Cardiac surgery
Introduction and cardiopulmonary bypass
In keeping with the move towards minimal access in other areas, coronary artery bypass grafting and various valvular procedures can now be performed ‘off-pump’ via small incisions on the beating heart without bypass. However, most cardiac surgery is still carried out on bypass, with a bloodless field created by clamping the ascending aorta just proximal to where the aortic cannula returns arterial blood from the bypass machine (see Fig. 44.1). Without coronary artery blood flow, myocardial ischaemic damage has to be mitigated by reducing metabolic demands by inducing diastolic arrest with a high-potassium cardioplegic solution (16 mmol/L KCl) (Table 44.1) and cooling (to between 4 and 12°C).
Table 44.1
Constituents of a typical infusate for cold cardioplegia (St Thomas’s solution)
| Constituent | Quantity |
| Sodium chloride | 110.0 mmol/L |
| Potassium chloride | 16.0 mmol/L |
| Magnesium chloride | 16.0 mmol/L |
| Calcium chloride | 1.2 mmol/L |
| Sodium bicarbonate | 10.0 mmol/L |
| Procaine | 16.0 mmol/L |
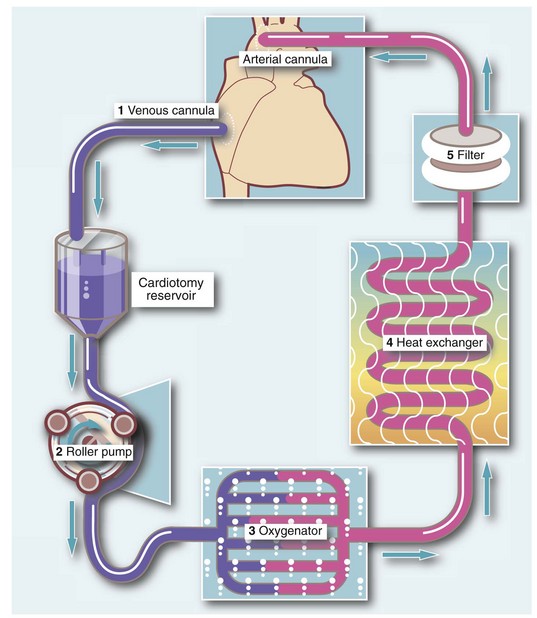
Fig. 44.1 The standard circuit for cardiopulmonary bypass
1. Systemic venous blood is siphoned into a reservoir by gravity from the right atrium (or from the superior and inferior vena cavae) via a venous cannula
2. Venous blood from the reservoir enters a roller pump
3. Blood is pumped under pressure through a membrane oxygenator
4. Oxygenated blood then passes through a heat exchanger to cool or rewarm the blood as necessary
5. The oxygenated and temperature-controlled blood finally passes through a filter and is returned to the systemic arterial circulation, usually via the ascending aorta by a roller pump
• Activation of the clotting cascade could occur, causing intravascular coagulation. This is prevented by anticoagulating with high-dose heparin (300 IU/kg) which is later reversed with protamine sulphate on coming off bypass
• Clotting factors and platelets are consumed in the extracorporeal circuit (mainly within the oxygenator) leading to a bleeding diathesis. Blood products may be needed to reverse this
• Activation of the complement cascade and other inflammatory mediators may result in systemic inflammatory response syndrome (SIRS) after bypass
Congenital cardiac disease
Types of congenital heart disease
Cyanotic heart disease
• Tetralogy of Fallot—the four features are ventricular septal defect (VSD), pulmonary stenosis, right ventricular hypertrophy and an aorta which overrides the ventricular septum, receiving blood from both ventricles
• Transposition of the great arteries—the pulmonary artery arises from the left ventricle and the aorta from the right ventricle
• Tricuspid atresia—absence of a functional tricuspid valve
• Truncus arteriosus—the pulmonary artery and aorta fail to develop separately
• Total anomalous pulmonary venous drainage—pulmonary venous blood drains into the right side of the heart
• Eisenmenger’s syndrome—increased pulmonary blood flow caused by a pre-existing left-to-right shunt (see next section) causes severe pulmonary hypertension later in life, which result in spontaneous reversal of the shunt so flow reverses to become right-to-left
Acyanotic heart disease
Acyanotic congenital heart disease may involve:
• A shunt from left to right sides of the heart (e.g. via an atrial or ventricular septal defect) or a ductus arteriosus which persists in its antenatal patent state (PDA)
• Failed or incomplete embryological development of parts of the heart or great vessels without shunting, e.g. coarctation of the aorta
Management of congenital heart disease
Palliating congenital cardiac disorders
When pulmonary blood flow is reduced (as in tricuspid atresia, tetralogy of Fallot or pulmonary artery stenosis), palliation aims to increase pulmonary flow by creating a shunt between the systemic arterial circulation and the pulmonary artery (see Fig. 44.2).
Acquired heart disease
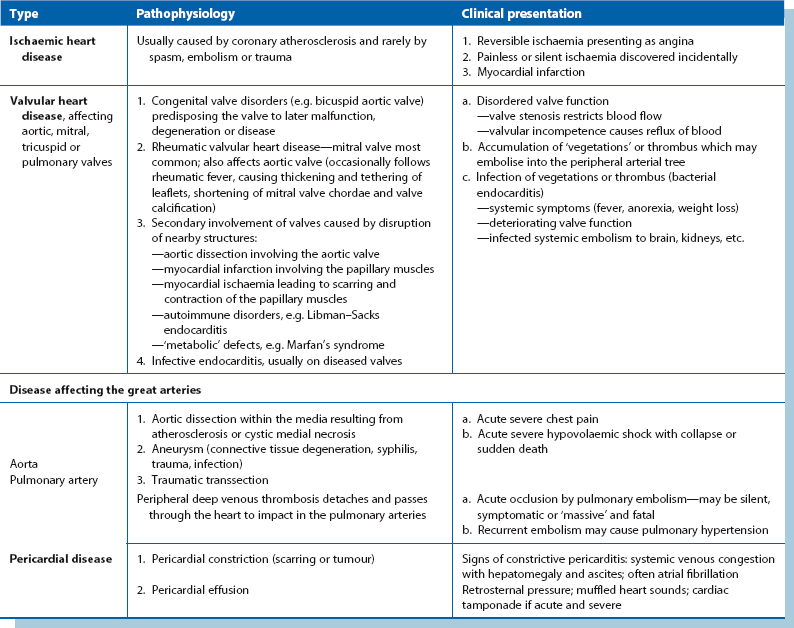
The types of acquired heart disease are listed in Table 44.2.
Coronary heart disease (see Table 44.3 for clinical presentations)
Risk factors for coronary atheroma (and atherosclerosis elsewhere) include unfavourable cholesterol and lipid profiles (often hereditary), cigarette smoking, diabetes, hypertension, obesity and a sedentary lifestyle (and perhaps a life of severe unrelieved stress). The presentations of coronary heart disease are outlined in Table 44.3.
Management of coronary artery disease
Coronary artery disease can be managed conservatively (‘medical management’) or by interventions employing percutaneous techniques or surgical bypass grafting. The anatomy of the coronary arteries is shown in Figure 44.3.
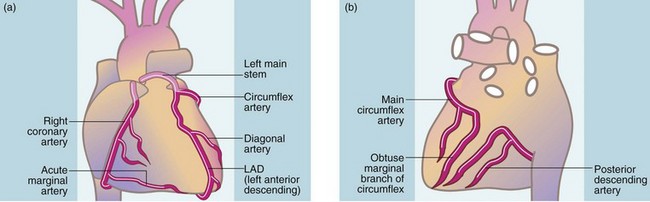
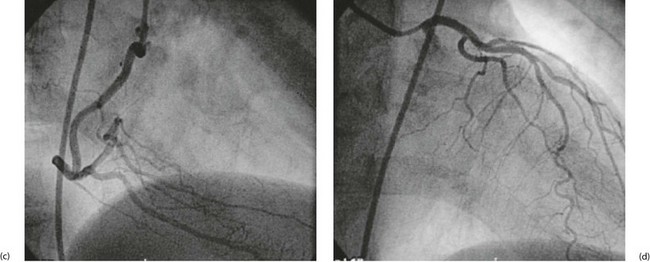
Fig. 44.3 Anatomy of the coronary arteries
(a) Anterior view of coronary arteries. (b) Posterior view of coronary arteries. (c–d) Selective coronary angiography (AP views as in (a)): (c) normal right coronary artery; (d) normal left coronary artery
Percutaneous angioplasty techniques: Percutaneous techniques to dilate stenoses or recanalise occluded coronary arteries have progressed since the early 1980s; these are usually performed by cardiologists. The mainstay of treatment is percutaneous transluminal coronary (balloon) angioplasty (PTCA) and insertion of intra-coronary artery stents. Stents are expensive, particularly drug-eluting stents (e.g. sirolimus or paclitaxel coated stents) aimed at minimising in-stent restenosis. Indications for the various stents still need to be clearly defined by randomised trials.
Coronary artery bypass grafting (CABG): Coronary artery bypass grafting is usually indicated in two categories of patient:
1. Those with symptomatic angina not relieved by medical therapy or who are intolerant of it. This is by far the largest group. Most are operated upon electively, but those with crescendo angina may need urgent or emergency surgery
2. Patients in categories believed to have a better prognosis after surgery than with medical therapy
Studies in the USA and Europe suggest improved survival after surgery is likely in patients with the following morphological characteristics:
• Stenosis of the left main stem coronary artery (before it bifurcates into anterior descending and circumflex arteries)
• Triple-vessel disease (i.e. disease of the right coronary, the left anterior descending and the circumflex arterial systems) plus impaired left ventricular contractility
• Two-vessel disease which includes a proximal stenosis in the left anterior descending coronary artery
The results of CABG are encouraging, with 85–90% relieved of angina without need for medication. A further 5% are substantially improved but require anti-anginal drug therapy.
Surgical technique (Fig. 44.4): The aim of CABG is to bypass occlusive disease and provide a new source of inflow for patent distal coronary arteries. Occlusive disease is usually in the proximal third of the epicardial coronary arteries. This fortunate morphology enables the distal end of bypass grafts to be anastomosed to patent recipient arteries beyond the main disease. Saphenous vein bypass grafts are employed as conduits from the ascending aorta or else a nearby left internal mammary artery is mobilised and anastomosed to the distal coronary artery.
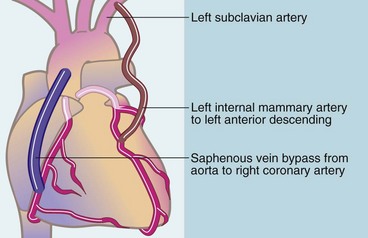
Fig. 44.4 Methods of coronary artery bypass grafting
Note that an explanted autogenous radial artery may be used as a bypass graft instead of saphenous vein
Prosthetic materials give poor results for CABG and are rarely used. Early conduits were exclusively reversed autologous long (great) saphenous vein. However, long-term patency is poor, with 50–70% occluding within 10 years of surgery. Long-term patency is better when the left internal mammary artery is grafted onto the left anterior descending coronary artery (see Fig. 44.4). This graft seems to be resistant to occlusion (90% patency at 10 years) and is the current choice for this site. Other arteries used for CABG including radial, right gastro-epiploic and inferior epigastric. A combination of left internal mammary grafting to left anterior descending artery and saphenous vein grafts to the other vessels remains the current surgical favourite.
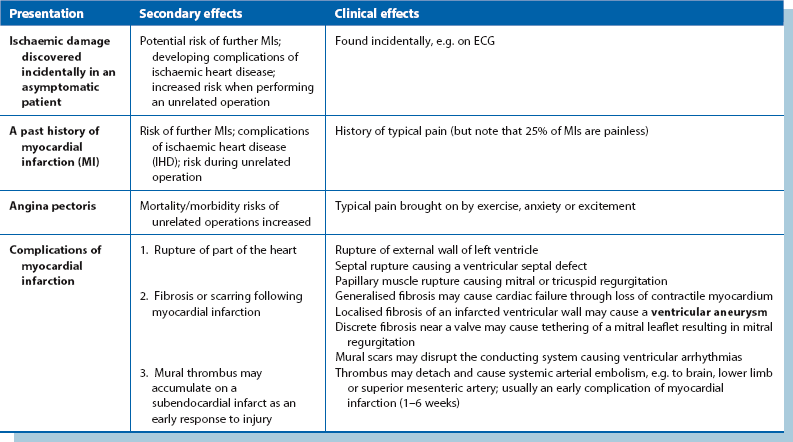
Other types of surgery for ischaemic heart disease: Other forms of surgery in addition to CABG may be required for complications of myocardial infarction. These carry a higher risk than isolated CABG surgery and include:
• Excision of a left ventricular aneurysm—mortality about 5%
• Replacement or repair of a mitral valve leaking as a result of ischaemia—mortality 5–8%
• Surgical identification and ablation of a ventricular arrhythmic focus—mortality 10–15%
• Emergency repair of a post-myocardial infarction ventricular septal rupture—mortality 20–40%
• Post-CABG heart failure necessitating ventricular support (intra-aortic balloon pump) or cardiac transplantation—mortality about 15% at 1 year
Valvular heart disease
Valve prostheses (Fig. 44.5)
The leaflets of prosthetic heart valves are constructed from artificial material or biological tissue. Various types of prosthetic valves are available (see Fig. 44.5). The mechanical demands on replacement heart valves are extreme. They must cause minimal restriction to blood flow when open, yet prevent reflux when closed; they must be biocompatible, non-thrombogenic, resistant to infection and, most demanding of all, capable of opening and closing 70 times a minute for many years without mechanical failure. Modern mechanical valves are durable but thrombogenic and generally require lifelong anticoagulation. Anticoagulation carries its own risk with a mortality of about 2% over 5 years. Even with effective anticoagulation, there is still a small risk of arterial embolism.

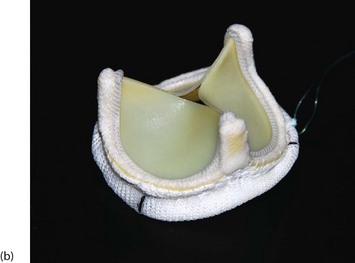
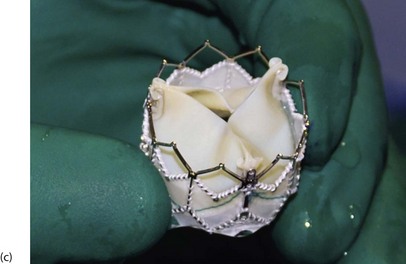
Fig. 44.5 Prosthetic valves
Three commonly used prosthetic heart valve are shown here. (a) A St. Jude bi-leaflet mechanical valve—used in 90% of patients requiring a mechanical prosthesis. Lifelong anticoagulation is mandatory for all patients receiving mechanical valve prosthesis. (b) An Edwards Magna Ease bovine pericardial stent mounted bioprosthesis. (c) An Edwards SAPIEN transcatheter bioprosthetic valve.
Disease of the thoracic aorta
Aortic dissection
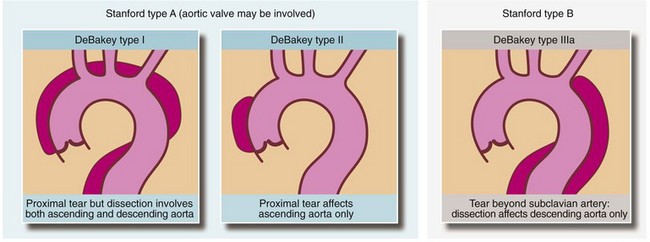
Dissection is classified according to the part affected; the most widely used method is the Stanford system. Type A indicates that only the ascending aorta is involved while type B is when any other part of the thoracic aorta is affected. An alternative classification method is that of DeBakey (see Fig. 44.6).
Thoracic aneurysms
Aneurysmal dilatation of the thoracic aorta may occur in the ascending part (Fig. 44.7), the arch or the descending part. There is a risk of rupture similar to abdominal aortic aneurysms and surgical intervention is usually recommended when the aneurysm reaches 6 cm diameter. Replacing the descending aorta, particularly when there is thoraco-abdominal disease, threatens the main blood supply of the spinal cord (the artery of Adamkiewicz at about T10) so that paraplegia complicates 10–30% of these operations. There is a growing trend towards endoluminal stent-grafting for appropriate thoracic aneurysms with the promise of lower morbidity and mortality.