22 Cardiothoracic surgery
Basic considerations
Pathophysiological assessment
Careful history and appropriate examination suggest the presence of possible cardiac pathology. The initial clinical assessment is then refined and specific investigations used to confirm and quantify any disease identified (Table 22.1).
Table 22.1 Specific assessments of cardiac pathophysiological status
| Investigation | Yield |
|---|---|
| ECG | |
| Resting | Rhythm; conduction abnormalities; atrial and ventricular hypertrophy; established ischaemic changes; evidence of previous myocardial infarction |
| Exercise | Exercise-induced ischaemic changes or arrhythmias |
| Chest X-ray | Cardiac enlargement; valvular calcification; evidence of pulmonary oedema (Kerley B lines, pleural effusion, interstitial marking, hilar flare); absent or enlarged cardiac or great vessel structures |
| Thallium isotope scan | Areas of low radio-uptake indicative of impaired myocardial perfusion |
| Echocardiography | |
| Precordial | Ventricular contractility; valvular stenoses, regurgitation or leaflet abnormalities; intracardiac morphology, including septal defects and intracardiac masses; pericardial effusion |
| Transoesophageal | Enhanced views of posterior cardiac structures (aortic and mitral valves, ascending aorta, great veins and posterior septae); posterior pericardial fluid collections |
| Cardiac catheterization | |
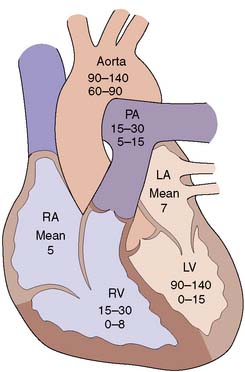
| Chamber pressures | Assess left and right ventricular function via determination of left ventricular end-diastolic pressure; atrial pressures in valve disease; transvalvular gradients (Fig. 22.1) |
| Angiography | Coronary arterial anatomy; intracardiac anatomy; trans-septal flow |
| O2 saturations | Intracardiac shunts |
| Cardiac output | Cardiac function and determination of secondary derived parameters, including peripheral and pulmonary vascular resistance |
Assessment of risk
Stroke
Stroke risk varies from 1% to over 10%, and is associated with intracardiac thrombus and severe atheromatous disease of the proximal aorta and carotids. Patients with evidence of peripheral vascular disease have a higher risk of stroke and those with high-grade symptomatic carotid disease may benefit from carotid endarterectomy prior to cardiac surgery (Ch. 21).
Specific aspects of surgical technique
Cardiopulmonary bypass (CPB)
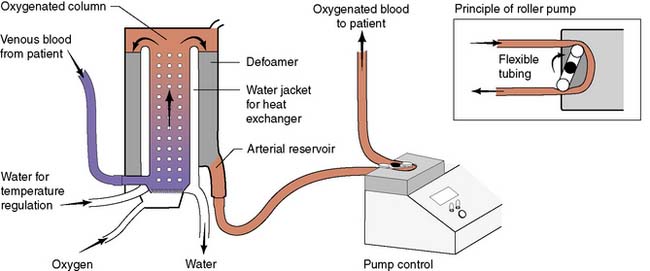
Modern cardiac and great vessel surgery became feasible with the development of cardiopulmonary bypass. Venous blood is drained via cannulae inserted into the right atrium or venae cavae and passes to a reservoir. It is then pumped through an oxygenator, which adds O2 and removes CO2, through a heat exchanger coil so that its temperature can be varied and finally, the blood is returned to the arterial circulation via a cannula in the ascending aorta or other suitable artery (femoral, axillary) (Figs 22.1, 22.2 and 22.3). Full anticoagulation with intravenous heparin is required to prevent blood clotting in the tubing, oxygenator and pump mechanisms. Roller or centrifugal pumps are used, as these minimize red cell trauma. Semipermeable membranes, or more commonly hollow fibres, form the blood–gas interface within the oxygenator. A trained perfusion technician controls the bypass machine.
Postoperative care
Complications
Other than death or stroke, established complications include:
• bleeding – multifactorial causes including hypothermia, platelet dysfunction, CPB and pharmacological (aspirin, clopidogrel)
• low cardiac output – poor myocardial protection, previous poor left ventricular (LV) function
• arrhythmias – atrial fibrillation occurs in up to 40%
Recovery time
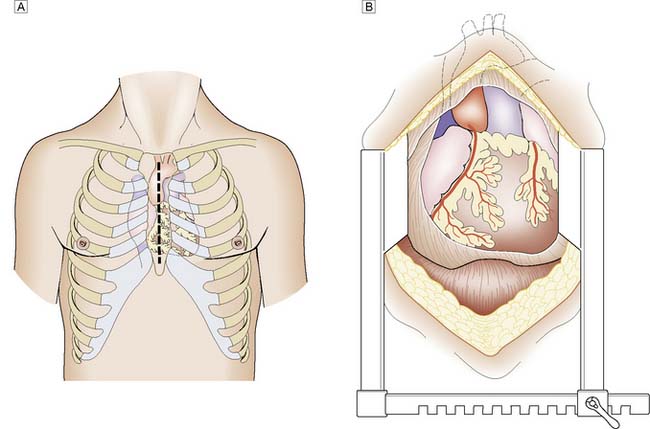
Patients undergoing routine elective coronary or valve surgery will usually leave acute hospital care within one week. Those requiring more extensive surgery or emergency procedures may take longer to recover. Most patients will have undergone a median sternotomy (Fig. 22.4). This wound heals quickly and, as the sternal edges are approximated securely by wire or heavy sutures, chest discomfort eases rapidly. Leg vein donor sites may take longer to heal, particularly around the knee. By 2 weeks the patient should be able to walk a few hundred metres, and by 3 months should have returned to full activity, including work.
Acquired cardiac disease
Surgical intervention may be required in the management of:
Ischaemic heart disease
Coronary artery disease (CAD)
Coronary artery atheroma (Ch. 21) results in narrowing of the vessels and most patients will present for surgery because of angina or previous myocardial infarction (MI).
Assessment
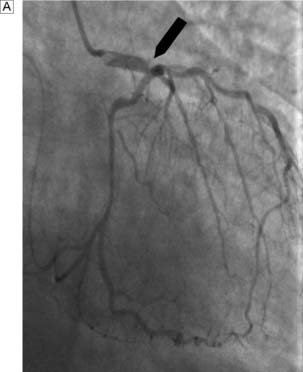
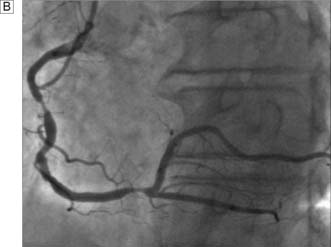
Exercise electrocardiography (ECG) is often used as an initial screening test for patients with suspected stable angina. Those with confirmed ischaemia then undergo coronary angiography and assessment of left ventricular function by means of angiography or echocardiography. Contrast medium is injected into the coronary circulation (Fig. 22.5) via a catheter that is usually inserted through the femoral or radial artery (Fig. 22.6). Images are obtained in several different planes so as to minimize the risk of missing eccentric lesions. Intervention is usually only advised for stenoses that exceed a 70% reduction in vessel diameter.
Coronary bypass
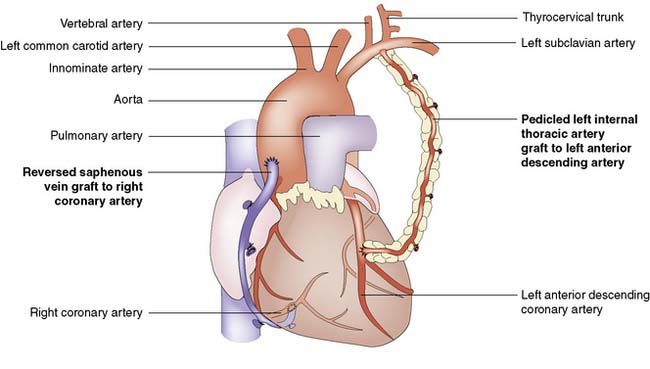
A coronary artery bypass graft (CABG) delivers blood to the distal coronary artery beyond a stenosis. If the distal artery is obliterated by atheroma, an endarterectomy procedure may be performed to restore the lumen. Originally, nearly all grafts comprised reversed segments of the long saphenous vein anastomosed proximally to the ascending aorta and distally to the coronary artery. Such grafts have patency rates of around 70% at 5 years and 40% at 10 years. Venous graft failure occurs as a result of intimal hyperplasia, which is thought to be, in part at least, a response to arterial pressure. The relatively high rate of vein graft failure stimulated interest in arterial grafts and led to the almost universal use of the internal thoracic artery (ITA). This is usually employed as a pedicled graft when it is left attached to the subclavian artery proximally, but can also be used as a free graft in the same manner as vein. ITA graft patency exceeds 90% at 5 years and 70% at 10 years. A common combination is to use the left ITA for the left anterior descending artery and vein grafts for the other vessels (Fig. 22.7).
Results
Uncomplicated coronary surgery should carry a 2–3% risk of mortality and a 1–2% risk of stroke. Angina is relieved completely in about 70% of cases, is significantly improved in the remainder, and recurs with a frequency of about 10% per year. Successful revascularization may also improve breathlessness if it is related to myocardial ischaemia, and survival is probably enhanced in patients with left main stem and triple vessel disease. The use of arterial conduits is associated with better graft patency and improved survival. Although there is a trend in that direction for patients with multiple arterial grafts followed up beyond 10 years, the added benefit over one ITA graft placed to the left anterior descending coronary is small. This may reflect the progression of native coronary disease. Secondary prevention is mandatory in all patients with CAD and includes antiplatelet medication (aspirin) and cholesterol reduction (statin) (EBM 22.1).
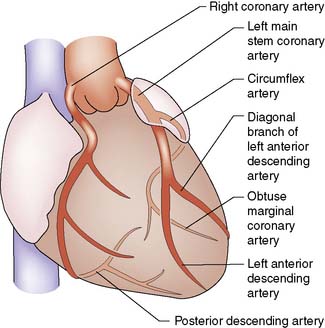
Summary Box 22.1 Coronary anatomy
• There are two coronary arteries (left and right), which have origin in the coronary sinuses: left or posterior sinus, right or anterior sinus
• The left main coronary artery passes behind the pulmonary trunk and divides into two large branches: the left anterior interventricular artery or left anterior descending (LAD), which supplies the anterior left ventricle and anterior two-thirds of the interventricular septum, and the circumflex coronary, which supplies the posterior and lateral walls of the left ventricle
• The right coronary artery passes down anteriorly in the right atrioventricular groove supplying the anterior right ventricle and acute marginal branches
• Either the right or circumflex may terminate as the posterior descending artery which supplies the inferior surface of both ventricles and the lower septum. This artery is then considered to be dominant
• The right, LAD and circumflex are each considered to be a ‘vessel system’. Disease within any one of these three vessels or its branches is termed single-vessel disease. Similarly, two- and three-vessel disease indicates involvement of two and three systems, respectively.
Surgery for the complications of coronary artery disease
Mitral valve regurgitation (MR)
Acute
Acute myocardial infarction involving a papillary muscle may cause this to rupture, causing gross regurgitation. The patient is usually very unwell with pulmonary oedema due to MR and low cardiac output due to infarction, and often requires emergency ventilation. Emergency mitral valve replacement and CABG is associated with a mortality of 15–40%, mainly due to poor ventricular function and secondary multiorgan failure (EBM 22.2).
22.2 Valve replacement
• Aortic valve replacement (AVR) is indicated for symptomatic patients with severe aortic stenosis (AS).
• AVR is indicated for patients with severe AS undergoing coronary artery bypass surgery (CABG) or surgery on the aorta or other heart valves.
• AVR is indicated for symptomatic patients with severe aortic regurgitation (AR) irrespective of left ventricular (LV) systolic function.
• AVR is indicated for asymptomatic patients with chronic severe AR and LV systolic dysfunction or while undergoing CABG or surgery on the aorta or other heart valves.
• Mitral valve (MV) surgery (repair if possible) is indicated in patients with symptomatic moderate or severe mitral stenosis.
• MV surgery is recommended for the symptomatic patient with acute severe mitral regurgitation (MR).
• MV surgery is beneficial for patients with chronic severe MR
Cardiac valvular disease
Surgical management
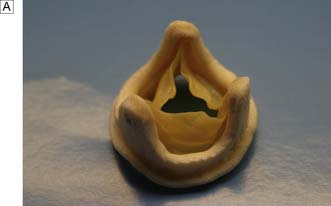
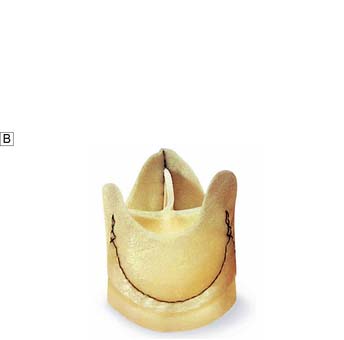
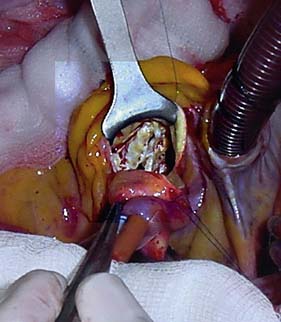
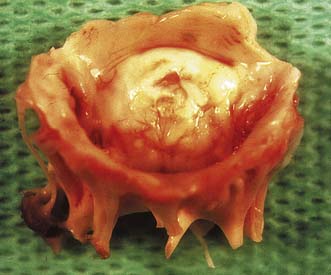
Options include valve replacement or repair. Replacement utilizes either a mechanical or a biological prosthesis. Mechanical valves have developed from the original ball-in-cage design through single disc designs to the current range of carbon bi-leaflet devices (Fig. 22.8). These should last indefinitely, but patients require lifelong warfarin to prevent thrombotic occlusion or embolism. Embolism risk is about 1–6% per year and is influenced by how accurately the INR is controlled. Mechanical valves produce audible clicks.
Biological valves are derived from:
• glutaraldehyde-preserved porcine aortic valves mounted on a frame (stent) (Fig. 22.9A)
• glutaraldehyde-preserved bovine pericardium formed into a three-leaflet valve and mounted on a stent
• glutaraldehyde-preserved porcine aortic unstented valves (Fig. 22.9B)
• human aortic root homografts removed from cadaveric hearts and preserved in antibiotic solution.
Aortic valve disease
Stenosis
Aortic stenosis is the commonest indication for valve surgery in the UK. Although rheumatic disease remains a common problem in underdeveloped countries, the most frequent aetiology in the Western world is calcific aortic stenosis which develops in the older population usually over 70 years. The normal aortic valve has three cusps but a congenital bicuspid valve usually calcifies from the sixth decade onwards (Fig. 22.10). Aortic stenosis causes left ventricular hypertrophy, effort angina, episodes of arrhythmia with syncope or even sudden death, and left ventricular failure.
Mitral valve disease
Stenosis
The timing of surgery is a matter of judgement but an echocardiographic calculated mitral valve area below 1 cm2 demonstrates severe stenosis and is an indication for surgery. Very occasionally, the patient may have echocardiographic evidence of leaflet fusion only, in which case percutaneous balloon valvuloplasty may be effective. Conservative surgery with separation of the fused leaflets (commissurotomy) and reconstruction of the valve is possible in some younger patients. Usually, however, there is extensive leaflet calcification, with involvement of the subvalvular apparatus. There is shortening and thickening of the papillary muscles and chordae tendinae, tethering the leaflets to the tips of the papillary muscles (Fig. 22.11). Valve replacement is therefore the only practical option.
Aortic aneurysm
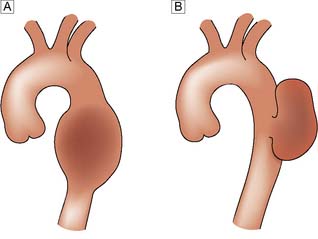
Tubulosaccular aneurysms
These are ‘true’ aneurysms that form either a fusiform (tubular) or a focal (saccular) type of swelling (Fig. 22.12). They are lined by layered thrombus and most are due to medial degeneration secondary to smoking and hypertension (Ch. 21).
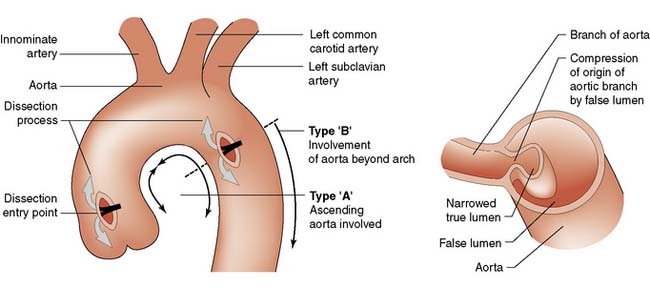
Aortic dissection
Dissections that originate distal to the left subclavian (Fig. 22.13 Type B), do not spread retrogradely to involve the aortic arch or ascending aorta, and are clinically stable, are usually managed conservatively by control of blood pressure, as the results of medical and surgical treatment are not different. Endovascular stent placement via the femoral artery under radiological control has an emerging role in this difficult situation and the decision to intervene on such patients is based on the development of rupture and organ/limb ischaemia.
In contrast, most patients with dissections that involve the ascending aorta (Fig. 22.13 Type A) or arch are offered emergency surgery to prevent rupture, stroke, MI and aortic valve incompetence. Surgery involves excising and replacing the portion of the aorta containing the entry point. This prevents more blood entering the false lumen and reapposes the layers of the aortic wall. Additional surgery to repair the aortic valve or to replace the aortic arch or descending aorta will be determined by individual circumstances.
Assessment
A patient with an incidentally discovered aneurysm should be thoroughly investigated, including tests of respiratory function and coronary angiography with contrast CT or MRI angiogram to fully delineate the extent of the aneurysm. Aneurysms that extend from the chest into the abdomen (thoracoabdominal aneurysm) require further investigations to clarify the relationship of the aneurysm to the renal and visceral vessels. Larger aneurysms (6 cm or greater) are more likely to rupture, and serial investigation will confirm whether or not an aneurysm is enlarging. Based on these considerations, a decision can then be taken regarding the potential benefit of surgery. In patients presenting with acute rupture of an aneurysm the diagnosis may have been made by one of the modalities described above or by transthoracic or transoesophageal echocardiography (Fig. 22.14). Surgery is recommended if they are potentially salvageable and considered likely to benefit from operative intervention.
Pericardial pathology
Pericardial constriction
Chronic pericardial inflammation, often from tuberculosis, may heal by intense fibrosis and calcification (Fig. 22.15). This leads to chronic tamponade and investigations should include echocardiography, right heart catheterization with record of chamber pressures and CT or preferably MRI. Surgery is undertaken via a median sternotomy to remove the parietal pericardium and any fibrotic visceral pericardium, and can be performed with or without CPB. Such surgery is difficult and can be accompanied by significant blood loss. The results are frequently disappointing because the patient may already have developed irreversible hepatic cirrhosis and myocardial function is poor.
Congenital cardiac disease
Coarctation of the aorta
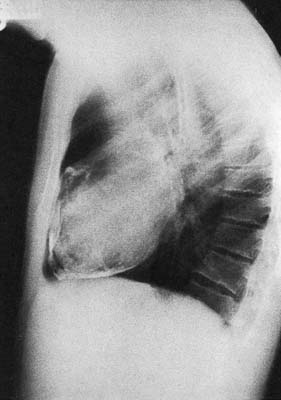
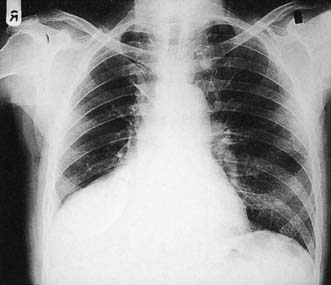
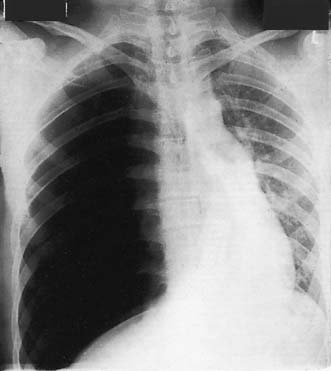
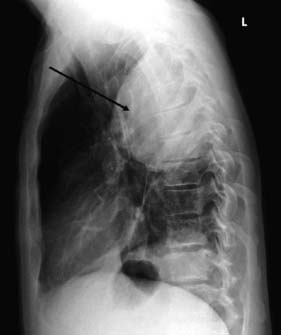
This condition is caused by a narrowing of the thoracic aorta, usually at the level of the ligamentum arteriosum. The lower body is perfused via extensive chest wall collaterals. Upper body hypertension develops and may lead to heart failure in infancy. Untreated adults develop hypertensive cerebrovascular and renal problems and accelerated coronary atheroma. Most children and young adults are asymptomatic and present with high blood pressure or an abnormal chest X-ray. The femoral pulses may be impalpable or weak and delayed, and a systolic murmur may be audible over the back. LVH is seen on the ECG and the chest X-ray shows an enlarged heart, reduced aortic knuckle and characteristic rib ‘notching’, caused by enlarged and tortuous intercostal arteries eroding the ribs near the posterior angles (Fig. 22.16). Balloon angioplasty has been used to dilate some coarctations in infants, but surgical correction is usually required. At operation, the left subclavian artery may be used as an onlay patch. Older children and adults are usually managed with a Dacron bypass graft. Surgical correction tends to reduce upper body hypertension in children. It is less effective in adults but pharmacological control of hypertension becomes more reliable. The operative risk is about 5%.
Thoracic surgery
Assessment
This is concerned with confirming the diagnosis, determining in oncological cases whether resection is appropriate, and establishing that the patient is fit for the intended surgical procedure. The principal investigations are summarized in Table 22.2. History can be instructive in suggesting advanced malignant disease and in providing evidence of the patient’s functional status.
| Investigation | Yield |
|---|---|
| ECG | |
| Resting | Rhythm; conduction abnormalities; atrial and ventricular hypertrophy; established ischaemic changes; evidence of previous myocardial infarction |
| Chest X-ray | |
| Posterior-anterior and lateral | Preliminary assessment of location of lesion; malignant involvement of phrenic nerve or ribs; presence of additional lesions or effusion; presence of pneumothorax or mediastinal air |
| Thoracic CT | Further refine radiological assessment of mass lesions as above; review mediastinum for enlarged nodes in bronchogenic carcinoma; inspect bronchi for dilatations in suspected bronchiectasis; determine areas of greatest disease in interstitial lung disease; locate intrathoracic collections; map out distribution of bullous/emphysematous lung disease |
| PET CT | Identify further disease elsewhere through metabolic uptake of 18F-fluorodeoxyglucose not identified by conventional CT |
| Upper abdominal CT | Exclude or confirm liver abnormalities; identify adrenal metastases |
| Upper abdominal ultrasound | Determine probable nature of cystic hepatic lesions; provide guidance for biopsy of hepatic or adrenal lesions; review diaphragm motion in cases of suspected diaphragmatic rupture or phrenic nerve paralysis |
| MRI | Useful for assessing relationship of tumour to adjacent neural structures, e.g. detecting possible intraspinal extension of paravertebral neurogenic tumours or involvement of brachial plexus by superior sulcus (Pancoast) tumours |
| Isotope scans | |
| Bone Lung |
Search for skeletal metastases; review chest wall for possible direct invasion by carcinoma Identify areas of low uptake indicative of impaired perfusion or ventilation |
| Pulmonary function tests | |
| FEV1 | Forced expiratory volume in 1 second; provides a measure of airway obstruction |
| FVC | Forced vital capacity; indicates presence of restriction of ventilation |
| CO transfer | Measures the diffusion capacity of the patient’s lungs |
| Walking test | Measures distance walked by the patient in a set time period (4 mins) and the perceived exercise level achieved as assessed by the final heart rate; useful as an indicator of functional status in patients with poor FEV1, as they may not comply well with the methodology of formal respiratory testing and hence underachieve |
| Arterial blood gas | Useful in demonstrating patients with CO2 retention who should be excluded from surgical consideration |
Bronchogenic carcinoma
Aetiology, pathology and presentation
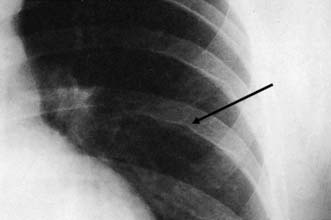
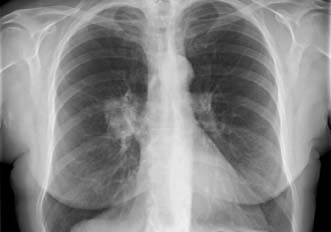
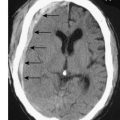
With the exception of alveolar cell carcinomas, which arise from cells lining the alveoli, Primary lung cancers arise within the bronchial epithelium and are hence termed bronchogenic carcinoma. They are described as peripheral or central, according to their location within the lung (Figs. 22.17, 22.18 and 22.19). Peripheral lesions may grow to 8 cm or more before causing local symptoms such as chest wall pain. Many are detected as incidental findings on a chest film taken for unrelated reasons, or for non-specific symptoms such as weight loss. Central lesions tend to occlude the airways, causing varying degrees of pulmonary collapse and consolidation (Fig. 22.19). Nodal spread occurs to the intralobar, hilar and mediastinal nodes, and thence to the scalene nodes. Metastases occur in bone, brain, liver, adrenals and lung. Local direct spread may involve the chest wall, vertebrae, trachea, oesophagus and great vessels.
As small cell lung cancer is regarded as a systemic disease at presentation, patients are not usually referred for surgery and are therefore treated with chemotherapy. All other varieties are resected if possible (EBM 22.3). Therefore, for surgical treatment purposes, bronchogenic carcinoma is categorized into small cell and non-small-cell. However, cell type is important as recent advances in pathology have found genetic mutations that identify tumours that may be sensitive to new chemotherapeutic agents.
• Patients with Stage I and II non-small cell lung cancer should be considered for curative surgery where possible.
• Lung resection should be as limited as possible without compromising cancer clearance. Lobectomy is the procedure of choice for fit patients.
• Every effort should be made to avoid a futile thoracotomy.
• Systematic lymph node dissection is recommended as offering the best compromise between accuracy of staging and containment of morbidity.
Assessment for pulmonary resection
Fitness for resection
Staging
Assessment of the potential for curative resection is determined by staging. Initial clinical assessment will normally filter out advanced disease and provide evidence of incurability because of local irresectability or disseminated disease (Table 22.3). Chest X-ray may reveal an elevated diaphragm, indicating phrenic nerve involvement, bone metastases or direct invasion of the rib cage. If an effusion is present, this should be aspirated; if malignant cells are noted on cytology, this would preclude resection.
Table 22.3 Clinical indicators of locally irresectable or incurable lung cancer
| Clinical finding | Pathological implication |
|---|---|
| Local inoperability | |
| Horner’s syndrome | Involvement of upper sympathetic chain |
| Hoarseness | Involvement of left recurrent laryngeal nerve |
| Upper body venous congestion | Involvement of superior vena cava |
| Severe shoulder/inner arm pain | Involvement of brachial plexus (Pancoast tumour) |
| Disseminated disease | |
| Scalene node enlargement | Nodal spread out of operative field |
| Hepatomegaly | Hepatic metastases |
| Focal bone pain | Bone metastases |
| Skin deposits | Cutaneous metastases |
| Behavioural/balance disturbance | Cerebral/cerebellar metastases |
| Headache |
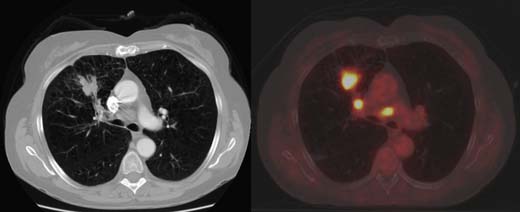
Contrast-enhanced thoracic and upper abdominal CT will clarify the nature and position of the pulmonary mass and should exclude other pulmonary lesions that might represent metastases or synchronous tumours. Mediastinal nodes < 1 cm in long axis are generally considered to be benign, but surgical sampling is necessary to confirm this. Where available, a combined thoracic CT/positron emission tomography (PET CT) scan is helpful in both locating and characterizing mediastinal lymph nodes (Fig. 22.18). A negative PET scan is highly accurate in predicting the absence of tumour involvement; a positive scan may indicate tumour but can also arise with inflammatory conditions, and, therefore, the positive glands must be sampled by mediastinoscopy. The liver and adrenals are common sites for metastases. Suspicious areas can be sampled by means of ultrasound-guided biopsy. Further investigations, such as bone or brain scans, will depend upon the clinical suspicion.
• Mediastinoscopy is used to sample the paratracheal and subcarinal lymph nodes. A low anterior cervical incision is made just above the jugular notch and the mediastinoscope used to create a passage in the pretracheal region. The lymph nodes are dissected and biopsied. EUS: Under Endoscopic Ultrasound guidance, fine needle aspiration of mediastinal nodes may be performed either transbronchially or transoesophageally.
• Mediastinotomy is used mainly to assess lymph nodes within the concavity of the aortic arch or anterior to the aorta, as these areas cannot be reached at mediastinoscopy. Access is gained via a short left anterior second interspace incision.
• Videothoracoscopy is a technique that allows the surgeon to inspect the pleural cavity, biopsy the primary lesion and sample the lower mediastinal and aortic arch lymph nodes. The extent of resection likely to be required can also be assessed in relation to the patient’s lung function. Videothoracoscopy may also reveal unforeseen causes of irresectability, such as pleural seedlings.
Resection
Lung tumours are normally removed en bloc with the surrounding parenchyma and local draining lymphatics. This involves either lobectomy or pneumonectomy. Occasionally, in unfit patients, small cancers are excised within a wedge or segment of lung but the risk of local recurrence is greater in these lung-sparing cases. An area of anterior chest wall directly invaded by tumour can be excised and replaced with synthetic patch, provided it is lateral to the posterior rib angles. Following assessment and surgical resection, the final pathological TNM stage (Table 22.4) is helpful in indicating prognosis and determining whether a patient might benefit from adjuvant therapy usually within the setting of a trial. Patients who are found to have positive mediastinal nodes following resection are routinely referred for adjuvant radiotherapy to the mediastinum in view of the high risk of recurrence in that area. Postoperative chemotherapy may improve 5-year survival across all resected stages by approximately 5%. Although still controversial, this form of adjuvant therapy is likely to become an increasingly common option for suitably fit patients. Operative mortality is about 2% for lobectomy and 6% for pneumonectomy.
| Tumour | |
| T1a | < 2cm |
| T1b | 2–3 cm |
| T2a | 3–5 cm |
| T2b | 5–7 cm or within 2 cm of main bronchus or partial lung collapse |
| T3 | > 7 cm, chest wall involvement, phrenic nerve involvement, whole lung collapse or > 1 tumour nodule in same lobe |
| T4 | direct involvement of mediastinum or tumour nodules > 1 lobe |
| Nodes | |
| N0 | No nodes |
| N1 | Local node involvement |
| N2 | Ipsilateral mediastinal nodes or sub carinal |
| N3 | Contralateral mediastinal nodes or supraclavicular nodes |
| Metastases | |
| M0 | No evidence of spread |
| M1a | Tumours in both lungs, malignant pericardial or pleural effusion |
| M1b | Distant metastases e.g. bone, adrenal, brain |
| Staging of lung cancer | |
| Stage 1A | T1a or T1b N0 M0 |
| 1B | T2a N0 M0 |
| Stage 2A | Any T1 or T2 N1, T2b N0 M0 |
| 2B | T2b N1 M0 or T3 N0 M0 |
| Stage 3A | Any T1 or T2 N2 M0, Any T3 N1 or N2, T4 N0 M0, T4 N1 M0 |
| 3B | Any T N3 M0 or T4 N2 M0 or T4 N3 M0 |
| Stage 4 | Any T or N with M1a or M1b |
Metastatic disease
Pulmonary metastases (Fig. 22.20) are the most common form of intrathoracic malignancy. A confirmatory diagnostic lung biopsy may be helpful for patients with no evident primary. A palliative pleurodesis in patients with associated pleural effusion can be achieved by instilling an irritant such as aluminium silicate powder (kaolin) into the pleural cavity.
Mediastinum
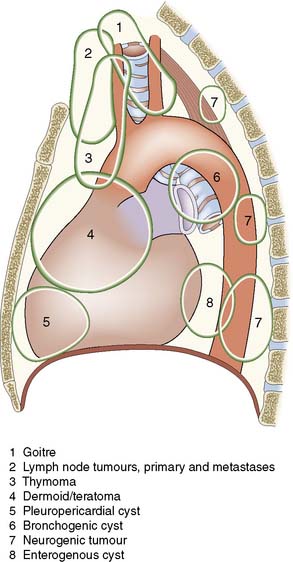
Mass lesions
Benign and malignant masses may arise in the mediastinum. Some clue to the likely diagnosis is provided by the location of the lesion (Fig. 22.21) within the mediastinum. Where the diagnosis is in doubt, tissue may be obtained by CT-guided needle biopsy. If this is either not feasible or is unsuccessful, a surgical biopsy can be obtained using mediastinotomy, mediastinoscopy or videothoracoscopy. The clinical features vary considerably, with some quite large masses being asymptomatic and identified on routine chest films. Non-specific symptoms include vague chest pain, cough, weight loss, fever and general malaise. Other lesions may cause direct pressure effects, such as tracheal compression by a retrosternal thyroid goitre or oesophageal compression by malignant lymphadenopathy. Thymomas may be identified during the evaluation of patients with myasthenia gravis and resection of these may improve their neurological symptoms.
Pneumothorax
However, the most common cause of pneumothorax is leakage of air from the lung, due either to a traumatic puncture wound or to spontaneous leakage from a large (bulla) or small (< 1 cm, ‘bleb’) air sac on the lung surface. Occasionally, the pulmonary leak point may have a flap valve mechanism that allows air out of but not back into the lung, causing a rapid build-up of pressure within the pleural cavity (tension pneumothorax – Fig. 22.23). This can be fatal, as the high intrapleural pressure completely flattens the ipsilateral lung while deviating the mediastinum to the opposite side, impeding venous return.
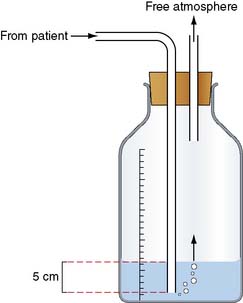
Management
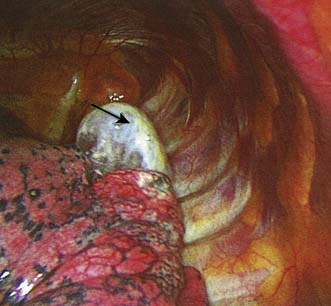
Initial management may involve aspiration or the insertion of a chest drain connected to an underwater seal into the pleural space (Fig. 22.24). This allows the lung to re-expand. In most cases of primary pneumothorax, air leakage stops within 48 hours or so, after which the drain can be removed. If the pneumothorax recurs or the air leakage does not stop, thoracoscopic surgery is indicated. The lung is inspected and any blebs or bullae are stapled. These are usually found at the apices of the upper or lower lobes (Fig. 22.22). Pleurodesis is then performed either by using an abrasion technique to scarify the parietal pleura, or a pleural strip (pleurectomy), or by insufflation of kaolin. Bullectomy and abrasion or pleurectomy carry about an 8% risk of further recurrent pneumothorax. This is reduced to 1–2% with kaolin insufflation, but as this technique involves leaving foreign material in the chest of a young person, it is usually kept in reserve for recurrent pneumothorax or for patients with no obvious culprit bulla or bleb.
Pleuropulmonary infection
Empyema
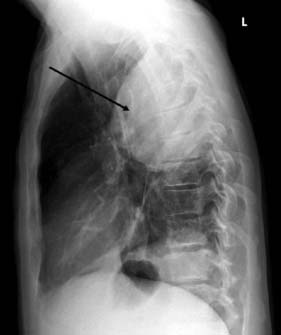
This is a collection of pus within the pleural cavity. It commonly follows pneumonia due to secondary infection of a reactive parapneumonic effusion. In the initial phase, the infected fluid is thin and may be completely evacuated by a low intercostal drain. The empyema quickly becomes thick and loculated as a result of the deposition of fibrin, and at this stage formal surgical drainage is required. The collection is typically placed posteriorly towards the base of the pleural cavity and causes a D-shaped shadow on the chest film (Fig. 22.25). Drainage in this phase may be achieved by videothoracoscopic techniques or by excising a 2 cm segment of rib over the lowest part of the empyema and suctioning and curetting the cavity clean. As dense fibrosis surrounds an empyema, drainage creates a fixed cavity. In elderly or unfit patients, a simple open tube drain is left in situ for many months, during which the cavity gradually shrinks and finally obliterates. In younger patients, open formal thoracotomy with decortication allows the fibrous cavity to be excised and any cortex over the lung removed. This returns more lung function to the patient and avoids prolonged open drainage, so that recovery is more rapid.