdominal and thoracic cavities also facilitates the return of blood to the right side of the heart.
Intercostals
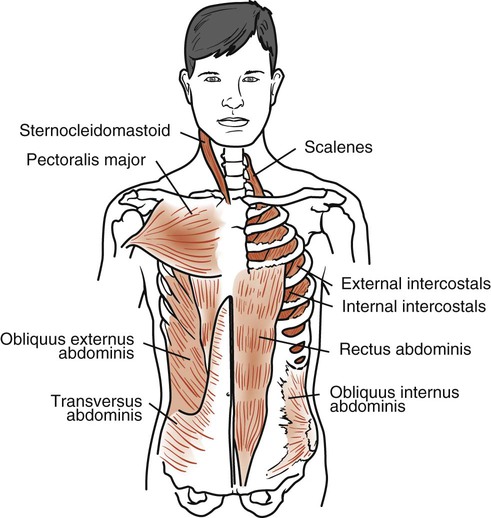
The external intercostals extend down and forward from the tubercles of the ribs (above) to the costochondral junction of the ribs (below), where they become continuous with the anterior intercostal membrane (Fig. 3-6). This membrane extends the muscle forward to the sternum. There are 11 external intercostal muscles on each side of the sternum. They are thicker posteriorly than anteriorly and thicker than the internal intercostal muscles. They are innervated by the intercostal nerves, and contraction draws the lower rib up and out toward the upper rib. This action increases the volume of the thoracic cavity.
Sternocleidomastoid
The sternocleidomastoid (SCM) muscles are strong neck muscles arising from two heads, one from the manubrium and one from the medial part of the clavicle (see Fig. 3-6). These two heads fuse into one muscle mass that inserts behind the ear into the mastoid process. It is innervated by the accessory nerve and the second cervical nerve. There are two of these muscles, one on each side of the neck. When one SCM contracts, it tilts the head toward the shoulder of the same side and rotates the face toward the opposite shoulder. If the two SCM muscles contract together, they pull the head forward into flexion. When the head is fixed, the muscles assist in elevating the sternum, increasing the anteroposterior diameter of the thorax.
The SCMs are the most important accessory muscles of inspiration. Their contractions can be observed in all patients during forced inspiration and in all patients who are dyspneic. These muscles become visually predominant in patients who are chronically dyspneic (see Chapter 5).
Scalenes
The anterior, medial, and posterior scalenes are three separate muscles that are considered a functional unit. They are attached superiorly to the transverse processes of the lower five cervical vertebrae and inferiorly to the upper surface of the first two ribs (see Fig. 3-6). They are innervated by their corresponding cervical spinal nerves. These muscles are primarily supportive neck muscles, but they can assist in respiration through reverse action. When their superior attachment is fixed, the scalenes act as accessory respiratory muscles and elevate the first two ribs during inspiration.
Pectoralis Major
The pectoralis major is a large muscle arising from the clavicle, the sternum, and the cartilages of all the true ribs (see Fig. 3-6). This muscle spreads across the anterior chest and inserts into the intertubercular sulcus of the humerus. It is innervated by the lateral and medial pectoral nerves and cervical nerves C5, C6, C7, C8, and T1. There are two of these muscles, one on each side of the body. This muscle rotates the humerus medially and draws the arm across the chest. During climbing and pull-ups, it draws the arms toward the trunk. During forced inspiration when the arms are fixed, it draws the ribs toward the arms, thereby increasing thoracic diameter.
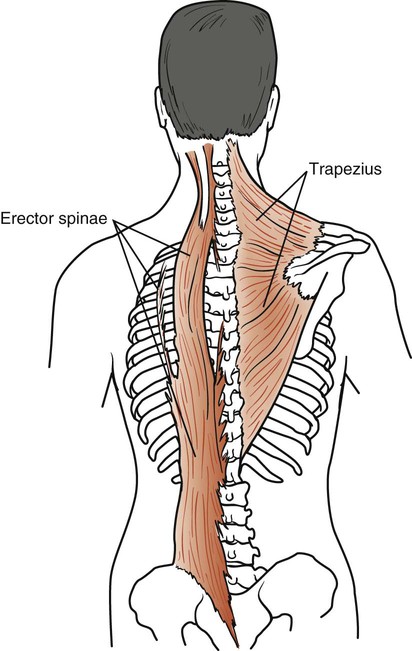
Trapezius
The trapezius consists of two muscles that form an extensive diamond-shaped sheet extending from the head down the back and out to both shoulders (Fig. 3-7). Its upper belly originates from the external occipital protuberance, curves around the side of the neck, and inserts into the posterior border of the clavicle. The middle part of the muscle arises from a thin diamond-shaped tendinous sheet, the supraspinous ligaments, and the spines of the upper thoracic region; it then runs horizontally and inserts into the spine of the scapula. Its lower belly arises from the supraspinous ligaments and the spines of the lower thoracic region, runs upward, and inserts into the lower border of the spine of the scapula. This large muscle is innervated by the external or spinal part of the accessory nerve and cervical nerves C3 and C4. Its main function is to rotate the scapulae during arm elevation and control gravitational descent of the arms. It also braces the scapulae and raises them, as when shrugging the shoulders. Its ability to stabilize the scapulae makes it an important accessory muscle in respiration. This stabilization enables the serratus anterior and pectoralis minor to elevate the ribs.
Erector Spinae
The erector spinae is a large muscle extending from the sacrum to the skull (see Figure 3-7). It originates from the sacrum, the iliac crest, and the spines of the lower thoracic and lumbar vertebrae. It separates into a lateral iliocostalis, an intermediate longissimus, and a medial spinalis column. This muscle mass inserts into various ribs and vertebral processes all the way up to the skull. It is innervated by the corresponding spinal nerves. These muscles extend, laterally flex, and rotate the vertebral column. They are considered accessory respiratory muscles through their extension of the vertebral column. In deep inspiration, these muscles extend the vertebral column, allowing further elevation of the ribs.
Expiration
Rectus Abdominis
The rectus abdominis rises from the pubic crest, extends upward, and inserts into the xiphoid process and the costal margin of the fifth, sixth, and seventh costal cartilages (see Fig. 3-6). It is innervated by corresponding spinal nerves and its action is considered within the context of the other abdominal muscles.
Obliquus Externus Abdominis
The obliquus externus abdominis arises in an oblique line from the fifth costal cartilage to the 12th rib (see Fig. 3-6). Its posterior fibers attach in an almost vertical line with the iliac crest. The other fibers extend down and forward and attach to the front of the xiphoid process, the linea alba, and the pubic symphysis. It is innervated by the lower six thoracic spinal nerves.
Obliquus Internus Abdominis
The obliquus internus abdominis originates from the lumbar fascia, the anterior two-thirds of the iliac crest, and the lateral two-thirds of the inguinal ligament (see Fig. 3-6). Its posterior fibers run almost vertically upward and insert into the lower borders of the last three ribs. The other fibers join an aponeurosis attached to the costal margin above, the linea alba in the midline, and the pubic crest below. It is innervated by the lower six thoracic nerves and the first lumbar spinal nerves.
Transversus Abdominis
The transversus abdominis arises from the inner surface of the lower six costal cartilages, the lumbar fascia, the anterior two-thirds of the iliac crest, and the lateral one-third of the inguinal ligament (see Fig. 3-6). It runs across the abdomen horizontally and inserts into the aponeurosis, extending to the linea alba. It is innervated by the lower six thoracic nerves and the first lumbar spinal nerves.
Action of the Abdominal Muscles
The four muscles of the abdomen work together to provide a firm but flexible wall to keep the abdominal viscera in position. The abdominal muscles exert a compressing force on the abdomen when the thorax and pelvis are fixed. This force can be used in defecation, urination, parturition, and vomiting. In forced expiration, the abdominal muscles help force the diaphragm back to its resting position and thus force air from the lungs. If the pelvis and vertebral column are fixed, the obliquus externus abdominis aids expiration further by depressing and compressing the lower part of the thorax. Patients with chronic obstructive pulmonary disease (COPD) have difficulty in exhalation, which causes them to trap air in their lungs. The continued contraction of the abdominal muscles throughout exhalation helps them force this air from the lungs. The abdominal muscles also play an important role in coughing. First, a large volume of air is inhaled, and the glottis is closed. The abdominal muscles then contract, raising intrathoracic pressure. When the glottis opens, the large difference in intrathoracic and atmospheric pressure causes the air to be expelled forcefully at tremendous flow rates (tussive blast). Individuals with weak abdominal muscles (from neuromuscular diseases, paraplegia, quadriplegia, or extensive abdominal surgery) often have ineffective coughs (see Chapters 6 and 32).
Upper Airways
Nose
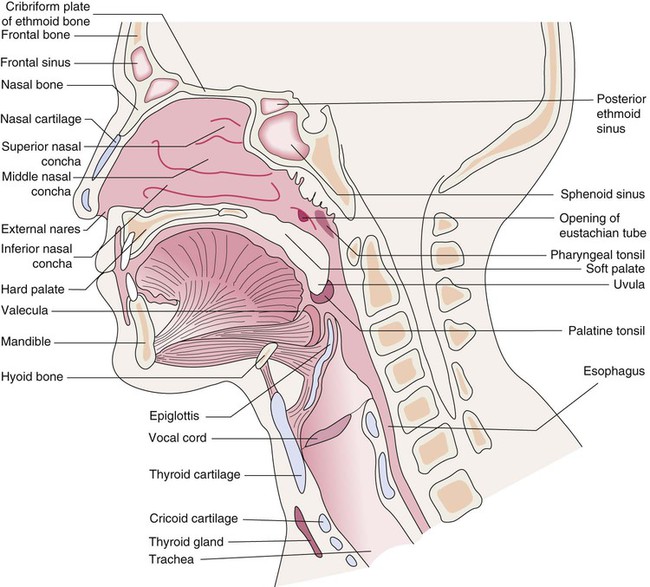
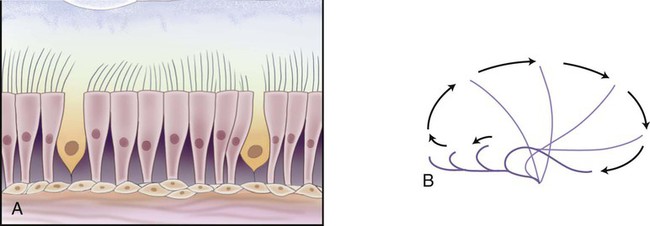
The anterior portion (vestibule) of the nasal cavity (Fig. 3-8) is lined with skin and coarse hairs (vibrissae) that entrap inhaled particles. The rest of the cavity and sinuses (with the exception of the olfactory region) are lined with respiratory mucous membrane. This membrane is composed of pseudostratified columnar ciliated epithelium (Fig. 3-9). It contains goblet cells, as well as mucous and serous glands that produce mucus and serous secretions. These secretions entrap foreign particles and bacteria. This mucus is then swept to the nasopharynx by the cilia at a rate of 5 to 15 mm/min, where it is swallowed or expectorated. The mucous membrane is vascular, with arterial blood supplied by branches of the internal and external carotid arteries. Venous drainage occurs through the anterior facial veins. The mucous membrane is thickest over the chonchae. As air is inhaled, it passes around and over the chonchae, whose vascular moist surfaces heat, humidify, and filter the inspired air. The mucous membrane may become swollen and irritated as a result of upper respiratory infections and may secrete copious amounts of mucus. Because this membrane is continuous with sinuses, auditory tubes, and lacrimal canaliculi, people with colds often complain of sinus headaches, watery eyes, earaches, and other symptoms. Secretions can be so copious that the nasal passages become completely blocked.
Pharynx
The nasopharynx is a continuation of the nasal cavities (see Fig. 3-8). It lies behind the nose and above the soft palate. With the exception of the soft palate, its walls are immovable, so its cavity is never obliterated as are the oropharynx and laryngopharynx. The nasopharynx communicates with the nasal cavity anteriorly through the posterior apertures of the nose. It communicates with the oropharynx and laryngopharynx through an opening, the pharyngeal isthmus, which is closed by elevations of the soft palate during swallowing.
The oropharynx extends from the soft palate to the epiglottis (see Fig. 3-8). It opens into the mouth anteriorly through the oropharyngeal isthmus. Its posterior walls lie on the bodies of the second and third cervical vertebrae. Laterally, two masses of lymphoid tissue—the palatine tonsils—may be seen. These tonsils form part of a circular band of lymphoid tissue surrounding the opening into the digestive and respiratory tracts.
The laryngopharynx lies behind the larynx and extends from the epiglottis above to the inlet of the esophagus below (see Fig. 3-8). The fourth to sixth cervical vertebrae lie behind the laryngopharynx. In front of the laryngopharynx are the epiglottis, the inlet of the larynx, and the posterior surfaces of the arytenoid and cricoid cartilages.
Larynx
The larynx is a complex structure composed of cartilages and cords moved by sensitive muscles (Fig. 3-10). It is located between the trachea and laryngopharynx, for which it forms an anterior wall. With its rapid closure it acts as a sphincteric valve, preventing food, liquids, and foreign objects from entering the airway. It controls airflow and at times closes so that thoracic pressure may be raised and the upper airways cleared by a propulsive cough when the larynx opens. Expiratory airflow vibrates as it passes over the contracting vocal chords, producing the sounds used for speech. (The larynx is not essential for speech. Humans can speak by learning to dilate the upper part of the esophagus so that air vibrates as it passes over that area; this is called esophageal speech.)
Lower Airways
Trachea
The trachea is a semirigid cartilaginous tube approximately 10 to 11 cm long and 2.5 cm wide. It lies in front of the esophagus and descends with a slight inclination to the right from the level of the cricoid cartilage (Fig. 3-11; see also Fig. 3-10). It travels behind the sternum into the thorax to the sternal angle (opposite the fifth thoracic vertebra), where it divides to form the right and left main stem bronchi. The tracheal wall is strengthened by 16 to 20 horseshoe-shaped cartilaginous rings. The open parts of the tracheal rings are completed by fibrous and elastic tissue and unstriated transverse muscle. This highly flexible part of the ring is positioned posteriorly. It indents or curves inward during coughing, which increases the velocity of expelled air. The cartilaginous rings lie horizontally one above the other, separated by narrow bands of connective tissue. The trachea is lengthened during hyperextension of the head; during swallowing, which raises the trachea; and during inspiration, when the lungs expand and pull the trachea downward. Its cross-sectional area becomes smaller with contraction of the unstriated transverse muscle fibers that complete the tracheal rings.
The bronchi of the airways continue to divide until there are approximately 23 generations (Table 3-1). The main, lobar, and segmental bronchi are made up of the first four generations. The walls contain U-shaped cartilage in the main bronchi. This cartilage becomes less well defined and more irregularly shaped as the bronchi continue to divide. In the segmental bronchi, the walls are formed by irregularly shaped helical plates with bands of bronchial muscle. The mucous membrane in these airways is essentially the same as that in the trachea, but the cells become more cuboidal in the lower divisions.
Table 3-1
Structural Characteristics of the Air Passages
| Generation (mean) | Number | Mean Diameter (mm) | Area Supplied | Cartilage | Muscle | Nutrition | Placement | Epithelium | |
| Trachea | 0 | 1 | 18 | Both lungs | U-shaped | From inferior thyroid, thoracic, and branches of bronchial arteries | |||
| Main bronchi | 1 | 2 | 13 | Individual lungs | Within connective tissue sheath alongside arterial vessels | ||||
| Lobar bronchi | 2 | 4 | 7 | Lobes | Irregular shape and helical plates | Helical bands | From the bronchial circulation | Columnar, ciliated | |
| ↓ | ↓ | ↓ | |||||||
| 3 | 8 | 5 | |||||||
| Segmental bronchi | 4 | 16 | 4 | Segments | |||||
| Small bronchi | 5 | 32 | 3 | Secondary lobules | |||||
| ↓ | ↓ | ↓ | |||||||
| 11 | 2000 | 1 | |||||||
| Bronchioles and terminal bronchioles | 12 | 4000 | 1 | Strong helical muscle bands | Embedded directly in the lung parenchyma | Cuboidal | |||
| ↓ | ↓ | ↓ | |||||||
| 16 | 65,000 | 0.5 | |||||||
| Respiratory bronchioles | 17 | 130,000 | 0.5 | Primary lobes | Muscle band between alveolar thin bands in alveolar septa | From the pulmonary circulation | Cuboidal to flat between the alveoli | ||
| ↓ | ↓ | ||||||||
| 19 | 500,000 | ||||||||
| Alveolar ducts | 20 | 1,000,000 | 0.3 | Alveoli | Lung parenchyma | Alveolar epithelium | |||
| ↓ | ↓ | ||||||||
| 22 | 4,000,000 | ||||||||
| Alveolar sacs | 23 | 8,000,000 | 0.3 | Lung parenchyma | Alveolar epithelium |
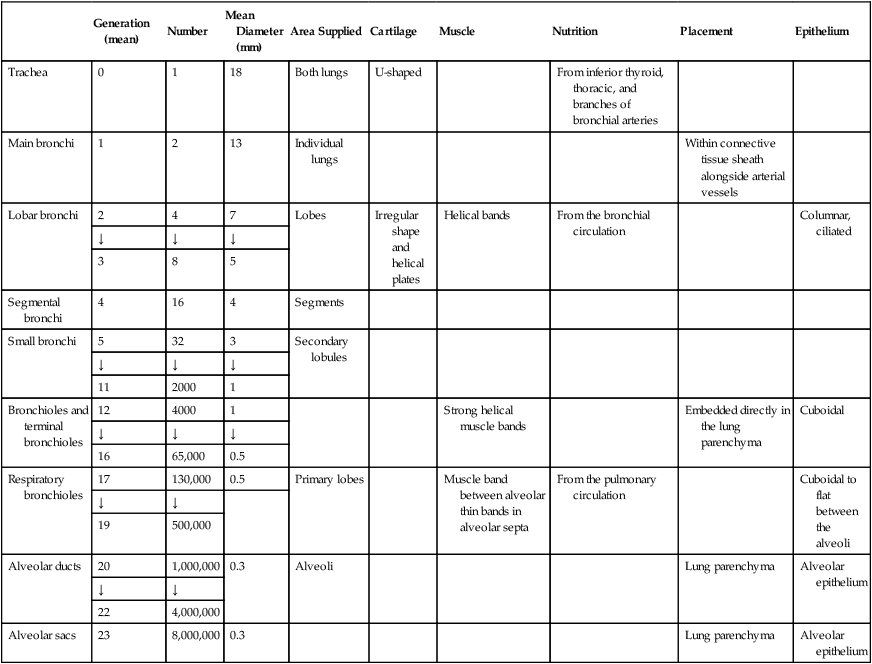
Adapted from Weibel ER: Morphometry of the human lung. New York, 1963, Springer.
Lungs
Surface Markings
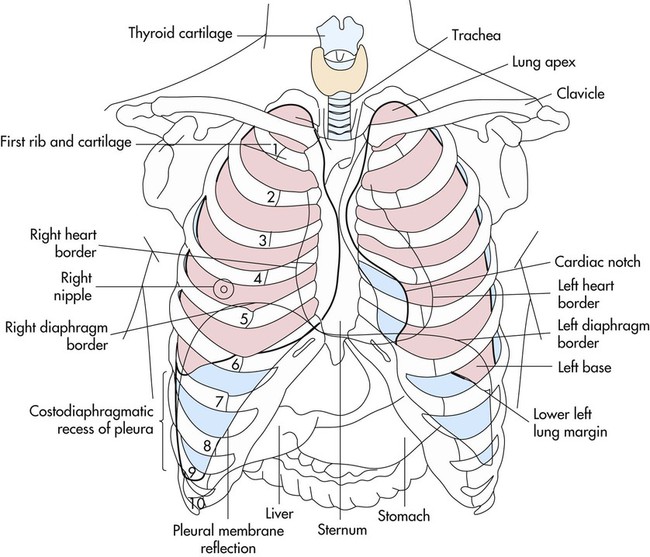
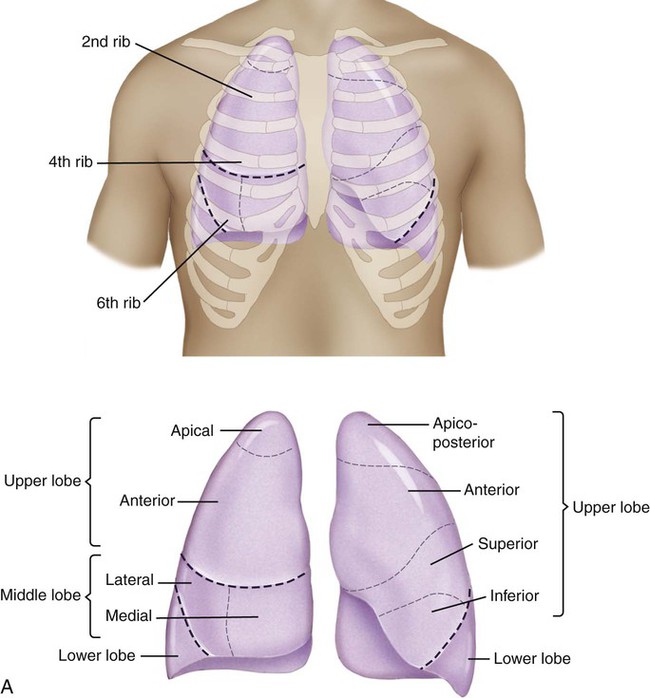
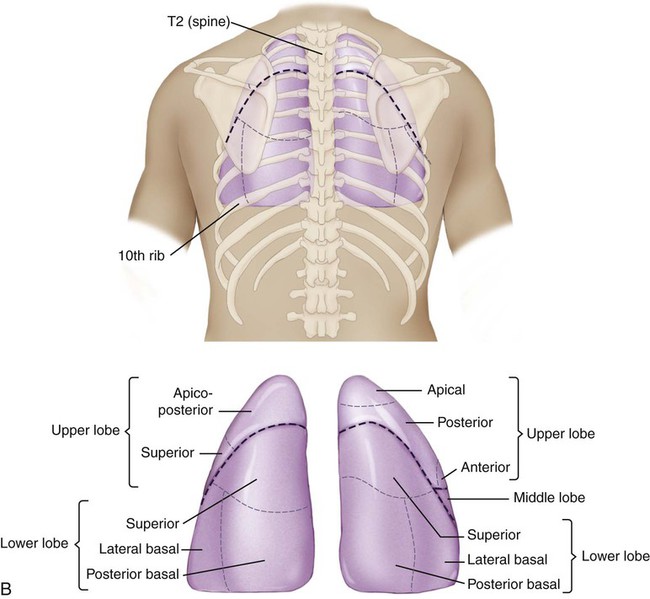
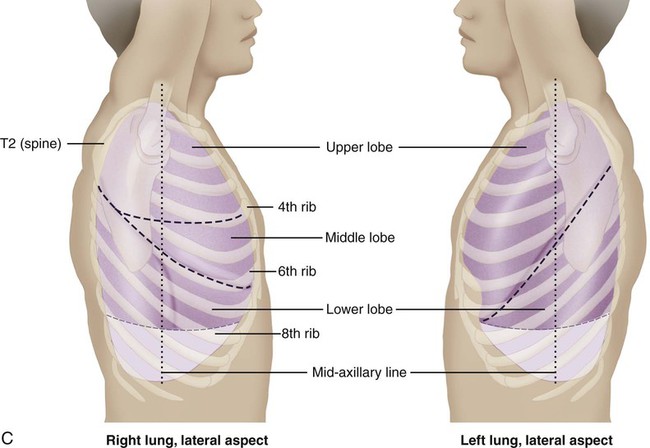
Surface markings of the lungs can be outlined on the chest with a basic knowledge of bony landmarks and of the gross anatomy of each lung (Table 3-2 and Fig. 3-12). The apices of both lungs extend 2 or 3 cm above the clavicles at the medial ends. The anteromedial border of the right lung runs from the sternoclavicular joint to the sternal angle and downward to the xiphisternum. The inferior border runs from the xiphisternum laterally to the sixth rib in the midclavicular line, the eighth rib in the midaxillary line, and the 10th rib in the midscapular line. The midscapular line runs downward from the inferior angle of the scapula with the arm at rest. The inferior border joins the posterior medial border of the lung 2 cm lateral to the tenth thoracic vertebra. The posterior medial border runs 2 cm lateral to the vertebral column from the seventh cervical vertebra to the tenth thoracic vertebra.
Table 3-2
Anatomic Arrangement of the Bronchopulmonary Segments
| Lobe | Right Lung: Bronchopulmonary Segments | Lobe | Left Lung: Bronchopulmonary Segments |
| Upper |
Anterior: Occupies basal area beneath the oblique fissure anteriorly
Superior: Occupies half the area from the oblique fissure downward* on the posterior aspect
Lateral: Extends from the junction of the middle lobe over the midaxillary area to occupy one-third the area inferior to the superior segment on the posterior aspect
Posterior: Occupies two-thirds of the area posteriorly beneath the superior segment
Medial: Occupies a space on the inner aspect of the right base†
Anterior: Occupies area inferior to the oblique fissure anteriorly
Superior: Occupies one-third of the basal area posteriorly from the oblique fissure downward
Lateral: Occupies the lateral half of the remaining two-thirds of the left lower lobe beneath the superior segment on the posterior aspect
Posterior: Occupies the medial portion of the remaining two-thirds of the left lower lobe beneath the superior segment on the posterior aspect
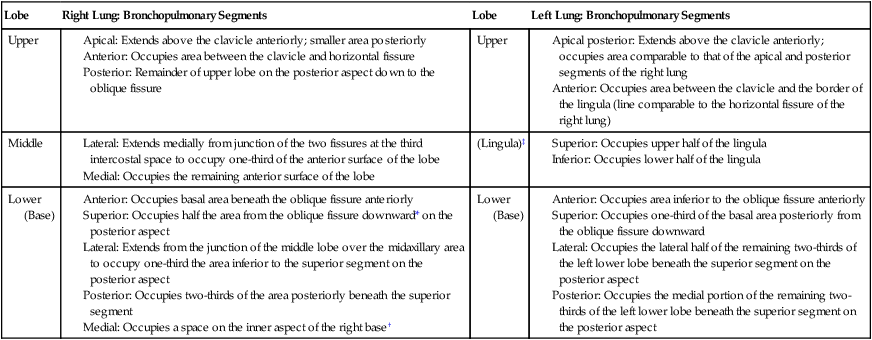
*This segment is best drained when the patient lies prone. Superior segments also called apical segments.
†Medial basal segment has no direct exposure to the chest wall; therefore it cannot be directly auscultated. This segment is best drained when the patient is positioned for the left lateral basal segment because of the comparable angle of its bronchus.
‡Lingula is not an area that is anatomically distinct from the right middle lobe; rather, it is anatomically part of the left upper lobe.
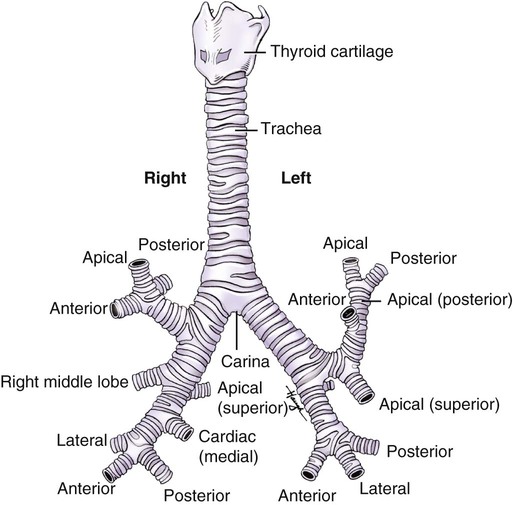
Bronchopulmonary Segments
The bronchopulmonary segments lie within the three lobes of the right lung and the two lobes of the left lung. There are 10 bronchopulmonary segments on the right and 8 on the left. Brief anatomic descriptions of the position of each lobe are provided in Table 3-2. Figure 3-12, A, illustrates the surface markings on the anterior view of the lungs and the position of the various bronchopulmonary segments within the major anatomic divisions provided by the fissures. Figure 3-12, B, shows some of these features from the lateral views. Figure 3-12, C, illustrates the surface markings and bronchopulmonary segments of the posterior aspect of the lungs.
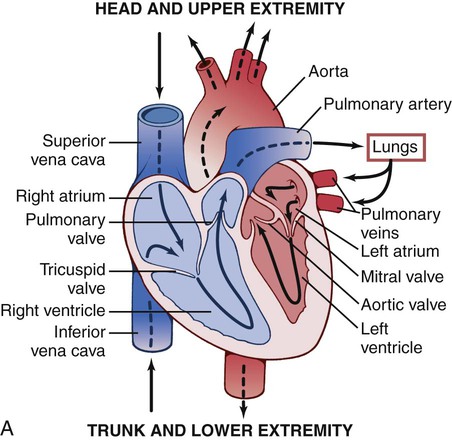
Heart
The heart is divided into right and left halves by an obliquely placed longitudinal septum (Fig. 3-13, A). Each half has two chambers: the atrium, which receives blood from veins, and the ventricle, which ejects blood into the arteries. The superior vena cava, inferior vena cava, and intrinsic veins of the heart deposit venous blood into the right atrium. Blood then passes through the tricuspid valve to the right ventricle. The right ventricle ejects the blood through the pulmonary valve into the pulmonary arteries, which are the only arteries in the body that contain deoxygenated blood. Pulmonary veins return the blood to the left atrium, and from there it passes through the mitral valve to the left ventricle. From the left ventricle it is ejected through the aortic valve into the main artery of the body, the aorta.
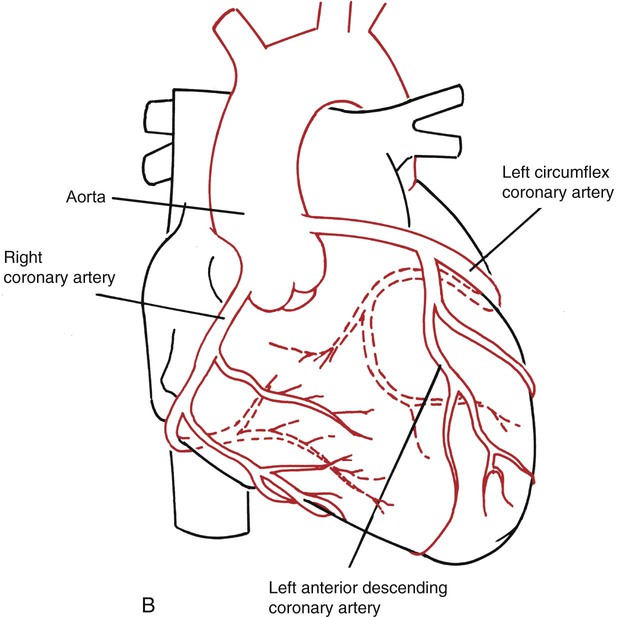
Heart Valves
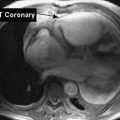
The arterial supply of the heart muscle is derived from the right and left coronary arteries, which arise from the aortic sinuses (Fig. 3-13, B). The left coronary artery (LCA) divides into the anterior descending artery and the left circumflex artery. These arteries supply most of the left ventricle, the left atrium, most of the ventricular septum, and in 45% of people, the sinoatrial (SA) node. The right coronary artery (RCA) supplies most of the right ventricle, the atrioventricular (AV) node, and in 55% of people, the SA node. Infarction of these arteries or their branches can cause interruption or cessation of the conduction system and death of the myocardial muscle in the area supplied by the artery. The severity of the infarction is dependent on the size of the artery and the importance of the area it supplies.
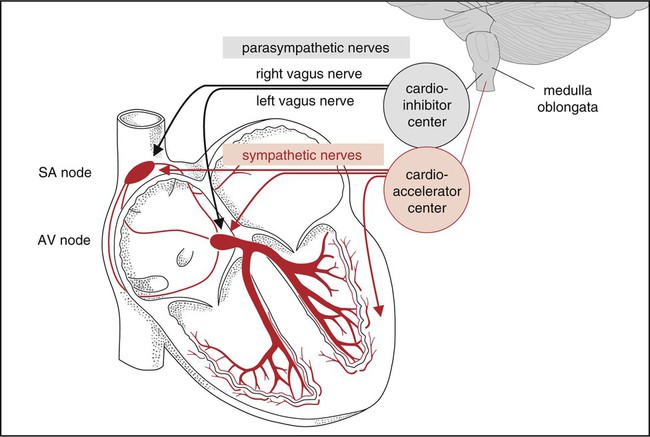
Innervation
Innervation of the heart involves a complex balance between its intrinsic automaticity and extrinsic nerves (Fig. 3-14). The SA and AV nodes provide the heart with an inherent ability for spontaneous rhythmic initiation of the cardiac impulse. The rate of this impulse formation is regulated by the autonomic nervous system (ANS), which also influences other phases of the cardiac cycle. It controls the rate of spread of the excitation impulse and the contractility of both atria and ventricles.
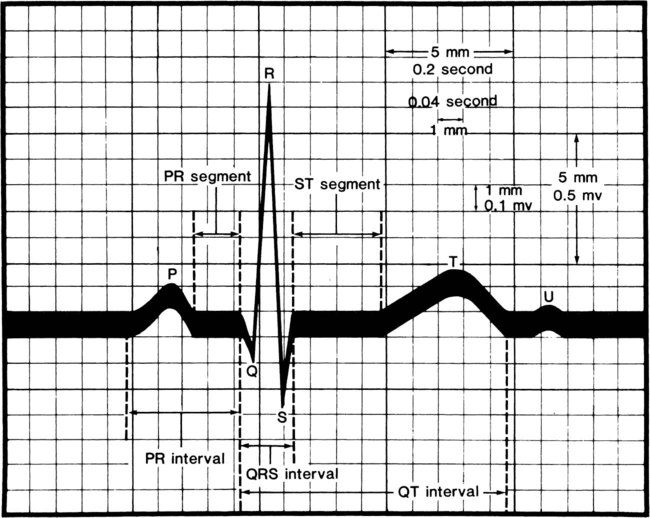
The muscle fibers of the heart are self-excitatory, which enables the heart to contract rhythmically and automatically. The normal pacemaker of the heart, the SA node, is located in the posterior wall of the right atrium. The concentric waves of excitation sent out by the SA node must travel through the AV node to reach the ventricles. This node is located in the floor of the right atrium, just above the insertion of the tricuspid valve. Its main function is to cause a 0.04-second delay in impulse transmission. This delay is beneficial for two reasons: it postpones ventricular excitation until the atria have had time to eject their contents into the ventricles, and it limits the number of signals that can be transmitted by the AV node. The AV node also has its own inherent rhythmicity, firing at a much slower rate than the SA node (40 to 60 beats per minute). Its main pathology is a result of occlusion of the right coronary artery, which supplies the AV node in 90% of cases. From the AV node arises a triangular group of fibers known as the AV bundle, or bundle of His. This bundle divides in the ventricular septum into two branches: the left bundle branch and the right bundle branch. Each of these bundles continues to divide into many fine nerve fibers that spread throughout the ventricles and terminate in the Purkinje fibers, which are continuous with the cardiac muscle. The waves of excitation pass through the bundle of His, down the bundle branches, and through the Purkinje fibers, which permeate the ventricles and cause them to contract. This wave of depolarization gives rise to the normal P-QRS-T configuration of the electrocardiogram (ECG) tracing (Fig. 3-15) (see Chapter 12). The P wave indicates atrial depolarization, the QRS complex indicates ventricular depolarization, and the T wave indicates ventricular repolarization. There is no wave indicating atrial repolarization because atrial repolarization is embedded in the QRS complex13,15 (see Physiology of the Electrical Excitation of the Heart and ECG Interpretation, Chapter 12).14,15
Systemic Circulation
The systemic vascular system is a complex series of branching blood vessels throughout the body. It provides nutrition and oxygen to, and removes waste products from, all tissues of the body. The driving force for this system is the heart. The vascular system has two major components: the peripheral and the pulmonary circulations.5
The microcirculation specifically consists of the metarterioles, the capillary bed, and the venules. The capillary wall is a semipermeable membrane that is responsible for the transfer of oxygen, nutrients, and waste between the circulation and tissue via the interstitial fluid (see Chapters 2 and 4). The capillary pores selectively allow molecules of different size to pass through them. This is an essential feature that regulates the movement of fluid in and out of the intravascular and extravascular compartments. This process is fundamental to maintaining and regulating normal hemodynamics.