Cardiovascular Anatomy and Segmental Approach to Imaging of Congenital Heart Disease
Embryologic Basis for the Segmental Approach to Heart Disease
Embryology of the heart is covered in Chapter 62. In this chapter, some embryologic events that are fundamental to understanding the segmental approach to heart disease are reiterated.
The heart develops from two simple epithelial tubes that fuse to form a single tube (Fig. 63-1) with the following components:
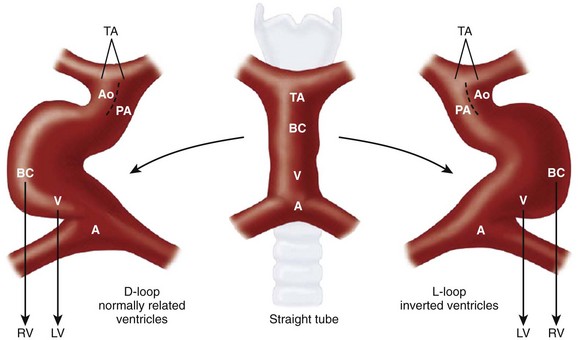
Figure 63-1 Bulboventricular looping of the primitive heart tube may occur to the right (D-looping) or to the left (L-looping).
Ao, aorta; BC, bulbus cordis; LV, left ventricle; PA, pulmonary artery; RV, right ventricle. (Modified from Van Praagh R, Weinberg PM, Matsuoka R, et al. Malposition of the heart and the segmental approach to diagnosis. In: Adams FH, Emmanouilides GC, eds. Moss’ heart diseases in infants, children and adolescents. 3rd ed. Baltimore: Williams & Wilkins; 1983.)
Sinus venosus consists of right and left horns. Each horn receives blood from three important veins: the umbilical vein, the common cardinal vein, and the vitelline vein.
Paired primitive atria will later fuse to form a common atrium.
The atrioventricular (AV) sulcus divides the common atrium and the primitive ventricle.
The primitive ventricle becomes the left ventricle (LV).
The interventricular sulcus divides the primitive ventricle and the bulbus cordis.
The bulbus cordis may be divided as follows: the proximal third gives rise to the body of the right ventricle (RV). The distal-most section is the truncus arteriosus, which develops into the aortic root and part of the pulmonary artery (PA). The remaining mid portion is the conus cordis, which connects the primitive RV to the truncus arteriosus. The conus cordis partitions to form the outflow tracts of the RV and LV.
Although the two ends of the heart tube remain relatively fixed, rapid growth of the middle section results in the development of a large S-shaped curve called the bulboventricular loop (see Fig. 63-1). As the heart tube grows and becomes longer, it usually bends to the right, termed D-looping by Van Praagh. D-looping is responsible for the proximal bulbus cordis (RV) lying anterior and to the right of the primitive ventricle (LV). If the heart tube loops to the left, termed L-looping, the RV will lie anterior and to the left of the LV.
In the heart tube stage, the primitive LV and the proximal bulbus cordis (primitive RV) are separated from the truncus arteriosus (which gives rise to both great arteries) by the conus or infundibulum. The conus consists of the subpulmonary and subaortic conus cushions. Normally, expansile growth of the subpulmonary conus occurs, causing it to protrude anteriorly on the left, carrying the pulmonary valve anteriorly, superiorly, and to the left of the aortic valve. Resorption of the subaortic conus occurs. Hence the aortic valve lies posterior, inferior, and right-sided, in direct fibrous contiguity with the mitral valve (Fig. 63-2). The anterior pulmonary artery rises above the anterior ventricle (RV) and leads to the posterior sixth arterial arch, which forms the branch pulmonary arteries. The posterior aorta originates above the posterior LV and leads to the anterior fourth arterial arch (which forms the aortic arch).
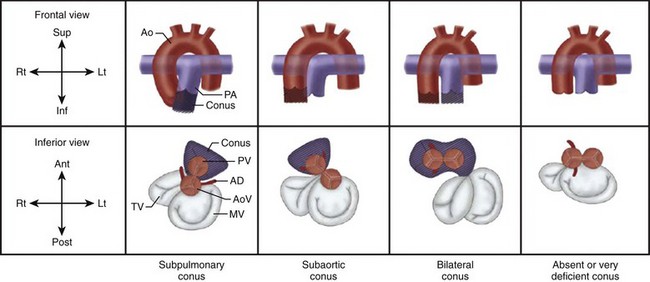
Figure 63-2 Normal and abnormal conal development.
A, Subpulmonary conus seen in normally related great arteries. B, Subaortic conus in typical transposition of the great arteries. C, Bilateral conus, as in double-outlet right ventricle. D, Absent or deficient conus, as in double-outlet left ventricle. On the side of the conus, the semilunar valve sits atop the muscular infundibulum, and no direct fibrous contiguity exists between the semilunar valve and the atrioventricular (AV) valve. On the side of the deficient conus, direct fibrous contiguity usually exists between the AV valve and the semilunar valve. Ant, anterior; Ao, aorta; AoV, aortic valve; Inf, inferior; Lt, left; MV, mitral valve; PA, pulmonary artery; Post, posterior; PV, pulmonary valve; Rt, right; Sup, superior; TV, tricuspid valve. (Modified from Van Praagh R, Weinberg PM, Matsuoka R, et al. Malposition of the heart and the segmental approach to diagnosis. In: Adams FH, Emmanouilides GC, editors: Moss’ heart diseases in infants, children and adolescents. 3rd ed. Baltimore: Williams & Wilkins; 1983.)
1. The development of the suprahepatic portion of the inferior vena cava (IVC) is closely linked to the growth of the liver, and thus the anatomic right atrium (RA) and the liver almost invariably develop on the same side of the body. This concept of visceroatrial situs is fundamental to the segmental approach.
2. Ventricular looping is independent of the visceroatrial situs. This phenomenon gives rise to the concept of concordance (RA-RV and left atrium [LA]-LV) and discordance (RA-LV and LA-RV).
3. Ventricular looping and the great arterial relationship are independent entities. The direction of bulboventricular looping and the development of the conotruncus are responsible for the ultimate relationship of the great arteries to each other and to the underlying ventricles and AV valves.
Segmental Approach to Diagnosis of Congenital Heart Disease
One can think of the heart as a three-level house (Fig. 63-3). The first level is the visceroatrial situs, the middle level is the ventricular loop, and the third level is the conotruncus. To describe it simply, the three levels are the atria, ventricles, and great arteries. The heart has two staircases: the AV ventricular junction and the ventriculoarterial junction. The levels represent the major cardiac segments. The staircases represent the connecting segments.
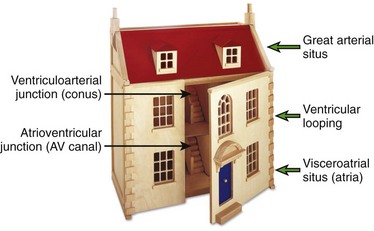
Figure 63-3 The “house” model of the heart.
The three levels (major cardiac segments) are the atria, ventricles, and great arteries. The house has two connecting walls with doors (connecting segments): the atrioventricular junction and the ventriculoarterial junction. The house has two entrances: the systemic veins and the pulmonary veins.
The segmental approach to heart disease comprises the following steps:
1. What is the anatomic type of each of the three major cardiac segments: the atria, the ventricles, and the great arteries?
2. How is each segment connected to the adjacent segment?
3. What are the associated anomalies involving the valves, atrial and ventricular septum, the great vessels, and the systemic and pulmonary veins?
4. How do the segmental combinations and connections, with or without the associated malformations, function?
Identification of the Major Cardiac Segments
Atrial Identification
The defining features of the morphologic RA (systemic venous atrium) and LA (pulmonary venous atrium) are based on their venous connections, as well as their appendage and pectinate muscle morphology. Using venoatrial connections for atrial identification is based on the fact that the sinus venosus, which carries the systemic venous return, is an integral part of the morphologic RA. Hence the morphologic RA receives the IVC and the superior vena cava (SVC) and the orifice of the coronary sinus. However, the SVC and coronary sinus have a high incidence of variation, which can be a source of diagnostic confusion. These variations include left SVC to an unroofed coronary sinus and bilateral SVC with the left SVC draining to an unroofed coronary sinus. In these cases, the SVC would appear to drain into the LA. In rare instances, even the IVC may drain into the coronary sinus, which may be unroofed, or the coronary sinus septum may be absent. In spite of this rare exception, the most reliable means of identifying the morphologic RA by cross-sectional imaging is by recognizing its connection to the IVC (Fig. 63-4). Even in the setting of an interrupted IVC, a suprahepatic segment of the IVC is present entering the RA, allowing accurate identification.
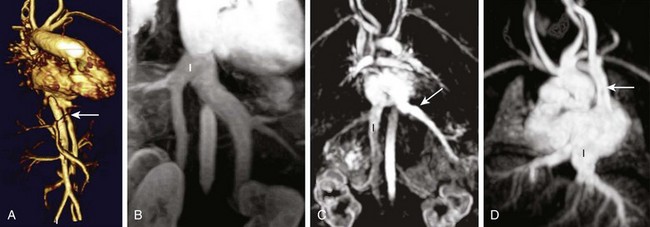
Figure 63-4 The inferior vena cava (IVC) as a marker of the morphologic right atrium (RA).
A, The IVC (arrow) and aorta are on the left and the IVC enters the left-sided atrium, which represents the morphologic RA. B, Bilateral IVC that fuse (I) prior to entering the right atrium. C, The right hepatic vein enters the IVC (I), which drains into the RA, while the remaining hepatic veins (arrow) drain separately into the left atrium. D, Left sided-interrupted IVC with azygos continuation. A suprahepatic segment of the IVC (I) drains into the left-sided right atrium in this patient with atrial situs inversus. A left superior vena cava also is present (arrow).
Ventricular Identification
1. Muscular connection between the free wall and the interventricular septum (moderator band) (Fig. 63-5, A)
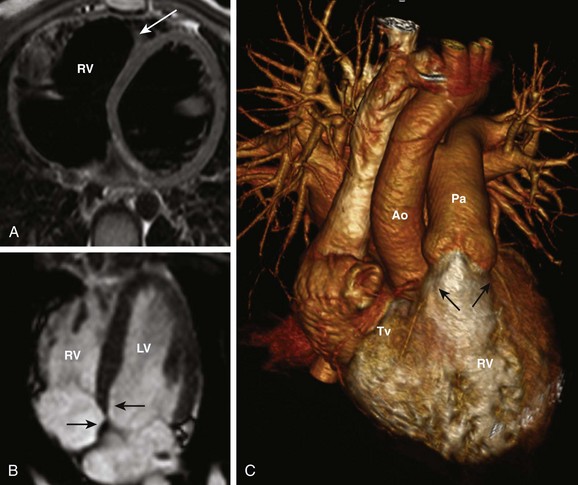
Figure 63-5 Right ventricular identification.
A, Moderator band of the right ventricle (RV) (arrow). B, The atrioventricular (AV) valve of the RV (tricuspid valve) is more apically displaced (arrow) than the AV valve of the left ventricle (LV) (mitral valve) (arrow). C, The conus (arrows) is a marker of the RV. It is a muscular cone of tissue that separates the AV valve (Tv) from the semilunar (pulmonary) valve on the same side. Ao, Aorta; Pa, pulmonary artery.
2. The septal attachment of the AV valve of the RV (tricuspid valve) is more apically placed relative to the LV (Fig. 63-5, B)
3. Presence of a conus/infundibulum; the infundibulum is identified as a muscular cone of tissue that separates the AV valve from the semilunar valve on the same side, resulting in a lack of fibrous contiguity between the two valves (Fig. 63-5, C)
The morphologic LV is identified by the following features:
1. Smooth surface of the interventricular septum without any muscular attachments to the free wall (Fig. 63-6)

Figure 63-6 Left ventricular identification.
A, Smooth septal surface of the left ventricle (LV) (arrow). B, The LV does not have a conus, resulting in mitral (Mv) to aortic (Ao) fibrous contiguity (arrow). RV, Right ventricle.
2. The septal attachment of the AV valve of the LV (mitral valve) is more cranially located relative to the RV (see Fig. 63-6, B)
3. Absence of a conus/infundibulum, resulting in fibrous contiguity between the AV valve and the semilunar valve on that side (see Fig. 63-6)
Great Arterial Identification
The pulmonary artery gives rise to branches to the lungs and no branches to the body. The aorta gives rise to branches to the body as well as the coronary arteries. A common vessel arising from the ventricles that gives rise to the coronaries and branches to the body as well the lungs is termed a common arterial trunk or truncus, and a segmental relationship is not assigned (labeled “X” for undetermined). On transverse cross-sectional imaging at the level of the outflow tract, the coronary artery origin is used to identify the aortic annulus. The intercoronary commissure of the aortic valve is pointed toward the right-left commissure of the pulmonary valve and is an important landmark for determining great arterial relationship (Fig. 63-7). In a solitus relationship of the great arteries, the aortic valve annulus lies posterior and to the right of the pulmonary valve annulus.
Analysis of the Three Major Cardiac Segments
First Major Segment: Visceroatrial Situs
Situs refers to the position of the atria and viscera relative to the midline. Three types of situs exist: solitus (S), inversus (I), and ambiguous (A) (Fig. 63-8). Heterotaxy is synonymous with situs ambiguous.

Figure 63-8 First major cardiac segment: visceroatrial situs.
The three types of visceroatrial situs: situs solitus, situs inversus, and situs ambiguous. CS, coronary sinus; IVC, inferior vena cava; LSVC, left-sided vena cava; RSCV, right-sided vena cava; SVC, superior vena cava. (Modified from Van Praagh R, Weinberg PM, Matsuoka R et al. Malposition of the heart and the segmental approach to diagnosis. In Adams FH, Emmanouilides GC, editors: Moss’ heart diseases in infants, children and adolescents, ed 3. Baltimore: Williams & Wilkins; 1983.)
1. Asplenia complex—Manifestations include bilateral three-lobed lungs with eparterial bronchi, a transverse or symmetric liver, a bilateral SVC, and bilateral triangular broad-based atrial appendages. The spleen is absent.
2. Polysplenia complex—Manifestations include bilateral two-lobed lungs with hyparterial bronchi, a transverse liver, multiple splenic fragments, and bilateral tubular atrial appendages. The renal to hepatic segment of the IVC is frequently absent, with associated azygos continuation.
3. Van Praagh has added a third syndrome of heterotaxy manifested by levocardia with a single right-sided spleen. Features of this syndrome are similar to that of right isomerism.
Second Major Segment: Ventricular Loop
Depending on the direction of ventricular looping during development, the RV may be located spatially on the right or left side of the heart (Fig. 63-9). If the bulboventricular loop occurs to the right, it is termed a D-loop, and the morphologic RV lies anterior and to the right of the morphologic LV. If the bulboventricular loop occurs to the left, it is termed an L-loop, and the morphologic RV lies anterior and to the left of the morphologic LV.

Figure 63-9 The second major cardiac segment: ventricular looping.
A, D-looping with the right ventricle (RV) lying anterior and to the right of the left ventricle (LV). B, L-looping, with the RV lying anterior and to the left of the LV. The RV is identified by the moderator band (red arrow), apical displacement of the AV valve (yellow arrow), and the conus (not shown).
Third Major Segment: Great Arterial Relationship
Normally the aortic annulus lies posterior, inferior, and to the right of the pulmonary valve annulus. This position is called solitus (S) (Fig. 63-10, A). In situs inversus, the aorta lies posterior and to the left of the pulmonary valve, termed inversus (I) (Fig. 63-10, B). Any other position of the aorta and pulmonary artery other than solitus or inversus is termed malposition. If the aorta lies to the right of the PA, it is called D-malposition (Fig. 63-10, C). If the aorta lies to the left of the PA, it is termed L-malposition (Fig. 63-10, D).

Figure 63-10 The third major cardiac segment: arterial relationship.
A, Solitus: the aortic annulus (Ao) lies posterior, inferior, and to the right of the pulmonary valve annulus (Pa). B, Inversus: the aorta lies posterior and to the left of the pulmonary valve. C, D-malposition: the aorta lies anterior and to the right of the pulmonary artery. D, L-malposition: the aorta lies anterior and to the left of the pulmonary artery.
Different Types of Human Hearts
The segmental possibilities at each level are as follows:
Atria: Solitus (S), inversus (I), and ambiguous (A)
Great arteries: Solitus (S), inversus (I), D-malposition, and L-malposition
Based on the various permutations and combinations of atrial, ventricular, and great arterial relationships, as well as on the anatomy of the conus, Van Praagh provided an overview of the diversity that exists in human hearts (Fig. 63-11). It becomes immediately apparent that a pattern-based approach or an approach based on connections or the direction of flow of blood will not do justice to the complexity of the disease process. The segmental approach not only takes into account the morphologic variations at each level but also provides structural landmarks to distinguish the variations from each other and determine their impact on physiology, thereby allowing informed decision making regarding management.

Figure 63-11 Van Praagh’s types of human hearts, based on segmental combinations of visceroatrial situs, ventricular looping, and great arterial situs.
The segmental combination is expressed as a set, within braces. For instance, a normal heart would be expressed as {S,D,S} for visceroatrial situs solitus, D-looping of the ventricles, and solitus relationship of the great arteries. The segmental connections and associated anomalies are expressed outside the braces. For instance, physiologically corrected transposition would be expressed as {S,L,L} transposition of the great arteries for solitus atria, L-looped ventricles, and L-malposition of the great arteries. If this patient also had straddling atrioventricular valve and an inlet ventricular septal defect (VSD), it would be expressed as {S,L,L} transposition of great arteries, straddling tricuspid valve, and inlet VSD. Ant, Anterior; LA, left atrium; LV, left ventricle; Post, posterior; R, right; RA, right atrium; RV, right ventricle. (Modified from Van Praagh R, Weinberg PM, Matsuoka R, et al. Malposition of the heart and the segmental approach to diagnosis. In: Adams FH, Emmanouilides GC, editors: Moss’ heart diseases in infants, children and adolescents. 3rd ed. Baltimore: Williams & Wilkins; 1983.)
Analysis of the Connecting Segments
First Connecting Segment: Atrioventricular Junction
The types of biventricular AV connections (Fig. 63-12) include:

Figure 63-12 Types of biventricular atrioventricular (AV) connections.
A, Concordant AV connections (right atrium-right ventricle [RA-RV] and left atrium-left ventricle [LA-LV]) B, Discordant AV connections (RA-LV and LA-RV). C, Balanced common AV canal with two symmetric sized ventricles. D, Straddling and overriding tricuspid valve (arrow) in a patient with D-looped ventricles. L, Left ventricle; R, right ventricle.
1. Concordant AV connections (RA-RV and LA-LV)
2. Discordant AV connections (RA-LV and LA-RV)
3. Straddling AV valve, in which attachment of the tensor apparatus of the valve to the opposite ventricle is abnormal
4. Overriding AV valve, in which the AV valve annulus crosses the interventricular septum and partly overlies the opposite ventricle
5. Overriding and straddling AV valve
6. Balanced common AV canal with two symmetric-sized ventricles
The types of univentricular AV connections (Fig. 63-13) include:

Figure 63-13 Types of univentricular atrioventricular (AV) connections.
A, Double inlet right ventricle (RV). B, Double inlet left ventricle (LV). C, Right dominant unbalanced common AV canal. D, Tricuspid atresia (arrow shows atretic tricuspid valve plane). E, Mitral atresia (arrow shows atretic fatty mitral valve plane). F, Severe mitral stenosis (arrow) in hypoplastic left heart syndrome. F, Fontan.
Second Connecting Segment: Conotruncus or Ventriculoarterial Junction
The development of the conotruncus is the most important variable in the genesis of outflow tract anomalies. The differential growth of the subpulmonary and subaortic conus cushions largely determines the relationship between the semilunar valves, between the semilunar valves and the ventricles, and between the semilunar valves and the atrioventricular valves. It also determines the presence of distal infundibular stenosis and the location of the ventricular septal defect in outflow tract anomalies. Based on conal development, the following anatomic types of conus (Fig. 63-14) may be recognized:

Figure 63-14 Types of ventriculoarterial connections based on conal development.
A, Concordant ventriculoarterial connection: solitus great arteries with subpulmonary conus. B, Double-outlet right ventricle (RV) with bilateral conus. C, Discordant ventriculoarterial connection: dextraposed transposition of the great arteries with subaortic conus. D, Double-outlet left ventricle (LV) with absent conus. A, Aorta; P, pulmonary artery. (From Krishnamurthy R. Embryologic basis and segmental approach to imaging of congenital heart disease. In: Ho V, Reddy GP, eds. Cardiovascular imaging. 1st ed. Philadelphia: Saunders Elsevier; 2010.)
1. Development of subpulmonary conus and resorption of the subaortic conus results in ventriculoarterial concordance and normal relationship of great arteries. The aorta lies posterior and to the right in a D-loop heart (solitus), and posterior and to the left in an L-looped heart (inversus).
2. Development of the subaortic conus and resorption of the subpulmonary conus results in ventriculoarterial discordance and transposition of the great arteries. Transposition means that the LV is connected to the main pulmonary artery and the RV is connected to the aorta. The aorta lies anterior and to the right in a D-looped heart (D-malposition) (Fig. 63-15) and anterior and to the left in an L-looped heart (L-malposition).

Figure 63-15 Conus in dextroposed transposition of the great arteries (D-TGA).
A and B, The aorta (Ao) lies anterior and to the right of the pulmonary artery (D-malposition). C, The right ventricle (RV) is connected to the aorta by a muscular infundibulum (black arrow), resulting in lack of fibrous contiguity between the tricuspid valve (long white arrow) and the aortic valve (short white arrow). D, The left ventricle (LV) is connected to the main pulmonary artery (Pa) with absence of an intervening conus, resulting in direct fibrous contiguity between the aortic and pulmonary valves (arrow). E, Three-dimensional volume rendering of a patient with D-TGA. H, heart.
3. Persistence and development of both the subaortic and subpulmonary conus leads to a double-outlet RV, meaning that both great vessels arise predominantly from the RV (Fig. 63-16). Variable development of the pulmonary and aortic conus may occur, resulting in variable location of the ventricular septal defect in relation to the great arteries.

Figure 63-16 Double outlet right ventricle (RV) with bilateral conus and side-by-side great arteries.
A, Three-dimensional volume rendering of double outlet RV showing both great arteries arising from the RV. B and C, Presence of a subpulmonary and subaortic conus (arrows). D, Side-by-side great arteries. Ao, Aorta; LV, left ventricle; PA, pulmonary artery.
4. Resorption of both the subpulmonary and subaortic conus results in double-outlet LV, meaning that both vessels arise predominantly from the LV.
Evaluation of Function
1. Pressure overload related to phenomena such as valvular stenosis, coarctation, and branch pulmonary artery stenosis
2. Volume overload related to phenomena such as valvular regurgitation and left to right shunts
3. Intermixing, in which there is mixing of oxygenated blood with deoxygenated blood before entering the systemic circulation; this phenomenon typically occurs in the setting of a common chamber, vessel, or valve or in a right-left shunt
4. Poor contractility of the myocardium related to conditions such as cardiomyopathy or ischemia
Illustrated Case Discussion
A case study of a segmental approach to imaging of CHD is illustrated in Figure 63-17.

Figure 63-17 Illustrative case study of segmental approach to imaging of congenital heart disease.
ao, Aortic outflow; av, aortic valve; cs, coronary sinus; F, Fontan; ivc, inferior vena cava; la, left atrium; lv, left ventricle; mv, mitral valve; pa, pulmonary artery; pv, pulmonary veins; ra, right atrium; rpa, right pulmonary artery; svc, superior vena cava; tv, tricuspid valve.
• Situs solitus of the atria (S): The coronary sinus and IVC enter the right-sided atrium, which therefore represents the morphologic RA. The pulmonary veins enter the morphologic LA. The RA connects to the right pulmonary artery via an atriopulmonary anastomosis. The SVC also connects to the right pulmonary artery.
• AV connection: The right AV valve (tricuspid valve) is atretic and fatty replaced. The left AV valve (mitral valve) is normal.
• D-looping of the ventricles: The large left-sided ventricle has a smooth septal surface consistent with a morphologic LV. The small blind-ending chamber to the right is the infundibular outlet chamber of the RV, which fills via a bulboventricular foramen.
• Ventriculoarterial connection: RV-PA outflow is atretic. Aortic outflow from the LV is normal.
• Solitus relationship of the great arteries (S): The aortic valve lies posterior and to the right of the atretic pulmonary valve.
Colvin, EV. Single ventricle. In: Garson A, Jr., Bricker JT, McNamara DG, eds. The science and practice of pediatric cardiology. Philadelphia: Lea and Febiger, 1990.
Krishnamurthy, R. Embryologic basis and segmental approach to imaging of congenital heart disease. In Ho V, Reddy GP, eds.: Cardiovascular imaging, 1st ed, Philadelphia: Saunders/Elsevier, 2010.
Shinebourne, EA, Macartney, FJ, Anderson, RH. Sequential chamber localization-logical approach to diagnosis in congenital heart disease. Br Heart J. 1976;38:327–340.
Van Praagh, R. Diagnosis of complex congenital heart disease: morphologic-anatomic method and terminology. Cardiovasc Intervent Radiol. 1984;7:115–120.
Van Praagh, R. The segmental approach to diagnosis in congenital heart disease. Birth Defects. 1972;8:4–23.
Van Praagh, S, Kreutzer, J, Alday, L, et al. Systemic and pulmonary venous connections in visceral heterotaxy, with emphasis on the diagnosis of the atrial situs: a study of 109 postmortem cases. In: Clark E, Takao A, eds. Developmental cardiology, morphogenesis and function. Mt. Kisco, NY: Futura, 1990.







