Chapter 22 Cardiopulmonary Bypass and the Anesthesiologist
GOALS AND MECHANICS OF CARDIOPULMONARY BYPASS
Venous return may be decreased deliberately (as is done when restoring the patient’s blood volume before coming off bypass) by application of a venous clamp. From the reservoir, blood is pumped to an oxygenator and heat exchanger unit before passing through an arterial filter and returning to the patient. Additional components of the circuit generally include pumps and tubing for cardiotomy suction, venting, and cardioplegia delivery and recirculation, as well as in-line blood gas monitors, bubble detectors, pressure monitors, and blood sampling ports. A schematic representation of a typical bypass circuit is depicted in Figure 22-1.
PHYSIOLOGIC PARAMETERS OF CARDIOPULMONARY BYPASS
The primary objective of CPB is maintenance of systemic perfusion and respiration. Controversy arises with the question of whether systemic oxygenation and perfusion should be “optimal or maximal.” Remarkably, after more than one-half century of CPB, there is continued disagreement regarding the fundamental management of extracorporeal circulation. Clinicians and investigators disagree on what are the best strategies for arterial blood pressure goals, pump flow, hematocrit, temperature, blood gas management, or mode of perfusion (pulsatile vs. nonpulsatile) (Box 22-1). Additional considerations of what is best relate to other goals of CPB: maintenance of homeostasis, facilitation of surgery, and avoidance of complications.1
Perfusion Pressure during Cardiopulmonary Bypass
Between mean arterial pressures (MAP) of 50 and 150 mmHg, cerebral autoregulation maintains a relatively constant blood flow and oxygen delivery. During hypothermic CPB, the lower limit of cerebral autoregulation may be as low as 20 to 30 mmHg,2 affording some additional protection against hypoperfusion. Increasing perfusion pressure to alleviate the risk of hypoperfusion may lead to greater embolic load and worse outcomes. Ultimately, the selection of perfusion pressure during CPB will need to be based on clinical outcome studies.
Pump Flow during Bypass
Most perfusion teams also monitor mixed venous saturation, targeting levels of 70% or greater. Unfortunately, this level does not guarantee adequate perfusion of all tissue beds, because some (muscle, subcutaneous fat) may be functionally removed from circulationduring CPB. Hypothermic venous saturation may overestimate end-organreserves. Regionalperfusion of various end-organs (brain, kidney, small intestine, pancreas, and muscle) has been quantified with a fluorescent microsphere technique.3 Cerebral blood flow was unchanged at higher pump flows. Renal perfusion was maintained at flows of 1.9 and 1.6 L/min/m2. Perfusion to the pancreas was constant at all flows, and small bowel perfusion varied linearly with pump flow. Muscle bed flows were decreased at all flows.
Bypass Temperature Management Strategy
Effects on Central Nervous System
Several groups of investigators have assessed the effect of normothermic temperatures during bypass on perioperative central nervous system events in cardiac surgery patients.4 Mild hypothermia provides some magnitude of cerebral protection during CPB, whereas mildly hyperthermic temperatures (>37°C) exacerbate and amplify the ischemic injury associated with CPB.
Temperature Monitoring
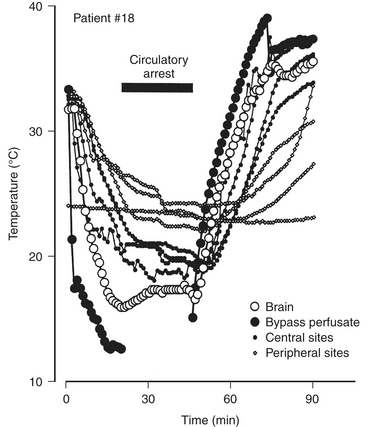
Because the brain is vulnerable to hyperthermic temperatures, it is important to use the temperature-monitoring site most likely to reflect cerebral temperature. The most commonly used sites in cardiac surgery patients include esophageal, nasopharyngeal, tympanic, pulmonary arterial, rectal, urinary bladder, subcutaneous (or muscle), and cutaneous sites. Unfortunately, none of these monitoring locations has been demonstrated to reflect cerebral temperature reliably. With exposure of the brain, investigators have placed a thermocouple directly in the cerebral cortex. Brain temperature was compared with values obtained from sensors in eight locations.5 Investigators found a poor concordance between cerebral temperature and values obtained at the other monitoring sites. Locations hypothesized to best reflect core temperature—tympanic membrane, esophagus, nasopharynx, pulmonary artery—sometimes overestimated cerebral temperature or underestimated brain temperature. Because of the substantial variability noted in central temperature readings (Fig. 22-2) and lack of the concordance of central temperature measures in every patient, the investigators recommended the use of at least three measures of central or core temperature.
Acid-Base Strategy
The pH-stat approach to acid-base balance maintains a pH of 7.40 and PCO2 of 40 mmHg when corrected for body temperature, typically requiring the addition of CO2 during hypothermic CPB. This method of blood gas management was generally favored until the mid 1980s because it was believed that the potent vasodilatory effects of CO2 would provide increased cerebral blood flow and thereby minimize the risk of cerebral ischemia during CPB. It is now recognized that pH-stat management during hypothermia produces passive cerebral vasodilation, impairs autoregulatory responses to blood pressure changes and metabolic demands in the brain, and does not improve overall oxygen balance. In contrast, α-stat management preserves autoregulation and the relationship between cerebral blood flow and metabolism. Neither blood gas strategy has any significant effect on hypothermic cerebral metabolism. The increased CBF seen with pH-stat may also increase the risk of cerebral embolization or produce a steal phenomenon.6
Fluid Management
Several studies have investigated the differences between colloid and crystalloid priming solutions. In general, crystalloid solutions lead to decreased colloid osmotic pressure with a resultant increase in extracellular water retention, irrespective of the osmolarity of the pump prime. Albumin, unlike a pure crystalloid prime, can decrease the interaction of blood components with the bypass circuit by coating the fluid pathway surfaces. In their meta-analysis of 21 controlled trials enrolling 1346 patients, Russell and associates showed a notably smaller drop in on-bypass platelet counts in patients treated with albumin in the pump prime.7
END-ORGAN EFFECTS OF CARDIOPULMONARY BYPASS
Myocardial Injury
Most coronary revascularization procedures are completed with the assistance of CPB. Although the completion of coronary anastomoses is facilitated by CPB (i.e., the surgeon can operate on a quiet, nonbeating heart), the heart is subjected to a series of events leading to ischemic myocardium during extracorporeal circulation. The operation, which is designed to preserve and improve myocardial function, is sometimes associated with myocardial damage (Box 22-2). The extent and incidence of this injury are dependent on the sensitivity and specificity of the diagnostic methods being used. However, most patients who undergo cardiac operations sustain some degree of myocardial injury. Although patients with normal ventricular function may tolerate these minor amounts of injury without detectable sequelae, those with impaired ventricular function preoperatively may not be able to tolerate the slightest injury. As the patient population for CPB continues to become older and have greater degrees of concomitant illness, understanding the physiology of and developing effective preventive strategies for myocardial injury during CPB are increasingly important. Because myocardial damage influences early and long-term results, the identification and control of factors associated with myocardial injury are critical to ensuring good outcomes. Although injury may be linked to anesthetic and surgical management, myocardial injury usually is thought to occur from inadequate myocardial protection during CPB.
Mechanisms
Certain specific events during CPB are associated with myocardial ischemia and injury (Table 22-1). These events lead to ischemia by increasing oxygen demands, decreasing oxygen supply, or a combination of both. When these factors are present together they potentiate myocardial damage. For example, the distended, fibrillating ventricle with a low perfusion pressure is particularly susceptible to damage.
Table 22-1 Factors Associated with Myocardial Injury during Cardiopulmonary Bypass
| Abnormal perfusate composition |
| Persistent ventricular fibrillation |
| Inadequate myocardial perfusion |
| Ventricular distention |
| Ventricular collapse |
| Coronary embolism |
| Catecholamines |
| Aortic cross-clamping |
| Reperfusion |
Myocardial Protection
Myocardial protection strategies can be summarized with four basic concepts:
Brain Injury
The brain is highly susceptible to injury during CPB. Many clinicians believe that cerebral injuries after cardiac surgery are the most devastating adverse outcomes associated with CPB. A study of 2400 patients undergoing elective coronary artery bypass grafting (CABG) from 24 U.S. centers reported that 6.1% of patients suffer adverse postoperative gross neurologic or psychiatric central nervous system events. These patients remain in the intensive care unit and hospital for greater periods of time, and 1 in every 3 surviving patients does not return home but requires continued long-term care and rehabilitation.8 (see chapter 23).
Renal Dysfunction
The effects of CPB on the renal system have significant health and economic impacts; however, despite intensive investigation into the pathogenesis and prevention of renal failure, there remains limited progress in the development of effective protective strategies in recent decades.9 Because intravascular volume depletion and hypoperfusion can lead to exacerbation of renal ischemia and accentuate the risk for postoperative acute renal failure, avoidance of nephrotoxic agents and close attention to intravascular volume, blood pressure, and cardiac output (CO) are central in the effort to reduce the occurrence of acute renal failure after cardiac surgery.
Gastrointestinal Effects
The effects of CPB on the gastrointestinal system are complex and not fully elucidated. Although most patients undergoing cardiac surgery do not suffer adverse changes in gastrointestinal function, subclinical perturbations including transient elevations in hepatocellular enzymes and hyperamylasemia have been observed after CPB. Although the incidence of gastrointestinal complications after CPB is low (range, 0.3% to 3.7%), they are associated with significant morbidity and remarkably high mortality (range, 11% to 67%) compared with cardiac surgery patients without postoperative gastrointestinal compromise. The frequently reported adverse gastrointestinal outcomes include gastroesophagitis, upper and lower gastrointestinal hemorrhage, hyperbilirubinemia, hepatic and splenic ischemia, colitis, pancreatitis, cholecystitis, diverticulitis, mesenteric ischemia, as well as intestinal obstruction, infarction, and perforation.10
Endocrine and Inflammatory Responses
Endocrine Response
Many other hormones increase during CPB. Vasopressin increases up to 20 times baseline levels during CPB. Some investigators suggest that vasopressin and angiotensin II are responsible for the elevations in SVR observed during CPB, and this may explain why vasopressin can be a useful drug to increase SVR on CPB in patients who are extremely vasodilated.11
Data from clinical and laboratory investigators now conclusively demonstrate the untoward affects of hyperglycemia in cardiac surgery. Aggressive control of perioperative glucose values represents the standard of care in cardiac surgery. Glucose values should be maintained at levels less than 200 mg/dL. Lower values (blood glucose 80 to 120 mg/dL) are likely superior to values of glucose more than 120 mg/dL. A protocol for management of glucose in cardiac surgery patients is outlined in Table 22-2.12
Table 22-2 Stanford University Adult Cardiac Anesthesia Continuous Insulin Infusion Protocol
| Bolus and start infusion as follows: | ||
|---|---|---|
| Blood Glucose (mg/dL) | Insulin Bolus Units | Units/hr |
| <125 | 0 | 0 |
| 125 to 175 | 5 | 1 |
| 175 to 225 | 10 | 2 |
| >225 | 15 | 3 |
| Measure blood glucose values every 30 minutes intraoperatively! | ||
| Insulin Titration | ||
| Blood Glucose | Treatment | |
| <75 | Stop insulin; D5W and recheck in 30 min; when > 150, restart at 50% of previous rate. | |
| 75-100 | Stop insulin; recheck in 30 min; restart at 50% of previous rate (unless < 0.25 U/hr). | |
| 101-125 | ||
Immunologic Inflammatory Response
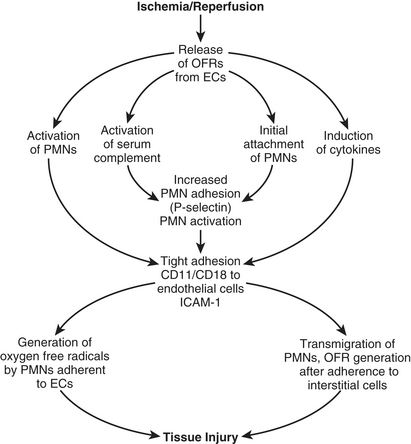
With initiation of CPB, complement is thought to be activated through the alternative pathway. Exposure of blood to the CPB circuit initiates activation of the coagulation cascade by means of the Hageman factor (XII). Although the coagulation cascade is initiated, it is not completed because of the presence of heparin. Activated factor XII (XIIa) results in plasmin generation. Plasmin activates complement by C1 (classical pathway), cleaves C3 (alternative pathway), and cleaves factor XII, which activates the kallikrein-kinin systems. Complement activation can also occur through the classical pathway by heparin-protamine complexes. Complement activation leads to direct membrane injury by complement subunits, neutrophil activation, enhancement of phagocytosis due to interaction of complement components and phagocytic cells, and release of lysosomal enzymes. The complement fragments are stimulants that induce changes in neutrophil behavior, causing neutrophil activation and migration and the promotion of adhesive and secretory events. These phenomena contribute to the reperfusion injury observed in cardiac surgery patients. Activated neutrophils attach to the endothelium, causing translocation of P-selectin from intracellular vesicles to the cell membrane and platelet-activating factor (PAF) synthesis. Endothelial membrane-bound PAF leads to increased neutrophil adhesion and activation and neutrophil adhesion protein (CD11/CD18) expression. Ultimately, increases in adhesion molecule emission in the endothelium and other tissue cell types (e.g., myocytes, alveolar cells, glomerular or renal tubular epithelium) result in transmigration of neutrophils into the interstitial space and release of large amounts of free radicals. These phenomena are schematically presented in Figure 22-3.
MANAGEMENT BEFORE BYPASS
An important objective of this phase is to prepare the patient for CPB (Box 22-3).13 This phase invariably involves two key steps: anticoagulation and vascular cannulation. Heparin is still the anticoagulant clinically used for CPB. Dose, method of administration, and opinions as to what constitutes adequate anticoagulation vary. Heparin must be administered before cannulation for CPB, even if cannulation must be done emergently. Failure to do so is to risk thrombosis in the patient and extracorporeal circuit. After heparin has been administered, a period of 3 to 5 minutes is customarily allowed for systemic circulation and onset of effect. Various confirmatory tests of actual achievement of anticoagulation are performed if time permits.
Vascular Cannulation
Arterial Cannulation
Reviews and clinical reports emphasize the importance of embolization as the major mechanism of focal cerebral injury in cardiac surgery patients.14 Intraoperative use of two-dimensional ultrasound to image the ascending aorta as a guide to selection of cross-clamping and cannulation sites is increasing. A femoral artery, rather than the ascending aorta, can be cannulated for systemic perfusion. Femoral cannulation is used when ascending aortic cannulation is considered relatively contraindicated, as in severe aortic atherosclerosis, aortic aneurysm or dissection, or known cystic medical necrosis. The anesthesiologist should seek evidence of cannula malposition by looking for unilateral blanching of the face, gently palpating carotid pulses, and checking for new unilateral diminution and by measuring blood pressure in both arms and checking for new asymmetries.
Venous Cannulation
Venous cannulation can be achieved using a single atrial cannula that is inserted into the right atrium and directed inferiorly (Fig. 22-4). Drainage holes are located in the inferior vena cava (IVC) and right atrium to drain blood returning from the lower extremities and the superior vena cava (SVC) and coronary sinus, respectively. This technique has the advantage of being simpler, faster and requiring only one incision; however, the quality of drainage can be easily compromised when the heart is lifted for surgical exposure. The bicaval cannulation technique, required in cases in which right atrial (RA) access is needed, involves cannulating the SVC and IVC (Fig. 22-5). Loops placed around the vessels can be tightened to divert all caval blood flow away from the heart. Blood returning to the right atrium from the coronary sinus will not be drained using this technique, so an additional vent or atriotomy is necessary.
Other Preparations
Before initiating CPB, the anesthesiologist should assess the depth of anesthesia and muscle relaxation. It is important to maintain paralysis to prevent patient movement that could result in dislodgment of bypass-circuit cannulae and prevent shivering as hypothermia is induced (with the attendant increases in oxygen consumption). It is difficult to determine the depth of anesthesia during the various stages of CPB. Because blood pressure, heart rate, pupil diameter, and the autonomic nervous system are profoundly affected by extracorporeal circulation (e.g., the heart is asystolic; blood pressure is greatly influenced by circuit blood flow; sweating occurs with rewarming), these variables do not reliably reflect the anesthetic state. Although hypothermia decreases anesthetic requirements, it is necessary to provide analgesia, unconsciousness, and muscle relaxation during CPB. With the initiation of bypass and hemodilution, blood levels of anesthetics and muscle relaxants will acutely decrease. However, plasma protein concentrations also decrease, which increases the free-fraction and active drug concentrations. Every drug has a specific kinetic profile during CPB, and kinetics and pharmacodynamics during CPB will vary greatly among patients. Many clinicians administer additional muscle relaxants and opioids at the initiation of CPB. A vaporizer for potent inhalation drugs may be included in the bypass circuit. A final inspection of the head and neck for color, symmetry, adequacy of venous drainage (neck vein and conjunctiva engorgement), and pupil equality is reasonable to serve as a baseline for the anesthetic state. A summary of preparatory steps to be accomplished during the pre-bypass phase is given in Table 22-3.
INITIATION AND DISCONTINUATION OF BYPASS SUPPORT: AN OVERVIEW
Initiation of Cardiopulmonary Bypass
Uncomplicated Initiation
Once all preparatory steps have been taken, the perfusionist progressively increases delivery of oxygenated blood to the patient’s arterial system, as systemic venous blood is diverted from the patient’s right side of the heart, maintaining the pump’s venousreservoir volume. After full flow is achieved, all systemic venous blood is (ideally) draining from the patient to the pump reservoir. The central venous pressure (CVP) and pulmonary arterial pressure (PAP) should decrease to near zero (2 to 5 mmHg), whereas systemic flow, arterial pressure, and oxygenation are maintained at desired values.Table 22-4 outlines tasks to be completed within 5 minutes of initiating CPB.
CVP = central venous pressure; PA = pulmonary artery; SVC = superior vena cava.
Preparation for Separation
Before discontinuation of CPB, conditions that optimize cardiac and pulmonary function must be restored. To a great extent this is achieved by reversing the processes and techniques used to initiate and maintain CPB (Table 22-5).
Hct = hematocrit; TEE = transesophageal echocardiography.
Potential for Patient Awareness
Patient movement before discontinuation of CPB is extremely disruptive and may be genuinely life-threatening if it results in cannula dislodgment or disruption of the procedure. Additional muscle relaxant should be administered. If awareness is suspected, supplemental amnestics or anesthetics should be administered during rewarming. Because sweating stops almost immediately on discontinuation of bypass, continued sweating after emergence from CPB may be a sign of awareness. Neurologic monitors such as the Bispectral Index are being used by some clinicians to help judge the depth of anesthesia during and after weaning from CPB.15
Correction of Metabolic Abnormalities and Arterial Oxygen Saturation
PERFUSION EMERGENCIES
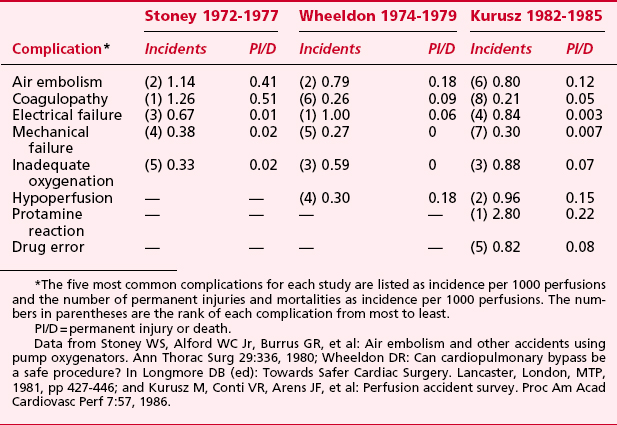
Accidents or mishaps occurring during CPB can quickly evolve into life-threatening emergencies (Box 22-4). Many of the necessary conditions of bypass (cardiac arrest, hypothermia, volume depletion) preclude the ability to resume normal cardiorespiratory function if an accident threatens the integrity of the extracorporeal circuit. Fortunately, major perfusion accidents occur infrequently and are rarely associated with permanent injury or death (Table 22-6). However, all members of the cardiac surgery team must be able to respond to perfusion emergencies to limit the likelihood of perfusion-related disasters.
Aortic or Arterial Dissection
After a dissection of the ascending aorta is diagnosed, immediate steps to minimize propagation must be taken. If it has occurred before CPB, the anesthesiologist should take steps to reduce MAP and the rate of rise of aortic pressure (dP/dt). If it occurs during CPB, pump flow and MAP are reduced to the lowest acceptable levels. Arterial perfusate is frequently cooled to profound levels (14° to 19°C) as rapidly as possible to decrease metabolic demand and protect vital organs. A different arterial cannulation site is prepared (e.g., the femoral artery is cannulated or the true aortic lumen is cannulated at a site more distal on the aortic arch). Arterial inflow is shifted to that new site in hopes that perfusing the true aortic lumen will reperfuse vital organs. The ascending aorta is cross-clamped just below the innominate artery, and cardioplegia is administered (into the coronary ostia or coronary sinus). The aorta is opened to expose the site of disruption, which is then resected and replaced. Reimplantation of the coronary arteries or aortic valve replacement, or both, may be necessary. The false lumina at both ends of the aorta are obliterated with Teflon buttresses, and the graft is inserted by end-to-end suture. With small dissections it is sometimes possible to avoid open repair by application of a partial occlusion clamp with plication of the dissection and exclusion of the intimal disruption. In some cases of arterial dissection during CPB, TEE has been found useful. Although provisional diagnoses were made on the basis of traditional signs, TEE allowed assessment of the origin and extent of dissection.16 Diagnosis of arterial dissection has also been assisted by presence of EEG asymmetry.
Massive Arterial Gas Embolus
In 1980, Mills and Ochsner17 suggested venoarterial perfusion as an alternative to hyperbaric therapy. Retrograde perfusion through the SVC cannula at 1.2 L/min at 20°C for 1 to 2 minutes was used in five of their eight patients with massive gas embolism. The goal was to flush air from the cerebral arterial circulation. None of the patients so treated had evidence of neurologic injury. Other reports using this technique have followed.
SPECIAL PATIENT POPULATIONS
Care of the Gravid Patient during Bypass
Studies assessing the effects of cardiac surgery and CPB on obstetric physiology and fetal well-being are lacking. However, several reviews and many case reports describe individual experience in caring for the gravid patient and fetus during cardiac surgery and extracorporeal circulation. These surveys and anecdotal reports, along with an understanding of the well-documented physiology of pregnancy and the effects of cardiac therapeutics on fetal physiology, can serve as a basis for a rational approach to care for the pregnant patient and fetus during cardiac surgery.18
Conducting the Bypass Procedure
The conditions of extracorporeal circulation—nonpulsatile blood flow, hypothermia, anemia, and requisite anticoagulation—will likely have a negative impact on fetal well-being during CPB. There are no studies that recommend a CPB management strategy in gravid patients. Recommendations are summarized (Table 22-7) for the management of bypass in pregnant patients.
Table 22-7 Recommendations for the Conduct of Extracorporeal Circulation in the Gravid Patient
| Variable | Recommended Value/Characteristic | Rationale |
|---|---|---|
| Blood flow | 3.0 L/min/m2 | Cardiac index normally is increased during pregnancy. |
| Blood pressure (MAP) | 60-70 mmHg | Uterine blood flow depends on maternal MAP. |
| Temperature | 32-34°C | Mild hypothermia decreases fetal oxygen requirements and is less likely to cause fetal arrhythmia. |
| Oxygenator type | Membrane | Membrane oxygenators are associated with fewer embolic phenomena than bubblers. |
| Hematocrit | 25%-27% | The quantity of oxygen carried in maternal blood (and therefore the oxygen available to the fetus) greatly depends on hemoglobin concentration. |
| Duration of perfusion | Minimized (?) | The duration of bypass is dictated by the complexity of the operative procedure. |
| Cardioplegia | ? | |
| Pulsatile perfusion | ? |
MAP = mean arterial pressure.
SUMMARY
1. Edmunds L.H. Cardiopulmonary bypass after 50 years. N Engl J Med. 2004;351:1603.
2. Newman M.F., Croughwell N.D., White W.D., et al. Effect of perfusion pressure on cerebral blood flow during normothermic cardiopulmonary bypass. Circulation. 1996;94:II353.
3. Slater J.M., Orszulak T.A., Cook D.J. Distribution and hierarchy of regional blood flow during hypothermic cardiopulmonary bypass. Ann Thorac Surg. 2001;72:542.
4. Mora C.T., Henson M.B., Weintraub W.S., et al. The effect of temperature management during cardiopulmonary bypass on neurological and neuropsychological outcomes in coronary revascularization patients. J Thorac Cardiovasc Surg. 1996;112:514.
5. Stone J.G., Young W.L., Smith C.R., et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology. 1995;82:344.
6. Kiziltan H.T., Baltali M., Bilen A., et al. Comparison of alpha-stat and pH-stat cardiopulmonary bypass in relation to jugular venous oxygen saturation and cerebral glucose-oxygen utilization. Anesth Analg. 2003;96:644.
7. Russell J.A., Navickis R.J., Wilkes M.M. Albumin versus crystalloid for pump priming in cardiac surgery: Meta-analysis of controlled trials. J Cardiothorac Vasc Anesth. 2004;18:429.
8. Roach G.W., Kanchuger M., Mora Mangano C.T., et al. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med. 1996;335:1857.
9. Mora Mangano C.T., Diamondstone L.S., Ramsay J.G., et al. Renal dysfunction following myocardial revascularization: Risk factors, adverse outcomes and hospital resource utilization. Ann Intern Med. 1998;128:194.
10. Perugini R.A., Orr R.K., Porter D., et al. Gastrointestinal complications following cardiac surgery: An analysis of 1477 cardiac surgery patients. Arch Surg. 1997;132:352.
11. Holmes C., Landry D., Granton J. Vasopressin and the cardiovascular system. Crit Care. 2004;8:15.
12. Ahmed Z., Lockhart C.H., Weiner M., Klingensmith G. Advances in diabetic management: Implications for anesthesia. Anesth Analg. 2005;100:666.
13. Jones R.H. The year in cardiovascular surgery. J Am Coll Cardiol. 2005;45:1517.
14. Wareing T.H., Davila-Roman V.G., Barzilai B., et al. Management of the severely atherosclerotic ascending aorta during cardiac operations: A strategy for detection and treatment. J Thorac Cardiovasc Surg. 1992;103:453.
15. Liu E., Dhara S. Monitoring oxygenator expiratory isoflurane concentrations and the bispectral index to guide isoflurane requirements during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2005;19:485.
16. Troianos C.A., Savino J.S., Weiss R.L. Transesophageal echocardiographic diagnosis of aortic dissection during cardiac surgery. Anesthesiology. 1991;75:149.
17. Mills N.L., Ochsner J.L. Massive air embolism during cardiopulmonary bypass: Causes, prevention, and management. J Thorac Cardiovasc Surg. 1980;80:708.
18. Pomini F., Mercogliano D., Cavalletti C., et al. Cardiopulmonary bypass in pregnancy. Ann Thorac Surg. 1996;61:259.